Abstract
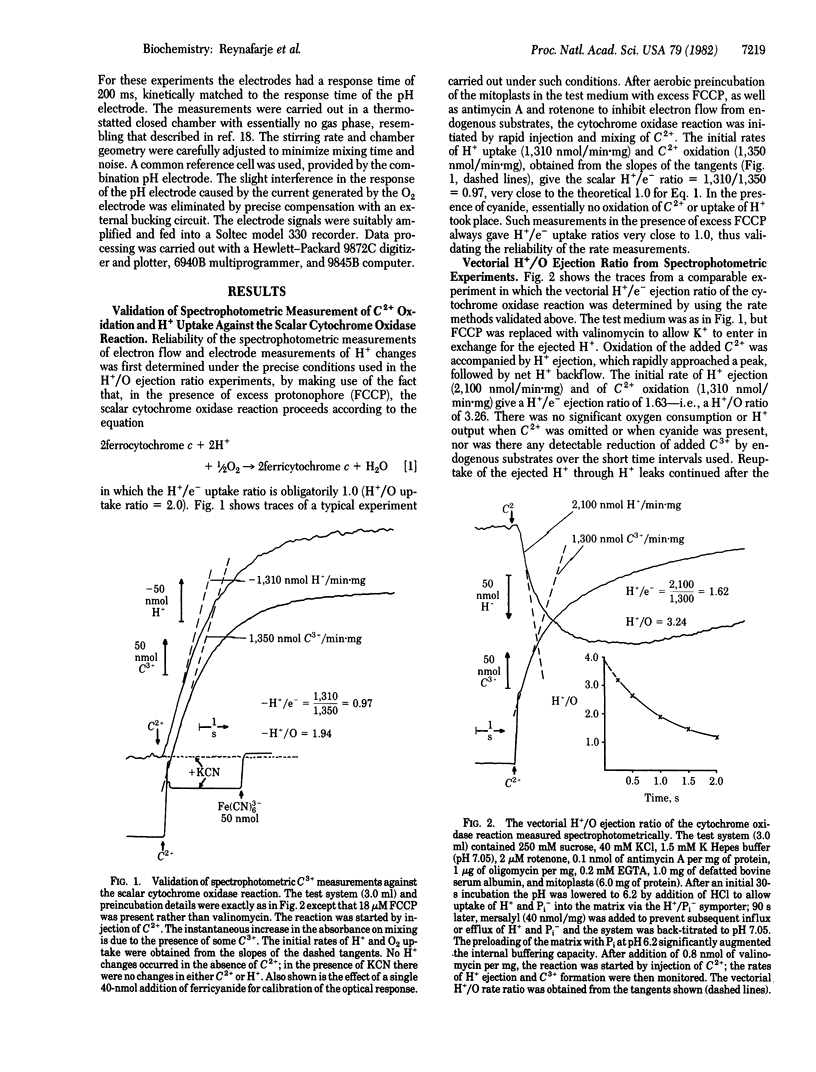
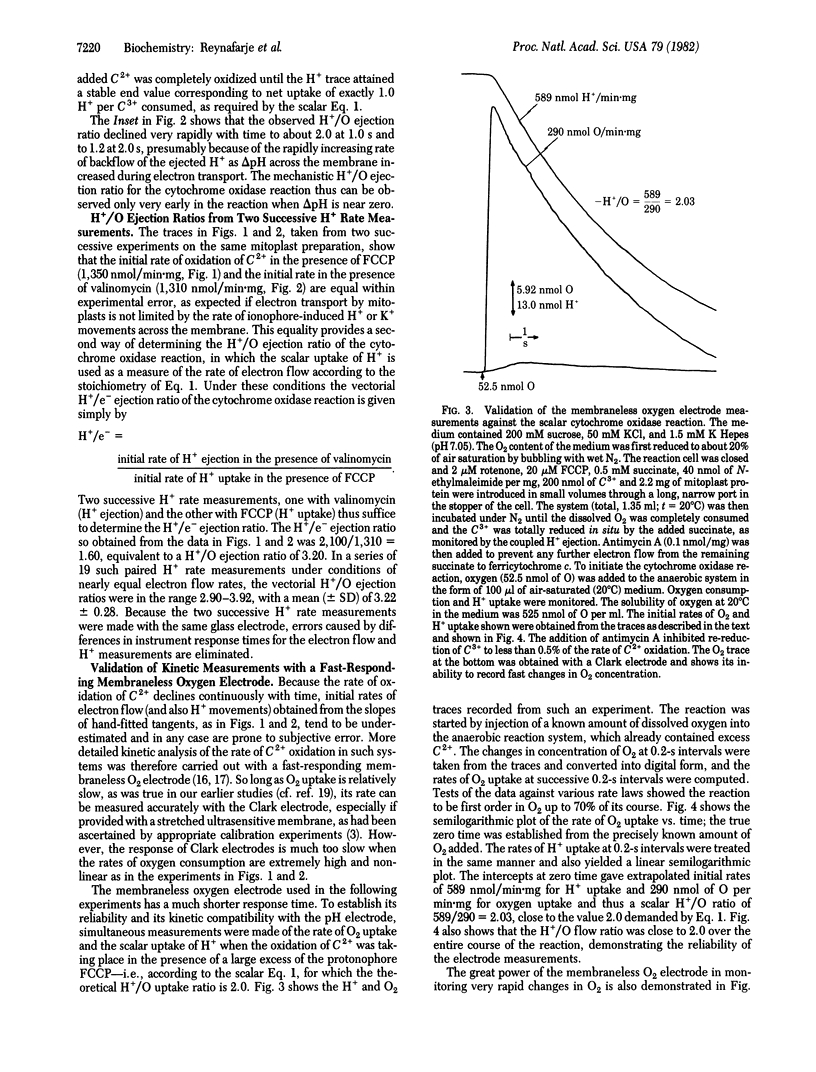
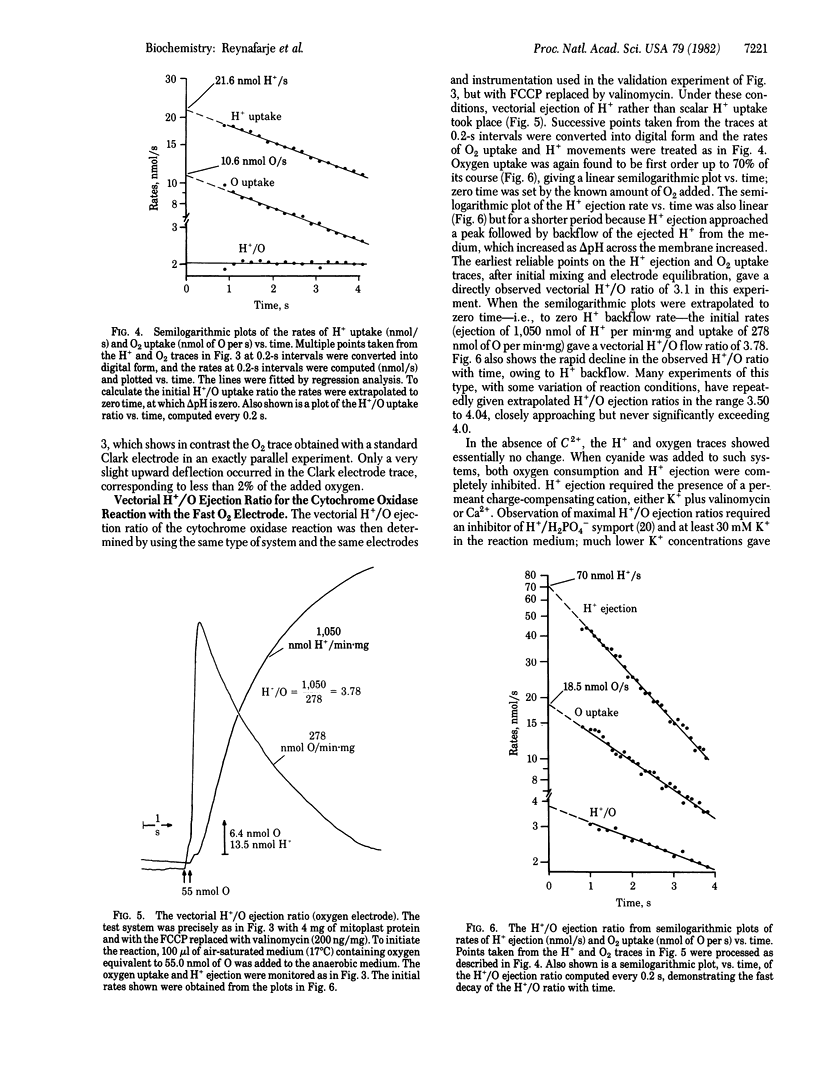
The mechanistic stoichiometry of vectorial H+ ejection coupled to electron transport from added ferrocytochrome c to oxygen by the cytochrome oxidase (EC 1.9.3.1) of rat liver mitoplasts was determined from measurements of the initial rates of electron flow and H+ ejection in the presence of K+ (with valinomycin). Three different methods of measuring electron flow were used: (a) dual-wavelength spectrophotometry of ferrocytochrome c oxidation, (b) uptake of scalar H+ for the reduction of O2 in the presence of a protonophore, and (c) a fast-responding membraneless oxygen electrode. The reliability of the rate measurements was first established against the known stoichiometry of the scalar reaction of cytochrome oxidase (2ferrocytochrome c + 2H+ + 1/2O2 leads to 2ferricytochrome c + H2O) in the presence of excess protonophore. With all three methods the directly observed vectorial H+/O ejection ratios in the presence of K+ + valinomycin significantly exceeded 3.0. However, because the rate of backflow of the ejected H+ into the mitoplasts is very high and increases with the increasing delta pH generated across the membrane, there is a very rapid decline in the observed H+/O ratio from the beginning of the reaction. Kinetic analysis of ferrocytochrome c oxidation by the mitoplasts, carried out with a fast-responding membraneless oxygen electrode, showed the reaction to be first order in O2 and allowed accurate extrapolation of the rates of O2 uptake and H+ ejection to zero time. At this point, at which there is zero delta pH across the membrane, the H+/O ejection ratio of the cytochrome oxidase reaction, obtained from the rates at zero time, is close to 4.0.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affolter H., Sigel E. A simple system for the measurement of ion activities with solvent polymeric membrane electrodes. Anal Biochem. 1979 Sep 1;97(2):315–319. doi: 10.1016/0003-2697(79)90078-2. [DOI] [PubMed] [Google Scholar]
- Al-Shawi M. K., Brand M. D. Steady-state H+/O stoichiometry of liver mitochondria. Biochem J. 1981 Dec 15;200(3):539–546. doi: 10.1042/bj2000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre A., Reynafarje B., Lehninger A. L. Stoichiometry of vectorial H+ movements coupled to electron transport and to ATP synthesis in mitochondria. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5296–5300. doi: 10.1073/pnas.75.11.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzone G. F., Pozzan T., Di Virgilio F. H+/site, charge/site, and ATP/site ratios at coupling site III in mitochondrial electron transport. J Biol Chem. 1979 Oct 25;254(20):10206–10212. [PubMed] [Google Scholar]
- Brand M. D., Harper W. G., Nicholls D. G., Ingledew W. J. Unequal charge separation by different coupling spans of the mitochondrial electron transport chain. FEBS Lett. 1978 Nov 1;95(1):125–129. doi: 10.1016/0014-5793(78)80066-0. [DOI] [PubMed] [Google Scholar]
- Brand M. D., Reynafarje B., Lehninger A. L. Re-evaluation of the H+/site ratio of mitochondrial electron transport with the oxygen pulse technique. J Biol Chem. 1976 Sep 25;251(18):5670–5679. [PubMed] [Google Scholar]
- Casey R. P., Chappell J. B., Azzi A. Limited-turnover studies on proton translocation in reconstituted cytochrome c oxidase-containing vesicles. Biochem J. 1979 Jul 15;182(1):149–156. doi: 10.1042/bj1820149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES P. W., GRENELL R. G. Metabolism and function in the cerebral cortex under local perfusion, with the aid of an oxygen cathode for surface measurement of cortical oxygen consumption. J Neurophysiol. 1962 Sep;25:651–683. doi: 10.1152/jn.1962.25.5.651. [DOI] [PubMed] [Google Scholar]
- Krab K., Wikström M. On the stoichiometry and thermodynamics of proton-pumping cytochrome c oxidase in mitochondria. Biochim Biophys Acta. 1979 Oct 10;548(1):1–15. doi: 10.1016/0005-2728(79)90182-8. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L., Reynafarje B., Alexandre A., Villalobo A. Respiration-coupled H+ ejection by mitochondria. Ann N Y Acad Sci. 1980;341:585–592. doi: 10.1111/j.1749-6632.1980.tb47200.x. [DOI] [PubMed] [Google Scholar]
- Lemasters J. J., Billica W. H. Non-equilibrium thermodynamics of oxidative phosphorylation by inverted inner membrane vesicles of rat liver mitochondria. J Biol Chem. 1981 Dec 25;256(24):12949–12957. [PubMed] [Google Scholar]
- Moyle J., Mitchell P. Cytochrome c oxidase is not a proton pump. FEBS Lett. 1978 Apr 15;88(2):268–272. doi: 10.1016/0014-5793(78)80190-2. [DOI] [PubMed] [Google Scholar]
- Papa S., Guerrieri F., Lorusso M., Izzo G., Boffoli D., Capuano F., Capitanio N., Altamura N. The H+/e- stoicheiometry of respiration-linked proton translocation in the cytochrome system of mitochondria. Biochem J. 1980 Oct 15;192(1):203–218. doi: 10.1042/bj1920203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen P. L., Greenawalt J. W., Reynafarje B., Hullihen J., Decker G. L., Soper J. W., Bustamente E. Preparation and characterization of mitochondria and submitochondrial particles of rat liver and liver-derived tissues. Methods Cell Biol. 1978;20:411–481. doi: 10.1016/s0091-679x(08)62030-0. [DOI] [PubMed] [Google Scholar]
- Reynafarje B., Brand M. D., Lehninger A. L. Evaluation of the H+/site ratio of mitochondrial electron transport from rate measurements. J Biol Chem. 1976 Dec 10;251(23):7442–7451. [PubMed] [Google Scholar]
- Reynafarje B., Lehninger A. L. The K+/site and H+/site stoichiometry of mitochondrial electron transport. J Biol Chem. 1978 Sep 25;253(18):6331–6334. [PubMed] [Google Scholar]
- Sigel E., Carafoli E. Quantitative analysis of the proton and charge stoichiometry of cytochrome c oxidase from beef heart reconstituted into phospholipid vesicles. Eur J Biochem. 1980 Oct;111(2):299–306. doi: 10.1111/j.1432-1033.1980.tb04942.x. [DOI] [PubMed] [Google Scholar]
- Sigel E., Carafoli E. The proton pump of cytochrome c oxidase and its stoichiometry. Eur J Biochem. 1978 Aug 15;89(1):119–123. doi: 10.1111/j.1432-1033.1978.tb20903.x. [DOI] [PubMed] [Google Scholar]
- Stucki J. W. The optimal efficiency and the economic degrees of coupling of oxidative phosphorylation. Eur J Biochem. 1980 Aug;109(1):269–283. doi: 10.1111/j.1432-1033.1980.tb04792.x. [DOI] [PubMed] [Google Scholar]
- Vercesi A., Reynafarje B., Lehninger A. L. Stoichiometry of H+ ejection and Ca2+ uptake coupled to electron transport in rat heart mitochondria. J Biol Chem. 1978 Sep 25;253(18):6379–6385. [PubMed] [Google Scholar]
- Wikström M., Krab K. Proton-pumping cytochrome c oxidase. Biochim Biophys Acta. 1979 Aug 17;549(2):177–122. doi: 10.1016/0304-4173(79)90014-4. [DOI] [PubMed] [Google Scholar]



