Abstract
Purpose
Understanding the extent of disease in asymptomatic patients with castration resistant prostate cancer is important when making treatment decisions and designing clinical trials. The ENTHUSE M0 (ENdoTHelin A USE) trial (NCT00626548) was a large phase III study comparing the endothelin A receptor antagonist zibotentan with placebo in patients with nonmetastatic, castration resistant prostate cancer. The study was stopped prematurely after early efficacy review indicated that it was unlikely to meet its co-primary objectives of improved overall and progression-free survival vs placebo. Screening failed in an unexpectedly high number of patients. We investigated this screening failure rate to promote better classification of patients thought to have nonmetastatic castration resistant prostate cancer and inform the design of future clinical trials in this setting.
Materials and Methods
The number of patients enrolled in and subsequently excluded from study was analyzed by geographic region and by the specialty of the investigating clinician (oncology or urology) who enrolled the study patients.
Results
Of 2,577 patients enrolled in a total of 350 hospital based centers in 39 countries screening failed in 1,155 (45%). The most common reason for screening failure was the detection of metastatic disease in 32% of all screened patients and in 71% of those in whom screening failed. The leading reasons for failed screening did not differ between investigator specialties overall or by geographic region.
Conclusions
The high frequency of asymptomatic metastasis in men thought to have nonmetastatic, castration resistant prostate cancer highlights the importance of periodic staging assessments for the condition. Optimal treatment modalities may differ for metastatic and nonmetastatic disease.
Keywords: prostate, prostatic neoplasms, neoplasm metastasis, mass screening, ZD4054
Current treatment options for asymptomatic CRPC vary depending on factors such as patient age, performance status and pivotally the presence or absence of radiographically detectable metastasis, which is most commonly located in bone. Strategies to alter the disease course in patients with asymptomatic CRPC without radiographically detectable metastasis are limited and none of them have a proven survival benefit. However, a phase III study of the human monoclonal antibody denosumab, which inhibits receptor activator of nuclear factor-κB ligand, showed statistically significantly prolonged bone metastasis-free survival in men with non-metastatic CRPC in favor of denosumab over placebo (median 4.2 months, HR 0.85, 95% CI 0.73–0.98, p = 0.028).1
After bone metastasis is present bone targeted therapy such as zoledronic acid2 and denosumab3 might be considered to prevent skeletal related events. Sipuleucel-T, an autologous cell immunotherapy, was recently approved by the United States FDA for patients with asymptomatic or minimally symptomatic CRPC with metastatic disease.4,5 Many other potential therapies, including MDV3100, abiraterone and TAK-700, are currently being studied in men with asymptomatic metastatic CRPC.6
There can be uncertainty about the presence of metastatic disease since in reality nonmetastatic CRPC is micrometastatic disease at levels below the detection limits of standard radiological imaging. Prospective analyses have been done on bone metastasis data from 2 phase III trials to study the natural history of nonmetastatic CRPC. In an analysis of 201 patients with nonmetastatic CRPC in the placebo arm of a prematurely terminated, randomized trial of zoledronic acid 33% had experienced bone metastasis and 21% had died by 2 years after trial entry.7 In the placebo arm of another phase III study 46% of 331 patients with nonmetastatic CRPC showed bone metastasis and 20% had died by 2 years after study entry.8 In each series higher baseline PSA and PSA velocity were associated with shorter time to bone metastasis development.7,8
Endothelin mediated signaling is implicated in prostate cancer progression and bone metastasis development.9–11 Consequently the specific endothelin A receptor antagonist zibotentan was investigated in patients with CRPC.12 In a phase II study zibotentan demonstrated anticancer activity in patients with metastatic CRPC, leading to the initiation of the large, multicenter, phase III ENTHUSE trial program. The ENTHUSE M0 study (NCT00626548) assessed zibotentan vs placebo in patients with non-metastatic CRPC and increasing PSA. Eligible patients were screened for metastasis by bone scan and CT/MRI. The study was stopped prematurely after an early efficacy review indicated that it was unlikely to meet its primary efficacy objectives of improved overall and progression-free survival vs placebo.
Screening failed in an unexpectedly high number of patients in the ENTHUSE M0 study. We investigated this high screening failure rate to better classify patients thought to have nonmetastatic CRPC and inform the design of future clinical trials in this setting.
MATERIALS AND METHODS
Study Design
In this phase III, international, double-blind, randomized, placebo controlled study patients were randomized 1:1 to zibotentan 10 mg once daily or matching placebo. The study protocol and consent forms were approved by appropriate ethical review boards. All patients provided written informed consent.
Patients
Eligible patients were 18 years old or older with histological or cytological confirmation of prostate adenocarcinoma, WHO performance status 0 to 1 and surgical or continuous medical castration with serum testosterone 2.4 nmol/l or less, or 70 ng/dl, which is the lower limit of quantification at many participating centers. Patients had been on stable treatment for 8 weeks and had no evidence of metastatic disease, local recurrence or pelvic lymph node disease (other than as described) on abdominopelvic CT/MRI and bone scan, which were done in all patients within 4 weeks before randomization, or on CT of the chest as clinically indicated. Patients with prostate cancer involving the pelvic lymph nodes were eligible for treatment only if disease was absent above the aortic bifurcation, and then if only a single lymph node was enlarged (no size limit applied) or multiple enlarged lymph nodes 2 cm or less in the short axis were identified, in accordance with RECIST (Response Evaluation Criteria in Solid Tumors), version 1.1.13 Also, patients had biochemical prostate cancer progression documented while castrate, defined as 2 or more consecutive or nonconsecutive PSA increases in 1 month or greater, including historical values, with 14 days or more between measurements. The highest PSA had to represent a relative increase of 50% or greater, or an absolute increase of 10 ng/ml or greater over the first of the 3 PSA values used for assessment with a minimum of 1.2 ng/ml in patients treated with radical prostatectomy and 5 ng/ml in all others.
Study exclusion criteria included definitive therapy to treat primary prostate cancer (prostatectomy, radiotherapy or cryotherapy) within 3 months of study entry, prior cytotoxic chemotherapy for recurrent prostate cancer with prior estramustine therapy allowed, prior targeted cancer therapy, intravenous bisphosphonates within 6 weeks before the start of study treatment, therapy with any investigational anticancer treatment within 4 weeks before starting study treatment, prior therapy with endothelin receptor antagonists or a family history of hypersensitivity to endothelin receptor antagonists, New York Heart Association stage II to IV cardiac failure or myocardial infarction within 6 months, QTc greater than 470 milliseconds, creatinine clearance less than 50 ml per minute by the Cockcroft-Gault formula or by 24-hour creatinine clearance, use of systemic retinoids or potent CYP450 inducers within 2 weeks of starting treatment with dexamethasone allowed if the investigator thought it necessary, hemoglobin less than 9 gm/dl and alanine aminotransferase or aspartate aminotransferase greater than 2.5 times ULN or serum bilirubin greater than 1.5 ULN.
Screening Failure
Screening was considered to have failed in men who were considered for study inclusion and consented but were found on examination not to fulfill eligibility criteria. Information on screening failure, including reasons for failure, was captured in a patient screening log kept by each participating investigator. For this analysis investigator specialty was classified as oncology or urology and investigator geographic region was classified by region (see table). Patient enrollment data and leading causes of screening failure are shown by region and investigator specialty. Patients could have more than 1 reason for screening failure.
Table 1.
Screening failure by region and investigator specialty
| No. Investigators | No. Enrolled Pts | No. Failed Screening (%) | |
|---|---|---|---|
| Asia Pacific: | 66 | 535 | 204 (38) |
| Oncology | 0 | 0 | 0 |
| Urology | 66 | 535 | 204 (38) |
| Australia: | 6 | 57 | 25 (44) |
| Oncology | 5 | 50 | 21 (42) |
| Urology | 1 | 7 | 4 (57) |
| Canada: | 23 | 285 | 159 (56) |
| Oncology | 4 | 62 | 38 (61) |
| Urology | 19 | 223 | 121 (54) |
| Central/South America: | 24 | 195 | 103 (53) |
| Oncology | 13 | 93 | 49 (53) |
| Urology | 11 | 102 | 54 (53) |
| Europe: | 138 | 1,183 | 509 (43) |
| Oncology | 34 | 307 | 126 (41) |
| Urology | 104 | 876 | 383 (44) |
| Middle East: | 14 | 54 | 35 (65) |
| Oncology | 6 | 18 | 11 (61) |
| Urology | 8 | 36 | 24 (67) |
| South Africa: | 6 | 78 | 44 (56) |
| Oncology | 2 | 7 | 4 (57) |
| Urology | 4 | 71 | 40 (56) |
| United States: | 28 | 190 | 76 (40) |
| Oncology | 12 | 95 | 34 (37) |
| Urology | 16
|
95
|
42 (41)
|
| Totals: | 305 | 2,577 | 1,155 (45) |
| Oncology | 76 | 632 | 283 (45) |
| Urology | 229 | 1,945 | 872 (45) |
RESULTS
Demographics
Patients
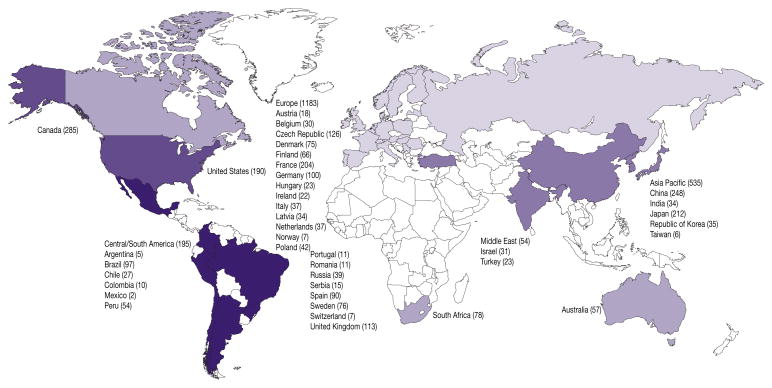
A total of 2,577 patients were enrolled at 350 hospital based centers in 39 countries as of January 14, 2011 (fig. 1). Of the patients 46% were from European centers while 21% were from East Asia, 11% from Canada, 8% Central America, 7% United States, 3% South Africa, and 2% each from Australia and the Middle East. Median age was 73 years (range 44 to 93). The population comprised predominantly white men (72.4%). The remaining patients were Asian (21.4%), black American (2.4%) and other (3.8%). All except 25 randomized patients underwent bone scan plus CT/MRI at baseline. Screening failed in 1,155 patients (45%) (table 1).
Figure 1.
Geographic distribution of enrolled patients
Investigators
Of the 305 participating investigators 229 (75%) and 76 (25%) were classified as urologists and oncologists, respectively (table 1). Most investigators were from the European (45%) and Asia Pacific (22%) regions. Urologists outnumbered oncologists in most regions with no investigators classified as oncologists in the Asia Pacific region. The balance of specialties approached parity in the United States, Central/South America and the Middle East. Australia was the only region where oncologists greatly outnumbered urologists. A mean of 8.4 patients were enrolled per investigator, ranging from 3.9 in the Middle East to 13 in South Africa. There was little difference between the specialties in the number of patients enrolled per investigator (table 1).
Screening Failure
Rates
The screening failure rate ranged from 38% in the Asia Pacific region to 65% in the Middle East. There was no difference in the rate between the specialties overall (each 45%) or in the geographic regions (table 1).
Reasons
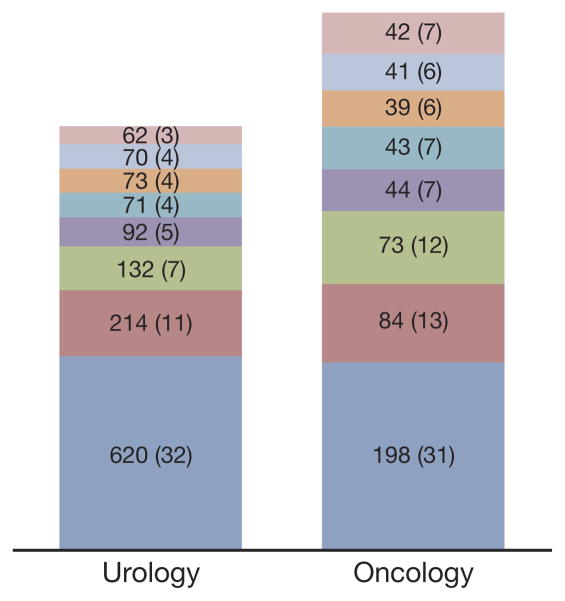
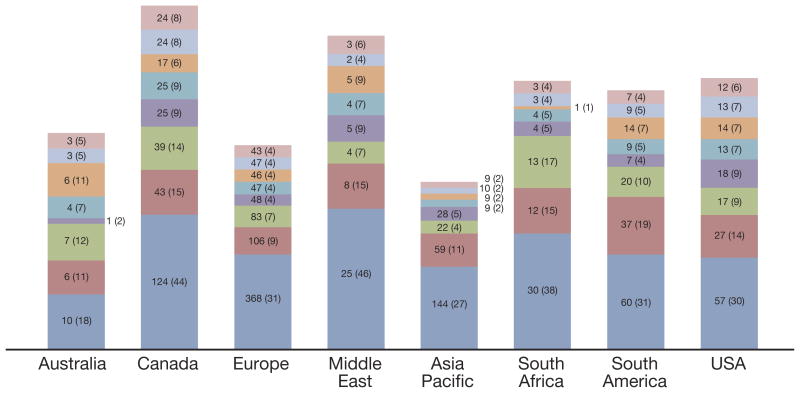
The most common reason for screening failure was the detection of metastatic disease, which was implicated in 71% of screening failures, that is 32% of enrolled patients (figs. 2 and 3). This was followed by creatinine clearance less than 50 ml per minute in 12% of enrolled patients, QTc greater than 470 milliseconds in 8% and testosterone greater than 70 ng/dl (noncastrate) in 5%. Some patients had more than 1 reason for screening failure. There was no clear difference between the specialties or among the regions in the reasons for failed screening (figs. 2 and 3).
Figure 2.
Leading reasons for screening failure by investigator specialty. Values indicate number of patients for whom reason was cited for failure. Values in parentheses indicate percent of total enrolled population. Some patients had more than 1 reason for failure. Pink bars indicate serum bilirubin greater than 1.5 ULN. Light blue bars indicate hemoglobin less than 9 gm/dl. Orange bars indicate no biochemical progression. Blue-green bars indicate alanine aminotransferase or aspartate aminotransferase greater than 2.5 ULN. Purple bars indicate testosterone greater than 70 ng/dl or noncastrate. Green bars indicate QTc interval greater than 470 milliseconds. Red bars indicate creatinine clearance less than 50 ml per minute. Dark blue bars indicate metastatic disease detected.
Figure 3.
Leading reasons for screening failure by region. Values indicate number of patients for whom reason was cited for failure. Values in parentheses indicate total enrolled population. Some patients had more than 1 reason for failure. Pink bars indicate serum bilirubin greater than 1.5 ULN. Light blue bars indicate hemoglobin less than 9 gm/dl. Orange bars indicate no biochemical progression. Blue-green bars indicate alanine aminotransferase or aspartate aminotransferase greater than 2.5 ULN. Purple bars indicate testosterone greater than 70 ng/dl or noncastrate. Green bars indicate QTc interval greater than 470 milliseconds. Red bars indicate creatinine clearance less than 50 ml per minute. Dark blue bars indicate metastatic disease detected.
DISCUSSION
The overall 45% screening failure rate in the ENTHUSE M0 study was higher than expected. Direct comparison with equivalent data from trials in similar populations1,7,14 is not possible since screening failure data are not published. The failure rate in patients with more advanced disease has generally been lower than these observed rates, including 12% to 35% in other phase III trials of CRPC5,15,16 and 23% in a recent phase III trial of denosumab in men with CRPC metastatic to bone.3
The main reason for the high screening failure rate in the ENTHUSE M0 study was the detection of metastatic disease on MRI, CT or bone scan, which occurred in almost a third of the patients screened. The unexpectedly high rate of metastatic disease in this trial suggests that a high proportion of men thought to have nonmetastatic CRPC may have had asymptomatic metastasis. As a result, it took longer than expected to recruit an adequate number of eligible patients for this study, prolonging the trial duration. Nonetheless, experience with this trial reveals that comprehensive imaging to evaluate the presence or absence of metastasis is mandatory for future clinical trials in this setting to avoid contaminating the study population with patients who have metastasis.
These findings raise the question of the consequences of not detecting metastatic disease in patients with presumed nonmetastatic CRPC. Such men would generally not be offered treatment that is indicated only for patients with metastatic disease. For example, docetaxel is only approved for meta-static CRPC,15,17 sipuleucel-T was recently approved by the FDA for asymptomatic or minimally symptomatic CRPC with metastatic disease4 and denosumab is currently approved at a dose of 120 mg subcutaneously every 4 weeks for confirmed bone metastasis.3 Accurate definition of metastatic status is important to allow the timely initiation of these treatments. Although no definitive data show that earlier introduction of these agents improves important clinical end points, the toxicity of these newly FDA approved agents is low, making their early introduction feasible in practice. In an era when urologists are discouraged from ordering bone scans and CT for newly diagnosed localized disease the screening failure rate in this study clearly illustrates that in the setting of asymptomatic CRPC metastasis is more common than generally supposed and more regular imaging should be considered even in the absence of symptoms.
In this study the rate of metastatic disease detection did not differ by the classification of investigator specialty (oncology vs urology). Thus, it is likely that these metastases go undetected as a result of common clinical practice among oncologists and urologists in the field of prostate cancer due to reliance on PSA measurement rather than imaging to make treatment decisions. The other reasons cited for screening failure showed little variation between the specialties and the overall screening failure rate was similar. Greater variation in the overall rate of screening failure was observed among geographic regions but the reasons underlying this variation are not apparent from the current analysis.
While this retrospective investigation of ENTHUSE M0 study screening failures provides interesting insight into the enrolled population, it has a number of limitations. Most notably available data on patient characteristics are limited for those in whom screening failed. When this study was designed, collecting data on those not randomized to study treatment was not considered relevant for inclusion in the protocol. Baseline data were obtained and reviewed to confirm eligibility but confirmation of ineligibility was not a requirement. Notably no data were available on absolute PSA, PSA kinetics or the site of detected metastasis, ie bone, lymph nodes or viscera.
Another topic worthy of comment relates to the eligibility criteria for lymph node disease. Since patients with pelvic lymph node metastasis below the aortic bifurcation were eligible to participate in this trial, the incidence of metastasis may have been even higher had these patients been considered. Furthermore, assessment of lymph node disease was based on RECIST guidelines13 and allowed patients to enter with multiple enlarged lymph nodes less than 2 cm in the short axis or a single enlarged lymph node of any size. However, the observed 45% screening failure rate may have been higher still if this criterion had been based on Prostate Cancer Working Group 2 guidelines, in which target lesion status is determined according to the long axis, ie the greatest diameter 2 cm or greater.18
This is not the first example of useful information emerging from a terminated study. The placebo arm of a phase III trial of zoledronic acid provided valuable information on the natural history of bone metastasis in CRPC cases, although the study was stopped early due to low accrual of primary end point events, namely detection of the first bone metastasis.7 The zoledronic acid study showed that median time to the first bone metastasis was not attained for 2 years. The conclusion was that disease and increasing PSA in these patients had a relatively indolent natural history.
Similar findings on the indolent nature of nonmetastatic CRPC were observed in the placebo arm of the previously published phase III atrasentan trial.8 Absolute PSA and PSA kinetics, ie PSA velocity and PSA doubling time, have served as prognostic markers to inform the design of clinical studies.19 In the Amgen 147 phase III bone metastasis prevention trial, which showed significant prolongation of bone metastasis-free survival for denosumab treatment vs placebo, these markers were used to select a higher risk population to calculate statistical power for the trial.1
CONCLUSIONS
The high frequency of asymptomatic metastasis in men thought to have nonmetastatic CRPC warrants the consideration of periodic staging studies in men with established nonmetastatic CRPC and by extension in those who progress from hormone sensitive nonmetastatic disease to CRPC. Defining the true extent of the disease will help inform patient treatment decisions since it may be advantageous to treat those with asymptomatic metastasis with agents approved for metastatic disease at the earliest possible opportunity. Whether this would lead to an improved clinical benefit or be a cost-effective option requires further investigation. Regardless, clinical trials done in the setting of nonmetastatic CRPC should factor the prevalence of asymptomatic metastasis into the recruitment design.
Acknowledgments
Dr. Zoë van Helmond, Mudskipper Bioscience, assisted with the manuscript.
Supported by AstraZeneca, SPORE (P50CA097186) and the Prostate Cancer Foundation.
Abbreviations and Acronyms
- CRPC
castration resistant prostate cancer
- CT
computerized tomography
- FDA
Food and Drug Administration
- MRI
magnetic resonance imaging
- PSA
prostate specific antigen
- QTc
corrected QT interval
- ULN
upper limit of normal
References
- 1.Smith MR, Saad F, Coleman R, et al. Denosumab and bone metastasis-free survival in men with castrate-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saad F, Gleason DM, Murray R, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with meta-static hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 3.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17:3520. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 5.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 6.Bishr M, Lattouf JB, Gannon PO, et al. Updates on therapeutic targets and agents in castration-resistant prostate cancer. Minerva Urol Nefrol. 2011;63:131. [PubMed] [Google Scholar]
- 7.Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23:2918. doi: 10.1200/JCO.2005.01.529. [DOI] [PubMed] [Google Scholar]
- 8.Smith MR, Cook R, Lee KA, et al. Disease and host characteristics as predictors of time to first bone metastasis and death in men with progressive castration-resistant nonmetastatic prostate cancer. Cancer. 2011;117:2077. doi: 10.1002/cncr.25762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gohji K, Kitazawa S, Tamada H, et al. Expression of endothelin receptor a associated with prostate cancer progression. J Urol. 2001;165:1033. [PubMed] [Google Scholar]
- 10.Nelson JB, Nguyen SH, Wu-Wong JR, et al. New bone formation in an osteoblastic tumor model is increased by endothelin-1 overexpression and decreased by endothelin A receptor blockade. Urology. 1999;53:1063. doi: 10.1016/s0090-4295(98)00658-x. [DOI] [PubMed] [Google Scholar]
- 11.Santini D, Galluzzo S, Zoccoli A, et al. New molecular targets in bone metastases. Cancer Treat Rev, suppl. 2010;36:S6. doi: 10.1016/S0305-7372(10)70013-X. [DOI] [PubMed] [Google Scholar]
- 12.James ND, Caty A, Payne H, et al. Final safety and efficacy analysis of the specific endothelin A receptor antagonist zibotentan (ZD4054) in patients with metastatic castration-resistant prostate cancer and bone metastases who were pain-free or mildly symptomatic for pain: a double-blind, placebo-controlled, randomized Phase II trial. BJU Int. 2010;106:966. doi: 10.1111/j.1464-410X.2010.09638.x. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) Eur J Cancer. 2009;45:228. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Nelson JB, Love W, Chin JL, et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer. 2008;113:2478. doi: 10.1002/cncr.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 16.Sternberg CN, Petrylak DP, Sartor O, et al. Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: the SPARC trial. J Clin Oncol. 2009;27:5431. doi: 10.1200/JCO.2008.20.1228. [DOI] [PubMed] [Google Scholar]
- 17.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 18.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin DW. Beyond PSA: utility of novel tumor markers in the setting of elevated PSA. Urol Oncol. 2009;27:315. doi: 10.1016/j.urolonc.2009.01.026. [DOI] [PubMed] [Google Scholar]