Synopsis
Glioblastoma remains one of the most difficult cancers to treat and represents the most common primary malignancy of the brain. While conventional treatments have found modest success in reducing the initial tumor burden, infiltrating cancer cells beyond the main mass are responsible for tumor recurrence and ultimate patient demise. Targeting the residual infiltrating cancer cells requires the development of new treatment strategies. The emerging field of cancer nanotechnology holds much promise in the use of multifunctional nanoparticles for the imaging and targeted therapy of GBM.. Nanoparticles have emerged as potential “theranostic” agents that can permit the diagnosis and therapeutic treatment of GBM tumors. A recent human clinical trial with magnetic nanoparticles has provided feasibility and efficacy data for potential treatment of GBM patients with thermotherapy. Here we examine the current state of nanotechnology in the treatment of glioblastoma and interesting directions of further study.
Keywords: Malignant Brain Tumors, Glioblastoma, Magnetic Nanoparticles, Nanoparticles, Convection-Enhanced Delivery, MRI, EGFR, Thermotherapy
Introduction
Glioblastoma (GBM), is the most common primary malignancy of the brain, as well as its most malignant 1. The median survival after radiation and chemotherapy ranges from 12 to 15 months, despite advances in surgery, radiation, and chemotherapy 2. GBM, tumors are nearly uniformly fatal due to local recurrence 3–5. Even for lesions amenable to gross surgical resection, infiltrating cancer cells beyond the boundaries of the enhancing lesion are responsible for tumor recurrence as well as radiation and chemotherapy resistance 6,7.
Cancer nanotechnology has recently emerged as a field which may provide answers to some of the difficulties encountered in treating GBM. Nanoparticles, defined as particles less than 100 nm in hydrodynamic size have been used in the treatment of various cancers 8. The use of biocompatible nanomaterials have permitted the fabrication of nanoparticles with capabilities that surpass those of conventional agents. Chemotherapy-loaded nanoparticles have resulted in sustained release formulations that can lower systemic toxicity and produce greater antitumor effects. Recently developed nanoparticles can cross the blood-brain barrier after systemic administration or be distributed in the brain by convection-enhanced delivery (CED) to target GBM cells therapeutically while harboring elements which may enable imaging of the particle and the target. The field has been moving at a rapid pace, enabling nanoparticles to be utilized in recent clinical trials 9. While not exhaustive, the list of nanoparticles being used in the treatment of experimental GBM includes polymeric particles, micelles 10, nanoshells 11, quantum dots 12, and magnetic iron-oxide nanoparticles (IONPs) 13. Nanotubes are another formulation of nanoparticle, being used to create structures that can trap diagnostic or therapeutic modalities within a cage. We will discuss the use of different nanoparticle formulations in strategies to image and treat GBM, including delivery schemes.
1.0 Magnetic Nanoparticles (MNPs)
Tags: Malignant Brain Tumors, Glioblastoma, GBM, Magnetic Nanoparticles, Nanoparticles, Convection-Enhanced Delivery, MRI, EGFR, Thermotherapy
1.1 MRI Contrast properties of MNPs
The base of the promise for “theranostic” nanoparticles with both therapeutic and diagnostic ability hinges on the idea that such nanoparticles will be able to image where the lesion is and treat it. Magnetic nanoparticles (MNPs) have attracted particular interest in this respect due to their unique paramagnetic properties that enable their detection by MRI 14,15. These MNPs have shown great potential as T1 or T2 contrast agents in MRI imaging 16,17, with superparamagnetic iron oxide-based nanoparticles (SPIOs) as the most commonly investigated type of MRI contrast agents 18. Since 1990, ultrasmall superparamagnetic iron oxide nanoparticles (USPIOs), smaller than 50nm, have been considered as an MRI contrast agent 19, and most of the MRI data regarding nanoparticles references these particles. USPIOs can be visualized in T2-weighted MRI sequences (T2 contrast agents) as a hypointense (dark) signal (negative contrast enhancement) or with T1-weighted MRI sequences (T1 contrast agents) as a hyperintense (bright) signal (positive contrast enhancement) 20–22.
USPIOs can provide contrast for a longer period of time 23, as compared to Gd-based contrast agents that are rapidly eliminated by the kidney 24,25. USPIOs are also taken up by tumor cells as well as by reactive phagocytic cells (e.g., microglia) found in brain tumors. The USPIOs can reside within brain tumors much longer than Gd-based agents, with a peak enhancement noted at 24–28 hours and persisting up to 72 hours after administration 26, 27. These agents may provide a safe alternative for patients at risk for nephrogenic systemic fibrosis, as preliminary studies have shown no adverse renal effects 27,28.
1.1.1 MNPs for Targeted Brain Tumor Imaging
Targeting of tumor cells can increase the benefits provided by nanoparticles as contrast agents. IONPs are taken up by GBM cells both in vivo and in vitro 29,30. Surface functionalization further enhances tumor uptake of these particles 31. Tumor-specific ligands conjugated to MNPs can further enhance the uptake within targeted tumor tissue (Figure 1) 32,33. Antibodies, peptides (including toxins), cytokines, and chemotherapeutic agents have been reported as possible MNP ligands 34. Amphiphilic triblock copolymer IONPs can be conjugated with a purified antibody that selectively binds to the epidermal growth factor receptor deletion mutant, EGFRvIII, which is solely expressed by a population of GBM tumors 35. Such nanoparticles exhibit MR contrast enhancement of GBM cells and can target these therapy-resistant cancer cells in vitro and in vivo.
Figure 1. Theranostic magnetic nanoparticles (MNPs) and tumor targeting.
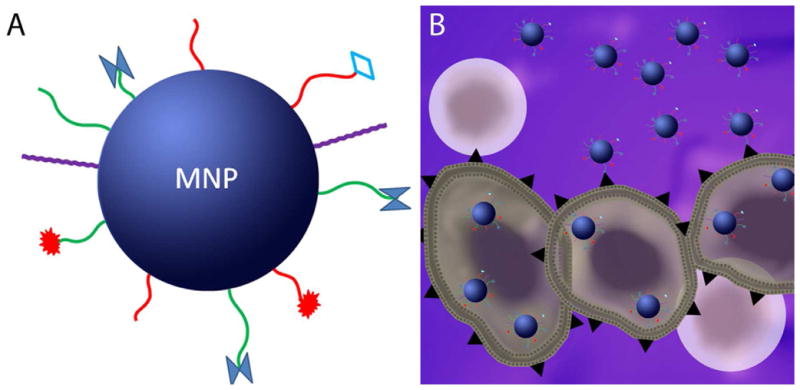
A., Illustration of a MNP with different functional groups on the surface which permit molecular targeting, imaging, enhanced plasma circulation times, and/or therapy. B., Illustration of MNPs functionalized with tumor cell specific ligands binding cancer cells (large irregular cells) instead of normal cells (in pink). Internalization of MNPs is shown in cancer cells as well.
Chlorotoxin, derived from scorpion venom, specifically binds to matrix metalloproteinase-2 (MMP-2), which is over-expressed on the surface of GBM cells 36,37. MMP-2 degrades the extracellular matrix during tumor invasion, and Chlorotoxin can be used to bind the MMP-2 and inhibit infiltration 38, 39. Chlorotoxin conjugated to MNPs can act as MRI contrast agents and the addition of a Cy5.5 molecule makes these suitable for use as an intraoperative fluorescent dye as well 40–42.
F3 is a small peptide that specifically binds to nucleolin over-expressed on proliferating endothelial cells of tumor cells and the associated vasculature 43. F3 coated IONPs can provide significant MRI contrast enhancement of intracranial rat-implanted tumors, compared with non-coated F3 nanoparticles, when administered intravenously 44.
A molecular MRI contrast agent, consisting of superparamagnetic iron-oxide nanoparticle coated with dextran, was functionalized with an anti-insulin-like-growth-factor binding protein 7 (anti-IGFBP7) single domain antibody and was found by both MRI and in vivo fluorescent imaging to target the vasculature of GBM cells 45.
Gadolinium has also been incorporated into some some therapeutic nanoparticles to enable them to be tracked using MRI. One group has designed nanoparticles containing gadolinium which are rapidly taken up by the GL-261 tumor cell line and show MRI contrast when these cells are then cultured in a chick embryo host46. Gadolinium nanoparticles functionalized with diethylenetriaminepentaacetic acid (DTPA) can also be used as a radiosensitizing agent 47. Fullarene magnetic nanotubes have been made such that gadolinium can be trapped within these structures to make them an effective contrast agent, along with whatever therapeutic modality is also associated with the fullerene cage48,49. It is also possible to internalize iron-oxide nanoparticles in these larger nanotube structures so that the magnetic properties of iron-oxide can be utilized, allowing the clinician to localize these particles to a particular area. This, together with surface targeting, can greatly increase the amount of intake and resultant therapeutic effect of these particles 50.
1.2 MNPs for Optical Delineation of Brain Tumors
While surgical intervention is not curative in GBM, obtaining a maximal resection is important for survival 51. The use of intraoperative MRI and neuronavigation have increased extent of resection and outcome 52–54. Recently, fluorescence-guided surgery after oral administration of 5-ALA has resulted in more complete resection of malignant gliomas 55,56. Laboratory studies have attempted to find ways to use optical aides to increase the contrast between normal and tumor tissue 57–59, and these methods have shown improvement in the extent of tumor resection in clinical use 60,61.
Fluorescent molecules have already been sucessfully incorporated into several nanoparticles. An IONP-Cy5.5 molecule has been used in many pre-clinical studies 40,41,62, giving it the dual benefits of MRI detection and possibly enhanced surgical contrast using the fluorescent properties of the particle. This also could lead to theranostic particles which could be injected pre-operatively to outline malignant tissue which would need to be resected at surgery.
1.3 MNPs for Stem Cell Tracking
The ability of MNPs to act as MRI contrast agents can be used to track stem cell tropism to malignant brain tumors in vivo. Intracranially administered neural stem cells (NSCs) have tropism for GBM tumors, making them attractive for tumor-targeting gene therapy 63, 64,65. Mesenchymal stem cells (MSCs) have also been found to migrate to tumor cells 66. By labeling these cells with IONPs, this migration can be visualized on MRI 67,68. Magnetically-labeled hematopoietic stem cells can also be tracked to gliomas in this fashion 69.
1.4 MNPs for Thermotherapy of GBM
One of the more unique features of MNPs is the ability to induce hyperthermia when exposed to alternating magnetic fields. Temperature elevations in the range of 41 °C and 46 °C can cause cells to undergo heat stress, resulting in protein denaturation, protein folding, aggregation, and DNA cross-linking 70. This process can induce apoptosis and heat shock protein (HSP) expression. At the tissue level, moderate hyperthermia causes changes in pH, perfusion, and oxygenation of the tumor microenvironment 71–74. These effects, combined with chemotherapy and radiation, can have a synergistic effect74, 100–103.
Hyperthermia can be induced in MNPs through the use of an appropriate alternating magnetic field (AMF) of the right amplitude and frequency to heat up the nanoparticles. A predictable and sufficient amount of heat known as the specific absorption rate (SAR) is produced. The MNPs utilize several different mechanisms to convert the magnetic energy into heat energy. Néel relaxation is caused by rapidly occurring changes in the direction of magnetic moments relative to crystal lattice. Brownian relaxation results from the physical rotation of MNPs within the medium in which they are placed. Both internal (Néel) and external (Brownian) sources of friction lead to a phase lag between applied magnetic field and the direction of magnetic moment, producing thermal losses (Figure 2).
Figure 2. Magnetic nanoparticle (MNP) response to alternating magnetic fields and thermotherapy.
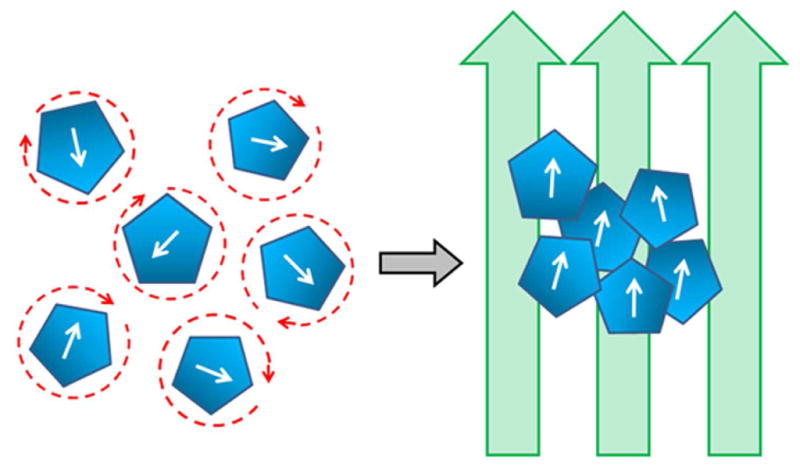
Application of applied magnetic fields (arrows) orients the MNPs on the right from their random orientation on the left in the absence of magnetic fields. Random orientation on the left produces thermal losses allowing for hyperthermia generation by the MNPs.
MNPs can be specifically engineered to maximize their suitability for hyperthermia, by producing greater saturation magnetization, optimal anisotropy, and larger size, within the constraints of nanoparticle production 75–77. MNPs suitable for thermotherapy can be made from a combination of various metals, including manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), zinc (Zn), magnesium (Mg) and their oxides 78–85. Ferrites of the various metals are frequently used in these settings, such as Cobalt ferrites (CoFe2O4), manganese ferrites (MnFe2O4), nickel ferrites (NiFe2O4), lithium ferrites (Li0.5Fe2.5O4), mixed ferrites of nickel–zinc–copper, and cobalt–nickel ferrites 81–87. There are also ferromagnetic NPs that are iron-based and have greater magnetic properties than IONPs 75. These Fe-based NPs produce greater hyperthermia effects at much lower concentrations than IONPs. FeNPs are comprised of an Fe core surrounded by an iron-oxide layer to permit stability. Nevertheless, owing to their lack of toxicity, excellent biocompatibility, and their capacity to be metabolized 88–90, iron oxide-based MNPs are actively being studied for thermotherapy of brain tumors.
MNP-based hyperthermia has been evaluated for feasibility in animal models and in human patients with malignant brain tumors. Dextran- or aminosilane-coated IONPs have been used for thermotherapy in a rodent GBM model 91 and in a human clinical trial in patients with recurrent GBM 9,92. Intratumoral injection of aminosilane-coated IONPs (core size 12 nm) and application of an AMF (100 kHz) in several sessions before and after adjuvant fractionated radiation therapy was given. With a high concentration of IONPs (>100 mg/ml), this achieved effective thermotherapy with a median peak temperature within the tumor of 51.2 °C. This Phase II clinical trial successfully demonstrated safety and efficacy of thermotherapy of malignant brain tumors with MNPs in humans, with a significant increase in overall survival as compared to a reference population. Further randomized studies will be required to validate the promise of this treatment modality.
2.0 Nanoparticlized Chemotherapeutic Agents
Tags: Malignant Brain Tumors, Glioblastoma, GBM, Nanoparticles, Convection-Enhanced Delivery, Chemotherapy
While few conventional chemotherapeutics have been proven effective in GBM, chemotherapeutics in a nanoparticle formulation offer possible advantages. These often can be targeted, evade the reticuloendothelial system for prolonged circulatory time, and can potentially cross the BBB better then standard chemotherapy agents. Polyethylene glycol-coated (PEG) coated paclitaxel (taxol) nanoparticles have been shown to offer superior bioavailability as compared to free paclitaxel with a survival advantage shown in a rodent glioma model 93. Poly(d,l-lactide-co-glycolide) (PLGA) nanoparticles are another form of biocompatible nanoparticles. Convection-enhanced delivery of these nanoparticles, loaded with camptothecin, has been shown to be efficacious in a rodent glioma model 94. While the controlled release offered by nanoparticles can reduce systemic toxicity and allow drug to be slowly released only when it has reached its target, there is also a need to ensure that an adequate dose is delivered to the lesion being treated. Nanoparticles have been developed which are thermosensitive, releasing their drug preferentially when the temperature has been increased 95. When delivered with gold nanorods, concurrent photothermal hyperthermia can release the drug from the heat sensitive nanoparticle, thus increasing efficacy.
3.0 Gene delivery with Nanoparticles
Tags: Malignant Brain Tumors, Glioblastoma, GBM, Nanoparticles, Convection-Enhanced Delivery, Gene therapy
The TCGA has revealed the multiple genetic aberrations in GBM tumors that can serve as therapeutic targets provide targets 96. Cationic solid lipid nanoparticles can be conjugated to PEGylated therapeutic c-Met siRNA and reduce human GBM tumor growth in a rodent model without significant toxicity 97. Another nanoparticle, containing the integrin binding motif, RGD, together with the PEG-PEI non-viral gene carrying nanoparticle, was able to deliver pORF-hTRAIL with increased efficiency and increase survival in a rodent glioma model 98.
4.0 Nanoparticles for Brachytherapy
Tags: Malignant Brain Tumors, Glioblastoma, GBM, Nanoparticles, Brachytherapy
Brachytherapy, where localized radiotherapy is delivered directly to a tumor, has been explored as a strategy with nanoparticles. In an orthotopic xenograft brain tumor model, a functionalized fullerene nanoparticle (177Lu-DOTA-f-Gd3N@C80) with radiolabeled lutetium 177 (177Lu) and tetraazacyclododecane tetraacetic acid (DOTA) provided an anchor to deliver effective brachytherapy and longitudinal imaging of the tumor 99. Internal fractionated radiation has also been achieved using a lipid nanoparticle formulation of radionucliides such as 188Re-SSS in the 9L rat glioma cell line.100
5.0 Gold Nanoparticle Phototherapy
Tags: Malignant Brain Tumors, Glioblastoma, GBM, Nanoparticles, Phototherapy, Gold nanoparticles
Gold nanoparticles can be designed as nanoshells, consisting of a spherical dielectric core nanoparticle surrounded by thin sheet metal 101. The size of each layer of the nanoshell can be tailored to enable it to have a peak light absorption at 800nm, in the near infrared range. Light in this region of the electromagnetic spectrum has minimal absorption by water and biological chromophores, allowing it to pass deep into tissues without losing much of its energy. This region of the electromagnetic spectrum is notable for minimal absorption by water and biological chromophores. Thus, light of this wavelength may penetrate deep into tissues with minimal disruption. This has enable researchers to produce such gold nanoparticles which can be activated by light and kill glioblastoma cells in vitro 102. One group has used macrophages loaded with gold nanoshells to deliver these particles to glioma spheroids to then be activated by near infrared light, inhibiting growth 103.
6.0 Malignant Brain Tumor Delivery of Nanoparticles
Tags: Malignant Brain Tumors, Glioblastoma, Magnetic Nanoparticles, Nanoparticles, Convection-Enhanced Delivery, Blood-brain barrier
Delivery of therapeutic agents to GBM tumors remains a formidable challenge. Systemic delivery is limited by the blood-brain barrier (BBB), non-specific uptake, nontargeted distribution, and systemic toxicity. We will examine the benefits and drawbacks of the use of systemic delivery, systemic delivery augmented by magnetic targeting, and direct infusion in the brain known as convection enhanced delivery (CED).
6.1 Systemic Delivery
The reticulo-endothelial system (RES) can significantly reduce the amount of nanoparticle available to treat the lesion by non-specific uptake in the liver, kidney, spleen, and circulating macrophages 104,105. This can be addressed by biocompatible surface coating of nanoparticles which can increase their circulation time 106. The BBB further obstructs delivery by preventing the entry of most particles from the circulation into the interstitial space of the brain. However, it is well-known that the vasculature in GBM is not phenotypically normal, due to open endothelial gaps and atypical angiogenesis, allowing more efflux of intravascular material into the tumor mass 107, 108,109. The enhanced permeability retention (EPR) effect is used to describe the selective extravasation of macromolecules, into the tumor interstitium through the hyper-permeable tumor vasculature 110. By attaching tumor-specific targeting ligands, delivery has been shown to be increased in a rodent model, as the extravasated treatment is more likely to be taken up by the lesion 44,111.
Integrins are over-expressed in GBM at the brain tumor border, and one of the integrin binding motifs is RGD. Conjugating this peptide to PEG and polyethylenimine (PEI) creates a nanoparticle which is targeted to GBM and was found to prolong survival in rodents implanted with human intracranial GBM xenografts 98. This same group was able to use their polyethylenimine-conjugated to DNA and myristic acid, a hydrophobic molecule which can enhance the ability of the polyethylenimine/DNA complexed nanoparticles to cross the BBB, thus showing a treatment effect in GBM tumor models.112
PLGA nanoparticles have been shown to cross the BBB. The use of surfactants such as poloxamer 188 (Pluronic F-68) or polysorbate 80 (Tween 80) can enhance the transport of the particles and increase the delivery of drugs conjugated to them and increase intracellular uptake113–115. A recent study demonstrated that conjugating transferrin, a protein known to be actively transported across the BBB, enhances the delivery of these particles to the brain, with an intact BBB as well as a disrupted BBB with an intracranial lesion 116.
The α-helical amphipathic peptide D[KLAKLAK]2 was originally designed as a synthetic antibacterial peptide that disrupts the bacterial cell membrane but is less toxic to eukaryotic cells. When conjugated to a mitochondrial peptide, CGKRK, IONP-derived nanoworms (due to their elongated shape), these particles localize to the mitochondria of tumor cells and cure tumors in a rodent tumor model. The nanoparticles could be seen to localize to the tumor on MRI 117.
6.2 Magnetic Targeting
The concept of magnetic targeting of malignant brain tumors has also been demonstrated in preclinical rodent models 118,119 as a method to enhance the systemic delivery of MNPs to malignant brain tumors. By using a magnetic field targeted to the region of interest, it has been shown that delivery of MNPs can be increased over the delivery to lesions when a magnetic field is not used 120. There are concerns in how efficacious the translation of this technique will be to human studies, as the depth of the lesions in the human brain will limit the ability to precisely target a lesion with a magnetic field119. Nevertheless, this remains an area for increased study.
In an effort to enhance the delivery and deposition of MNPs into malignant brain tumors, many studies have examined using strategies to open the BBB. Focal ultrasound (FUS) represents a non-invasive technique which can selectively disrupt the BBB and increase the EPR effect in a targeted region of the brain 121, 122,123. FUS and magnetic targeting have been used synergistically to enhance the delivery and the deposition of chemotherapy (epirubicin)- loaded MNPs into tumor-bearing animals. Epirubicin delivery and brain tumor accumulation was significantly enhanced by the combined FUS/magnetic targeting approach of epirubicin-MNPs 124.
6.3 Convection-Enhanced Delivery (CED)
Convection-enhanced delivery (CED), where bulk flow is used to distribute infusate throughout the brain with a pressure gradient, is a well-established technique for delivery of molecules to the brain125. This bypasses the BBB, allowing targeted delivery of infusate to the parenchyma of a region of interest through a catheter. A pump is connected to each infusion catheter in order to ensure a positive pressure gradient during delivery for convection of molecules through the interstitium of the brain. The pressure gradient created by the pump greatly augments the delivery that would be achieved by the use of simple diffusion alone 126.
The size of nanoparticles makes them optimal to be delivered with CED. Penetration of nanoparticles through the extracellular matrix (ECM) in the brain is possible due to the larger effective pore size of the ECM (50 nm) 127. CED of dextran-coated maghemite MNPs have recently been depicted by MRI in a normal rat brain model 128, showing that these particles could be directly imaged and tracked. They also showed that increased viscosity of the infusate increased efficacy of delivery and reduced leakback.
Imaging the infusate in CED is critical for ensuring adequate drug delivery to regions of interest. Valuable feedback can be gained from tracking infusate delivered into the brain to enable clinicians to properly plan further treatments and avoid pitfalls, such as placement of catheters near sulci or ventricles 129,130. Trials of conventional chemotherapeutics have failed to show significant benefit with CED, and lack of adequate drug delivery is often cited as the reason for this 131. While progress has been made using surrogate tracers such as Gd-DTPA132, directly imaging the therapeutic particle would provide even more accurate information.
We have studied the CED of theranostic MNPS in mice (Figure 3) 35. This particle consisted of an IONP core, coated by polymer and conjugated to an EGFRvIII antibody, specific for a subset of GBM tumors. We both assessed the ability of the nanoparticles to localize to and image the lesion treated as well as its treatment effect. CED enabled a broad distribution of the nanoparticles in the region of the tumor and the surrounding brain, and repeat imaging showed that this effect remained for days after the nanoparticle delivery.
Figure 3. Convection-enhanced delivery (CED) of magnetic nanoparticles (MNPs) in the rodent brain.
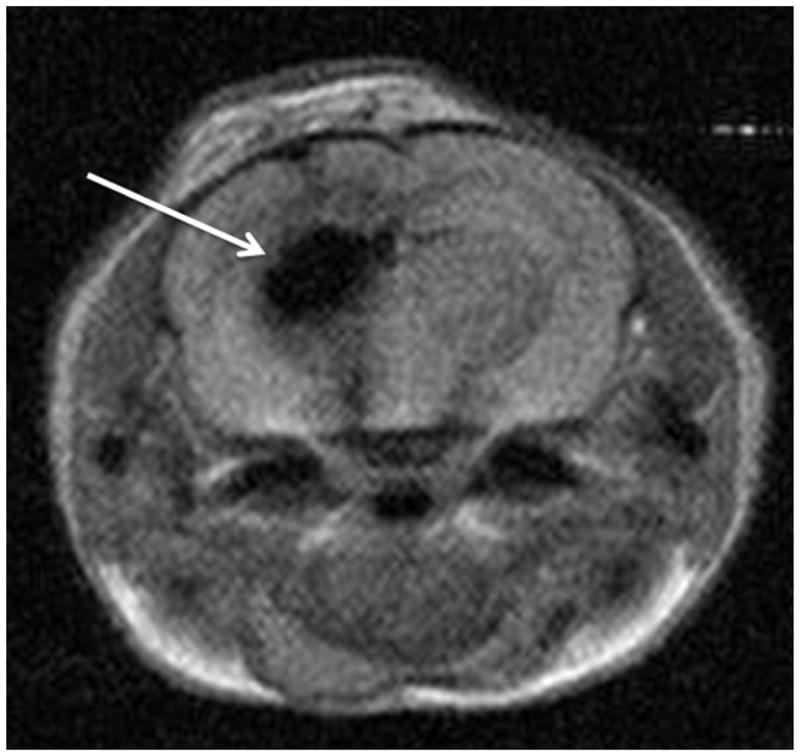
Magnetic resonance imaging of a rodent brain depicting the hypointense (dark) area in the brain that represents distribution of MNPs after CED with no leakback.
Future Studies
While researchers have made great strides in developing nanoparticles that address the difficulties in treating GBM, many challenges still remain. In the use of magnetic nanoparticles for thermotherapy and magnetic targeting, clinical equipment needs to be further developed and improved133 to make these cost effective and freely available for further clinical trials. Phase III studies will need to be undertaken to prove their effectiveness. In addition, drug delivery remains an issue with nanoparticles, and as further targeting motifs are studied, delivery of these particles will be enhanced, further expanding their possible effectiveness.
Conclusions
Nanotechnology has quickly become a very promising tool in the ongoing research to tackle the difficulties in treating GBM. We expect translational research to continue to elucidate further uses for this technology as these various particles come to widespread clinical use.
Key Points.
GBM remains a difficult tumor to treat due to its infiltrative nature
Nanoparticles present a new way to approach infiltrating cells
Magnetic nanoparticles can be used as magnetic resonance imaging contrast agents and therapeutic agents, including the use of thermotherapy
Nanoparticlized chemotherapeutics can be more efficacious than conventional chemotherapeutic agents due to their ability to target GBM cells
Gene delivery through the use of nanoparticles may be a safe option to deliver therapeutic genes to tumor cells
Brachytherapy delivered by radioactive nanoparticles can provide long term focused radiation therapy to these lesions
Gold nanoparticles can be used to treat tumors through phototherapy, where deep penetrating near-infrared light can be used to inhibit tumor growth
Nanoparticles can be delivered safely systemically or by bulk flow using convection-enhanced delivery directly to the tumor
Magnetic targeting can be used to enhance the delivery of magnetic nanoparticles, by directing the delivered particles to the area of interest
Acknowledgments
Financial support:
AB: American Association of Neurological Surgeons/Congress of Neurological Surgeons Section on Tumors Brainlab International Fellowship
CGH: NIH (NS053454), the Georgia Cancer Coalition, Distinguished Cancer Clinicians and Scientists Program, the Robbins Scholar Award, and the Dana Foundation.
Footnotes
Conflicts of interest:
EKN, AB, MK, CGH: No conflicts to report
Disclosures:
EKN: No funding to disclose
MK: No funding to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Edjah Nduom, Email: enduom@emory.edu.
Alexandros Bouras, Email: alexandros.bouras@emory.edu.
Milota Kaluzova, Email: mkaluzo@emory.edu.
References
- 1.Brat DJ, Prayson RA, Ryken TC, et al. Diagnosis of malignant glioma: role of neuropathology. J Neurooncol. 2008;89:287–311. doi: 10.1007/s11060-008-9618-1. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Legler JM, Ries LA, Smith MA, et al. Cancer surveillance series [corrected]: brain and other central nervous system cancers: recent trends in incidence and mortality. J Natl Cancer Inst. 1999;91:1382–90. doi: 10.1093/jnci/91.16.1382. [DOI] [PubMed] [Google Scholar]
- 4.Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345:1008–12. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 5.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–9. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 6.Kelly PJ, Daumas-Duport C, Kispert DB, et al. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987;66:865–74. doi: 10.3171/jns.1987.66.6.0865. [DOI] [PubMed] [Google Scholar]
- 7.Demuth T, Berens ME. Molecular mechanisms of glioma cell migration and invasion. J Neurooncol. 2004;70:217–28. doi: 10.1007/s11060-004-2751-6. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi CU, Ryozi, Tasaki A. Ultra-fine particles: exploratory science and technology. Westwood, NJ: Noyes Publications; 1997. p. 2. [Google Scholar]
- 9.Maier-Hauff K, Ulrich F, Nestler D, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol. 2010 doi: 10.1007/s11060-010-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L, Venkatraman SS, Yang Y-Y, et al. Polymeric micelles anchored with TAT for delivery of antibiotics across the blood-brain barrier. Biopolymers. 2008;90:617–623. doi: 10.1002/bip.20998. [DOI] [PubMed] [Google Scholar]
- 11.Loo C, Lin A, Hirsch L, et al. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technology in Cancer Research & Treatment. 2004;3:33–40. doi: 10.1177/153303460400300104. [DOI] [PubMed] [Google Scholar]
- 12.Xing Y, Chaudry Q, Shen C, et al. Bioconjugated quantum dots for multiplexed and quantitative immunohistochemistry. Nature Protocols. 2007;2:1152–1165. doi: 10.1038/nprot.2007.107. [DOI] [PubMed] [Google Scholar]
- 13.Provenzale JM, Silva GA. Uses of Nanoparticles for Central Nervous System Imaging and Therapy. American Journal of Neuroradiology. 2009;30:1293–1301. doi: 10.3174/ajnr.A1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain TK, Richey J, Strand M, et al. Magnetic nanoparticles with dual functional properties: drug delivery and magnetic resonance imaging. Biomaterials. 2008;29:4012–21. doi: 10.1016/j.biomaterials.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun C, Lee JS, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev. 2008;60:1252–65. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corot C, Robert P, Idee JM, et al. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev. 2006;58:1471–504. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Lodhia J, Mandarano G, Ferris N, et al. Development and use of iron oxide nanoparticles (Part 1): Synthesis of iron oxide nanoparticles for MRI. Biomed Imaging Interv J. 2010;6:e12. doi: 10.2349/biij.6.2.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorek DL, Chen AK, Czupryna J, et al. Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Ann Biomed Eng. 2006;34:23–38. doi: 10.1007/s10439-005-9002-7. [DOI] [PubMed] [Google Scholar]
- 19.Weissleder R, Elizondo G, Wittenberg J, et al. Ultrasmall superparamagnetic iron oxide: characterization of a new class of contrast agents for MR imaging. Radiology. 1990;175:489–93. doi: 10.1148/radiology.175.2.2326474. [DOI] [PubMed] [Google Scholar]
- 20.Pan D, Caruthers SD, Hu G, et al. Ligand-directed nanobialys as theranostic agent for drug delivery and manganese-based magnetic resonance imaging of vascular targets. J Am Chem Soc. 2008;130:9186–7. doi: 10.1021/ja801482d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Na HB, Lee JH, An K, et al. Development of a T1 contrast agent for magnetic resonance imaging using MnO nanoparticles. Angew Chem Int Ed Engl. 2007;46:5397–401. doi: 10.1002/anie.200604775. [DOI] [PubMed] [Google Scholar]
- 22.Bridot JL, Faure AC, Laurent S, et al. Hybrid gadolinium oxide nanoparticles: multimodal contrast agents for in vivo imaging. J Am Chem Soc. 2007;129:5076–84. doi: 10.1021/ja068356j. [DOI] [PubMed] [Google Scholar]
- 23.Bourrinet P, Bengele HH, Bonnemain B, et al. Preclinical safety and pharmacokinetic profile of ferumoxtran-10, an ultrasmall superparamagnetic iron oxide magnetic resonance contrast agent. Invest Radiol. 2006;41:313–24. doi: 10.1097/01.rli.0000197669.80475.dd. [DOI] [PubMed] [Google Scholar]
- 24.Aime S, Caravan P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging. 2009;30:1259–67. doi: 10.1002/jmri.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abraham JL, Thakral C. Tissue distribution and kinetics of gadolinium and nephrogenic systemic fibrosis. Eur J Radiol. 2008;66:200–7. doi: 10.1016/j.ejrad.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Varallyay P, Nesbit G, Muldoon LL, et al. Comparison of two superparamagnetic viral-sized iron oxide particles ferumoxides and ferumoxtran-10 with a gadolinium chelate in imaging intracranial tumors. AJNR Am J Neuroradiol. 2002;23:510–9. [PMC free article] [PubMed] [Google Scholar]
- 27.Neuwelt EA, Varallyay CG, Manninger S, et al. The potential of ferumoxytol nanoparticle magnetic resonance imaging, perfusion, and angiography in central nervous system malignancy: a pilot study. Neurosurgery. 2007;60:601–11. doi: 10.1227/01.NEU.0000255350.71700.37. discussion 611–2. [DOI] [PubMed] [Google Scholar]
- 28.Neuwelt EA, Hamilton BE, Varallyay CG, et al. Ultrasmall superparamagnetic iron oxides (USPIOs): a future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney Int. 2009;75:465–74. doi: 10.1038/ki.2008.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore A, Marecos E, Bogdanov A, Jr, et al. Tumoral distribution of long-circulating dextran-coated iron oxide nanoparticles in a rodent model. Radiology. 2000;214:568–74. doi: 10.1148/radiology.214.2.r00fe19568. [DOI] [PubMed] [Google Scholar]
- 30.Zimmer C, Weissleder R, Poss K, et al. MR imaging of phagocytosis in experimental gliomas. Radiology. 1995;197:533–8. doi: 10.1148/radiology.197.2.7480707. [DOI] [PubMed] [Google Scholar]
- 31.Villanueva A, Canete M, Roca AG, et al. The influence of surface functionalization on the enhanced internalization of magnetic nanoparticles in cancer cells. Nanotechnology. 2009;20:115103. doi: 10.1088/0957-4484/20/11/115103. [DOI] [PubMed] [Google Scholar]
- 32.Rhyner MN, Smith AM, Gao X, et al. Quantum dots and multifunctional nanoparticles: new contrast agents for tumor imaging. Nanomedicine (Lond) 2006;1:209–17. doi: 10.2217/17435889.1.2.209. [DOI] [PubMed] [Google Scholar]
- 33.Peng XH, Qian X, Mao H, et al. Targeted magnetic iron oxide nanoparticles for tumor imaging and therapy. Int J Nanomedicine. 2008;3:311–21. doi: 10.2147/ijn.s2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Remsen LG, McCormick CI, Roman-Goldstein S, et al. MR of carcinoma-specific monoclonal antibody conjugated to monocrystalline iron oxide nanoparticles: the potential for noninvasive diagnosis. AJNR Am J Neuroradiol. 1996;17:411–8. [PMC free article] [PubMed] [Google Scholar]
- 35.Hadjipanayis CG, Machaidze R, Kaluzova M, et al. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010;70:6303–12. doi: 10.1158/0008-5472.CAN-10-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soroceanu L, Gillespie Y, Khazaeli MB, et al. Use of chlorotoxin for targeting of primary brain tumors. Cancer Res. 1998;58:4871–9. [PubMed] [Google Scholar]
- 37.Lyons SA, O’Neal J, Sontheimer H. Chlorotoxin, a scorpion-derived peptide, specifically binds to gliomas and tumors of neuroectodermal origin. Glia. 2002;39:162–73. doi: 10.1002/glia.10083. [DOI] [PubMed] [Google Scholar]
- 38.Deshane J, Garner CC, Sontheimer H. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J Biol Chem. 2003;278:4135–44. doi: 10.1074/jbc.M205662200. [DOI] [PubMed] [Google Scholar]
- 39.Veiseh O, Gunn JW, Kievit FM, et al. Inhibition of tumor-cell invasion with chlorotoxin-bound superparamagnetic nanoparticles. Small. 2009;5:256–64. doi: 10.1002/smll.200800646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veiseh O, Sun C, Fang C, et al. Specific targeting of brain tumors with an optical/magnetic resonance imaging nanoprobe across the blood-brain barrier. Cancer Res. 2009;69:6200–7. doi: 10.1158/0008-5472.CAN-09-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veiseh O, Sun C, Gunn J, et al. Optical and MRI multifunctional nanoprobe for targeting gliomas. Nano Lett. 2005;5:1003–8. doi: 10.1021/nl0502569. [DOI] [PubMed] [Google Scholar]
- 42.McFerrin MB, Sontheimer H. A role for ion channels in glioma cell invasion. Neuron Glia Biol. 2006;2:39–49. doi: 10.1017/S17440925X06000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christian S, Pilch J, Akerman ME, et al. Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J Cell Biol. 2003;163:871–8. doi: 10.1083/jcb.200304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reddy GR, Bhojani MS, McConville P, et al. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clin Cancer Res. 2006;12:6677–86. doi: 10.1158/1078-0432.CCR-06-0946. [DOI] [PubMed] [Google Scholar]
- 45.Tomanek B, Iqbal U, Blasiak B, et al. Evaluation of brain tumor vessels specific contrast agents for glioblastoma imaging. Neuro Oncol. 2012;14:53–63. doi: 10.1093/neuonc/nor183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faucher L, Guay-Begin AA, Lagueux J, et al. Ultra-small gadolinium oxide nanoparticles to image brain cancer cells in vivo with MRI. Contrast Media Mol Imaging. 2011;6:209–18. doi: 10.1002/cmmi.420. [DOI] [PubMed] [Google Scholar]
- 47.Mowat P, Mignot A, Rima W, et al. In vitro radiosensitizing effects of ultrasmall gadolinium based particles on tumour cells. J Nanosci Nanotechnol. 2011;11:7833–9. doi: 10.1166/jnn.2011.4725. [DOI] [PubMed] [Google Scholar]
- 48.Fillmore HL, Shultz MD, Henderson SC, et al. Conjugation of functionalized gadolinium metallofullerenes with IL-13 peptides for targeting and imaging glial tumors. Nanomedicine (Lond) 2011;6:449–58. doi: 10.2217/nnm.10.134. [DOI] [PubMed] [Google Scholar]
- 49.Leung K. TAMRA-IL-13-Conjugated functionalized gadolinium metallofullerene (Gd3N@C80(OH)-26(CH2CH2COOH)-16), Molecular Imaging and Contrast Agent Database (MICAD) Bethesda (MD): 2004. [PubMed] [Google Scholar]
- 50.Lu YJ, Wei KC, Ma CC, et al. Dual targeted delivery of doxorubicin to cancer cells using folate-conjugated magnetic multi-walled carbon nanotubes. Colloids Surf B Biointerfaces. 2012;89:1–9. doi: 10.1016/j.colsurfb.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Sanai N, Polley MY, McDermott MW, et al. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115:3–8. doi: 10.3171/2011.2.jns10998. [DOI] [PubMed] [Google Scholar]
- 52.Senft C, Franz K, Blasel S, et al. Influence of iMRI-guidance on the extent of resection and survival of patients with glioblastoma multiforme. Technol Cancer Res Treat. 2010;9:339–46. doi: 10.1177/153303461000900404. [DOI] [PubMed] [Google Scholar]
- 53.Mehdorn HM, Schwartz F, Dawirs S, et al. High-field iMRI in glioblastoma surgery: improvement of resection radicality and survival for the patient? Acta Neurochir Suppl. 2011;109:103–6. doi: 10.1007/978-3-211-99651-5_16. [DOI] [PubMed] [Google Scholar]
- 54.Willems PW, Taphoorn MJ, Burger H, et al. Effectiveness of neuronavigation in resecting solitary intracerebral contrast-enhancing tumors: a randomized controlled trial. J Neurosurg. 2006;104:360–8. doi: 10.3171/jns.2006.104.3.360. [DOI] [PubMed] [Google Scholar]
- 55.Hadjipanayis CG, Jiang H, Roberts DW, et al. Current and future clinical applications for optical imaging of cancer: from intraoperative surgical guidance to cancer screening. Semin Oncol. 2011;38:109–18. doi: 10.1053/j.seminoncol.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Meir EG, Hadjipanayis CG, Norden AD, et al. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60:166–93. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moore GE, Peyton WT, et al. The clinical use of fluorescein in neurosurgery; the localization of brain tumors. J Neurosurg. 1948;5:392–8. doi: 10.3171/jns.1948.5.4.0392. [DOI] [PubMed] [Google Scholar]
- 58.Britz GW, Ghatan S, Spence AM, et al. Intracarotid RMP-7 enhanced indocyanine green staining of tumors in a rat glioma model. J Neurooncol. 2002;56:227–32. doi: 10.1023/a:1015035213228. [DOI] [PubMed] [Google Scholar]
- 59.Ozawa T, Britz GW, Kinder DH, et al. Bromophenol blue staining of tumors in a rat glioma model. Neurosurgery. 2005;57:1041–7. doi: 10.1227/01.neu.0000180036.42193.f6. discussion 1041–7. [DOI] [PubMed] [Google Scholar]
- 60.Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 61.Eljamel MS, Goodman C, Moseley H. ALA and Photofrin fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: a single centre Phase III randomised controlled trial. Lasers Med Sci. 2008;23:361–7. doi: 10.1007/s10103-007-0494-2. [DOI] [PubMed] [Google Scholar]
- 62.Kircher MF, Mahmood U, King RS, et al. A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer Res. 2003;63:8122–5. [PubMed] [Google Scholar]
- 63.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97:12846–51. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang SY, Liu H, Zhang JN. Gene therapy of rat malignant gliomas using neural stem cells expressing IL-12. DNA Cell Biol. 2004;23:381–9. doi: 10.1089/104454904323145263. [DOI] [PubMed] [Google Scholar]
- 65.Benedetti S, Pirola B, Pollo B, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–50. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- 66.Hamada H, Kobune M, Nakamura K, et al. Mesenchymal stem cells (MSC) as therapeutic cytoreagents for gene therapy. Cancer Sci. 2005;96:149–56. doi: 10.1111/j.1349-7006.2005.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu X, Hu J, Zhou L, et al. In vivo tracking of superparamagnetic iron oxide nanoparticle-labeled mesenchymal stem cell tropism to malignant gliomas using magnetic resonance imaging. Laboratory investigation. J Neurosurg. 2008;108:320–9. doi: 10.3171/JNS/2008/108/2/0320. [DOI] [PubMed] [Google Scholar]
- 68.Tang C, Russell PJ, Martiniello-Wilks R, et al. Concise review: Nanoparticles and cellular carriers-allies in cancer imaging and cellular gene therapy? Stem Cells. 2010;28:1686–702. doi: 10.1002/stem.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arbab AS, Janic B, Knight RA, et al. Detection of migration of locally implanted AC133+ stem cells by cellular magnetic resonance imaging with histological findings. FASEB J. 2008;22:3234–46. doi: 10.1096/fj.07-105676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goldstein LS, Dewhirst MW, Repacholi M, et al. Summary, conclusions and recommendations: adverse temperature levels in the human body. International Journal of Hyperthermia. 2003;19:373–384. doi: 10.1080/0265673031000090701. [DOI] [PubMed] [Google Scholar]
- 71.Hildebrandt B, Wust P, Ahlers O, et al. The cellular and molecular basis of hyperthermia. Critical Reviews in Oncology Hematology. 2002;43:33–56. doi: 10.1016/s1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 72.Wust P, Hildebrandt B, Sreenivasa G, et al. Hyperthermia in combined treatment of cancer. Lancet Oncology. 2002;3:487–497. doi: 10.1016/s1470-2045(02)00818-5. [DOI] [PubMed] [Google Scholar]
- 73.Suto R, Srivastava PK. A MECHANISM FOR THE SPECIFIC IMMUNOGENICITY OF HEAT-SHOCK PROTEIN-CHAPERONED PEPTIDES. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 74.Santos-Marques MJ, Carvalho F, Sousa C, et al. Cytotoxicity and cell signalling induced by continuous mild hyperthermia in freshly isolated mouse hepatocytes. Toxicology. 2006;224:210–8. doi: 10.1016/j.tox.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 75.Hadjipanayis CG, Bonder MJ, Balakrishnan S, et al. Metallic iron nanoparticles for MRI contrast enhancement and local hyperthermia. Small. 2008;4:1925–9. doi: 10.1002/smll.200800261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mehdaoui B, Meffre A, Carrey J, et al. Optimal size of nanoparticles for magnetic hyperthermia: a combined theoretical and experiemental study. Adv Funct Mat. 2011 [Google Scholar]
- 77.Dennis CL, Jackson AJ, Borchers JA, et al. Nearly complete regression of tumors via collective behavior of magnetic nanoparticles in hyperthermia. Nanotechnology. 2009;20:395103. doi: 10.1088/0957-4484/20/39/395103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee JH, Jang JT, Choi JS, et al. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nature Nanotechnology. 2011;6:418–22. doi: 10.1038/nnano.2011.95. [DOI] [PubMed] [Google Scholar]
- 79.Wijaya A, Brown KA, Alper JD, et al. Magnetic field heating study of Fe-doped Au nanoparticles. Journal of Magnetism and Magnetic Materials. 2007;309:15–19. [Google Scholar]
- 80.Sharma R, Chen CJ. Newer nanoparticles in hyperthermia treatment and thermometry. Journal of Nanoparticle Research. 2009;11:671–689. [Google Scholar]
- 81.Pradhan P, Giri J, Samanta G, et al. Comparative evaluation of heating ability and biocompatibility of different ferrite-based magnetic fluids for hyperthermia application. Journal of Biomedical Materials Research Part B-Applied Biomaterials. 2007;81B:12–22. doi: 10.1002/jbm.b.30630. [DOI] [PubMed] [Google Scholar]
- 82.Kim D-H, Thai YT, Nikles DE, et al. Heating of Aqueous Dispersions Containing MnFe(2)O(4) Nanoparticles by Radio-Frequency Magnetic Field Induction. Ieee Transactions on Magnetics. 2009;45:64–70. [Google Scholar]
- 83.Kaman O, Pollert E, Veverka P, et al. Silica encapsulated manganese perovskite nanoparticles for magnetically induced hyperthermia without the risk of overheating. Nanotechnology. 2009;20 doi: 10.1088/0957-4484/20/27/275610. [DOI] [PubMed] [Google Scholar]
- 84.Atsarkin VA, Levkin LV, Posvyanskiy VS, et al. Solution to the bioheat equation for hyperthermia with La1-xAgyMnO3-nanoparticles: The effect of temperature autostabilization. International Journal of Hyperthermia. 2009;25:240–247. doi: 10.1080/02656730802713565. [DOI] [PubMed] [Google Scholar]
- 85.Bae S, Lee SW, Takemura Y, et al. Dependence of frequency and magnetic field on selfheating characteristics of NiFe2O4 nanoparticles for hyperthermia. Ieee Transactions on Magnetics. 2006;42:3566–3568. [Google Scholar]
- 86.Kim DH, Lee SH, Kim KN, et al. Temperature change of various ferrite particles with alternating magnetic field for hyperthermic application. Journal of Magnetism and Magnetic Materials. 2005;293:320–327. [Google Scholar]
- 87.Kim DH, Lee SH, Kim KN, et al. In vitro and in vivo characterization of various ferrites for hyperthermia in cancer-treatment. In: Li PZKCCW, editor. Bioceramics. Vol. 17. Key Engineering Materials; 2005. pp. 827–830. [Google Scholar]
- 88.Huber DL. Synthesis, properties, and applications of iron nanoparticles. Small. 2005;1:482–501. doi: 10.1002/smll.200500006. [DOI] [PubMed] [Google Scholar]
- 89.Pradhan P, Giri J, Banerjee R, et al. Cellular interactions of lauric acid and dextran-coated magnetite nanoparticles. Journal of Magnetism and Magnetic Materials. 2007;311:282–287. [Google Scholar]
- 90.Luis Corchero J, Villaverde A. Biomedical applications of distally controlled magnetic nanoparticles. Trends in Biotechnology. 2009;27:468–476. doi: 10.1016/j.tibtech.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 91.Jordan A, Scholz R, Maier-Hauff K, et al. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J Neurooncol. 2006;78:7–14. doi: 10.1007/s11060-005-9059-z. [DOI] [PubMed] [Google Scholar]
- 92.Maier-Hauff K, Rothe R, Scholz R, et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: results of a feasibility study on patients with glioblastoma multiforme. J Neurooncol. 2007;81:53–60. doi: 10.1007/s11060-006-9195-0. [DOI] [PubMed] [Google Scholar]
- 93.Jiang X, Xin H, Sha X, et al. PEGylated poly(trimethylene carbonate) nanoparticles loaded with paclitaxel for the treatment of advanced glioma: in vitro and in vivo evaluation. Int J Pharm. 2011;420:385–94. doi: 10.1016/j.ijpharm.2011.08.052. [DOI] [PubMed] [Google Scholar]
- 94.Sawyer AJ, Saucier-Sawyer JK, Booth CJ, et al. Convection-enhanced delivery of camptothecin-loaded polymer nanoparticles for treatment of intracranial tumors. Drug Deliv Transl Res. 2011;1:34–42. doi: 10.1007/s13346-010-0001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Agarwal A, Mackey MA, El-Sayed MA, et al. Remote triggered release of doxorubicin in tumors by synergistic application of thermosensitive liposomes and gold nanorods. ACS Nano. 2011;5:4919–26. doi: 10.1021/nn201010q. [DOI] [PubMed] [Google Scholar]
- 96.Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jin J, Bae KH, Yang H, et al. In Vivo Specific Delivery of c-Met siRNA to Glioblastoma Using Cationic Solid Lipid Nanoparticles. Bioconjug Chem. 2011;22:2568–72. doi: 10.1021/bc200406n. [DOI] [PubMed] [Google Scholar]
- 98.Zhan C, Meng Q, Li Q, et al. Cyclic RGD-Polyethylene Glycol-Polyethylenimine for Intracranial Glioblastoma-Targeted Gene Delivery. Chem Asian J. 2012;7:91–6. doi: 10.1002/asia.201100570. [DOI] [PubMed] [Google Scholar]
- 99.Shultz MD, Wilson JD, Fuller CE, et al. Metallofullerene-based nanoplatform for brain tumor brachytherapy and longitudinal imaging in a murine orthotopic xenograft model. Radiology. 2011;261:136–43. doi: 10.1148/radiol.11102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vanpouille-Box C, Lacoeuille F, Belloche C, et al. Tumor eradication in rat glioma and bypass of immunosuppressive barriers using internal radiation with (188)Re-lipid nanocapsules. Biomaterials. 2011;32:6781–90. doi: 10.1016/j.biomaterials.2011.05.067. [DOI] [PubMed] [Google Scholar]
- 101.Hirsch LR, Gobin AM, Lowery AR, et al. Metal nanoshells. Ann Biomed Eng. 2006;34:15–22. doi: 10.1007/s10439-005-9001-8. [DOI] [PubMed] [Google Scholar]
- 102.Bernardi RJ, Lowery AR, Thompson PA, et al. Immunonanoshells for targeted photothermal ablation in medulloblastoma and glioma: an in vitro evaluation using human cell lines. J Neurooncol. 2008;86:165–72. doi: 10.1007/s11060-007-9467-3. [DOI] [PubMed] [Google Scholar]
- 103.Baek SK, Makkouk AR, Krasieva T, et al. Photothermal treatment of glioma; an in vitro study of macrophage-mediated delivery of gold nanoshells. J Neurooncol. 2011;104:439–48. doi: 10.1007/s11060-010-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nie S, Xing Y, Kim GJ, et al. Nanotechnology applications in cancer. Annu Rev Biomed Eng. 2007;9:257–88. doi: 10.1146/annurev.bioeng.9.060906.152025. [DOI] [PubMed] [Google Scholar]
- 105.Peer D, Karp JM, Hong S, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–60. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 106.Gref R, Minamitake Y, Peracchia MT, et al. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–3. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 107.van der Sanden BP, Rozijn TH, Rijken PF, et al. Noninvasive assessment of the functional neovasculature in 9L-glioma growing in rat brain by dynamic 1H magnetic resonance imaging of gadolinium uptake. J Cereb Blood Flow Metab. 2000;20:861–70. doi: 10.1097/00004647-200005000-00013. [DOI] [PubMed] [Google Scholar]
- 108.Vajkoczy P, Menger MD. Vascular microenvironment in gliomas. J Neurooncol. 2000;50:99–108. doi: 10.1023/a:1006474832189. [DOI] [PubMed] [Google Scholar]
- 109.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Son YJ, Jang JS, Cho YW, et al. Biodistribution and anti-tumor efficacy of doxorubicin loaded glycol-chitosan nanoaggregates by EPR effect. J Control Release. 2003;91:135–45. doi: 10.1016/s0168-3659(03)00231-1. [DOI] [PubMed] [Google Scholar]
- 111.Agemy L, Friedmann-Morvinski D, Kotamraju VR, et al. Targeted nanoparticle enhanced proapoptotic peptide as potential therapy for glioblastoma. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:17450–5. doi: 10.1073/pnas.1114518108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li J, Gu B, Meng Q, et al. The use of myristic acid as a ligand of polyethylenimine/DNA nanoparticles for targeted gene therapy of glioblastoma. Nanotechnology. 2011;22:435101. doi: 10.1088/0957-4484/22/43/435101. [DOI] [PubMed] [Google Scholar]
- 113.Tahara K, Kato Y, Yamamoto H, et al. Intracellular drug delivery using polysorbate 80-modified poly(D,L-lactide-co-glycolide) nanospheres to glioblastoma cells. J Microencapsul. 2011;28:29–36. doi: 10.3109/02652048.2010.522258. [DOI] [PubMed] [Google Scholar]
- 114.Gelperina S, Maksimenko O, Khalansky A, et al. Drug delivery to the brain using surfactant-coated poly(lactide-co-glycolide) nanoparticles: influence of the formulation parameters. Eur J Pharm Biopharm. 2010;74:157–63. doi: 10.1016/j.ejpb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 115.Wohlfart S, Khalansky AS, Gelperina S, et al. Efficient chemotherapy of rat glioblastoma using doxorubicin-loaded PLGA nanoparticles with different stabilizers. PLoS One. 2011;6:e19121. doi: 10.1371/journal.pone.0019121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chang J, Paillard A, Passirani C, et al. Transferrin Adsorption onto PLGA Nanoparticles Governs Their Interaction with Biological Systems from Blood Circulation to Brain Cancer Cells. Pharm Res. 2011 doi: 10.1007/s11095-011-0624-1. [DOI] [PubMed] [Google Scholar]
- 117.Agemy L, Friedmann-Morvinski D, Kotamraju VR, et al. Targeted nanoparticle enhanced proapoptotic peptide as potential therapy for glioblastoma. Proc Natl Acad Sci U S A. 2011;108:17450–5. doi: 10.1073/pnas.1114518108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chertok B, Moffat BA, David AE, et al. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials. 2008;29:487–96. doi: 10.1016/j.biomaterials.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chertok B, David AE, Huang Y, et al. Glioma selectivity of magnetically targeted nanoparticles: a role of abnormal tumor hydrodynamics. J Control Release. 2007;122:315–23. doi: 10.1016/j.jconrel.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pulfer SK, Ciccotto SL, Gallo JM. Distribution of small magnetic particles in brain tumor-bearing rats. J Neurooncol. 1999;41:99–105. doi: 10.1023/a:1006137523591. [DOI] [PubMed] [Google Scholar]
- 121.Hynynen K, McDannold N, Vykhodtseva N, et al. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J Neurosurg. 2006;105:445–54. doi: 10.3171/jns.2006.105.3.445. [DOI] [PubMed] [Google Scholar]
- 122.Pardridge WM. Drug and gene delivery to the brain: the vascular route. Neuron. 2002;36:555–8. doi: 10.1016/s0896-6273(02)01054-1. [DOI] [PubMed] [Google Scholar]
- 123.Muldoon LL, Soussain C, Jahnke K, et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007;25:2295–305. doi: 10.1200/JCO.2006.09.9861. [DOI] [PubMed] [Google Scholar]
- 124.Liu HL, Hua MY, Yang HW, et al. Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc Natl Acad Sci U S A. 2010;107:15205–10. doi: 10.1073/pnas.1003388107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bobo RH, Laske DW, Akbasak A, et al. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91:2076–80. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Allard E, Passirani C, Benoit JP. Convection-enhanced delivery of nanocarriers for the treatment of brain tumors. Biomaterials. 2009;30:2302–18. doi: 10.1016/j.biomaterials.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 127.Thorne RG, Nicholson C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc Natl Acad Sci U S A. 2006;103:5567–72. doi: 10.1073/pnas.0509425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Perlstein B, Ram Z, Daniels D, et al. Convection-enhanced delivery of maghemite nanoparticles: Increased efficacy and MRI monitoring. Neuro Oncol. 2008;10:153–61. doi: 10.1215/15228517-2008-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sampson JH, Brady ML, Petry NA, et al. Intracerebral infusate distribution by convection-enhanced delivery in humans with malignant gliomas: descriptive effects of target anatomy and catheter positioning. Neurosurgery. 2007;60:ONS89–98. doi: 10.1227/01.NEU.0000249256.09289.5F. discussion ONS98–9. [DOI] [PubMed] [Google Scholar]
- 130.Varenika V, Dickinson P, Bringas J, et al. Detection of infusate leakage in the brain using real-time imaging of convection-enhanced delivery. J Neurosurg. 2008;109:874–80. doi: 10.3171/JNS/2008/109/11/0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sampson JH, Archer G, Pedain C, et al. Poor drug distribution as a possible explanation for the results of the PRECISE trial. J Neurosurg. 2010;113:301–9. doi: 10.3171/2009.11.JNS091052. [DOI] [PubMed] [Google Scholar]
- 132.Asthagiri AR, Walbridge S, Heiss JD, et al. Effect of concentration on the accuracy of convective imaging distribution of a gadolinium-based surrogate tracer. J Neurosurg. 2011;115:467–73. doi: 10.3171/2011.3.JNS101381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Silva AC, Oliveira TR, Mamani JB, et al. Application of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatment. Int J Nanomedicine. 2011;6:591–603. doi: 10.2147/IJN.S14737. [DOI] [PMC free article] [PubMed] [Google Scholar]


