Abstract
Understanding stroke-induced changes to the motor control of the more affected arm of people with stroke may lead to more effective rehabilitation interventions that improve function. Reaching movements of the more affected arm in persons with stroke are slow, segmented and indirect. Such changes may be related to a reduced capacity to transmit motor commands in the presence of neuromotor noise. In tasks requiring both speed and accuracy, transmission capacity can be characterized by the linear relationship between movement time and task difficulty (Fitts’ law). This study quantified Fitts’ slope and intercept coefficients in stroke during reaching tasks and their relationship to kinematic measures of path accuracy (directness), trajectory corrections (segmentation), and planning strategy (skewness). We compared Fitts’ slope and intercept and kinematics among the more and less affected arm of twenty persons with stroke and the non-dominant arm of ten healthy persons. Slope and intercept were significantly increased in the more affected arm of the group with stroke and related to clinical measurements of motor impairment and tone. For both the more and less affected arm of the group with stroke, increased slopes and intercepts were correlated to more indirect, segmented, and positively skewed movement. Our findings suggest that stroke results in greater neuromotor noise which has consequences on both motor execution and planning. Individuals with stroke demonstrate substantially more deviation from straight-line paths than controls, despite using more conservative strategies (i.e., leftward shift of velocity profile) and extensive feedback control (i.e., segmentation).
Keywords: Fitts, motor control, CVA, hemiparesis, spasticity, accuracy, speed-accuracy
INTRODUCTION
Reaching has been defined as the voluntary positioning of the hand at or near a desired location so that it may interact with the environment (Carr and Shepherd, 1999). The ability to reach is an essential component of activities of daily living such as feeding, dressing and grooming. Eighty-five percent of those with stroke experience impairment of the upper extremity (UE) in the acute phase and forty percent continue to have chronic UE impairments (Parker et al., 1986).
Three sequential stages (target localization, movement planning, and movement execution) underlie the production of goal-directed arm movement. van Beers and colleagues (2004) described 1) localization as the process by which “locations of the target and hand are derived from sensory information”, 2) planning as “the selection of motor commands that can produce the movement from the initial to the target position” and 3) execution as the process where “the planned motor commands are sent to the muscles so the movement is actually made”. In healthy individuals, reaching tasks that require both speed and accuracy are classically characterized by a bell-shaped velocity profile with two phases: (1) an initial adjustment that rapidly covers distance and (2) a slower homing-in phase (e.g., Todor and Cisneros, 1985). As accuracy demands increase, the profile skews to the left (i.e., peak velocity occurs earlier in movement) and the duration of the deceleration phase is extended which indicates an increase in feedback control (Todor and Cisneros, 1985). Fitts’ law (Fitts, 1954) describes the tradeoff between speed and accuracy of aiming, pointing, and grasping movements as a linear relationship between movement time and the log of the ratio between the distance to the target and the width of the target (Equation 1):
| Equation 1 |
Here MT is the duration of the movement, A is its amplitude (i.e., distance), and W is the width of the target in the direction of movement; a (intercept) and b (slope) are experimentally determined from regression. The logarithmic term in equation 1 represents the accuracy requirement of the task and is often called the Index of difficulty (ID).
Fitts’ theory (1954) proposes that the neuromotor system behaves like a stochastic communication channel with a transmission capacity limited by a signal dependent noise. Recently, Harris and Wolpert (1998) demonstrated that Fitts’ law is consistent with “minimum variance” movement planning, a scheme by which movements (and their speeds) are planned according to their anticipated variability (due to noise) and the required task accuracy. In reaching to a target, the maximum allowable noise is related to the target width (i.e., spatial tolerance) and the signal size is related to the movement speed. From cortical neurons to motor units of muscles, noise is an inherent property of all parts of the motor system (De Jong and Galen, 1997). Noise during the movement execution has been identified as the primary cause of movement variability in healthy individuals (van Beers et al., 2004).
Studies which have manipulated external noise (e.g., physical perturbations, force fields) have demonstrated that such noise results in reduced accuracy and altered trajectories (Kawato, 1999). Fitts’ law has only been examined in the less affected arm of individuals with a stroke with results suggesting an increase in movement time when compared to healthy individuals (Haaland and Harrington, 1989). In our study, we selected to study the more affected arm of people with stroke since it is this arm that interferes with function and understanding its motor control may lead to the development of effective rehabilitation strategies. We propose that the neuronal damage and the resulting impairments from stroke will result in greater internal noise (e.g., reduced transmission capacity) which can be quantified using Fitts’ law.
Assessment of speed and accuracy tradeoffs during a Fitts’ task may be useful in quantifying potential decreases in transmission capacity following a stroke but it does not, by itself, provide insight into potentially altered mechanisms underlying trajectory formation. Given that external noise has been shown to alter the trajectory formation in healthy individuals, this study also assessed the characteristics of the end-point path and trajectory (i.e., velocity profiles), in addition to the speed-accuracy relationship of reaching in people with stroke. Such an approach allows one to draw associations between transmission capacity and the kinematic aspects of movement.
In this study we assessed the effect of target size and distance on reaching performance across three arm conditions: the 1. more and 2. less affected arm in persons with stroke and the 3. non-dominant arm of otherwise healthy persons (control group). We hypothesized that transmission capacity would be reduced (i.e., increases in Fitts’ slope) and such an effect would relate to the degree of motor impairment and characteristic changes in the reaching trajectory.
METHODS
Subjects
Twenty older adults (Mean=60.9, SD=6.1, Range=49–72 years, 13 males and 7 females) were recruited from the community with the following inclusion criteria: 1) a minimum of one year post-stroke, 2) present with hemiparesis secondary to first cerebrovascular accident (CVA), 3) able to provide informed consent, 4) able to follow one and two step commands and 5) able to voluntarily flex/abduct their shoulder 45 degrees and extend their elbow 30 degrees. Subjects with stroke were excluded if they presented with hemispatial neglect as confirmed by the line bisection test (Schenkenberg et al., 1980). As our central purpose was to identify the effect of stroke on reaching performance, we selected to use the less active non-dominant arm of healthy subjects for control comparison. Ten right-handed healthy adults of similar age (Mean=61.0, SD=9.0, Range=51–77 years) and gender (6 males and 4 females) were recruited from the community. Musculoskeletal or neurological conditions (in addition to the CVA for the subjects with stroke) that would affect upper extremity function were exclusion criteria for all subjects. The characteristics of the subjects with stroke are described in Table 1. The study protocol was approved by local university and hospital ethics committees. The more affected arm in subjects with stroke was assessed for motor impairment by the upper extremity portion of the Fugl-Meyer scale (Fugl-Meyer et al., 1975) and for the resistance to passive movement (i.e., tone) by the Modified Ashworth Scale (MAS) (Bohannon and Smith, 1987).
Table 1.
Characteristics of subjects with stroke (n=20)
| Code | Sex/Age (yrs) | Age (yrs) | Time since stroke (yrs) | Fugl-Meyer Upper Extremity Score (/66) | MAS Score (/4) | Hand dominance prior to stroke | Lesion Side/Location/Type (Taken from chart review) |
|---|---|---|---|---|---|---|---|
| SR01 | M | 57 | 6 | 19 | 1 | R | Left/MCA/Hemorrhagic |
| SR02 | F | 64 | 4 | 64 | 1 | R | Left/Posterior Putamen/Hemorrhagic |
| SR03 | M | 67 | 5 | 59 | 0 | R | Right/Intracerebral/Hemorrhagic |
| SR04 | M | 66 | 4 | 64 | 1 | R | Left/Putamen/Hemorrhagic |
| SR05 | F | 60 | 3 | 26 | 1+ | R | Right/Internal Carotid Artery/Ischemic |
| SR08 | M | 59 | 5 | 18 | 3 | R | Left/periventricular/Ischemic |
| SR09 | F | 59 | 2 | 38 | 3 | R | Right/MCA/Ischemic |
| SR10 | M | 57 | 8 | 62 | 0 | L | Right/Internal Carotid Artery/Ischemic |
| SR11 | M | 59 | 1 | 25 | 1 | R | Left/Internal Capsule/Lacunar |
| SR12 | M | 58 | 5 | 36 | 1 | R | Left/Anterior Cerebral Artery/Ischemic |
| SR13 | M | 63 | 11 | 41 | 1 | L | Left/Carotid Artery/Ischemic |
| SR14 | F | 67 | 2 | 62 | 0 | R | Right/Subarachnoid/Hemorrhagic |
| SR15 | F | 69 | 2 | 34 | 1+ | R | Left/Internal Capsule/Ischemic |
| SR16 | M | 50 | 1 | 57 | 0 | R | Right/Cerebellum/Hemorrhagic |
| SR17 | M | 61 | 5 | 15 | 1 | R | Left/Basal Ganglia/Ischemic |
| SR18 | M | 72 | 4 | 18 | 4 | R | Right/Carotid/Ischemic |
| SR19 | M | 56 | 3 | 14 | 1+ | L | Left/Frontal corona radiata/Hemmorrhagic |
| SR20 | F | 57 | 7 | 55 | 1 | R | Left/Anterior cerebral artery/Ischemic |
| SR21 | F | 49 | 1 | 44 | 2 | R | Left/Basal Ganglia/Hemorrhagic |
| SR22 | M | 66 | 7 | 13 | 1 | R | Left/Internal Capsule/Ischemic |
|
| |||||||
| Mean±SD | 60.9±6.0 | 4.3±2.6 | 38.2±19.0 | 1.3±1.1 | Note: MCA=Middle Cerebral Artery | ||
Reaching Task
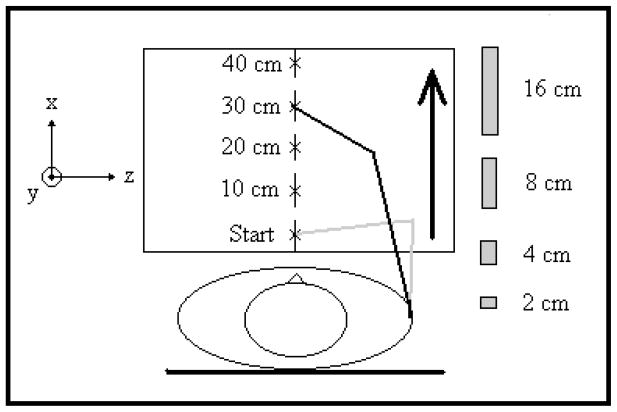
Subjects performed reaching movements while sitting in a chair (seat height = 44cm). The starting position and targets were located on the top of a table set to the height of the xyphoid (i.e., chest). We randomized combinations of target distance (10, 20, and 40 cm) and width (2, 4, 8, 16 cm). For those with a more limited range of motion (n=6), a 30 cm distance replaced the 40 cm distance. The start position of the subject’s pointer (index finger tip or index distal interphalangal joint if the subject was unable to extend the interphalangal joint) was standardized for subjects so that their elbow and shoulders were positioned in mid-range. Targets were placed along the mid-sagittal plane (Figure 1).
Figure 1.
Target Locations and Sizes. Targets were placed on top of a table at 10, 20, and 30 (or 40) cm distances from the start position. Reaching movements were to targets of widths 2,4,8, and 16 cm.
For subjects with stroke, their more affected arm was tested first to minimize potential fatigue over the session. The subjects were instructed: “At the sound of the tone, reach to the target as fast as you can. Make sure that you touch the target.” For each task (specific combination of distance and target width), the subject was given practice trials (minimum of three) until they found a maximal speed that could be used to consistently touch the target. At this time, three consecutive movements were recorded. Movements were recorded as hits if any part of the pointer touched the target. If a reaching movement missed the target, the entire acclimatization and recording process were restarted for the task, so that all captured reaching movements would be the result of equivalent movement strategies. This blocked design of each task was undertaken to reduce the variability during movement planning (van Beers et al., 2004).
Kinematic analysis
Pointer movements were recorded at 60 Hz with an infrared emitting diode (attached to the tip of the pointing finger) using a three-dimensional optoelectronic system (Northern Digital) and then low pass (zero phase) filtered at 10 Hz. Movement initiation and cessation were identified from velocity profiles. Movement initiation was identified by an algorithm which determined when the forward velocity rose ten standard deviations above the pre-movement mean velocity and then a backwards local minima search identified initiation. Movement was arrested when the forward velocity fell below zero and while the pointer was in contact with the target. These algorithms were verified by visual inspection of position and velocity profiles.
Across trials, the relative reliability [intra-class correlations, ICC(1,1); Shrout and Fleiss, 1979] and measurement agreement [standard error of measurement, SEM; Eliasziw et al., 1994] of movement time was high: control subjects (ICC=0.98, %SEM=10.5), less affected arm of stroke subjects (ICC=0.98, %SEM=11.6), and more affected arm of stroke subjects (ICC=0.98, %SEM=20.9). Linear regressions between movement time and task ID determined Fitts’ slope and intercept coefficients. Individual, not pooled data was used for the regression (e.g., regression of all the data for the more affected arm of subject SR01).
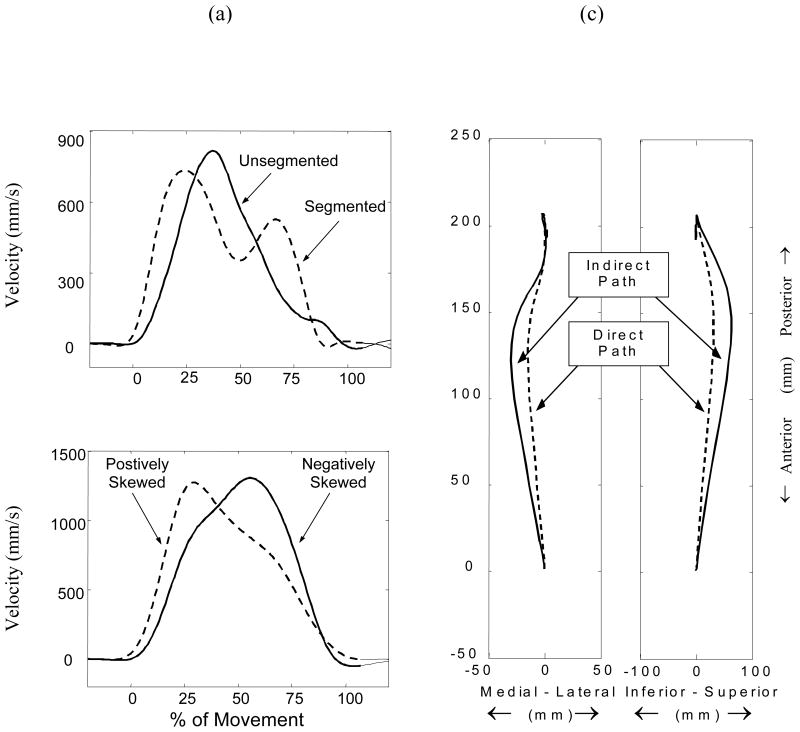
Kinematic descriptors of skewness, directness, and segmentation were gleaned from the path and velocity profiles of each trial. Deceleration times are extended to accommodate increasing accuracy requirements and result from a strategy developed during movement planning (e.g., Milner and Ijaz, 1990) and we used a statistical definition of skewness (Zar, 1999) to measure this shift in the velocity profile. Straight-line (i.e., direct) reference paths are adapted by motor plans in an attempt to minimize kinematic errors (Wolpert et al., 1995); we used directness, the ratio of the direct to the actual path (Bastian et al., 1996), to quantify the ability to execute the desired straight path. Segmentation was defined by the number of velocity peaks and troughs (i.e., minima and maxima) and was used to estimate the total number of movements (the initial movement plus subsequent corrective movements), to complete the movement task (e.g., Trombly, 1992). Possible variations of kinematic descriptors are shown in Figure 2.
Figure 2.
Kinematic descriptors of reaching movements. The velocity profile can have changes in segmentation (2a) and skewness (2b). The reaching path can have changes in its directness (2c). Note: all the descriptors of the kinematic profile are independent of distance and velocity.
Statistical analysis
Statistical analyses were performed on Fitts’ coefficients and the mean values (across trials) of kinematic variables. The effect of arm condition (more affected arm of stroke subjects, less affected arm of stroke subjects, and control subjects) on the slope and intercept coefficients was determined using ANOVAs followed by post-hoc Duncan’s multiple comparison tests. Relationships between Fitts’ coefficients and motor impairment (Fugl-Meyer) were evaluated by Pearson correlations. Tone (MAS) was non-normally distributed. Therefore, its relationships with Fitts’ coefficients were assessed using Spearman correlations.
The effect of arm condition on reaching kinematics (skewness, segmentation, and directness) was also evaluated using ANOVAs followed by post-hoc Duncan multiple comparison tests. Finally, within each arm condition, relationships between Fitts’ coefficients and kinematics were evaluated by Pearson correlations. A significance level of 0.05 was used for all statistical analyses.
RESULTS
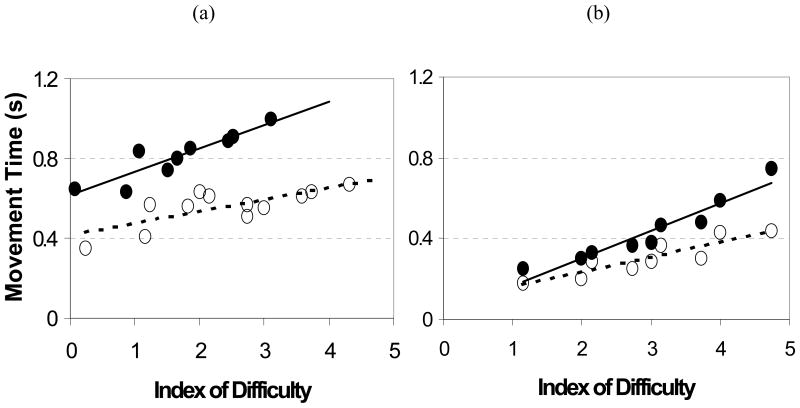
For the control subjects and the more and less affected arm of subjects with stroke, movement time increased linearly with task difficulty. The quality of regression fit was not reduced in the six cases that the 30 cm target distance substituted the 40 cm distance. There was a significant effect of arm condition on both the intercept, F(2,47)=5.67, p=0.002, and the slope, F(2,47)=3.37, p=0.026. The mean intercepts of the controls (0.198 seconds) were about one-half of the less affected arm (0.367 seconds) and one-fifth of the more affected arm (0.953 seconds) of subjects with stroke, respectively; the mean intercept of the more affected arm was significantly different than both the control and less affected conditions. The post-hoc Duncan test found the mean slope of the more affected arm (0.239 seconds/ID) to be significantly greater, four times, than the other two conditions (control arm =0.063 seconds/ID, less affected arm =0.059 seconds/ID). Regression lines are shown for the more and less affected arm of a typical severely impaired (low FM) (Figure 3a) and mildly impaired (high FM) (Figure 3b) subject.
Figure 3.
Movement Time versus Index of Difficulty for the less (empty circles and dashed line) and more (solid circles and line) affected arm of persons with stroke. (3a) Low Fugl-Meyer score (SR01 – FM=19). (3b) High Fugl-Meyer Score (SR20 – FM=55). Notice that as the impairment reduces, the slope and intercept of the more and less affected lines become more similar.
For the correlations between Fitts’ coefficients and clinical measures, significant correlations were identified between tone-slope (Spearman r=0.587, p=0.013) and motor impairment-intercept (Pearson r=−0.571, p=0.013) for the more affected arm of subjects with stroke. No other significant correlations were identified.
Table 2 shows the effects of arm condition on each of the kinematic descriptors. The shapes of the velocity profiles for the non-dominant arm of control subjects were consistent with other studies of healthy adults of speed/accuracy tasks in the literature (e.g. Smyrnis et al., 2000). Movements were characteristically direct, slightly negatively skewed, and unsegmented. Movements of the less affected arm of stroke subjects were similarly direct and segmented but were positively skewed. In addition to being less direct, movements of the more affected arm of stroke subjects were significantly more positively skewed and segmented.
Table 2.
Effect of Side on Kinematic descriptors
| Arm condition | Control (n=10) | Stroke (n=20) | |
|---|---|---|---|
| Less Affected | More Affected | ||
| Skewnessa | −2.679 | 5.377 | 50.870 |
| Directnessa | 0.832 | 0.867 | 0.751 |
| Segmentationa | 1.792 | 2.105 | 9.249 |
Post-hoc multiple comparison test significantly differentiated descriptor associated with the more affected arm from all other arm groups.
For the correlations between Fitts’ coefficients and kinematics, significant correlations were found between Fitts’ slope and directness in the more affected arm of subjects with stroke. In addition, significant correlations of the Fitts’ intercept and reaching kinematics (most notably, skewness) were found for the more and less affected arm of subjects with stroke, but not for the control group (see Table 3).
Table 3.
Correlations of Fitts’ Coefficeints with Kinematic Descriptors
| Arm Condition | Control (n=10) | Stroke (n=20) | |
|---|---|---|---|
| Less Affected | More Affected | ||
| Fitts’ Intercept | |||
| Skewness | −0.276 | 0.579* | 0.617** |
| Directness | −0.195 | 0.581* | −0.138 |
| Segmentation | 0.339 | 0.636** | 0.488* |
| Fitts’ Slope | |||
| Skewness | 0.373 | 0.457 | 0.051 |
| Directness | 0.202 | 0.204 | −0.491* |
| Segmentation | 0.034 | 0.450 | 0.109 |
correlation significant at p<0.05;
correlation significant at p<0.01
DISCUSSION
Increased neuromotor noise following stroke
Discrete reaching movements for both the more and less affected arm of individuals with stroke adhered to Fitts’ law in that movement time is modulated and increased with task difficulty and could be decomposed into accuracy dependent (slope) and independent (intercept) components. Fitts’ law interprets the slope as an indicator of the signal-dependent noise that limits neuromotor transmission capacity (Fitts, 1954). Transmission capacity (i.e., slope−1) decreases with age (e.g., Pohl et al., 1996) and is higher for fine motor movements (i.e., finger versus arm) (Langolf et al., 1976). Our finding of a 75% decrease of transmission capacity in the more affected arm is consistent with population vector coding models which have demonstrated that signals become noisier with the removal of neurons and have correctly predicted more variable movement following stroke (Reinkensmeyer et al., 2003). Population vector coding explains neural activity in several motor areas impaired by stroke, including the primary motor cortex (e.g., Georopolous et al., 1982), premotor cortex (Caminiti et al., 1991), area V of parietal cortex (Kalaska et al., 1983), and the cerebellum (Fortier et al., 1989).
In Fitts’ tasks, the intercept is the movement time at a theoretical zero Index of Difficulty and results from increased movement time which is an offset (i.e., bias) at all IDs. The 400% greater value of the intercept of the more affected arm of individuals with stroke was related to the severity of motor impairments. Impairments found in stroke such as abnormal motor unit recruitment and discharge rate (Gemperline et al., 1995; Rosenfalck and Andreassen, 1980), increased joint viscoelasticity (McCrea et al., 2003a), and longer times to develop/reduce torque (McCrea et al., 2003b) may contribute to the greater movement time across all IDs. Tone contributed to motor noise as reflected by its relationship to both the slope and intercept and suggests that hypertonia may result in more variable and slower responses to descending motor commands. van Beers et al (2004) suggested that a constant noise could be extracted from the movement time during reaching, and could result from factors such as background motorneuron activity or co-contraction.
Consequences of increased neuromotor noise on trajectory formation
Arm motion is regulated by the sum of feedforward and feedback controller motor commands. In healthy neuromuscular systems, ballistic reaching movements are preplanned (i.e., feedforward) and executed largely without visual (Flowers, 1976) and proprioceptive feedback (Bizzi and Polit, 1979). Following stroke, there is an increased reliance on the feedback control of reaching (Trombly, 1992). In our study, kinematic descriptors demonstrated greater skewness and segmented profiles during movements of the more affected arm (indicates a largely feedback mediated strategy) while the less direct path suggests that the initial movement is not on target and corrective adjustments are required. These kinematic features are not mutually exclusive as more conservative movement planning of speed-accuracy tradeoffs (i.e., increased movement time) may compensate for indirect movements.
The relationships between neuromotor noise and kinematics suggest that neuromotor noise effects both the planning and execution stages for persons with stroke. In the more affected arm, both increased skewness (conservative planning strategy) and segmentation (trajectory corrections) were related to the intercept, while reduced directness (path accuracy and execution) was related to increased transmission capacity (i.e., slope−1). Wallace and Newell (1983) found that in healthy subjects, there is an increased reliance on sensory feedback for increasing accuracy requirement suggesting that there is a gradual shift from a feedforward to a feedback model of control depending upon the difficulty of the task (Siegel, 1977). The steeper slope in the Fitts’ relation, together with the increased skewness and segmentation of profiles for the more affected arm of subjects with stroke, suggests that this shift from feedforward to feedback loop control occurs at reduced levels of accuracy for movement of the more affected arm. We postulate that increased neuromotor noise causes the error of the executed movement to exceed the tolerance of a planned trajectory so that subsequent corrective submovements are necessary during the task (evidenced by segmentation); consequently conservative strategies are employed.
Studies of healthy individuals suggest that the feedforward controller specifies a motor command using an inverse (i.e., internal) model of the arm. An internal model is trained (learned) by minimizing the difference between the actual movement and its prediction. The formation and calibration internal models may be impeded by damage to areas of the brain where the model is formed, and also by the increased sensory noise which would result in poorer estimations of the arm’s actual behaviour (Saunder and Knill, 2004). There is some evidence that there is a reduced ability to form an appropriate internal model following stroke, including altered feedforward control of the passive intersegmental joint torques during reaching movements (Beer et al., 2000) which would result in joint in-coordination (Levin, 1996) and poorer anticipatory control of arm movements to perturbations (Takahashi and Reinkensmeyer, 2003).
Effect of slower movement on the speed-accuracy trade-off
We would argue that the observed changes in movement trajectory and reductions in transmission capacity (i.e., Fitts coefficients) associated with the more affected arm result from the neuromotor pathology and cannot be explained simply by the slower movement of the more affected arm. First, note that matching speeds between a stroke and a control group is not an option as Fitts’ law only emerges when the speed is maximal for that individual for a given index of difficulty. Second, our correlational analysis showed that individuals with stroke were not simply moving slower with similar movement strategies to the control group. For example, slower movements of the more affected arm were associated with greater corrective responses (e.g., segmentation), while slower movements for the control subjects did not exhibit similar movement strategies.
Limitations
We simultaneously compared differences in all three arm conditions via ANOVAs which was a statistically conservative approach (rather than paired t-tests between the more affected and less affected arm and t-tests between the less affected and control arm) that may have reduced our ability to detect differences of the less affected arm relative to the control arm. For example, mean values of kinematic descriptors and Fitts’ intercept of the less affected arm of the subjects with stroke lay between the control values and values of the more affected arm of the subjects with stroke. Haaland and Harrington (1989) reported an increase in movement time of the corrective component of the less affected arm of individuals with stroke when compared to healthy individuals during a reaching task for their left CVA group but not right CVA group. The smaller differences between the less affected arm of subjects with stroke and that of controls might be detected when controlling for lesion location. Such an analysis, however, is multifactorial [effects of hemisphere (i.e., left versus right lesion), pre-stroke dominance (i.e., dominant side versus non-dominant side affected), lesion volume, affected substrate(s)] and would require a substantially larger sample.
Clinical implications
This discrete reaching task was selected as it represents a functional movement of daily living. Fitts’ paradigm and its ability to assess neuromotor transmission capacity may serve as a useful tool for making inferences about the recovery of the nervous system following injury. Our results suggest that reduced transmission capacity has consequences for both motor execution and planning. Individuals with stroke demonstrated substantially more deviation from straight-line paths than controls, despite using more conservative strategies (i.e., leftward shift of velocity profile) and extensive feedback control (i.e., segmentation).
Acknowledgments
The authors would like to thank the Canadian Institute of Health Research (#63617) and Michael Smith Foundation of Health Research for salary support to JJE and the Rick Hansen Neurotrauma Initiative for a studentship to PHM.
References
- Bastian AJ, Martin TA, Keating JG, Thach WT. Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J Neurophysiol. 1996;76:492–509. doi: 10.1152/jn.1996.76.1.492. [DOI] [PubMed] [Google Scholar]
- Beer RF, Dewald JP, Rymer WZ. Deficits in the coordination of multijoint arm movements in patients with hemiparesis: evidence for disturbed control of limb dynamics. Exp Brain Res. 2000;131:305–319. doi: 10.1007/s002219900275. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Smith MB. Inter rater reliability of a modified Ashworth Scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- Bizzi E, Polit A. Processes controlling visually evoked movements. Neuropsychologia. 1979;17:203–213. doi: 10.1016/0028-3932(79)90011-3. [DOI] [PubMed] [Google Scholar]
- Brunnstrom S. Movement Therapy in Hemiplegia. Harper and Row; New York: 1970. [Google Scholar]
- Caminiti R, Johnson PB, Galli C, Ferraina S, Burnod Y, Urbano A. Making arm movements within different parts of space: The premotor and motor cortical representation of a coordinate system for reaching visual targets. J Neuroscience. 1991;11:1182–97. doi: 10.1523/JNEUROSCI.11-05-01182.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr J, Shepherd R. Reaching and Manipulation. In: Carr J, Shepherd R, editors. Neurological Rehabilitation, Optimizing Motor Performance. Oxford: Reed Educational and Professional Publishing Ltd; 1999. pp. 126–153. [Google Scholar]
- De Jong WP, Van Galen GP. Are speed/accuracy trade-offs caused by neuromotor noise, or not? Behav Brain Res. 1997;20:306–307. [Google Scholar]
- Eliasziw M, Young SL, Woodbury MG, Fryday-Field K. Statistical methodology for the assessment of interrater and intrarater reliability. Phys Ther. 1994;74:89–100. doi: 10.1093/ptj/74.8.777. [DOI] [PubMed] [Google Scholar]
- Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psych. 1954;47:381–391. [PubMed] [Google Scholar]
- Flowers KA. Visual “closed-loop” and “open-loop” characteristics of voluntary movement in patients with Parkinsonism and intention tremor. Brain. 1976;99:269–310. doi: 10.1093/brain/99.2.269. [DOI] [PubMed] [Google Scholar]
- Fortier PA, Kalaska JF, Smith AM. Cerebellar neuronal activity related to whole-arm reaching movements in the monkey. J Neurophysiology. 1989;62:198–211. doi: 10.1152/jn.1989.62.1.198. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. Poststroke hemiplegic patient: evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Gemperline JJ, Allen S, Walk D, Rymer WZ. Characteristics of motor unit discharge in subjects with hemiparesis. Muscle Nerve. 1995;18:1101–1114. doi: 10.1002/mus.880181006. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Delong MR, Crutcher MD. Relations between parameters of step-tracking movements and single cell discharge in the globus pallidus and subthalamic nucleus of the behaving monkey. J Neurosci. 1983;3:1586–1598. doi: 10.1523/JNEUROSCI.03-08-01586.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL. Hemispheric control of the initial and corrective components of aiming movements. Neuropsychologia. 1989;27:961–969. doi: 10.1016/0028-3932(89)90071-7. [DOI] [PubMed] [Google Scholar]
- Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature. 1998;394:780–784. doi: 10.1038/29528. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Caminiti R, Goergopoulos AP. Cortical mechanisms related to the direction of two-dimensional arm movements: Relations in parietal area 5 and comparison with motor cortex. Exp Brain Res. 1983;51:247–260. doi: 10.1007/BF00237200. [DOI] [PubMed] [Google Scholar]
- Kawato Internal models for motor control and planning. Curr Opin Neurobiol. 1999;9:718–727. doi: 10.1016/s0959-4388(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Langolf GD, Chaffin DB, Foulke JA. An investigation of Fitts’ law using a wide range of movement amplitudes. Journal of Motor Behavior. 1976;8:113–128. doi: 10.1080/00222895.1976.10735061. [DOI] [PubMed] [Google Scholar]
- Levin MF. Interjoint coordination during pointing movements is disrupted in spastic hemiparesis. Brain. 1996;119:281–293. doi: 10.1093/brain/119.1.281. [DOI] [PubMed] [Google Scholar]
- McCrea PH, Eng JJ, Hodgson AJ. Linear Spring-Damper Model of the Hypertonic Elbow: Reliability and Validity. J Neurosci Methods. 2003a;128:120–128. doi: 10.1016/s0165-0270(03)00169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea PH, Eng JJ, Hodgson AJ. Time and magnitude characteristics of force generation are impaired in both upper extremities with chronic stroke. Muscle Nerve. 2003b;28:46–53. doi: 10.1002/mus.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TE, Ijaz MM. The effect of accuracy constraints on three-dimensional movement kinematics. Neuroscience. 1990;35:365–374. doi: 10.1016/0306-4522(90)90090-q. [DOI] [PubMed] [Google Scholar]
- Parker VM, Wade DT, Langton Hewer R. Loss of arm function after stroke: measurement, frequency, and recovery. Int Rehabil Med. 1986;8:69–73. doi: 10.3109/03790798609166178. [DOI] [PubMed] [Google Scholar]
- Pohl PS, Winstein CJ, Fisher BE. The locus of age-related movement slowing: Sensory processing in continuous goal-directed aiming. Journal of Gerontology: Psychological Sciences. 1996;51B:94–102. doi: 10.1093/geronb/51b.2.p94. [DOI] [PubMed] [Google Scholar]
- Reinkensmeyer DJ, Iobbi MG, Kahn LE, Kamper DG, Takahashi CD. Modeling Reaching Impairment After Stroke Using Population Vector Model of Movement Control That Incorporates Neural Firing-Rate Variability. Neural Computation. 2003;15:2619–42. doi: 10.1162/089976603322385090. [DOI] [PubMed] [Google Scholar]
- Rosenfalck A, Andreassen S. Impaired regulation of force and firing pattern of single motor units in patients with spasticity. J Neurol Neurosurg Psychiatry. 1980;43:897–906. doi: 10.1136/jnnp.43.10.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JA, Knill DC. Visual Feedback Control of Hand Movements. J Neuroscience. 2004;24:3223–3234. doi: 10.1523/JNEUROSCI.4319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkenberg T, Bradford DC, Ajax ET. Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology. 1980;30:509–517. doi: 10.1212/wnl.30.5.509. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Smyrnis N, Evdokimidis I, Constantinidis TS, Kastrinakis G. Speed-accuracy trade-off in the performance of pointing movements in different directions in two-dimensional space. Exp Brain Res. 2000;34:21–31. doi: 10.1007/s002210000416. [DOI] [PubMed] [Google Scholar]
- Takahashi C, Reinkensmeyer DJ. Hemiparetic stroke impairs anticipatory control of arm movement. Exp Brain Res. 2003;149:131–40. doi: 10.1007/s00221-002-1340-1. [DOI] [PubMed] [Google Scholar]
- Todor JI, Cisneros J. Accommodation to increased accuracy demands by the right and left hands. J Mot Behav. 1985;17:355–372. doi: 10.1080/00222895.1985.10735354. [DOI] [PubMed] [Google Scholar]
- Trombly C. Deficits of Reaching in Subjects with Left Hemiparesis: A Pilot Study. American Journal of Occupational Therapy. 1992;46:887–897. doi: 10.5014/ajot.46.10.887. [DOI] [PubMed] [Google Scholar]
- van Beers RJ, Haggard P, Wolpert DM. The Role of Execution Noise in Movement Variability. J Neurophysiology. 2004;91:1050–63. doi: 10.1152/jn.00652.2003. [DOI] [PubMed] [Google Scholar]
- Wallace SA, Newell KM. Visual control of discrete aiming movements. QJ Exp Psychol A. 1983;35A:311–321. doi: 10.1080/14640748308402136. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. Are arm trajectories planned in kinematic or dynamic coordinates – an adaptation study. Exp Brain Res. 1995;103:460–470. doi: 10.1007/BF00241505. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. 4. Prentice-Hall Inc; Upper Saddle River, New Jersey: 1999. [Google Scholar]