Abstract
Interactions between disseminated tumor cells (DTCs) and stromal cells in the microenvironment are critical for tumor colonization of distal organs. Recent studies have shown that vascular cell adhesion molecule-1 (VCAM-1) is aberrantly expressed in breast cancer cells and mediates pro-metastatic tumor-stromal interactions. Moreover, the usefulness of VCAM-1 to DTCs in two different organs –lung and bone– is based on distinct mechanisms. In the lungs, VCAM-1 on the surface of cancer cells binds to its counter-receptor, the α4β1 integrin (also known as very-late antigen, VLA-4), on metastasis-associated macrophages, triggering VCAM-1 mediated activation of the PI3K growth and survival pathway in the cancer cells. In the bone marrow, cancer cell VCAM-1 attracts and tethers α4 integrin-expressing osteoclast progenitors to facilitate their maturation into multinucleated osteoclasts that mediate osteolytic metastasis. These findings highlight the importance of direct interactions between DTCs and stromal cells during tumor dissemination and draw attention to the possibility of targeting the α4 integrin-VCAM-1 interactions in metastatic breast cancer. Anti-α4 integrin inhibitors have been developed to treat various diseases driven by massive leukocyte infiltrates and have gained FDA approval or are undergoing clinical trials. Testing these drugs against tumor-stromal leukocyte interactions may provide a new strategy to suppress lung and bone relapse of breast cancer.
Background
Over the last several decades, early detection, surgical removal and targeted therapy have improved treatment outcome for primary breast tumors. However, breast cancer still has a high mortality rate which is primarily due to metastatic disease. Lung, bone, brain and liver are the most common sites of distant dissemination (1, 2). Metastasis from primary sites to distal organs is a complex process that involves a series of sequential steps: invasion and intravasation of tumor cells from the primary tumor sites to enter the circulation, extravasation of these circulating tumor cells (CTCs) into distant tissues, and final colonization of the seeded organ (3, 4). Even though CTCs are present in large numbers in the bloodstream, only a small proportion of these cells succeed in forming distal metastasis (1, 5). As envisioned in the `seed and soil' hypothesis (6, 7), the organ tropism of metastasis depends on the complex interplay between tumor cells and the unique microenvironments of different distal organs. The underlying mechanisms of this interplay are just beginning to be elucidated. Two recent studies on a cell surface molecule that mediate cell adhesion, termed vascular cell adhesion molecule-1 (VCAM-1), provide new insights into these mechanisms. When aberrantly expressed in breast cancer cells, VCAM-1 mediates distinct tumor-stromal interactions that are unique to lung and bone microenvironments and facilitate metastasis to these sites (8, 9). Little is known about the function of VCAM-1 in other cancers. However, VCAM-1 expression has been reported in gastric and renal carcinomas, and melanomas (10–12), where it might plays roles similar to those recently reported in breast cancer (8, 9). The new work identifies VCAM-1 as an interesting new player in tumor progression and a target worth considering for potential therapies against lung and bone metastasis of breast cancer and other cancers.
VCAM-1 as an adhesion molecule in endothelial cells
VCAM-1 is an immunoglobulin (Ig)-like adhesion molecule with seven extracellular Ig domains that are mainly expressed in endothelial cells (13, 14). While expressed at low level on resting endothelial cells, VCAM-1 is strongly induced by several inflammatory cytokines (15, 16). VCAM-1 binds with high affinity to the integrins α4β1 (also known as very-late antigen, VLA-4) and α4β7. α4β1 in particular is a well-studied counter-receptor expressed on the surface of several cell types of the hematopotietic lineage including lymphocytes, monocyte/macrophages and eosinophils (17). VCAM-1 plays a critical role in the inflammatory response by recruiting leukocytes to acute and chronic inflammatory sites (15, 16). In addition to mediating leukocyte adhesion on endothelial cells, VCAM-1 can activate signaling pathways to facilitate leukocyte passage from blood to tissue. The short intracellular tail of VCAM-1 interacts with Ezrin (also known as cytovillin or villin-2), a member of the ERM (Ezrin/Radixin/Moesin) protein family (18). ERM are cytoplasmic adaptor proteins that link various transmembrane proteins to the actin cytoskeleton (18). In endothelial cells, VCAM-1 clustering, either by antibody cross-linking or integrin binding, triggers the activation of Rac1, which is a Rho-like GTPase (19). The activation of Rac1 results in the rearrangement of the cytoskeletal network, which is thought to remodel the tight junctions between vascular endothelial cells and consequently facilitate transendothelial migration. Circulating melanoma cells expressing α4 integrin have been shown to interact with VCAM-1 on endothelial cells to facilitate extravasation and metastasis in distal organs (20–22). Therefore, in addition to passive entrapment of tumor cells by size restriction (4), capillary beds may actively participate in the extravasation into disseminated sites of CTCs via the adhesion molecules on vascular endothelial cells.
Role of VCAM-1 in lung metastasis of breast cancer
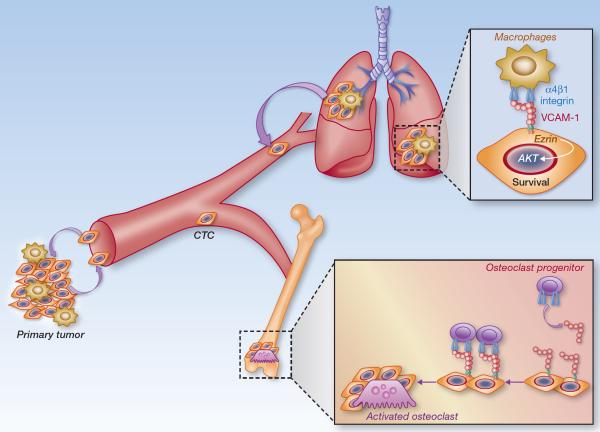
Studies combining animal models and analyses of clinical samples identified a lung metastatic gene signature (LMS) as a set of 18 genes whose expression is associated with lung relapse in estrogen receptor-negative (ER−) tumors and mediates lung metastasis in xenograft models (23). Understanding the molecular mechanisms of how the LMS genes mediate lung metastasis can provide novel treatment modalities for lung metastasis of breast cancer. VCAM-1 is among these 18 genes, and its expression in breast cancer cells enhances the ability to form metastases in the lungs (8, 23). The obvious question is why do cancer cells with a high propensity to form lung metastasis abnormally overexpress a vascular endothelial adhesion molecule? Our work aiming at question indicates that the high expression of VCAM-1 in tumor cells does not influence tumor cell intravasation from the primary sites, the number of CTCs in blood stream, or the CTC extravasation into the lungs. Instead, VCAM-1 promotes lung colonization by providing juxtacrine survival signaling that protects tumor cells from death (Figure 1).
Figure 1.
VCAM-1 expressed in breast cancer cells mediates lung and bone metastasis by interacting with stromal leukocytes that express α4 integrin counter-receptors. In primary tumor sites and in the pulmonary parenchyma, interactions with macrophages initiate juxtacrine stimulation through the C-terminal tail of VCAM-1 and the adaptor protein Ezrin, which enhances PI3K-Akt cell survival signaling. In the bone marrow, cancer cell VCAM-1 attracts and tethers myeloid osteoclast progenitor cells to facilitate their maturation and stimulate osteolytic metastasis.
Similar to its adhesive function in endothelial cells, VCAM-1 expressed on the surface of tumor cells tethers leukocytes that express α4 integrins. Analyses on leukocyte subpopulations in lung metastatic lesions indicate that tumor-associated monocytes and macrophages prominently express α4 integrins, but also express higher level of α4 integrins compared to T and B lymphocytes. Macrophages have been shown to play important roles in primary tumor growth and lung metastasis at different steps of the lung metastatic process, including promoting tumor angiogenesis, tumor invasion at the edge, tumor cell intravasastion into blood stream and lung colonization (24, 25). Most of these studies have been done on breast tumors and show that the crosstalk between tumor cells and tumor-associated macrophages (TAMs) is mediated by various soluble factors secreted by tumor cells and TAMs. However, recent studies have shown that depleting lung macrophages or preventing the recruitment of macrophages into lung by blocking CCL2-CCR2 signaling in mouse models significantly decreases lung metastasis, via unknown molecular mechanisms (26, 27). The recent work on VCAM-1 provides one such mechanism (8). Tumor cells entering the lung parenchyma are immediately surrounded by macrophages, probably as a result of an innate immune response. VCAM-1 itself does not appear to play an active role in recruiting macrophages to the vicinity of tumor cells. However, the close proximity of macrophage and tumor cells subsequently facilitates contact between the α4 integrins and VCAM-1. This direct contact initiates juxtacrine stimulation by clustering VCAM-1 on tumor cells to activate pro-survival AKT signaling. VCAM-1 engagement by α4 integrins recruits Ezrin to the VCAM-1 cytoplasmic tail, leading to phosphorylation of Ezrin on tyrosine. Once activated, Ezrin serves as an adaptor that binds both PI3K and its downstream mediator, AKT, leading to activation of AKT-mediated cell survival signaling (8). Similar survival advantage from VCAM-1 is observed in TAM-enriched primary tumor sites when lung metastatic tumor cells were back-seeded into primary tumors (8). Therefore, α4 integrin-expressing TAMs create a favorable microenvironment for high VCAM-1 expressing tumor cells in lungs and also in areas of primary tumor infiltration. A direct interaction between lung macrophages and DTCs via VCAM-1 evokes tumor cell pro-survival signaling and promotes lung metastasis.
Role of VCAM-1 in bone metastasis of breast cancer
Another set of studies identified a role of VCAM-1 in osteolytic bone metastasis by breast cancer cells that have emerged from latency in the bone marrow (9). Although high expression of VCAM-1 is observed in clinical tissue samples of lung metastasis and bone metastases compared to brain metastasis tissues, VCAM-1 did not directly support the survival of bone metastatic breast cancer cells in the marrow in mouse models (8). However, VCAM-1 was highly expressed in aggressive clones that emerged from an indolent bone metastatic breast cancer cell line (9). Hyperactive NF-kB signaling appears to drive VCAM-1 expression in these cells. Regardless, a high level of VCAM-1 expression mediates the aggressive osteolytic phenotype of these “post-dormancy” breast cancer cells. VCAM-1 also mediated bone metastasis in other mouse and human breast cancer cell lines. Notably, systemic administration of blocking antibodies targeting either α4 integrin or VCAM-1 decreased bone metastasis in these mouse models (9).
VCAM-1 expression had no effect on the proliferation of breast cancer cells in the bone marrow (9). So how does VCAM-1 facilitate bone metastasis in these “post-dormancy” cells that egressed from the indolent stage? Bone metastasis proceeds with the outgrowth of micrometastases in the bone marrow and areas adjacent to the bone matrix. As the lesion grows, it engages osteoclasts in the destruction of bone matrix, causing the release of bound TGF-β and other growth factors that further stimulate the cancer cells to release more osteoclast activating factors. This is referred to as the “vicious cycle” of bone metastasis (28, 29). Thus, osteolytic metastasis is dependent on the differentiation of osteoclast precursors and their maturation into multinucleated osteoclasts. Bone metastatic cells have been shown to increase the activity of osteoclast progenitors through RANKL (receptor activator of nuclear factor kB ligand) (29, 30). The study by Lu et al. showed that osteoclast progenitors in the bone marrow express α4β1 integrin. The tethering of these cells to the VCAM-1 rich surface of the cancer cells apparently stimulates osteoclast differentiation by facilitating cell fusion into multinucleated, mature osteoclasts (Figure 1) (9).
Clinical-Translational Advances
The prevention and treatment of metastatic breast cancer remain a challenge. The recent findings on the roles of VCAM-1 in lung and bone metastasis provide one potential target to control the disease. Drugs that disrupt the binding of α4 intergrins to VCAM-1 have already been in development to treat diseases involving massive influx of leukocytes into inflammatory sites (31, 32). Natalizumab, a monoclonal antibody against α4 integrin, is an FDA-approved drug for the treatment of relapsing multiple sclerosis (MS) and inflammatory bowel disease (IBD). Furthermore, small molecule inhibitors against α4 integrin are in clinical trials. Based on the highlighted studies, these drugs have potential usefulness in treating metastatic breast cancer by interrupting α4 intergrin-VCAM-1 interactions.
Natalizumab
Natalizumab (TYSABRI™, Elan Pharmaceuticals and Biogen Idec) is a humanized monoclonal antibody that binds to α4 integrin chains and prevents formation of the α4β complexes. Natalizumab is generated by fusing the complementary determining regions of antibody AN100226m and human immunoglobulin (IgG4) framework, in order to reduce immunogenicity and increase the half-life of the drug in patients (33, 34). Its efficacy in treating MS was preclinically demonstrated using an animal model of autoimmune encephalomyelitis (EAE) (35). Cerebral microvessels in these animals, as well as in MS patients, display high VCAM-1 expression prior to perivascular leukocyte infiltration (36). AN100226m suppressed and reversed the disease progression in the EAE animal model (37). Natalizumab can block α4 integrin binding to both MAdCAM-1 and VCAM-1, the two adhesion molecules mediating leukocyte trafficking in gut-associated lymphoid organs and inflammatory sites (15, 38, 39). According to the public accessible website from U.S. National Institute of Health (ClinicalTrials.gov), Biogen Idec additionally conducted a clinical trial testing the utility of natalizumab as a potential therapy for multiple myeloma (MM) (40). In animal models of MM, anti-α4 integrin treatment decreases the growth of MM in bone marrow (41–43). This suppressive effect is due to the disruption of α4 integrin interactions between tumor and bone marrow stromal cells as well as tumor cells and extracellular matrix proteins (43–47).
Although no significant adverse events were observed in earlier clinical trial studies of natanizumab, a small proportion of patients developed progressive multifocal leukoencephalopathy (48). This is a rare but fatal neurological condition that occurs in patients with severe immune deficient condition (49). Therefore, Natalizumab is not approved in combination with immunossupressive agents. Immunosuppression is a common adverse effect of many chemotherapeutic agents used to treat breast cancer, creating a significant challenge for the potential use of natalizumab to treat metastatic breast cancer.
Small molecule inhibitors against α4 integrin
Small molecule inhibitors against a4 integrin have been developed, largely by structure based design. They are in clinical testing for its efficacy in treating autoimmune diseases, but still in much more preliminary stage than antibody based therapy. Importantly, these small molecules have several advantages over antibody based therapy. Although the human IgG4 framework used to humanize natalizumab does not activate the complement cascade and has low affinity to Fc receptors, antibodies against natalizumab still emerged in 11% of MS patients and 7% of IBD patients in the clinical trials (50, 51). This problem is largely averted with small molecules. Furthermore, long-term treatment is required for autoimmune diseases and potentially in breast cancer patients to with metastasis. Natalizumab needs to be administered intravenously over one hour every four weeks, whereas small molecule inhibitors of α4 integrin make oral bioavailability possible (52). Despite these advantages over Natalizumab, the application of small molecule inhibitors against α4 integrin target a biologically important interaction and therefore would need to be closely monitored for possible adverse reactions. Currently, an orally active α4 integrin antagonist, AJM300, reportedly is under clinical trials in IBD patient (53) and a non-oral small inhibitor, ELND002, is in phase I clinical trials in MS patients (54).
Disrupting α4 integrin-VCAM-1 interactions between cancer cells and stromal components of the tumor microenvironment offers a possibility to suppress the outgrowth of disseminated breast cancer cells. Additional studies are needed in order to better identify the efficacy of this approach, but the recent reports (8, 9) provide encouraging evidence in support of this possibility.
Footnotes
The authors disclose no conflicts of interest.
References
- 1.Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 3.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald IC, Groom AC, Chambers AF. Cancer spread and micrometastasis development: quantitative approaches for in vivo models. Bioessays. 2002;24:885–93. doi: 10.1002/bies.10156. [DOI] [PubMed] [Google Scholar]
- 6.Fidler IJ, Yano S, Zhang RD, Fujimaki T, Bucana CD. The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol. 2002;3:53–7. doi: 10.1016/s1470-2045(01)00622-2. [DOI] [PubMed] [Google Scholar]
- 7.Langley RR, Fidler IJ. The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer. 128:2527–35. doi: 10.1002/ijc.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Q, Zhang XH, Massague J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell. 20:538–49. doi: 10.1016/j.ccr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M, et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell. 20:701–14. doi: 10.1016/j.ccr.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruco LP, de Laat PA, Matteucci C, Bernasconi S, Sciacca FM, van der Kwast TH, et al. Expression of ICAM-1 and VCAM-1 in human malignant mesothelioma. The Journal of pathology. 1996;179:266–71. doi: 10.1002/(SICI)1096-9896(199607)179:3<266::AID-PATH592>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 11.Ding YB, Chen GY, Xia JG, Zang XW, Yang HY, Yang L. Association of VCAM-1 overexpression with oncogenesis, tumor angiogenesis and metastasis of gastric carcinoma. World journal of gastroenterology : WJG. 2003;9:1409–14. doi: 10.3748/wjg.v9.i7.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin J, Kim J, Ryu B, Chi SG, Park H. Caveolin-1 is associated with VCAM-1 dependent adhesion of gastric cancer cells to endothelial cells. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2006;17:211–20. doi: 10.1159/000094126. [DOI] [PubMed] [Google Scholar]
- 13.Vonderheide RH, Tedder TF, Springer TA, Staunton DE. Residues within a conserved amino acid motif of domains 1 and 4 of VCAM-1 are required for binding to VLA-4. J Cell Biol. 1994;125:215–22. doi: 10.1083/jcb.125.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cybulsky MI, Fries JW, Williams AJ, Sultan P, Eddy R, Byers M, et al. Gene structure, chromosomal location, and basis for alternative mRNA splicing of the human VCAM1 gene. Proc Natl Acad Sci U S A. 1991;88:7859–63. doi: 10.1073/pnas.88.17.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–90. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 16.Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi-Rosso G, et al. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59:1203–11. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- 17.Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, et al. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–84. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 18.Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 11:276–87. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Wetering S, van den Berk N, van Buul JD, Mul FP, Lommerse I, Mous R, et al. VCAM-1-mediated Rac signaling controls endothelial cell-cell contacts and leukocyte transmigration. Am J Physiol Cell Physiol. 2003;285:C343–52. doi: 10.1152/ajpcell.00048.2003. [DOI] [PubMed] [Google Scholar]
- 20.Cardones AR, Murakami T, Hwang ST. CXCR4 enhances adhesion of B16 tumor cells to endothelial cells in vitro and in vivo via beta(1) integrin. Cancer Res. 2003;63:6751–7. [PubMed] [Google Scholar]
- 21.Taichman DB, Cybulsky MI, Djaffar I, Longenecker BM, Teixido J, Rice GE, et al. Tumor cell surface alpha 4 beta 1 integrin mediates adhesion to vascular endothelium: demonstration of an interaction with the N-terminal domains of INCAM-110/VCAM-1. Cell Regul. 1991;2:347–55. doi: 10.1091/mbc.2.5.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao J, Qi Q, Yang Y, Gu HY, Lu N, Liu W, et al. Inhibition of alpha(4) integrin mediated adhesion was involved in the reduction of B16-F10 melanoma cells lung colonization in C57BL/6 mice treated with gambogic acid. Eur J Pharmacol. 2008;589:127–31. doi: 10.1016/j.ejphar.2008.04.063. [DOI] [PubMed] [Google Scholar]
- 23.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–24. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4:e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sterling JA, Edwards JR, Martin TJ, Mundy GR. Advances in the biology of bone metastasis: how the skeleton affects tumor behavior. Bone. 2011;48:6–15. doi: 10.1016/j.bone.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 11:411–25. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–49. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 31.Comi G. Treatment of multiple sclerosis: role of natalizumab. Neurol Sci. 2009;30(Suppl 2):S155–8. doi: 10.1007/s10072-009-0147-2. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt KJ, Buning J, Jankowiak C, Lehnert H, Fellermann K. Crohn's targeted therapy: myth or real goal? Curr Drug Discov Technol. 2009;6:290–8. doi: 10.2174/157016309789869083. [DOI] [PubMed] [Google Scholar]
- 33.Mountain A, Adair JR. Engineering antibodies for therapy. Biotechnol Genet Eng Rev. 1992;10:1–142. doi: 10.1080/02648725.1992.10647886. [DOI] [PubMed] [Google Scholar]
- 34.Leger OJ, Yednock TA, Tanner L, Horner HC, Hines DK, Keen S, et al. Humanization of a mouse antibody against human alpha-4 integrin: a potential therapeutic for the treatment of multiple sclerosis. Hum Antibodies. 1997;8:3–16. [PubMed] [Google Scholar]
- 35.Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–6. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 36.Lee SJ, Benveniste EN. Adhesion molecule expression and regulation on cells of the central nervous system. J Neuroimmunol. 1999;98:77–88. doi: 10.1016/s0165-5728(99)00084-3. [DOI] [PubMed] [Google Scholar]
- 37.Kent SJ, Karlik SJ, Cannon C, Hines DK, Yednock TA, Fritz LC, et al. A monoclonal antibody to alpha 4 integrin suppresses and reverses active experimental allergic encephalomyelitis. J Neuroimmunol. 1995;58:1–10. doi: 10.1016/0165-5728(94)00165-k. [DOI] [PubMed] [Google Scholar]
- 38.Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. Role of alpha 4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol. 1994;152:3282–93. [PubMed] [Google Scholar]
- 39.Hemler ME, Elices MJ, Parker C, Takada Y. Structure of the integrin VLA-4 and its cell-cell and cell-matrix adhesion functions. Immunol Rev. 1990;114:45–65. doi: 10.1111/j.1600-065x.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 40.Elan Pharmaceuticals . ClinicalTrialsgov [Internet] National Library of Medicine (US); Bethesda (MD): 2011. A Study of ELND002 in Patients With Relapsing Forms of Multiple Sclerosis. [cited 2012 Jun 13]. Available from: http://clinicaltrialsgov/show/NCT01318421 NLM Identifier: NCT01318421. [Google Scholar]
- 41.Podar K, Zimmerhackl A, Fulciniti M, Tonon G, Hainz U, Tai YT, et al. The selective adhesion molecule inhibitor Natalizumab decreases multiple myeloma cell growth in the bone marrow microenvironment: therapeutic implications. Br J Haematol. 155:438–48. doi: 10.1111/j.1365-2141.2011.08864.x. [DOI] [PubMed] [Google Scholar]
- 42.Olson DL, Burkly LC, Leone DR, Dolinski BM, Lobb RR. Anti-alpha4 integrin monoclonal antibody inhibits multiple myeloma growth in a murine model. Mol Cancer Ther. 2005;4:91–9. [PubMed] [Google Scholar]
- 43.Mori Y, Shimizu N, Dallas M, Niewolna M, Story B, Williams PJ, et al. Anti-alpha4 integrin antibody suppresses the development of multiple myeloma and associated osteoclastic osteolysis. Blood. 2004;104:2149–54. doi: 10.1182/blood-2004-01-0236. [DOI] [PubMed] [Google Scholar]
- 44.Kibler C, Schermutzki F, Waller HD, Timpl R, Muller CA, Klein G. Adhesive interactions of human multiple myeloma cell lines with different extracellular matrix molecules. Cell Adhes Commun. 1998;5:307–23. doi: 10.3109/15419069809040300. [DOI] [PubMed] [Google Scholar]
- 45.Uchiyama H, Barut BA, Chauhan D, Cannistra SA, Anderson KC. Characterization of adhesion molecules on human myeloma cell lines. Blood. 1992;80:2306–14. [PubMed] [Google Scholar]
- 46.Michigami T, Shimizu N, Williams PJ, Niewolna M, Dallas SL, Mundy GR, et al. Cell-cell contact between marrow stromal cells and myeloma cells via VCAM-1 and alpha(4)beta(1)-integrin enhances production of osteoclast-stimulating activity. Blood. 2000;96:1953–60. [PubMed] [Google Scholar]
- 47.de la Fuente MT, Casanova B, Garcia-Gila M, Silva A, Garcia-Pardo A. Fibronectin interaction with alpha4beta1 integrin prevents apoptosis in B cell chronic lymphocytic leukemia: correlation with Bcl-2 and Bax. Leukemia. 1999;13:266–74. doi: 10.1038/sj.leu.2401275. [DOI] [PubMed] [Google Scholar]
- 48.Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 366:1870–80. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 49.Major EO. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med. 61:35–47. doi: 10.1146/annurev.med.080708.082655. [DOI] [PubMed] [Google Scholar]
- 50.Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GP, Libonati MA, et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348:15–23. doi: 10.1056/NEJMoa020696. [DOI] [PubMed] [Google Scholar]
- 51.Ghosh S, Goldin E, Gordon FH, Malchow HA, Rask-Madsen J, Rutgeerts P, et al. Natalizumab for active Crohn's disease. N Engl J Med. 2003;348:24–32. doi: 10.1056/NEJMoa020732. [DOI] [PubMed] [Google Scholar]
- 52.Sugiura T, Kageyama S, Andou A, Miyazawa T, Ejima C, Nakayama A, et al. A Novel, Orally Active Alpha 4 Integrin Antagonist, AJM300 Prevents the Development of Experimental Colitis Induced by Adoptive Transfer of IL-10 Deficient CD4+ T Cells in Mice. J Pharmacol Exp Ther. 2012 doi: 10.1124/jpet.112.193946. [DOI] [PubMed] [Google Scholar]
- 53.Takazoe M, Watanabe M, Kawaguchi T, Matumoto T, Oshitani N, Hiwatashi N, et al. Digestive Disease Week. Chicago, IL: 2009. Oral alpha-4 integrin inhibitor (AJM300) in patients with active Crohn's disease: a randomized, double-blind, placebo-controlled trial. [Google Scholar]
- 54.Biogen Idec . ClinicalTrialsgov [Internet] National Library of Medicine (US); Bethesda (MD): 2008–2011. Study of Natalizumab in Relapsed/Refractory Multiple Myeloma. Available from: http://clinicaltrialsgov/show/NCT00675428 NLM Identifier: NCT00675428. [Google Scholar]