Abstract
Background
It is standard practice to administer a cholinesterase inhibitor (e.g., neostigmine) at the end of a surgical case to reverse suspected effects of neuromuscular blocking agents regardless of whether such residual effects are present. The authors hypothesized that cholinesterase inhibition when given the in absence of neuromuscular blockade (NB) would decrease upper airway dilatory muscle activity and consequently upper airway volume.
Methods
The authors measured genioglossus and diaphragm electromyograms during spontaneous ventilation in anesthetized, tracheostomized rats before and after administration of neostigmine (0.03, 0.06, or 0.12 mg/kg), after recovery of the train-of-four ratio (quadriceps femoris muscle) to unity after NB (n = 18). For comparison, the authors made the same measurements in rats that had no previous NB (n = 27). In intact anesthetized rats, the authors measured upper airway volume and end-expiratory lung volume by magnetic resonance imaging before and after 0.12 mg/kg neostigmine (n = 9).
Results
Neostigmine treatment in rats that had fully recovered from NB based on the train-of-four ratio caused dose-dependent decreases in genioglossus electromyogram (to 70.3 = 7.6, 49.2 = 3.2, and 39.7 = 2.3% of control, respectively), decreases in diaphragm electromyogram (to 103.1 ± 6.5, 83.1 ± 4.7, and 68.7 ± 7.3% of control), and decreases in minute ventilation to a nadir value of 79.6 ± 6% of preneostigmine baseline. Genioglossus electromyogram effects were the same when neostigmine was given with no previous NB. Neostigmine caused a decrease in upper airway volume to 83 ± 3% of control, whereas end-expiratory lung volume remained constant.
Conclusions
The cholinesterase inhibitor neostigmine markedly impairs upper airway dilator volume, genioglossus muscle function, diaphragmatic function, and breathing when given after recovery from vecuronium-induced neuromuscular block.
PARTIAL neuromuscular transmission failure (train-of-four [TOF] ratio at the adductor pollicis muscle: 0.5– 0.9) evokes dysphagia,1 aspiration,1 and a decrease in the rate of maximum airflow during inspiration2,3 by partial inspiratory upper airway obstruction.4 These signs and symptoms1– 4 can be difficult to detect5 because they may be present even with a magnitude of muscle weakness insufficient to evoke dyspnea or a decrease in vital capacity,3–5 respiratory drive,4,6 or lung volume,4 suggesting that upper airway muscles are more vulnerable to NB than the respiratory “pump” muscles.4
Cholinesterase inhibitors (ChEIs; e.g., neostigmine) are clinically useful to improve skeletal muscle function when receptors are partially blocked due to lingering effects of neuromuscular blocking agents.7,8 Therefore, it is recommended to reverse effects of neuromuscular blocking agents at the end of a surgical procedure by ChEI administration.7,8 This approach increases skeletal muscle force reliably in patients presenting with residual neuromuscular blockade (NB) from short- or intermediate-acting neuromuscular blocking drugs.9 One possible drawback to this treatment practice is that ChEI-based reversal agents, in high doses having been applied to humans by some anesthesiologists,7,10 can cause neuro-muscular transmission failure when administered to patients who have already recovered from NB.11–14 ChEIs may cause neuromuscular transmission failure by desensitization of acetylcholine receptors,13 depolarization block of neuromuscular transmisssion,12 or open channel block.15 Neuromuscular transmission impairment of respiratory muscles could increase the risk of developing respiratory complications.
The objective for this preclinical study in rats was to study the dose–response relation of ChEIs on genioglossus muscle and diaphragmatic activity to maintain normal upper airway dimensions.16 We hypothesized that neostigmine, when given after recovery from NB (based on TOF ratio), would decrease upper airway dilatory muscle activity and consequently upper airway volume. We further aimed to explore whether giving neostigmine to rats that had recovered from NB would be as detrimental as giving neostigmine to rats that had received no previous NB treatment.
Materials and Methods
Fifty-seven adult male Sprague-Dawley rats (300 – 400 g, Harlan Sprague-Dawley; Indianapolis, IN) were used in these experiments. All procedures were approved by local animal care and use committees (Harvard Medical School, Boston, MA), and every effort was made to minimize the numbers of rats used and their suffering.
Experimental Preparation
Electromyographic recording electrodes were inserted during isoflurane (Baxter Healthcare Corporation, Deer-field, IL) anesthesia into the diaphragm and the genioglossus (one on each side of the midline by open surgery). The trachea was carefully transected (protocols 1 and 2 only) and cannulated proximally, and a femoral artery and vein were cannulated for measurement of arterial blood pressure and administration of drugs and fluid, respectively.17 To assess the degree of NB, the femoral nerve was stimulated and the evoked muscular response was measured by accelerometry of the quadriceps femoris muscle (TOF-Watch SX Monitor; Organon International, Oss, The Netherlands; as depicted in figures E1 and E2 on the Anesthesiology Web site at http://www.anesthesiology.org).
Measurements and Data Analysis
Electromyographic signals were amplified with a Grass Polygraph (Grass Instruments, Quincy, MA), filtered (100 Hz low pass, 10 kHz high pass), rectified, and integrated on a moving-time-average basis with a time constant of 100 ms. Tonic genioglossus activity was defined as nadir (expiratory) genioglossus activity during expiration minus genioglossus activity measured after euthanasia of the rat. This approach was used to discriminate between electrical noise and the small tonic genioglossus signal. Phasic genioglossus activity was defined as peak inspiratory genioglossus activity minus nadir expiratory activity of the same respiratory cycle. Tracheal airflow was recorded by using a pneumotachograph (Fleisch 00; Gould Medical Ltd., Lutterworth, United Kingdom) and differential pressure transducer (PT5; Grass Instruments) attached to the tracheostomy cannula. We also measured continuously end-tidal carbon dioxide with an infrared carbon dioxide analyzer. All signals were digitized and analyzed off-line with Axotape and Clampfit software (Molecular Devices, Sunnyvale, CA).
Protocols
Pilot Study
In a pilot study (n = 6 rats), we administered 0.06 mg/kg neostigmine (Sicor Pharmaceuticals Inc., Irvine, CA) and glycopyrrolate (American regent Inc., Shirley, NY) during pancuronium (Bexter, Deer-field, IL)– evoked NB (target NB = TOF ratio 0.2– 0.5) to determine whether the typical mg/kg dose used in humans would be appropriate in rats.
Protocol 1
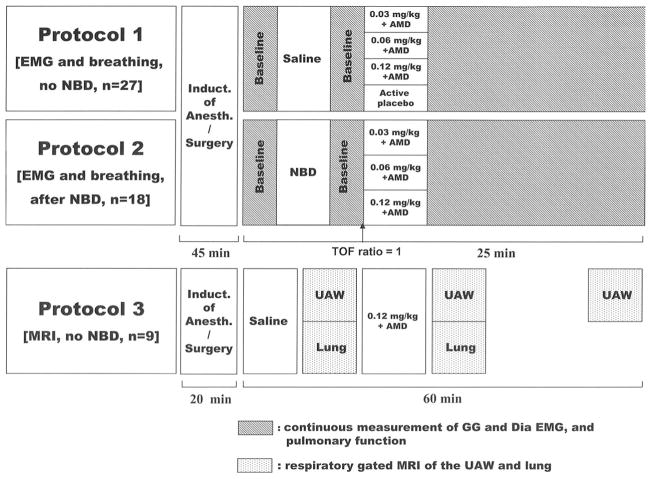
Neuromuscular blocking drugs were not given in this protocol. We recorded in 27 rats genioglossus and diaphragm electromyogram, tidal volume, respiratory rate, end-tidal carbon dioxide, electrocardiogram, and arterial blood pressure before and after injection of saline or the test drug, neostigmine (0.03, 0.06, or 0.12 mg/kg; fig. 1). Cholinesterase-based NB reversal protocols typically include coadministration of an antimuscarinic drug (e.g., atropine or glycopyrrolate) to decrease the likelihood of autonomic side effects.18 Therefore, in our study, neostigmine was always given with either atropine (50% of neostigmine dose, n = 11) or glycopyrrolate (25% of neostigmine dose, n = 12). An antimus-carinergic drug (atropine [n = 2] or glycopyrrolate [n = 2]; both from American Regent Inc., Shirley, NY) alone was given as an “active control” (fig. 1). Arterial blood gas analyses were made before and 2 min after neostigmine injection.
Fig. 1.
Protocols. Protocol 1: Assessment of neostigmine effects on respiratory muscle function and breathing. We did not administer neuromuscular blocking drugs (NBDs) in this protocol. Protocol 2: Assessment of respiratory muscle function and breathing effects of neostigmine when administered after recovery of the train-of-four (TOF) ratio to unity from vecuronium-induced neuromuscular blockade. Protocol 3: Magnetic resonance imaging (MRI) of upper airway volume and end-expiratory lung volume before and after neostigmine. Active control = glycopyrrolate or atropine. AMD = antimuscarinergic drug; Dia = diaphragm; EMG = electromyogram; GG = genioglossus muscle; UAW = upper airway.
Protocol 2
Protocol 2 was the same as protocol 1 but after full recovery from vecuronium (Organon Inc., Roseland, NJ) administration (134 ± 47 μg/kg, n = 18) as defined by a TOF ratio of unity.
Protocol 3
We assessed the effects of neostigmine (0.12 mg/kg) administered with glycopyrrolate (0.03 mg/kg) on upper airway volume (n = 6) and end-expiratory lung volume (n = 3). All magnetic resonance imaging sessions were conducted at 4.7 T (Biospec 47/40; Bruker BioSpin, Karlsruhe, Germany; fig. 1). For upper airway imaging, each rat was placed into the scanner in the supine position with a respiratory sensor located on its back at the level of the upper abdomen. A series of three magnetic resonance images were taken immediately before and after injection and 45 min later.
For lung imaging, additional electrodes were applied for cardiac gating, at both forepads and at the left rear pad. Animals were put head-first into the scanner with the upper airway or thorax centered with respect to the radiofrequency birdcage coil (7 cm ID).
Statistical Analysis
For testing the main two hypotheses, i.e., a neostigmine evoked decrease in (1) genioglossus electromyogram and (2) upper airway volume, we applied paired t tests for comparison of values of variables obtained at baseline and after administration of 0.06 mg/kg neostigmine. Bonferroni-Holm adjustments were used for correction of the α error for multiple testing. Subsequently, we tested for dose–response relations of neostigmine on the different metrics of respiratory function. Neostigmine-evoked changes in genioglossus electromyogram, diaphragm electromyogram, tidal volume, and respiratory rate (percentage preneostigmine) were tested separately for protocols 1 and 2 by one-way analysis of variance (Scheffé post hoc test). Estimates of the dose–response relation of neostigmine on the genioglossus muscle were taken from least squares linear regression of the logarithm of each dose against a probit transformation of the genioglossus electromyogram depression.
To test for differences in the effects of neostigmine at the genioglossus and diaphragm, we applied within each protocol (1 and 2) two-way repeated-measures analysis of variance. We selected raw values of the variables “before neostigmine injection” and “after neostigmine injection” as within-subject variables, and put in the model “neostigmine dose” and “muscle” (genioglossus vs. diaphragm) as between-subject factors.
Finally, to further analyze possible differences in respiratory muscle effects of neostigmine between protocols (protocol 1 [placebo] vs. protocol 2 [precurarization]), we pooled the data from protocols 1 and 2 and applied another two-way repeated-measures analysis of variance. Mean and SEM were used to summarize continuous variables (additional information available on the Anesthesiology Web site at http://www.anesthesiology.org).
Results
Fifty-seven rats were included in this study, and experiments were successfully completed in all but one rat. Accordingly, data from 56 rats are presented.
Pilot Study
Pancuronium at a dose of 118 ± 54 μg/kg was required to achieve NB, which produced a nadir TOF ratio of 0.2– 0.3 that remained in the range between 0.2 and 0.5 over a period of approximately 5 min. In parallel, phasic electromyogram of the genioglossus and diaphragm decreased to nadir values of 10 ± 3% and 20 ± 10%, respectively, of baseline. After administration of 0.06 mg/kg neostigmine and 0.015 mg/kg glycopyrrolate, genioglossus and diaphragm electromyogram recovered to baseline values within 4 min in all rats. Therefore, doses recommended to be used for reversal of NB in humans can be applied on a mg/kg basis to rats for reversal of NB. In the main study, we then halved and doubled this dose to establish a dose–response curve.
Protocol 1: Neostigmine Effects on Respiratory Muscle Function and Breathing without Previous Neuromuscular Blocking Drug Administration
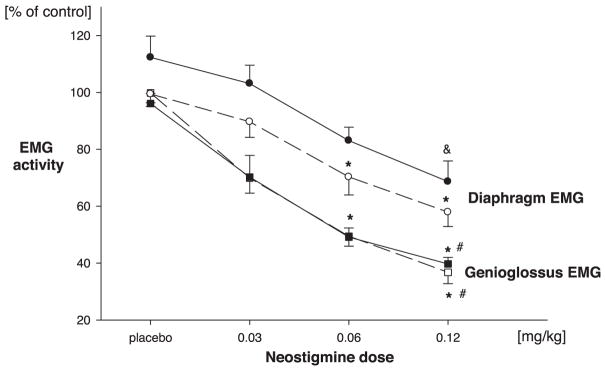
Neostigmine decreased genioglossus activity in a dose-dependent fashion (F = 14.0, P < 0.0001), with a mean nadir value after the highest dose of neostigmine (0.12 mg/kg) of 36.8 ± 4% of baseline (figs. 2 and 3). The duration of the effect of 0.06 mg/kgneostigmine is depicted in figure 4. Time from neostigmine injection until recovery of genioglossus electromyogram to baseline increased significantly (F = 4.7, P = 0.023) with neostigmine dose from 7 ± 4 min up to 25 ± 7 min. Under baseline conditions, tonic genioglossus activity amounted to 4 ± 0.4% of its phasic electromyogram activity. Tonic genioglossus activity was not affected by neostigmine (102 ± 0.8%; 0.06 mg/kg).
Fig. 2.
Peak effects of neostigmine on genioglossus electromyogram (EMG) (squares) and the diaphragm EMG (circles). Solid lines, closed symbols = neostigmine given without previous vecuronium administration (protocol 1); dashed lines, open symbols = neostigmine given after recovery of the train-of-four ratio from neuromuscular blockade (protocol 2). Values are given in percent of control values observed before neostigmine injection. * P < 0.05, dose effect, same muscle, same protocol (vs. 0.03 mg/kg). # P < 0.05, dose effect significantly lower than diaphragm (same protocol). & P < 0.05 versus baseline (before neostigmine injection, paired t test).
Fig. 3.
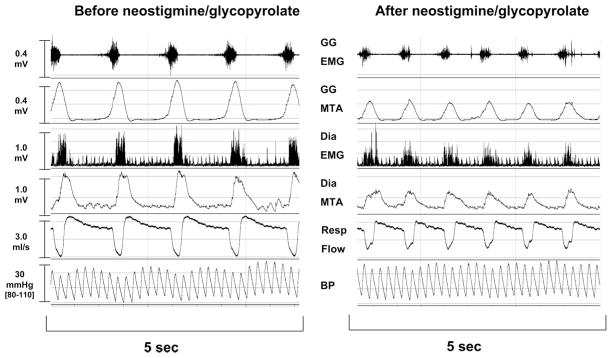
Typical response to administration of neostigmine. Genioglossus (GG) electromyogram (EMG; raw signal and moving time average [MTA]), diaphragm (Dia) EMG (rectified raw signal and MTA), respiratory (Resp) flow, and blood pressure (BP) (from top to bottom) before and after administration of 0.06 mg/kg neostigmine without previous administration of neuro-muscular blocking drug (protocol 1). Twenty seconds after neostigmine, phasic activity of the genioglossus and diaphragm were markedly decreased.
Fig. 4.
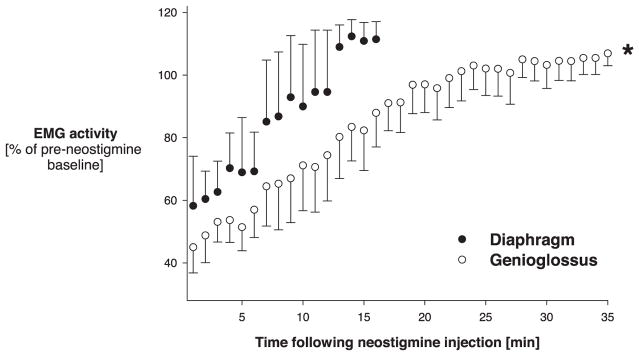
Time course of recovery of the genioglossus (open circles) and diaphragm (closed circles) electromyogram (EMG) from 0.06 mg/kg neostigmine. No vecuronium had been given previously (protocol 1). Values of variables were averaged every minute until maximum recovery. Diaphragm and genioglossus EMG varied on a breath-by-breath basis (fig. 3). EMG recovery was significantly faster at the diaphragm compared with the genioglossus muscle. * P < 0.05 for between-groups effects (genioglossus vs. diaphragm).
Diaphragmatic activity decreased dose dependently (F = 8.6, P < 0.01) after neostigmine (nadir electromyogram after 0.12 mg/kg neostigmine: 57.9 ± 5.0% of baseline; figs. 2 and 3). The peak effect of neostigmine was significantly smaller on the diaphragm compared with the genioglossus (F = 5.8, P = 0.02; fig. 3).
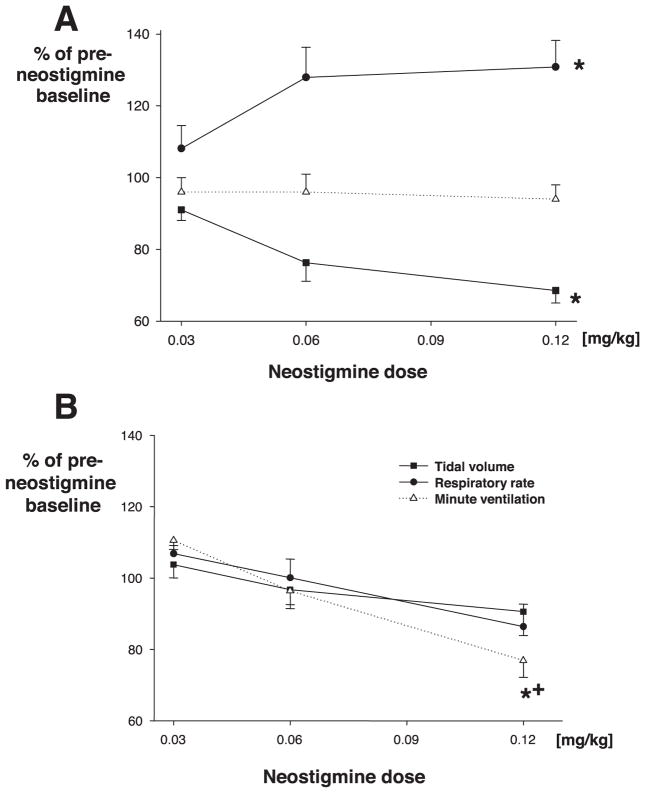
Tidal volume decreased (F = 9.2, P < 0.01) significantly with increasing neostigmine doses to a nadir of 68.6 ± 3.5% of baseline, whereas the respiratory rate increased up to 138 ± 7.4% of baseline, such that minute ventilation remained constant (fig. 5A). Time to recovery of the respiratory rate and tidal volume mirrored almost exactly the recovery of the diaphragmatic electromyogram.
Fig. 5.
Peak effects of neostigmine on minute ventilation, respiratory rate, and tidal volume in rats. (A) Effect of neostigmine on minute ventilation after recovery from neuromuscular blockade. Neostigmine administered after recovery from vecuronium neuromuscular block impaired minute ventilation in tracheostomized rats. (B) Effect of neostigmine (no previous neuromuscular blockade). Rats developed rapid shallow breathing, but minute ventilation remained constant. * P < 0.05 versus baseline. + P < 0.05 for between-groups effects (with vs. without previous vecuronium injection).
The type of antimuscarinergic drug (atropine vs. glycopyrrolate) coadministered with neostigmine did not affect measures of respiratory function. Neither atropine nor glycopyrrolate when given alone affected genioglossus or diaphragm electromyogram. Arterial blood pressure (systolic: 127 ± 4 mmHg, diastolic: 102 ± 3.8 mmHg), arterial partial pressure of oxygen (230 ± 7.6 mmHg) and carbon dioxide (35 ± 2 mmHg, values before neostigmine injection) were not significantly different.
Neostigmine given after recovery from NB affected neither TOF ratio (96.5 ± 11% of preneostigmine values) nor twitch height (96.2 ± 5.5% of preneostigmine values).
Protocol 2: Effects of Neostigmine Administered after Recovery from Neuromuscular Blockade
The prevecuronium value for TOF ratio amounted to 1.00 ± 1.08. Neostigmine given after recovery of the TOF ratio from NB did not affect TOF ratio, but dose-dependently decreased inspiratory (phasic) genioglossus electromyogram and diaphragm electromyogram to a nadir of 39.7 ± 2.3% of values observed immediately before injection (F = 7.6, P < 0.01). Peak effect at the genioglossus muscle was observed 13.2 ± 1.1 s after neostigmine injection.
As in protocol 1, neostigmine effects on genioglossus were significantly greater (F = 15.8, P < 0.0001) compared with the effects on the diaphragm, which decreased activity only after injection of the highest dose (to 74.6 ± 29% of values observed immediately before injection; P = 0.024, paired t test; fig. 3). Nonetheless, minute ventilation decreased dose dependently (F = 4.7, P = 0.022) after neostigmine injection to a nadir value of 76.9 ± 5% of preneostigmine baseline (fig. 5B). Minute ventilation decrements were short-lived (at most 25 ± 3 s) and were not associated with a measurable increase in end-tidal carbon dioxide. The time of recovery of minute ventilation was dependent on neostigmine dose (F = 7.3, P = 0.01). After recovery of the TOF ratio to unity from NB, genioglossus electromyographic activity was significantly higher (135 ± 19% of baseline) compared with the diaphragm electromyogram (95 ± 9% of baseline; P < 0.05, paired t test).
Comparison of Effects of Neostigmine Given with and without Previous Neuromuscular Blockade (Analysis of Pooled Data from Protocols 1 and 2)
Previous administration of vecuronium tended to ameliorate effects of neostigmine on diaphragm electromyogram (F = 2.74, P = 0.1), but not its effects on genioglossus electromyogram (F = 0.23, P = 0.88). At the time of neostigmine injection, end-tidal carbon dioxide concentration was higher in animals pretreated with vecuronium, amounting to 54.7 ± 3.3 mmHg (protocol 2) versus 44 ± 2.6 mmHg (protocol 1; P = 0.012, t test); these values did not change during the time between neostigmine injection and measurement of its peak effect at the respiratory muscles.
The effects of neostigmine on minute ventilation differed significantly between groups (F = 4.7, P = 0.036; fig. 5), with ventilation only impaired with previous administration of vecuronium. Respiratory rate was significantly (F = 22.76, P < 0.0001) lower in rats recovering from vecuronium (138 ± 7.4% of baseline [protocol 1] vs. 86 ± 6% [protocol 2]; F = 37.6, P < 0.0001).
Protocol 3: Effects of Neostigmine on Airway Volume Magnetic Resonance Imaging
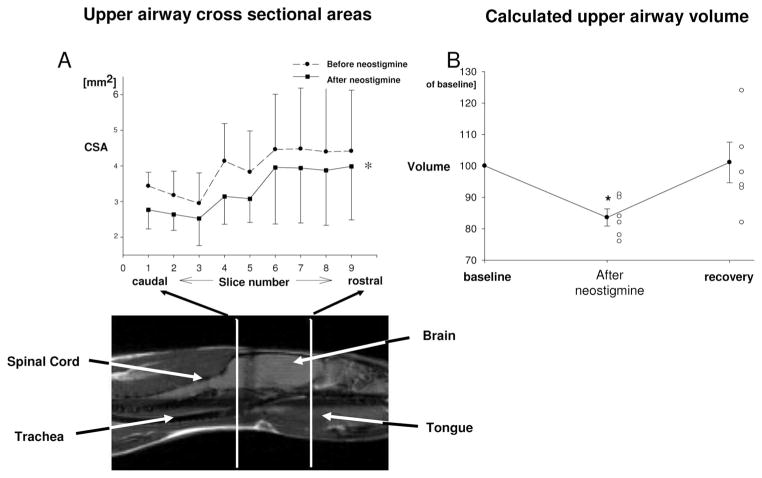
Neostigmine at 0.12 mg/kg (and glycopyrrolate at 0.03 mg/kg) decreased upper airway volume from 36.4 ± 10.3 mm3 to 30.6 ± 8.7 mm3, as depicted in figure 6. Post block recovery values of upper airway size amounted to 101 ± 7% of baseline values.
Fig. 6.
Effects of 0.12 mg/kg neostigmine on upper airway structure. (A) Cross-sectional areas (CSAs) of rat upper airway. The sagittal view shows the rat in the prone position. Measurements were made from the junction of the soft and hard palate (cranial margin) down to 9 mm below (area of the vocal cords). CSAs are significantly decreased after injection of neostigmine. (B) Upper airway volume at baseline, immediately after injection of neostigmine and 45 min after injection (recovery). Individual data (open symbols) and mean ± SEM (closed symbols with error bars). Upper airway volume was significantly decreased with neostigmine, and mean values recovered 45 min after drug injection.
Lung volume did not change after injection of reversal agents (7.0 ± 0.5 ml before vs. 6.9 ± 0.4 ml).
Discussion
This study showed that neostigmine administration after recovery from NB impaired genioglossus and diaphragmatic function in a dose-dependent fashion. Effects were significantly greater in the upper airway dilator muscle compared with the respiratory pump muscle. Neostigmine-evoked upper airway muscle dysfunction was associated with a decrease in upper airway volume, whereas end-expiratory lung volume was unaffected. Previous administration of vecuronium introduced a brief decrease of minute volume in response to neostigmine that was not present in untreated rats.
Effects of neuromuscular blocking drugs are muscle dependent,19 and it has been suggested that upper airway dilator muscles are particularly susceptible to low doses of nondepolarizing neuromuscular blocking drugs.1,20 Our findings suggest that the upper airways may also be especially vulnerable to an overabundance of acetylcholine at the neuromuscular junction; we found that doses of neostigmine that affected neither the TOF ratio nor the twitch height of the quadriceps femoris muscle nonetheless markedly impaired genioglossus muscle function and that this impairment was significantly greater than that of the diaphragm. It is possible that TOF ratio measurements are not sensitive enough to detect small degrees of skeletal muscle dysfunction from partial paralysis.21,22 Indeed, a decrease in upper esophageal sphincter resting tone,1 peak inspiratory flow,3 and end-inspiratory upper airway volume4 can occur in some volunteers recovering from NB, even with recovery of the TOF ratio to 0.9 –1.1,3,4 Neuromuscular blocking drugs (both nondepolarizing and depolarizing compounds including neostigmine11) produce a progressive failure of neuromuscular transmission with increasing rates of stimulation.11,23 The TOF stimulation test uses a stimulation rate of 2 Hz. By contrast, the firing frequency of the genioglossus during normal inspiration is higher (15–25 Hz),24 such that decreases in genioglossus activity during partial paralysis may exist under conditions where TOF ratio and twitch height are normal. This might explain why the phasic genioglossus muscle activity was more susceptible to neostigmine than the quadriceps femoris muscle function assessed by intermittent stimulation with 2 Hz.
We also found that neostigmine decreased genioglossus electromyogram more than the diaphragm electromyogram, which may be due to a number of factors including but not limited to differences in discharge rate, chemosensitivity, blood flow, fiber size, and acetylcholine receptor density. Although our study does not address these mechanisms, we speculate that the low firing frequency of the diaphragm (diaphragm: 8 –13 Hz; genioglossus: 15–25 Hz)24 during quiet breathing may account for the lower vulnerability of the diaphragm to neostigmine.
Animals breathed spontaneously throughout, and mild hypercapnia was present in rats after recovery of the TOF ratio from vecuronium, when neostigmine was injected. Hypercapnia might explain why genioglossus activity at time of neostigmine injection was higher than the diaphragm electromyogram in these rats. In fact, the activating effect of carbon dioxide on respiratory muscles is stronger at the genioglossus compared with the diaphragm.25 In animals recovering from vecuronium NB, neostigmine significantly and dose-dependently decreased minute volume. Because the rats were tracheostomized in our study, these effects on breathing were not accounted for by airway obstruction and were likely caused by direct impairment of pump muscle function. We speculate that decrements in minute volume in the presence of neostigmine may have been a consequence of an impaired chemoreceptive ventilatory response by an unidentified mechanism, such as release of endogenous opioids during hypercapnia.26
We observed that neostigmine effects on diaphragmatic function were less intense, and pathologic breathing did not occur, if the neuromuscular blocking drug vecuronium had been given previously. Regarding the mechanism of this observation, we speculate from our data that some residual effects of vecuronium might have been present at the diaphragm at the time of injection of reversal agents (i.e., at the time of recovery of the TOF ratio to unity), which protected the muscle from (neostigmine-evoked) depolarizing neuromuscular block.27 In fact, after recovery from vecuronium-evoked NB, at the time of injection of neostigmine, diaphragm electromyograms showed some decrease from baseline in our study, whereas genioglossus electromyograms had recovered to significantly higher values at this time.
In contrast to studies showing that upper airway muscles are susceptible to NB,1–4 some data suggest that upper airway patency can be stable in humans during partial NB in volunteers.28 D’Honneur et al.28 did not find signs and symptoms of upper airway collapse during partial paralysis at a TOF ratio of 0.5. Although it is unclear why the results differ between studies, possible explanations include adaptive neuromuscular mechanisms such as posttetanic potentiation and the genioglossus negative pressure reflex. In fact, D’Honneur et al.28 decreased the upper airway pressure progressively every three respiratory cycles, such that the maximum challenge to the airway dilator muscles should have been reached with a delay of approximately 2 min after initiation of the pressure drop. Accordingly, we speculate that partial paralysis may impair the “passive”29 inspiratory airway function during (forced) inspiration, whereas the dynamic upper airway dilator muscle response that compensates for pharyngeal mechanical loads when the muscles are put under stress29 may be less affected. This suggestion is also supported by the observation that phasic reflexes are resistant to the effects of neuromuscular blocking agents.30
Our study provides some insight into the mechanism of the observed ChEI-evoked upper airway volume decrease. When applied directly into the brain, neostigmine has central ventilatory effects that are similar to those observed in our study.31,32 However, it is unlikely that central effects of neostigmine account quantitatively for its effects on breathing observed in our study. In contrast to physostigmine, which is a tertiary amine that crosses the blood–brain barrier freely, neostigmine, a quaternary compound, would not be expected to move freely across this barrier. This difference has been demonstrated in rats.33 The effects of neostigmine on upper airway size could potentially be mediated by NB of the upper airway dilator muscles or by an increase in upper airway stiffness. The latter mechanism is unlikely to account quantitatively for the neostigmine-evoked decrease in upper airway diameter. Increased upper airway stiffness can be the consequence of tracheal traction as a result of increased lung volume34 or increased tonic upper airway dilator muscle activity. Our data show that in rats that breathe by the physiologic route (i.e., rats that were not tracheostomized; protocol 3), neostigmine did not affect end-expiratory lung volume as shown by three-dimensional magnetic resonance imaging. Moreover, neostigmine did not affect tonic genioglossus activity, one means of affecting airway stiffness. By contrast, neostigmine decreased markedly phasic genioglossus activity, and this effect at the upper airway dilator muscle was significantly greater than the impairment of diaphragmatic function. Therefore, we suggest that the observed decrease in inspiratory upper airway volume was caused, at least in part, by a neostigmine-evoked weakness of upper airway dilator muscles.
Our data show that ChEIs not only do not protect, but actually impair upper airway integrity and ventilation when administered to rats after full recovery from NB. The degree of ChEI-evoked impairment of upper airway function observed in this study is likely to be physiologically significant. Decreases in genioglossus activity and upper airway volume observed in this study are similar to that reported in humans during residual NB at a TOF ratio of 0.5– 0.8,4 which is known to put patients at risk for postoperative respiratory complications35 when NB drugs of long duration have been used. However, it is unclear whether the same doses of neostigmine (0.03–0.12 mg/kg) administered in rats have comparable respiratory side effects in humans.
Reversal of residual NB is an important goal in terms of patients’ postoperative safety, because reversal is associated on average with a decreased risk of 24-h postoperative morbidity and mortality.36 By contrast, omitting antagonism introduces a significant risk of residual paralysis even with short-acting neuromuscular blocking agents.37 Therefore, it is important to state that reversal of an existing NB improves patients’ safety, a statement that is supported by our pilot data. Neostigmine restored genioglossus and diaphragmatic function to baseline values within 4 min in all rats. By contrast, if the current findings in rats are relevant in humans, our data would support the view that partial paralysis should be first identified, and then reversed only if detected, particularly when high doses (0.04 –0.07 mg)7,10 are given.
Limitations
This preclinical study was not designed to mimic clinical practice. Species-dependent effects of neuromuscular blocking agents have been reported,38 and rats are believed to be more resistant to depolarizing neuromuscular blocking drugs than humans.38 Therefore, the dose–response curve of ChEIs with respect to genioglossus muscle electromyogram may be different in humans compared with this study in rats. Ultimately, dose–response studies in humans are required to define the risk– benefit ratio on the upper airway muscles of ChEI when given for treatment of NB. In fact, it has already been shown that the even the TOF ratio can decrease when 0.04 mg/kg neostigmine is given to humans after recovery of the TOF ratio from NB.14 A patient fully recovered from neuromuscular block will occasionally receive neostigmine, and it would be interesting to see how our results translate to what happens in human volunteers.
In summary, our data show that neostigmine markedly impairs upper airway dilator volume, genioglossus muscle function, diaphragmatic function, and breathing when given after recovery from vecuronium-induced neuromuscular block.
Acknowledgments
Received from the Division of Sleep Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts. Submitted for publication February 21, 2007. Accepted for publication July 6, 2007. Supported by grants from the National Institute of Health (Beeson Award K23 AG024837-01), Nos. P50 HL 60292 and RO1-HL73146 (National Institutes of Health, Bethesda, Maryland); and Organon International Inc., Roseland, New Jersey. Organon did not participate in generation of the protocol, data analysis and interpretation, or writing.
The authors thank Sebastian Zaremba, cand. med. (Research Associate, Brigham and Women’s Hospital, Boston, Massachusetts), for his helpful assistance with data analysis during revision of the manuscript.
Footnotes
Additional material related to this article can be found on the Anesthesiology Web site. Go to http://www.anesthesiology.org, click on Enhancements Index, and then scroll down to find the appropriate article and link. Supplementary material can also be accessed on the Web by clicking on the “ArticlePlus” link either in the Table of Contents or at the top of the Abstract or HTML version of the article.
Information on purchasing reprints may be found at www.anesthesiology.org or on the masthead page at the beginning of this issue. Anesthesiology’s articles are made freely accessible to all readers, for personal use only, 6 months from the cover date of the issue.
References
- 1.Sundman E, Witt H, Olsson R, Ekberg O, Kuylenstierna R, Eriksson LI. The incidence and mechanisms of pharyngeal and upper esophageal dysfunction in partially paralyzed humans: Pharyngeal videoradiography and simultaneous manometry after atracurium. Anesthesiology. 2000;92:977–84. doi: 10.1097/00000542-200004000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Eikermann M, Groeben H, Bunten B, Peters J. Fade of pulmonary function during residual neuromuscular blockade. Chest. 2005;127:1703–9. doi: 10.1378/chest.127.5.1703. [DOI] [PubMed] [Google Scholar]
- 3.Eikermann M, Groeben H, Husing J, Peters J. Accelerometry of adductor pollicis muscle predicts recovery of respiratory function from neuromuscular blockade. Anesthesiology. 2003;98:1333–7. doi: 10.1097/00000542-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Eikermann M, Vogt FM, Herbstreit F, Vahid-Dastgerdi M, Zenge MO, Ochterbeck C, de Greiff A, Peters J. The predisposition to inspiratory upper airway collapse during partial neuromuscular blockade. Am J Respir Crit Care Med. 2007;175:9–15. doi: 10.1164/rccm.200512-1862OC. [DOI] [PubMed] [Google Scholar]
- 5.Viby-Mogensen J, Jorgensen BC, Ording H. Residual curarization in the recovery room. Anesthesiology. 1979;50:539–41. doi: 10.1097/00000542-197906000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson LI. Reduced hypoxic chemosensitivity in partially paralysed man: A new property of muscle relaxants? Acta Anaesthesiol Scand. 1996;40:520–3. doi: 10.1111/j.1399-6576.1996.tb04482.x. [DOI] [PubMed] [Google Scholar]
- 7.Bevan DR, Donati F, Kopman AF. Reversal of neuromuscular blockade. Anesthesiology. 1992;77:785–805. doi: 10.1097/00000542-199210000-00025. [DOI] [PubMed] [Google Scholar]
- 8.Klingenmaier CH, Bullard R, Thompson D, Watson R. Reversal of neuromuscular blockade with a mixture of neostigmine and glycopyrrolate. Anesth Analg. 1972;51:468–72. [PubMed] [Google Scholar]
- 9.Baurain MJ, Hoton F, D’Hollander AA, Cantraine FR. Is recovery of neuromuscular transmission complete after the use of neostigmine to antagonize block produced by rocuronium, vecuronium, atracurium and pancuronium? Br J Anaesth. 1996;77:496–9. doi: 10.1093/bja/77.4.496. [DOI] [PubMed] [Google Scholar]
- 10.Sacan O, White PF, Tufanogullari B, Klein K. Sugammadex reversal of rocuronium-induced neuromuscular blockade: A comparison with neostigmine-glycopyrrolate and edrophonium-atropine. Anesth Analg. 2007;104:569–74. doi: 10.1213/01.ane.0000248224.42707.48. [DOI] [PubMed] [Google Scholar]
- 11.Churchill-Davidson HC, Christie TH. The diagnosis of neuromuscular block in man. Br J Anaesth. 1959;31:290–301. doi: 10.1093/bja/31.7.290. [DOI] [PubMed] [Google Scholar]
- 12.Payne JP, Hughes R, Al Azawi S. Neuromuscular blockade by neostigmine in anaesthetized man. Br J Anaesth. 1980;52:69–76. doi: 10.1093/bja/52.1.69. [DOI] [PubMed] [Google Scholar]
- 13.Yost CS, Maestrone E. Clinical concentrations of edrophonium enhance desensitization of the nicotinic acetylcholine receptor. Anesth Analg. 1994;78:520–6. doi: 10.1213/00000539-199403000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Caldwell JE. Reversal of residual neuromuscular block with neostigmine at one to four hours after a single intubating dose of vecuronium. Anesth Analg. 1995;80:1168–74. doi: 10.1097/00000539-199506000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Legendre P, Ali DW, Drapeau P. Recovery from open channel block by acetylcholine during neuromuscular transmission in zebrafish. J Neurosci. 2000;20:140–8. doi: 10.1523/JNEUROSCI.20-01-00140.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brennick MJ, Trouard TP, Gmitro AF, Fregosi RF. MRI study of pharyngeal airway changes during stimulation of the hypoglossal nerve branches in rats. J Appl Physiol. 2001;90:1373–84. doi: 10.1152/jappl.2001.90.4.1373. [DOI] [PubMed] [Google Scholar]
- 17.Chamberlin NL, Eikermann M, Fassbender P, White DP, Malhotra A. Genioglossus premotoneurons and the negative pressure reflex in rats. J Physiol. 2007;579:515–26. doi: 10.1113/jphysiol.2006.121889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammond J, Wright D, Sale J. Pattern of change of bronchomotor tone following reversal of neuromuscular blockade: Comparison between atropine and glycopyrrolate. Br J Anaesth. 1983;55:955–9. doi: 10.1093/bja/55.10.955. [DOI] [PubMed] [Google Scholar]
- 19.Hemmerling TM, Schmidt J, Hanusa C, Wolf T, Schmitt H. Simultaneous determination of neuromuscular block at the larynx, diaphragm, adductor pollicis, orbicularis oculi and corrugator supercilii muscles. Br J Anaesth. 2000;85:856–60. doi: 10.1093/bja/85.6.856. [DOI] [PubMed] [Google Scholar]
- 20.Isono S, Kochi T, Ide T, Sugimori K, Mizuguchi T, Nishino T. Differential effects of vecuronium on diaphragm and geniohyoid muscle in anaesthetized dogs. Br J Anaesth. 1992;68:239–43. doi: 10.1093/bja/68.3.239. [DOI] [PubMed] [Google Scholar]
- 21.Waud BE, Waud DR. The relation between tetanic fade and receptor occlusion in the presence of competitive neuromuscular block. Anesthesiology. 1971;35:456–64. doi: 10.1097/00000542-197111000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Eikermann M, Gerwig M, Hasselmann C, Fiedler G, Peters J. Impaired neuromuscular transmission after recovery of the train-of-four ratio. Acta Anaesthesiol Scand. 2007;51:226–34. doi: 10.1111/j.1399-6576.2006.01228.x. [DOI] [PubMed] [Google Scholar]
- 23.Gissen AJ, Katz RL. Twitch, tetanus and posttetanic potentiation as indices of nerve-muscle block in man. Anesthesiology. 1969;30:481–7. doi: 10.1097/00000542-196905000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Saboisky JP, Gorman RB, De Troyer A, Gandevia SC, Butler JE. Differential activation among five human inspiratory motoneuron pools during tidal breathing. J Appl Physiol. 2007;102:772–80. doi: 10.1152/japplphysiol.00683.2006. [DOI] [PubMed] [Google Scholar]
- 25.Haxhiu MA, van Lunteren E, Mitra J, Cherniack NS. Responses to chemical stimulation of upper airway muscles diaphragm in awake cats. J Appl Physiol. 1984;56:397–403. doi: 10.1152/jappl.1984.56.2.397. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg HE, Tarasiuk A, Rao RS, Kupferman M, Kane N, Scharf SM, Kuperferman M. Effect of chronic resistive loading on ventilatory control in a rat model. Am J Respir Crit Care Med. 1995;152:666–76. doi: 10.1164/ajrccm.152.2.7633724. [DOI] [PubMed] [Google Scholar]
- 27.Szalados JE, Donati F, Bevan DR. Effect of d-tubocurarine pretreatment on succinylcholine twitch augmentation and neuromuscular blockade. Anesth Analg. 1990;71:55–9. doi: 10.1213/00000539-199007000-00009. [DOI] [PubMed] [Google Scholar]
- 28.D’Honneur G, Lofaso F, Drummond GB, Rimaniol JM, Aubineau JV, Harf A, Duvaldestin P. Susceptibility to upper airway obstruction during partial neuromuscular block. Anesthesiology. 1998;88:371–8. doi: 10.1097/00000542-199802000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol. 2007;102:547–56. doi: 10.1152/japplphysiol.00282.2006. [DOI] [PubMed] [Google Scholar]
- 30.Smith CM, Budris AV, Paul JW. Quantification of phasic and tonic stretch reflexes: Effects of neuromuscular blocking agents. J Pharmacol Exp Ther. 1962;136:267–75. [PubMed] [Google Scholar]
- 31.Douglas CL, Bowman GN, Baghdoyan HA, Lydic R. C57BL/6J and B6. V-LEPOB mice differ in the cholinergic modulation of sleep and breathing. J Appl Physiol. 2005;98:918–29. doi: 10.1152/japplphysiol.00900.2004. [DOI] [PubMed] [Google Scholar]
- 32.Lydic R, Douglas CL, Baghdoyan HA. Microinjection of neostigmine into the pontine reticular formation of C57BL/6J mouse enhances rapid eye movement sleep and depresses breathing. Sleep. 2002;25:835–41. doi: 10.1093/sleep/25.8.835. [DOI] [PubMed] [Google Scholar]
- 33.Stark P, Boyd ES. Effects of cholinergic drugs on hypothalamic self-stimulation response rates of dogs. Am J Physiol. 1963;205:745–8. doi: 10.1152/ajplegacy.1963.205.4.745. [DOI] [PubMed] [Google Scholar]
- 34.Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol. 1988;65:2124–31. doi: 10.1152/jappl.1988.65.5.2124. [DOI] [PubMed] [Google Scholar]
- 35.Berg H, Roed J, Viby-Mogensen J, Mortensen CR, Engbaek J, Skovgaard LT, Krintel JJ. Residual neuromuscular block is a risk factor for postoperative pulmonary complications: A prospective, randomised, and blinded study of postoperative pulmonary complications after atracurium, vecuronium and pancuronium. Acta Anaesthesiol Scand. 1997;41:1095–103. doi: 10.1111/j.1399-6576.1997.tb04851.x. [DOI] [PubMed] [Google Scholar]
- 36.Arbous MS, Meursing AE, van Kleef JW, de Lange JJ, Spoormans HH, Touw P, Werner FM, Grobbee DE. Impact of anesthesia management characteristics on severe morbidity and mortality. Anesthesiology. 2005;102:257–68. doi: 10.1097/00000542-200502000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Tramer MR, Fuchs-Buder T. Omitting antagonism of neuromuscular block: Effect on postoperative nausea and vomiting and risk of residual paralysis: A systematic review. Br J Anaesth. 1999;82:379–86. doi: 10.1093/bja/82.3.379. [DOI] [PubMed] [Google Scholar]
- 38.Zaimis EJ. Motor end-plate differences as a determining factor in the mode of action of neuromuscular blocking substances. J Physiol. 1953;122:238–51. doi: 10.1113/jphysiol.1953.sp004995. [DOI] [PMC free article] [PubMed] [Google Scholar]