Abstract
EGFR-mutant lung cancers eventually become resistant to treatment with EGFR tyrosine kinase inhibitors (TKIs). The combination of EGFR-TKI afatinib and anti-EGFR antibody cetuximab can overcome acquired resistance in mouse models and human patients. Since afatinib is also a potent HER2 inhibitor, we investigated the role of HER2 in EGFR-mutant tumor cells. We show in vitro and in vivo that afatinib plus cetuximab significantly inhibits HER2 phosphorylation. HER2 overexpression or knockdown confers resistance or sensitivity, respectively, in all studied cell line models. Fluorescent in situ hybridization analysis revealed that HER2 was amplified in 12% of tumors with acquired resistance versus only 1% of untreated lung adenocarcinomas. Notably, HER2 amplification and EGFR T790M were mutually exclusive. Collectively, these results reveal a previously unrecognized mechanism of resistance to EGFR TKIs and provide a rationale to assess the status and possibly target HER2 in EGFR mutant tumors with acquired resistance to EGFR TKIs.
Keywords: EGFR mutations, lung cancer, EGFR tyrosine kinase inhibitors, erlotinib, afatinib, cetuximab, HER2 amplification, EGFR T790M, acquired resistance
Introduction
About 10–30% of tumors from non-small cell lung cancer (NSCLC) patients harbor somatic activating mutations in the gene encoding the epidermal growth factor receptor (EGFR) (1–3). Tumors with the most common alterations, exon 19 deletions and exon 21 point mutations (L858R), are initially responsive to EGFR tyrosine kinase inhibitors (TKIs) such as gefitinib or erlotinib (4, 5) but eventually acquire resistance. Upon disease progression, more than half of the cases harbor a second-site mutation in EGFR, T790M, which alters binding of drug to the ATP-binding pocket (6–8). The optimum treatment for patients with acquired resistance remains unclear.
Afatinib (BIBW2992) is a selective and potent irreversible inhibitor of EGFR and the related ERBB-family member, HER2, with IC50 values of 0.5 and 14 nM, respectively (9). We previously showed in preclinical models that dual inhibition of mutant EGFR with afatinib and the anti-EGFR monoclonal antibody, cetuximab, could overcome T790M-mediated resistance (10). The combination depleted levels of both phosphorylated and total EGFR. The role of HER2 was not investigated. The promising preclinical data led to an ongoing phase IB/II trial with highly encouraging results. Eighteen confirmed partial radiographic responses were observed in the first 45 evaluable patients with acquired resistance, leading to a 40% response rate (95% CI 0.23–0.50) (11). By contrast, erlotinib plus cetuximab or afatinib alone in a similar cohort of patients showed 0% and 7% response rates (RRs), respectively (12, 13) suggesting that the combination of afatinib and cetuximab was synergistic.
Here, given the effect of afatinib on HER2, we investigated the role of HER2 in mediating the sensitivity of EGFR mutant tumor cells to EGFR TKIs. We uncovered HER2 amplification as an unrecognized mechanism of acquired resistance that occurs in a subset of tumors lacking the EGFR T790M mutation.
Results
Effect of afatinib and cetuximab on HER2 in models of acquired resistance to erlotinib
In previous studies of the combination of afatinib and cetuximab, we utilized transgenic mouse lung tumors and H1975 NSCLC cell line xenografts. In both of these models, the TKI resistant T790M mutation was present de novo in cis with a drug-sensitive EGFR mutation (10). Here, we used PC9/BRc1 cells that recapitulate the acquisition of resistance; they were clonally derived from drug-sensitive PC-9 cells (exon 19 deletion) and acquired a secondary T790M mutation by long-term passage in culture (14, 15). Consistent with our prior studies, the combination of afatinib and cetuximab in PC9/BRc1 xenografts led to greater growth inhibition than either drug alone (Fig. 1A).
Figure 1. Effects of combination therapy with afatinib and cetuximab in in vitro and in vivo models of acquired resistance.
(A) Athymic nude mice with PC9/BRc1 tumors were administered vehicle, afatinib, cetuximab, or afatinib plus cetuximab. Tumor volume was determined at the indicated times after the onset of treatment. Points, values from five mice per group; bars, SE. *, P < 0.05, for the combination of afatinib plus cetuximab versus either afatinib or cetuximab alone. (B) PC9/BRc1 cells were plated in soft agar and treated with erlotinib (E), cetuximab (C), afatinib (A), or either combination of erlotinib plus cetuximab or afatinib plus cetuximab for 8 days, after which the absorbance was measured according to the manufacturer’s protocol. Data are means ± SD of triplicates from an experiment that was repeated a total of 3 times with similar results. (C) Cells were serum starved for 12 hours prior to treatment with the indicated drugs for 8 hours, after which cell lysates were subjected to immunoblot analysis with antibodies to the indicated proteins.
To model treatment in vitrowe examined the effects of the anti-EGFR agents on cell growth in three dimensional colony formation assays. As expected for T790M-harboring cells, erlotinib had minimal effect on the growth of PC9/BRc1 colonies (Fig 1B top). Cetuximab modestly inhibited the growth of these cells in a dose-dependent manner, but erlotinib had no additive effect (Fig. 1B top). By contrast, the combination of afatinib and cetuximab together inhibited the growth of PC9/BRc1 cells to a significantly greater extent compared with either drug alone (Fig. 1B bottom). Thus, results from the colony formation assays were reflective of data derived from in vivo xenograft models.
We next used immunoblotting studies to examine the effects of various anti-EGFR agents in PC9/BRc1 cells on levels of phosphorylated EGFR, HER2, HER3, and downstream signaling molecules, AKT and ERK. After 8 hours, cetuximab alone, erlotinib alone, or the combination each minimally inhibited phosphorylated levels of these proteins (Fig. 1C). By contrast, the combination of afatinib plus cetuximab significantly decreased phosphorylated levels of all of the signaling molecules (Fig. 1C).
Interestingly, afatinib alone inhibited levels of phosphorylated HER2 to a greater extent than EGFR or HER3. Similar results were obtained using a separate resistant clone, PC9/BRc4 cells, which harbors the T790M mutation (Supplementary Figs. 1A, B). Comparable outcomes were also derived from other EGFR mutant lines with T790M-mediated acquired resistance, i.e. H3255/XLR and HCC827/R1 cells (14) (Supplementary Figs. 1A, B). Incidentally, we noted that PC9/BRc1 cells express total HER2 at a higher level than parental PC9 cells upon 12-hour serum starvation (Supplementary Fig. 1C).
We further examined the status of EGFR signaling pathway proteins in vivo after treatment with the combination of drugs for varying amounts of time. In tumor lysates derived from PC9/BRc1 xenografts, dual inhibition for 8 hours depleted levels of both phospho-EGFR and total EGFR, as previously reported (10) (Fig. 2A). The effect of treatment on levels of total EGFR was greater in vivo than in vitro (Fig. 2A vs. Fig. 1C). Levels of phospho-HER2 and -HER3 were also diminished but became reactivated after 48 hours of treatment (Fig. 2A).
Figure 2. Role of HER2 in mediating acquired resistance to EGFR inhibition.
(A) Tumor lysates from PC9/BRc1 xenograft models treated for the indicated times with the combination of afatinib plus cetuximab were subjected to immunoblot analyses with antibodies against the indicated proteins. (B) 200 µg of tumor lysate from CCSP-rtTA/EGFRL858R+T790M (C/L+T) mice treated with afatinib/cetuximab for 5 days and samples from untreated controls were hybridized to phospho-RTK arrays (R&D Systems, ARY-014) in accordance to the manufacturer’s protocol. Phosphorylated levels of EGFR, HER2, and HER3 were quantified using Protein array analyzer for ImageJ and normalized to positive control signals on the arrays. Data are presented as mean ± sem (n≥3). (C) Co-immunopreciptiation of HER2 and mutant EGFR L858R+T790M in transgenic mouse lung tumors driven by mutant EGFR. IgG was used as immune-precipitation control. (D) PC9/BRc1 cells were transfected with siRNAs (scramble, EGFR siRNA sequences 1–2, or HER2 siRNA sequences 1–3) for 120 hours, after which cells were harvested and subjected to immunoblot analysis with antibodies against the indicated proteins (left), or cell viability was assessed as described in Methods (right). Data are expressed as a percentage of the value for cells transfected with scramble siRNA and are means of triplicates from experiments that were repeated a total of 3 times with similar results. (E) PC9/BRc1 cells were transfected with scramble or HER2 siRNAs for 24 hours, after which cells were harvested and incubated in 96 well plates for 24 hours prior to treatment with afatinib for 72 hours. Points, mean of triplicates from experiments that were repeated a total of three times with similar results; bars, SD.
Similar results were obtained using transgenic animals that express human EGFRL858R+T790M in lung epithelia (16). Here, tumor lysates from animals treated with afatinib/cetuximab for five days displayed lower levels of phospho-EGFR, -Her2, and tyrosine-phosphorylated protein in general compared to untreated controls (Fig. 2B, Supplementary Figs. 2A and 2B). The difference in Her3 phosphorylation upon treatment was not as pronounced (Fig. 2B, Supplementary Fig. 2A). Immunoprecipitation studies using an L858R-specific antibody in tumor lysates showed that Her2 co-precipitated with mutant EGFR (Fig. 2C), suggesting that these family members heterodimerize in L858R + T790M-driven mouse lung tumors.
Finally, to characterize a functional role for HER2 in resistant cells, we determined the effect of decreased HER2 expression on survival and drug-sensitivity of PC9/BRc1 cells (Figs. 2D and 2E). Knockdown of HER2 using two different short-interfering RNAs (siRNAs) led to decreased survival compared to controls, but the decrease was not as extensive as that seen upon knockdown of EGFR (Fig. 2D). Similar results were obtained with other EGFR-resistant lines (Supplementary Fig. 2C). Knockdown of HER2 also increased the sensitivity of PC9/BRc1 cells to afatinib (Fig. 2E). Taken together, these data suggest that inhibition of HER2 may play an important role in the efficacy of afatinib and cetuximab in TKI-resistant EGFR-mutant lung adenocarcinomas.
HER2 and sensitivity of EGFR TKI-resistant cells to panitumumab plus afatinib
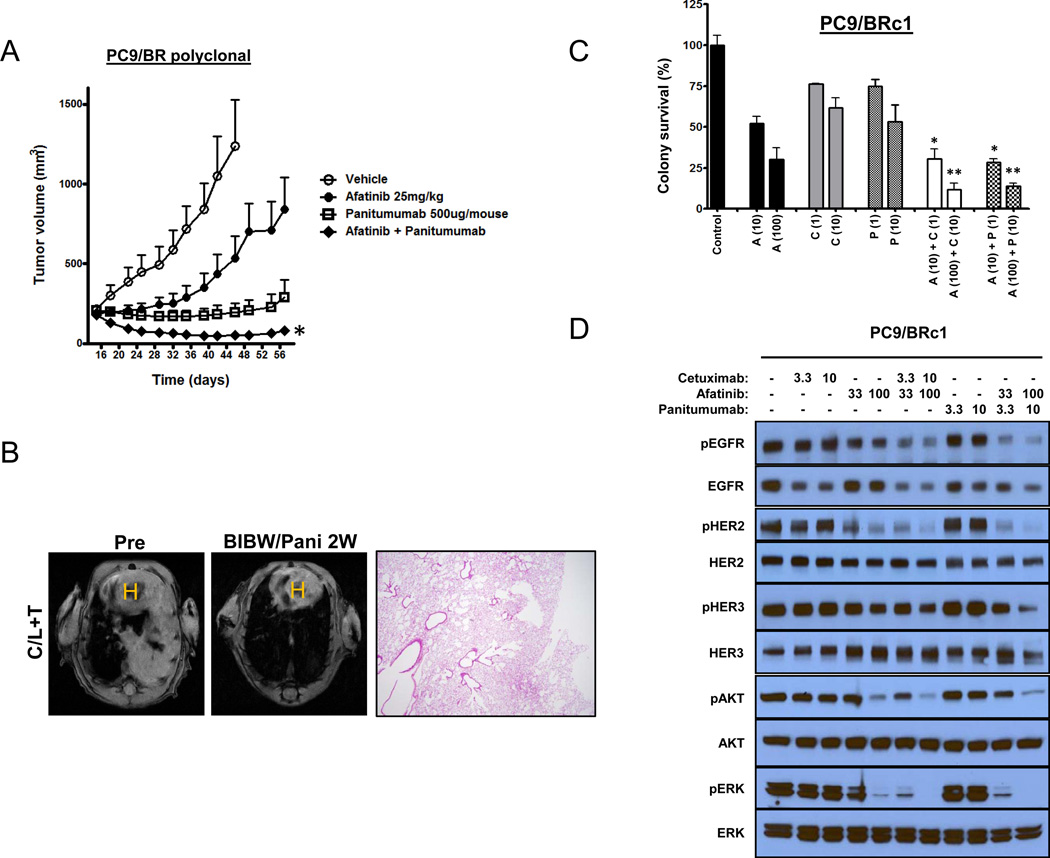
Cetuximab is a human-murine chimeric antibody of the IgG1 isotype approved for use in colorectal and head and neck cancers. In humans, it may activate the complement pathway and mediate antibody-dependent cellular cytotoxicity (ADCC). Panitumumab is a related but fully-humanized anti-EGFR antibody of the IgG2a subtype that may not induce complement pathway activation or ADCC (16); it is approved for the treatment of colorectal cancers. To determine if panitumumab could substitute for cetuximab, we treated PC9/BR polyclonal cells in vivo and in vitro with afatinib, panitumumab, or the combination. We obtained similar results to those with cetuximab in xenografts (Fig. 3A) and in soft agar assays (Fig. 3B). The drug combination was also highly effective at eradicating T790M-driven lung tumors in EGFRL858R+T790M transgenic mice (Fig. 3C). Immunoblot analysis of cells treated in vitro revealed that the combination of afatinib and panitumumab inhibited both EGFR and HER2 phosphorylation and down-regulated EGFR expression similar to the combination of afatinib with cetuximab (Fig. 3D). Collectively, these data show that unique anti-EGFR antibodies with different isotypes can synergize with afatinib to overcome T790M-mediated resistance and that inhibition of HER2 may play an important role in the efficacy of the combination.
Figure 3. The effects of combined therapy with afatinib and panitumumab in EGFR-mutant mouse models and in vitro.
(A) Athymic nude mice with PC9/BR polyclonal tumors were administered vehicle, afatinib, panitumumab, or afatinib plus panitumumab. Points, values from five mice per group; bars, SE. *, P < 0.05, for the combination of afatinib plus panitumumab versus either afatinib or panitumumab alone. (B) MRI images of lungs from a tumor-bearing C/L+T mouse pretreatment and after treatment with the combination of afatinib plus panitumumab (pani) for 2 weeks (2W). H&E-stained section from a treated C/L+T mouse (right panels) (C) PC9/BRc1 cells were plated in soft agar and treated with afatinib (B), cetuximab (C), panitumumab (P), or either a combination of afatinib plus cetuximab or afatinib plus panitumumab for 8 days, after which absorbance levels were measured. Data are means ± SD of triplicates from an experiment that was repeated a total of 3 times with similar results. (D) Cells were serum starved for 12 hours prior to treatment with the indicated drugs for 8 hours, after which cell lysates were subjected to immunoblot analysis with antibodies against the indicated proteins.
Amplification of HER2 occurs in EGFR mutant tumors with acquired resistance to EGFR-TKIs
To explore whether Her2 levels are altered in erlotinib-resistant lung tumors, we performed quantitative RT-PCR on lung tumor samples and adjacent normal lung from EGFR L858R mice in which long-term erlotinib treatment gave rise to resistant tumors, as previously described (17). Seven of nineteen (37%) erlotinib-resistant tumors showed >2-fold increase in Her2 expression compared to normal lung. Only one tumor also harbored the T790M mutation, while three other T790M-positive tumors had Her2 expression levels comparable to adjacent lung tissue (Supplementary Figure 3).
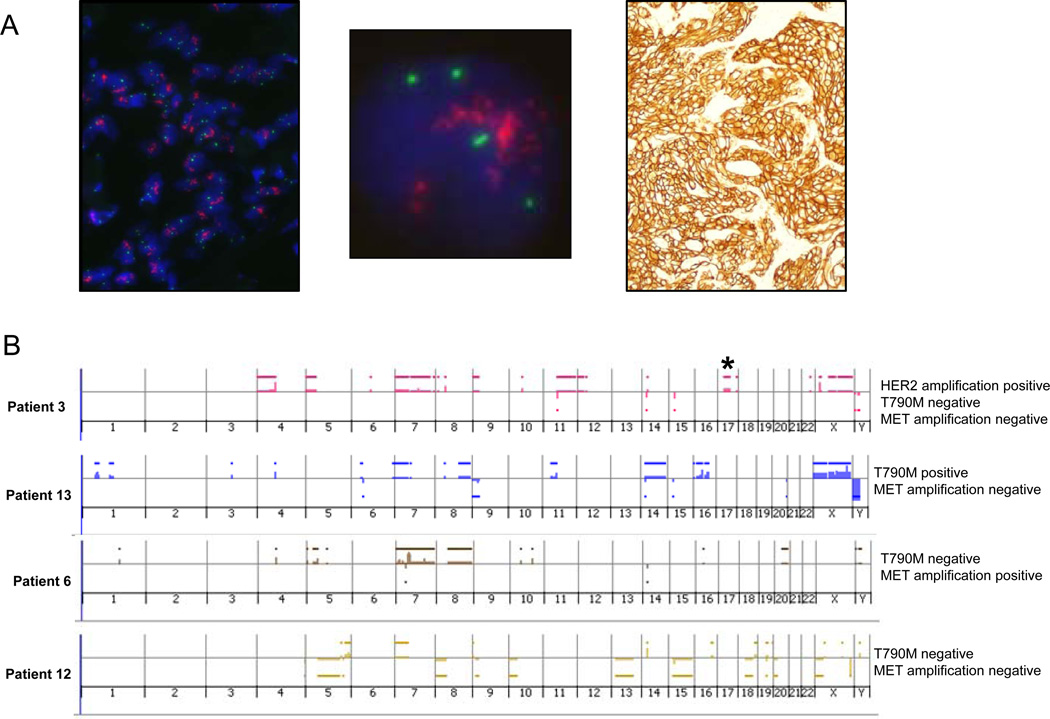
To assess the potential clinical relevance of HER2 status on lung adenocarcinomas with EGFR mutations, we performed standard fluorescent in situ hybridization (FISH) analysis for HER2 on tumor samples from patients with acquired resistance to gefitinib or erlotinib (Fig. 4A). HER2 amplification was observed in 3 of 26 samples (12%). The HER2:CEP17 ratios were 2.2, 2.7, and 5.9, respectively. These specimens also showed strong HER2 positivity by immunohistochemical staining. Notably, all three cases were EGFR T790M-negative. We also conducted Sequenom testing for major and minor mutations in KRAS and PIK3CA; results were negative for all tested mutations in all samples. Histologic examination revealed no evidence of morphologic changes such as epithelial-mesenchymal transition or small cell lung cancer, which has been observed in some cases of acquired resistance (18), (19). Given that 17 of 26 samples analyzed were EGFR T790M positive, HER2 amplification was exclusive of the EGFR T790M mutation (Fisher’s Exact Test, p=0.02, Table 1). Unfortunately, baseline untreated samples were not available in the three patients with T790M-negative, HER2-amplified tumors to determine if HER2 amplification existed pre-treatment. We further re-examined array comparative genomic hybridization data from 12 patients with acquired resistance (20) and found that one sample (8%), which was EGFR T790M-negative, had a focal amplicon at 17q21, consistent with HER2 amplification (Fig. 4B). This sample was also negative for amplification of the gene encoding the MET tyrosine kinase, which constitutes another mechanism of acquired resistance (20). Finally, we performed HER2 FISH on 99 untreated lung adenocarcinomas. Only one (1%) tumor displayed HER2 amplification (HER2: CEP17 ratio: 2.9; notably this tumor also had an EGFR mutation) (3/26 vs 1/99; p=0.03; Fisher’s exact test). Collectively, these results demonstrate that HER2 amplification is detected in a subset of EGFR-TKI-resistant lung cancers; in human lung tumors, such amplification occurs in the absence of EGFR T790M.
Figure 4. HER2 amplification in EGFR T790M-negative tumors with acquired resistance.
(A) HER2 FISH (left (low magnification), middle (high magnification) panels) from a tumor specimen with acquired resistance to erlotinib. HER2 (red) and CEP17 (green). The PathVysion HER2 probe kit (Abbott) was used for HER2 FISH analysis. The tumor nuclei show multiple, clustered HER2 signals (red) and 2–4 chromosome 17 centromere signals (green), indicating high level HER2 amplification in this case (ratio of Her-2/chromosome17 signals > 2.2). Immunohistochemistry (right panel) was performed on the same case using the HercepTest kit (DAKO), showing strong positive staining for HER2 (3+), according to standard scoring criteria used in breast cancer. (B) Array comparative genomic hybridization data from 12 patients with acquired resistance to gefitinib or erlotinib analyzed using a 60-mer oligonucleotide array platform (Agilent).18 Asterisk denotes amplification in HER2, occurring in 1 of 12 samples. This sample was negative for both T790M mutation and MET amplification. Lower panels show representative examples without HER2 amplification.
Table 1.
EGFR T790M and HER2 status in 26 EGFR-mutant tumors from patients with acquired resistance to erlotinib or gefitinib.
| Gene status | HER2 amp + | HER2 amp − |
|---|---|---|
| EGFR T790M + | 0 | 17 |
| EGFR T790M − | 3 | 6 |
HER2 contributes to the antitumor effects of EGFR-TKIs in drug-sensitive EGFR mutant lung adenocarcinoma cells
Having implicated HER2 as a potential mediator of resistance in EGFR TKI-resistant cells, we investigated the role of HER2 in mediating the sensitivity of parental EGFR mutant cells to erlotinib. We used PC9 (exon 19 deletion), HCC827 (exon 19 deletion), and H3255 (L858R) cells, none of which harbor EGFR T790M. Compared to controls, cells treated with increasing concentrations of erlotinib displayed reduced levels of phosphorylated HER2 as well as HER3, AKT, and ERK (Fig. 5A). Depletion of HER2 expression with siRNAs furthermore inhibited the growth of all three EGFR mutant cell lines. However, the degree of growth inhibition was less than that observed with knockdown of EGFR itself (Fig. 5B, Supplementary Fig. 4A) or with that observed in cells more dependent upon HER2, such as BT474 breast cancer cells (Supplementary Fig. 4B). Collectively, these data demonstrate that HER2 affects the sensitivity of EGFR mutant lung adenocarcinoma cells to EGFR TKIs.
Figure 5. HER2 levels affect the sensitivity of EGFR-mutant NSCLC cells to EGFR TKIs.
(A) Cells were serum starved for 12 hours prior to treatment with the indicated concentrations of erlotinib for 8 hours, after which cell lysates were subjected to immunoblot analysis with antibodies against the indicated proteins. (B) PC9 cells were transfected with scramble or HER2 siRNAs for 24 hours, after which cells were harvested and incubated in 96 well plates for 24 hours prior to treatment with afatinib for 72 hours. Points, mean of triplicates from experiments that were repeated a total of three times with similar results; bars, SD. (C) Cells stably transfected with either empty vector (Mock) or a HER2 expression vector (clones 1–3) were harvested and subjected to immunoblot analysis with antibodies to the indicated proteins. (D, E) Cells were incubated for 24 hours prior to treatment with erlotinib (D) or afatinib (E) for 72 hours, after which cell viability was assessed as described in Methods. Points, mean of triplicates from experiments that were repeated a total of three times with similar results; bars, SD. (F, G) Cells were serum starved for 12 hours prior to treatment with erlotinib (F) or afatinib (G) for 8 hours, after which cell lysates were subjected to Immunoblot analysis with antibodies against the indicated proteins.
Finally, to confirm that HER2 overexpression causes resistance to erlotinib in EGFR-mutant lung adenocarcinomas, we introduced wild-type HER2 cDNAs into EGFR-TKI sensitive PC9 cells (Fig. 5C) and performed standard growth inhibition assays (Fig. 5D, E). HER2 overexpression (>50-fold above baseline, as per densitometry assessment) conferred resistance to erlotinib (Fig. 5D) but not afatinib (Fig. 5E). Similar results were seen when HER2 cDNAs were expressed in HCC827 cells (Supplementary Fig. 5A, B). Compared to control (mock-infected) cells, erlotinib failed to inhibit phosphorylation of HER2, AKT and ERK, in PC9/HER2 clones (Fig. 5F, G). We did not introduce HER2 into cells harboring the T790M mutation as the data from our clinical samples showed that these events were mutually exclusive. Collectively, these data indicate that HER2 overexpression can mediate resistance to EGFR-TKIs in EGFR mutant NSCLC.
Discussion
All patients with metastatic EGFR mutant lung cancer ultimately develop resistance to EGFR-TKIs gefitinib and erlotinib. The most commonly observed mechanism involves acquisition of cells harboring a second-site mutation, T790M (6–8). We previously demonstrated in clinically relevant mouse lung tumor models that the combination of afatinib plus cetuximab could overcome T790M-mediated resistance, due to depletion of both phosphorylated and total EGFR (10). This drug combination has recently shown promising efficacy in patients with EGFR mutant tumors and acquired resistance to gefitinib or erlotinib (11). Because afatinib inhibits HER2 in addition to EGFR, here, we investigated the role of HER2 in mediating sensitivity/resistance to EGFR TKIs in this setting.
Through the study of preclinical in vitro and in vivo models as well as human tissues, we identify a major role for HER2 in mediating sensitivity and resistance to EGFR TKIs. First, in multiple models of acquired resistance, levels of phosphorylated HER2 decrease after treatment with either afatinib or cetuximab alone and more dramatically with the combination. The same observations were made with afatinib plus an alternative anti-EGFR antibody, panitumumab. Second, HER2 is dephosphorylated in drug-sensitive EGFR mutant cells after treatment with erlotinib. Third, HER2 overexpression or knockdown confers resistance or sensitivity, respectively, in cell line models. Fourth, HER2 is amplified in both murine and human tumors with acquired resistance to erlotinib. Notably, in humans samples, EGFR T790M and HER2 amplification appear mutually exclusive.
Previous studies have postulated conflicting data on the role of EGFR heterodimers in mediating sensitivity to EGFR TKIs in EGFR mutant lung cancer. Through comparison of immunoprecipitates of phosphoinositide 3-kinase (PI3K) between gefitinib-sensitive and –resistant NSCLC cell lines, a strong correlation was identified between ErbB3/HER3 expression in NSCLC cell lines and sensitivity to gefitinib, raising the possibility that ErbB3 is used to couple EGFR to the PI3K-AKT pathway in gefitinib-sensitive NSCLC cell lines harboring WT and mutant EGFRs (15). Alternatively, through study of receptor down-regulation, data suggests that mutant EGFRs, especially the L858R/T790M variant, have a propensity to heterodimerize with HER2, which allows for evasion of CBL-mediated ubiquitinylation and subsequent lysosomal degradation (21). Our findings support the notion that HER2 influences EGFR TKI sensitivity in EGFR mutant cells, although we did not exclude any role for HER3. We further hypothesize that EGFR mutant cells can become resistant either by acquiring the T790M mutation which enhances HER2 heterodimerization in the absence of HER2 amplification or by acquiring HER2 amplification in the absence of a second-site mutation. Future biochemical studies and or genetic studies using ErbB2 or ErbB3 knockouts in the context of EGFR mutant lung cancers may help shed further light on this issue.
In the present study, we found that HER2 amplification is a rare event in untreated lung adenocarcinomas, occurring in 1% of samples as assessed by FISH. By contrast, others group have reported that HER2 may be amplified, even up to 22.8% of NSCLCs (22–25). This high figure appears inconsistent with other datasets. For example, microarray-based genomic copy number analyses have shown that HER2 copy number gain occurred in only 10 of 628 (1.6%) NSCLCs (26). In our own genomic studies, HER2 gene copy gain occurred in only 2 of 199 (1%) lung adenocarcinomas, neither of which harbored EGFR mutations (27). Further systematic analyses of HER2 status in both untreated NSCLCs and those with acquired resistance to EGFR TKIs are warranted.
Activation of HER2 signaling was recently reported to cause resistance to cetuximab alone in patients with colorectal cancer (28, 29). In one of the studies (29), introduction of HER2 into another EGFR drug-sensitive lung cancer cell line, HCC827, failed to affect drug-sensitivity, consistent with our findings (Supplementary Fig. 5). These data suggest that the effects of HER2 expression or activity on sensitivity/resistance to anti-EGFR drugs may depend on cell line lineage or other as yet undefined genetic determinants. The studies in colon cancer suggest that the combination of afatinib plus cetuximab could also be efficacious in overcoming acquired resistance to cetuximab in that disease.
In summary, our data suggest that the ErbB family member, HER2, plays a significant role in mediating sensitivity of EGFR mutant lung tumors to anti-EGFR therapy. We identify HER2 amplification as a new mechanism of acquired resistance to EGFR TKIs in EGFR-mutant NSCLC tumors, occurring independently of the EGFR T790M secondary mutation. This observation could explain why the combination of afatinib/cetuximab induces responses in some but not all patients without T790M-mediated acquired resistance (30). If the 12% prevalence of HER2 amplification in this clinical setting is verified in future studies, this would place it as one of the most common acquired resistance mechanisms after the EGFR T790M mutation (Fig. 6). Hopefully, this knowledge will eventually lead to improved therapeutic outcomes for patients with EGFR mutant lung cancer.
Figure 6. Frequency of mechanisms of acquired resistance to EGFR-TKIs in EGFR mutant lung cancer.
Methods
Cell culture and derivation of TKI-resistant lines
EGFR mutant PC9 cells (del E746-A750) or HCC827 cells (del E746-A750) were cultured in RPMI media (ATCC) supplemented with 10% heat inactivated fetal bovine serum (Gemini Bio Products). These lines have been used as reagents in the Pao Lab since 2005 (7), (31). Cell lines were re-genotyped multiple times to confirm the presence of known EGFR mutations using standard Sanger sequencing. Cells were grown in a humidified incubator with 5% CO2 at 37°C. Resistant cells were derived as previously described (14). Briefly, parental cells were cultured with increasing concentrations of TKIs starting with the IC30. Doses were increased in a stepwise pattern when normal cell proliferation patterns resumed. Fresh drug was added every 72–96 hours. Resistant cells were maintained initially as polyclonal populations under constant TKI selection. Clonal resistant cells were isolated by limiting dilution.
Xenograft studies
Nude mice (nu/nu; Harlan Laboratories) were used for in vivo studies and were cared for in accordance with guidelines approved by the MSKCC Institutional Animal Care and Use Committee and Research Animal Resource Center. Eight-week-old female mice were injected s.c. with 10 million PC9/BRc1 cells. When tumors reached ~150 mm3animals were randomized to receive vehicle alone, cetuximab (1 mg/mouse twice per week, intraperitoneally; Bristol-Myers Squibb/ImClone/Eli Lilly) or panitumumab (500 ug/mouse twice per week, intraperitoneally; Amgen), afatinib (25 mg/kg daily, orally; drug synthesized by the MSKCC Organic Synthesis Core, or a combination of both afatinib and either cetuximab or panitumumab. Achievable afatinib serum levels at this dose range from 80–667 nM (9); the standard dose in humans is 40 mg po qd; at 45mg, the Cmax is 141 nM (32). Mice were observed daily throughout the treatment period for signs of morbidity/mortality. Tumors were measured twice weekly using calipers, and volume was calculated using the formula: length × width2 × 0.52. Body weight was also assessed twice weekly. Tumor samples were collected within 8 hours of the last treatment. Each sample was cut in halves; one half was preserved in 4% paraformaldehyde and one half was flash frozen in liquid nitrogen and stored at −80°C until further use.
Transgenic Mouse Studies
All animals were kept in pathogen-free housing under guidelines approved by the Yale or VICC Institutional Animal Care and Use Committees. The generation of EGFRL858R+T790M mice was previously described (10). Doxycycline was administered by feeding mice with doxycycline-impregnated food pellets (625 ppm; Harlan-Teklad). Afatinib was suspended in 0.5% (w/v) methylcellulose and injected intraperitoneally at the dose of 25 mg/kg daily for 5 days. Cetuximab was administered i.p. at 1 mg/mouse 2 times/week. Panitumumab was administered i.p. at 500 µg/mouse 2 times/week.
Soft agar assay
The colony-forming capacity of PC9/BRc1 cells was assessed using the CytoSelect 96-Well In Vitro Tumor Sensitivity Assay (Soft Agar Colony Formation) Kit (Cell Biolabs Inc.), according to manufacturer’s protocol. Briefly, 50 µL of Base Agar Matrix Layer was dispensed into each well of a 96-well tissue culture plate. Cells (5 × 103) in 75 µL of Cell Suspension/Agar Matrix Layer were dispensed into each well. The cells were treated with 50 µL of culture medium containing various drugs. After incubation for 8 days, 125 µL of the 1 × Matrix Solubilization Buffer was added to solubilize the agar matrix completely, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide was added to each well. The absorbance was then recorded on a SpectraMax fluorometer at 570 nm.
Immunoblotting
Cells were washed with cold PBS and lysed for 30 minutes with RIPA buffer (150mM Tris.HCl pH 7.5, 150mM NaCl, 1% NP-40 substitute, 0.1% SDS) supplemented with protease inhibitor cocktail (Roche), 40mM sodium fluoride, 1mM sodium orthovanadate, and 1µM okadaic acid. Protein levels were quantified with Bradford Reagent (Bio-Rad) and equal amounts were loaded for SDS-PAGE using 4–20% acrylamide precast gels (Invitrogen), followed by transfer to PVDF membranes. Membranes were blotted with pEGFR (Y1068, CST, cat#2234), total EGFR (BD Biosciences, cat# 610016), EGFRL858R (CST, cat# 3197), pHER2 (Y1248, CST, cat# 2247), total HER2 for mouse (Millipore, cat#06-562), pHER3 (Y1197, CST, cat# 4561), total HER3 for mouse (NanoTools, cat#0237), pAKT (S473, CST, cat#9271), total AKT (CST, cat#9272), pERK (T202/204, cat#9101), total ERK (cat# 9102), Pro-surfactant Protein C (Abcam, cat#ab90716) and actin (Sigma-Aldrich) followed by HRP conjugated secondary antibodies or pY-HRP (R&D Systems, part#841403). All antibodies were purchased from Cell Signaling Technology, unless noted. Signals were detected with Western blotting detection reagents (Perkin Elmer, NEL10500NEA).
Growth inhibition assay
Cellular growth inhibition was measured with CellTiter Blue Reagent (Promega, G8081) as per the manufacturer’s instructions using cells plated in triplicate at a density of 2000 cells per well. Fluorescence was measured on a SpectraMax fluorometer. Growth inhibition was calculated as percentage of vehicle-treated wells ± SD.
RNA interference
Cells were plated at 50% to 60% confluence in 6-cm dishes and then incubated for 24 hours before transient transfection for the indicated times with siRNAs mixed with Lipofectamine reagent (Invitrogen). siRNAs specific for EGFR (MQ-003114-03) and HER2 (MQ-003126-04) mRNA were obtained from Dharmacon.
FISH analysis
Assessment of HER2 gene copy number was performed on formalin-fixed paraffin-embedded specimens. The Vysis PathVysion HER-2 DNA Probe Kit (Abbott Laboratories, Abbott Park, IL) was used following standard manufacturer’s protocol. At least 40 cells were analyzed for each case by 2 reviewers and were classified as amplified if the HER2/CEP17 ratio per cell was > 2, or homogeneously staining regions with >15 copies in >10% of the cells were present. Cases with a ratio between 1.8 and 2.2 were initially considered as borderline range prompting further review and recounting of wider areas to confirm their status as amplified or not amplified.
Cell transfection
A pBabe-puro vector encoding wild-type HER2 was kindly provided by Carlos Arteaga (30).The pBabe-puro/WT-HER2 vector and the pVSV-G vector (Clontech, Palo Alto, CA, USA) for production of the viral envelope were introduced into GP2-293 cells (80% confluence in a 10-cm dish) with the use of FuGENE6 transfection reagent. After 48h, viral particles released into the culture medium were concentrated by centrifugation at 15,000g for 3 h at 4°C. The resulting pellet was then suspended in fresh RPMI 1640 medium and used to infect PC9 and HCC827 cells as previously described (33).
Quantitative RT-PCR
Mouse lung tissue samples were crushed and RNA was extracted from the tissue powders using TRIzol reagent (Invitrogen). RNA was DNase-treated (Invitrogen) to eliminate any contaminating DNA. cDNA was made from 600ng of DNase-treated RNA using the Superscript III First-Strand Synthesis System for RT-PCR (Invitrogen). Resulting cDNA was diluted 10-fold and 2 µl were used for quantitative RT-PCR analysis using the mouse Her2 and surfactant protein C TaqMan Gene Expression Assays (Applied Biosystems). Reactions were performed in a 10 µl volume using the TaqMan Universal PCR Master Mix, No AmpErase UNG (Applied Biosystems) and run in triplicates on the Applied Biosystems ViiA7 Real-Time PCR System.
Immunoprecipitation
Lung tissue containing tumors from mutant EGFR L858R+T790M transgenic mice were pulverized and lysed in co-immunoprecipitation buffer containing 150 mM NaCl, 50 mM Tris HCl and 1% NP40 plus the Halt protease and phosphotase inhibitor cocktail (Thermo Scientific). After pre-clearing lysates with protein A/G plus beads, 500 µg of protein was incubated with 5 µg of antibody against EGFR L858R overnight at 4°C. Lysates were further incubated with 40 µl of 50% protein A/G plus beads for 1 hour at 4°C, followed by 5 washes with lysis buffer. Immunoblotting was performed as described above.
Statistical analysis
Quantitative data for western blot and RTK arrays are presented as means ± SD and were analyzed by Student’s 2-tailed t test. A value of p < 0.05 was considered statistically significant.
Supplementary Material
Statement of Significance.
Because all EGFR-mutant lung adenocarcinomas eventually develop resistance to TKI therapy, understanding mechanisms of acquired resistance may improve clinical outcomes. These results implicate HER2 as a novel protein involved in the sensitivity or resistance of EGFR-mutant lung cancer and provide a rationale to assess the status of and possibly target HER2 in such tumors.
Acknowledgements
We acknowledge Monica Red Brewer for critical reading of the manuscript.
Grant support
We acknowledge support from the NIH/National Cancer Institute (NCI) grants R01-CA121210 (WP), P01-CA129243 (WP, ML, MK), U54-CA143798 (WP), R00CA131488 (KP), R01CA120247 (KP) and from the Uehara Memorial Foundation (KT). Additional support was received from the Vanderbilt-Ingram Cancer Center Core grant (P30-CA68485) to WP, Uniting Against Lung Cancer (KP) and the American Italian Cancer Foundation (VP).
Abbreviations
- EGFR
epidermal growth factor receptor
- TKI
tyrosine kinase inhibitor
- HER2/3
human epidermal growth factor receptor 2/3
- NSCLC
non-small cell lung cancer
- ATP
adenosine triphosphate
- RR
response rate
- ADCC
antibody-dependent cellular cytotoxicity
- BR
BIBW2992 resistant
- BRc1
BIBW2992-resistant clone 1
- FISH
fluorescent in situ hybridization
Footnotes
Disclosure of potential conflicts of interest
V.A.M. has consulted for Astellas, Boehringer-Ingelheim, Clovis, and Genentech and currently is an employee of Foundation Medicine. W.P. has consulted for Clovis, MolecularMD, Bristol-Myers Squibb, and AstraZeneca and has research funding from Clovis, Symphogen, Bristol-Myers Squibb, and AstraZeneca. Y.J. has received research funding from Boehringer-Ingelheim and Roche/Genentech. G.J.R. has consulted for Chugai, Ariad, Tragara, Daiichi, Novartis, Abbott Molecular and has research funding from GSK, Boehringer-Ingelheim, Novartis, Chugai, Infinity, BMS, and Pfizer. M.G.K. has consulted for Pfizer, Boehringer Ingelheim, and Genentech/Roche and has research funding from Pfizer and Boehringer Ingelheim. Rights to a patent application for EGFR T790M testing were licensed on behalf of V.A.M., K.A.P, W.P. to MolecularMD.
References
- 1.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. The New England journal of medicine. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 2.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 3.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. The New England journal of medicine. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 5.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatinpaclitaxel in pulmonary adenocarcinoma. The New England journal of medicine. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. The New England journal of medicine. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 7.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS medicine. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arcila ME, Oxnard GR, Nafa K, Riely GJ, Solomon SB, Zakowski MF, et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:1169–1180. doi: 10.1158/1078-0432.CCR-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac LR, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27:4702–4711. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regales L, Gong Y, Shen R, de Stanchina E, Vivanco I, Goel A, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest. 2009;119:3000–3010. doi: 10.1172/JCI38746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yelena Y, Janjigian HJMG, Leora Horn, Egbert F Smit, Yali Fu, Fei Wang, Mehdi Shahidi, Louis Denis, William Pao, Vincent A Miller. Activity and tolerability of afatinib (BIBW 2992) and cetuximab in NSCLC patients with acquired resistance to erlotinib or gefitinib. ASCO. 2011 abst 7525.2011. [Google Scholar]
- 12.Janjigian YY, Azzoli CG, Krug LM, Pereira LK, Rizvi NA, Pietanza MC, et al. Phase I/II trial of cetuximab and erlotinib in patients with lung adenocarcinoma and acquired resistance to erlotinib. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:2521–2527. doi: 10.1158/1078-0432.CCR-10-2662. [DOI] [PubMed] [Google Scholar]
- 13.Miller VA, Hirsh V, Cadranel J, Chen Y-M, Park K, Kim S-W, Caicun Z, Oberdick M, Shahidi M, Yang Phase IIB/III double-blind randomized trial of afatinib (BIBW 2992, an irreversible inhibitor of EGFR/HER1 and HER2) + best supportive care (BSC) versus placebo + BSC in patients with NSCLC failing 1–2 lines of chemotherapy and erlotinib or gefitinib (LUX-Lung 1) Annals of Oncol. 2010;21 [Google Scholar]
- 14.Chmielecki J, Foo J, Oxnard GR, Hutchinson K, Ohashi K, Somwar R, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Science translational medicine. 2011;3:90ra59. doi: 10.1126/scitranslmed.3002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 16.Yan L, Hsu K, Beckman RA. Antibody-based therapy for solid tumors. Cancer J. 2008;14:178–183. doi: 10.1097/PPO.0b013e318172d71a. [DOI] [PubMed] [Google Scholar]
- 17.Politi K, Fan PD, Shen R, Zakowski M, Varmus H. Erlotinib resistance in mouse models of epidermal growth factor receptor-induced lung adenocarcinoma. Disease models & mechanisms. 2010;3:111–119. doi: 10.1242/dmm.003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zakowski MF, Ladanyi M, Kris MG. EGFR mutations in small-cell lung cancers in patients who have never smoked. The New England journal of medicine. 2006;355:213–215. doi: 10.1056/NEJMc053610. [DOI] [PubMed] [Google Scholar]
- 19.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Science translational medicine. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bean J, Brennan C, Shih JY, Riely G, Viale A, Wang L, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shtiegman K, Kochupurakkal BS, Zwang Y, Pines G, Starr A, Vexler A, et al. Defective ubiquitinylation of EGFR mutants of lung cancer confers prolonged signaling. Oncogene. 2007;26:6968–6978. doi: 10.1038/sj.onc.1210503. [DOI] [PubMed] [Google Scholar]
- 22.Cappuzzo F, Ligorio C, Janne PA, Toschi L, Rossi E, Trisolini R, et al. Prospective study of gefitinib in epidermal growth factor receptor fluorescence in situ hybridization-positive/phospho-Akt-positive or never smoker patients with advanced non-small-cell lung cancer: the ONCOBELL trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:2248–2255. doi: 10.1200/JCO.2006.09.4300. [DOI] [PubMed] [Google Scholar]
- 23.Cappuzzo F, Varella-Garcia M, Shigematsu H, Domenichini I, Bartolini S, Ceresoli GL, et al. Increased HER2 gene copy number is associated with response to gefitinib therapy in epidermal growth factor receptor-positive non-small-cell lung cancer patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:5007–5018. doi: 10.1200/JCO.2005.09.111. [DOI] [PubMed] [Google Scholar]
- 24.Pugh TJ, Bebb G, Barclay L, Sutcliffe M, Fee J, Salski C, et al. Correlations of EGFR mutations and increases in EGFR and HER2 copy number to gefitinib response in a retrospective analysis of lung cancer patients. BMC cancer. 2007;7:128. doi: 10.1186/1471-2407-7-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varella-Garcia M, Mitsudomi T, Yatabe Y, Kosaka T, Nakajima E, Xavier AC, et al. EGFR and HER2 genomic gain in recurrent non-small cell lung cancer after surgery: impact on outcome to treatment with gefitinib and association with EGFR and KRAS mutations in a Japanese cohort. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2009;4:318–325. doi: 10.1097/JTO.0b013e31819667a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chitale D, Gong Y, Taylor BS, Broderick S, Brennan C, Somwar R, et al. An integrated genomic analysis of lung cancer reveals loss of DUSP4 in EGFR-mutant tumors. Oncogene. 2009;28:2773–2783. doi: 10.1038/onc.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertotti A, Migliardi G, Galimi F, Sassi F, Torti D, Isella C, et al. A Molecularly Annotated Platform of Patient-Derived Xenografts ("Xenopatients") Identifies HER2 as an Effective Therapeutic Target in Cetuximab-Resistant Colorectal Cancer. Cancer discovery. 2011;1:508–523. doi: 10.1158/2159-8290.CD-11-0109. [DOI] [PubMed] [Google Scholar]
- 29.Yonesaka K, Zejnullahu K, Okamoto I, Satoh T, Cappuzzo F, Souglakos J, et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Science translational medicine. 2011;3:99ra86. doi: 10.1126/scitranslmed.3002442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang SE, Narasanna A, Perez-Torres M, Xiang B, Wu FY, Yang S, et al. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer cell. 2006;10:25–38. doi: 10.1016/j.ccr.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 31.Gong Y, Somwar R, Politi K, Balak M, Chmielecki J, Jiang X, et al. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS medicine. 2007;4:e294. doi: 10.1371/journal.pmed.0040294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eskens FA, Mom CH, Planting AS, Gietema JA, Amelsberg A, Huisman H, et al. A phase I dose escalation study of BIBW 2992, an irreversible dual inhibitor of epidermal growth factor receptor 1 (EGFR) and 2 (HER2) tyrosine kinase in a 2-week on-2-week off schedule in patients with advanced solid tumours. British journal of cancer. 2008;98:80–85. doi: 10.1038/sj.bjc.6604108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takezawa K, Okamoto I, Nishio K, Janne PA, Nakagawa K. Role of ERK-BIM and STAT3-survivin signaling pathways in ALK inhibitor-induced apoptosis in EML4-ALK-positive lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:2140–2148. doi: 10.1158/1078-0432.CCR-10-2798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.