Abstract
Accumulating evidence suggests that subjective cognitive complaints (SCC) may indicate subtle cognitive decline characteristic of individuals with preclinical Alzheimer’s disease (AD). In this study, we sought to build upon previous studies by associating SCC and amyloid-β deposition using Positron Emission Tomography with Pittsburg Compound B (PiB-PET) in cognitively normal older individuals. One-hundred thirty one subjects (mean age 73.5 ± 6) were administered three subjective cognitive questionnaires and a brief neuropsychological battery. A relationship between a subjective memory complaints composite score and cortical PiB binding was found to be significant, even after controlling for depressive symptoms. By contrast, there were no significant relationships between objective cognitive measures of memory and executive functions and cortical PiB binding. Our study suggests that SCC may be an early indicator of AD pathology detectable prior to significant objective impairment.
Keywords: preclinical Alzheimer’s disease, early detection, amyloid imaging, subjective cognitive complaints
1. Introduction
As the field positions itself for forthcoming secondary prevention trials, the discovery of sensitive indicators of preclinical Alzheimer’s disease (AD) is at a premium. Increasingly, it has been suggested that subjective cognitive complaints (SCC), at the stage of preclinical AD, may indicate initial cognitive decrements that are otherwise undetectable with standardized objective tests of cognitive performance (Reisberg et al., 2008). The importance of SCC as a diagnostic criterion has already been recognized in MCI (Albert et al., 2011; Petersen et al., 1999), though evidence suggests that self-reported concerns become less reliable as patients progress towards dementia, whereas informant-report and objective cognitive measures become more accurate (Roberts, Clare, & Woods, 2009). Thus, there may be an optimal timeframe in which SCC contribute to our ability to identify individuals at-risk for cognitive decline and eventual AD dementia, but the role of SCC in the early detection of AD remains to be fully elucidated.
Several previous population-based studies have not found associations between SCC and objective cognitive testing in non-demented individuals, raising the possibility that individuals with SCC represent the “worried well,” rather than an at-risk group (Bassett & Folstein, 1993; Gagnon et al., 1994). In fact, it is well-established that SCC relate more strongly to depressive symptomatology and personality traits than objective cognitive impairment (Comijs, Deeg, Dik, Twisk, & Jonker, 2002; Minett, Da Silva, Ortiz, & Bertolucci, 2008; Smith, Petersen, Ivnik, Malec, & Tangalos, 1996). However, several studies, including our previous work, have considered the effects of depression and have still found a relationship between SCC and objective cognitive functioning in individuals who were non-demented (Amariglio, Townsend, Grodstein, Sperling, & Rentz, 2011; Lam, Lui, Tam, & Chiu, 2005; Snitz, Morrow, Rodriguez, Huber, & Saxton, 2008).
Longitudinal studies that have used SCC as a predictor of future cognitive decline in CN individuals have also been mixed. For example, no relationship was found between SCC and cognitive decline after two years in a large community dwelling sample (Jorm et al., 1997), but a relationship in the same sample was revealed after the third year of follow-up (Jorm, Christensen, Korten, Jacomb, & Henderson, 2001). Additionally, several other studies were unable to find relationships between SCC and decline on objective cognitive measures, though longitudinal follow-up and sample size were limited (Flicker, Ferris, & Reisberg, 1993; Taylor, Miller, & Tinklenberg, 1992). By contrast, others have found that SCC predict future cognitive decline (Dik et al., 2001; Geerlings et al., 1999; Hohman, Beason-Held, Lamar, & Resnick, 2011; Schmand, Jonker, Hooijer, & Lindeboom, 1996; Schofield, Jacobs, Marder, Sano, & Stern, 1997; Wang et al., 2004). These previous studies have varied in the instruments used to assess SCC, ranging from a single question regarding memory function to more detailed series of questions probing multiple aspects of subjective cognitive assessment.
The advent of novel neuroimaging techniques has provided another method to assess the potential utility of SCC in early detection of AD. Studies relating SCC with AD biomarker evidence in CN individuals have revealed associations between decreased gray matter volume (Jessen et al., 2006; Saykin et al., 2006; van Norden et al., 2008) and cerebral hypometabolism in parietotemporal and parahippocampal regions (Mosconi et al., 2008), suggesting that SCC may coincide with early pathological changes, prior to objective cognitive impairment. The relationship between amyloid-β burden, a pathological hallmark of AD, and SCC has also been investigated. Several studies have not found a relationship with amyloid deposition using Pittsburgh Compound B Positron Emission Tomography (PiB-PET) imaging (Chetelat et al., 2010; Rodda et al., 2010), and another only found a relationship in APOE 4 carriers (Rowe et al., 2010). More recently, an association between PiB retention and SCC has been described in CN individuals. Specifically, individuals who reported their memory was worse than their peers were more likely to have increased PiB uptake (Perrotin, Mormino, Madison, Hayenga, & Jagust, 2012). By contrast, no relationship was found with PiB uptake when individuals were asked if there had been a change in their memory from 20 years ago.
In this study, we sought to add to previous studies by associating amyloid-β deposition on PiB-PET imaging with a wide variety of SCC items in CN older individuals. We administered three separate questionnaires that measure self-appraisal of cognitive abilities in everyday life to assess multiple SCC items. We hypothesized that subjective memory complaints, in particular, would demonstrate a relationship with amyloid deposition because previous findings have suggested that subtle decline in episodic memory is the most reliable predictor of preclinical AD (Collie & Maruff, 2000; Rentz et al., 2011).
2. Materials and methods
2.1. Subjects
One hundred thirty one subjects (age: 73.5 ±6.0, years of education: 16.1 ±2.9, 53% women) enrolled in the Harvard Aging Brain Study at the Center for Alzheimer Research and Treatment at the Brigham and Women’s Hospital (BWH) and Massachusetts General Hospital (MGH) were studied using protocols and informed consent procedures approved by the Partners Human Research Committee.
Subjects were clinically normal, defined by a Clinical Dementia Rating (CDR) (Morris, 1993) score of 0, a Mini Mental State Exam (MMSE) (Folstein, Folstein, & McHugh, 1975) score of greater than or equal to 28 and a Geriatric Depression Scale (GDS) score of less than 11 (Yesavage et al., 1983). A detailed review of medical history and functional performance as well as physical and neurological examinations confirmed their status as clinically normal. None of the participants had a history of alcoholism, drug abuse, head trauma or current serious medical or psychiatric illness.
2.2. Behavioral Data
2.2.1 Subjective Cognitive Measures
Subjects were administered three different questionnaires that measured SCC including: 1) The Everyday Cognition (E-Cog) scale, a 39-item measure, scored on a Likert scale (1 = “Better or no change” to 4 =“Consistently much worse”), which was specifically developed to assess cognitive abilities in older adults (S. T. Farias et al., 2008). Questions are framed in the context of current performance compared to 10 years ago. The scale contains six domain-specific factors that include Everyday Memory (MEM), Language (LANG), Visuospatial Abilities (VIS), Planning (PLAN), Organization (ORG), and Divided Attention (ATT). There is both an informant-rated and self-rated version of the E-Cog composed of identical questions, but only the informant version has been formally validated (Farias, personal communication, 10 April 2012). The self-rated version was used for this study and the average score for each subscale was used for the statistical analyses. 2) The Memory Functioning Questionnaire (MFQ) is a 63-item questionnaire scored on a Likert Scale (1 = “Serious problem” to 7= “Never a problem”) (Gilewski, Zelinski, & Schaie, 1990). The scale has been formally validated and is divided into several subscales, based on a factor analysis, that include General Frequency of Forgetting (GenFF) (“How would you rate your memory in terms of the kinds of problems you have?), Seriousness of Forgetting (SF) (“When you actually forget in the following situations, how serious of a problem do you consider the memory failure to be?”), Retrospective Functioning (RF) (“How is your memory compared to the way it was in the past”), and Mnemonics Usage (MU) (“How often do you use the following techniques to remind yourself about things?). The average score of each subscale was used for the statistical analyses. 3) Finally, subjects were administered a set of 7 yes or no questions that have been used previously in large epidemiological studies to assess cognitive changes in older individuals, though not formally validated (Amariglio, et al., 2011; Go et al., 1997). Answers on these questions (0=No, 1=Yes) were added together to create a summary score that was used in statistical analyses.
In order to test our specific hypothesis that subjective memory complaints would be the most indicative of early AD pathology, we created an a priori, subjective memory complaint composite score (SMC composite) by combining the three subjective memory subscales (MFQ: GenFF; E-Cog: MEM; 7 Questions Sum). Of note, we selected the GenFF subscale, the most reliable subscale of the MFQ, rather than the other three subscales (i.e., SF, RF, MU) for our primary analysis. Raw scores from all three measures were converted to z-scores for each subject. As mentioned previously, higher scores on the E-Cog and 7 Questions indicate greater SCC, whereas lower scores on the MFQ indicated greater SCC. Thus, inverse z-scores from the MFQ were used for the composite. All three z-scores were subsequently averaged to create a SMC composite score, which was normally distributed, and with higher z-scores indicating greater SCC.
2.2.2 Neuropsychological evaluation
In addition to the SCC questionnaires, subjects were administered an extensive battery of neuropsychological (NP) tests that covered the cognitive realms of attention, executive functions, memory, language, and visuospatial processing. Specific NP tests analyzed in this study included the Mini-Mental State Exam (MMSE) (Folstein, et al., 1975), Letter-Number Sequencing (Wechsler, 1997), Trails A and B (Reitan, 1979), 30-Item Boston Naming Test (BNT) (Kaplan, Goodglass, & Weintraub, 1983), Visual Form Discrimination Test (Benton, Varney, Hamsher, & Spreen, 1983), and the 6-Trial Selective Reminding Test (SRT) (Masur et al., 1989).
2.3 Positron emission tomography
PiB PET acquisitions were performed as described previously (Gomperts et al., 2008; Johnson et al., 2007). Following a transmission scan, 8.5–15 mCi 11C-PiB was injected as a bolus and followed immediately by a 60-min dynamic acquisition. PiB PET data were reconstructed and corrected for attenuation. Each frame was evaluated to verify adequate count statistics and absence of head motion. The Logan graphical analysis method (Logan et al., 1996; Price et al., 2005) with cerebellar cortex as the reference tissue input function was used to evaluate specific PiB retention expressed as the distribution volume ration (DVR) (Archer et al., 2006; Fagan et al., 2006; Johnson, et al., 2007; Lopresti et al., 2005). We calculated the DVR in an aggregate of amyloid-vulnerable cortical regions-of-interest (ROI). As in previous studies (Gomperts, et al., 2008; Johnson, et al., 2007; Rentz et al., 2010), the ROIs were defined according to the automated anatomical labeling (AAL) parcellation scheme (Tzourio-Mazoyer et al., 2002) applied to data warped to the Montreal Neurological Institute (MNI) standard space using Statistical Parametric Mapping Version 8 (SPM8) software.
As in earlier studies (Johnson, et al., 2007; Rentz, et al., 2011), we chose an a priori threshold of amyloid positivity that is somewhat arbitrary, since a rigorous definition will likely require longitudinal follow-up. We split the CN group based on a cut point that represents the local minimum of a density plot. The cut point (DVR= 1.25) differs slightly from previous reports and is a somewhat higher, more conservative threshold that classifies fewer CN as PiB-positive, reflecting the considerably larger available sample compared to our previous studies (Gomperts, et al., 2008; Johnson, et al., 2007; Rentz, et al., 2010). Most importantly, all analyses were also performed using PiB as a continuous variable without the use of a threshold.
2.4 Statistical analysis
Group comparisons on the behavioral measures comparing PiB-positive and PiB-negative individuals were performed using 2-sample Mann-Whitney Tests, except for gender which used a χ2 analysis. We chose a cut-point for amyloid PiB-positive to be a global mean PiB ≥1.25 (n = 34). PiB-negative was defined as a global mean PiB < 1.25 (n = 97). We performed an a priori multiple regression analyses relating PiB retention (DVR, cerebellar reference) as a continuous variable in amyloid-vulnerable cortical regions with the SMC composite as the dependent variable. Even though our sample included individuals who did not report clinically significant depression, we included the Geriatric Depression Scale as a covariate in our models because it was significantly associated with all the SCC subscales. Age (r = 0.095, p = 0.281), years of education (r = −0.06, p = 0.48) and gender (r = 0.11, p = 0.21) were not significantly correlated with SCC items or PiB retention and were thus not included in our models. Individual subscales from each of the measures were associated with PiB retention in post-hoc analyses. For all subscales that had a skewed distribution, partial Spearman’s rank correlations were performed (E-Cog subscales and 7 Questions). The Geriatric Depression Scale was controlled for in the partial correlations. Significance was set a priori as p < 0.05. All data were analyzed using SPSS v18.0.
3. Results
3.1 Characteristics of the sample
The sample had an average age of 74 years (range: 66–87 years). There was no significant difference between the proportion of men and women in the sample (women: 53%). Group scores on the SCC questionnaires and NP tests for the whole sample, and by high and low PiB groups, are presented in Table 1.
Table 1.
Sample characteristics.
| All subjects | PiB-Negative | PiB-Positive | Difference Statistic | |
|---|---|---|---|---|
| Subjects, n | 131 | 97 | 34 | |
| Age, years | 73.5(±6.0) | 72.7 (±5.9) | 75.5(±6.0) | 0.38 |
| Sex, % female | 53.0 | 51.5 | 57.1 | 0.23 |
| Education, years | 16.1(±2.9) | 16.3(±2.9) | 15.6(±1.0) | 0.01* |
| MMSE | 29.3(±0.7) | 29.4(±0.7) | 29.0(±0.7) | 0.00* |
| GDS | 2.7(±1.3) | 2.8(±2.8) | 2.6(±2.5) | 0.92 |
| SMC Composite | −0.01(±0.82) | −0.15(±0.74) | 0.23(±0.85) | 0.015* |
| E-Cog | ||||
| Memory | 1.6 (±0.5) | 1.5(±0.4) | 1.6(±0.5) | 0.13 |
| Language | 1.3(±0.4) | 1.3(±0.4) | 1.4(±0.6) | 0.05 |
| Visuospatial | 1.2(±0.3) | 1.2(±0.3) | 1.2(±0.4 | 0.19 |
| Planning | 1.1(±0.3) | 1.1(±0.2) | 1.1(±0.3) | 0.98 |
| Organization | 1.3(±0.4) | 1.3(±0.4) | 1.4(±0.4) | 0.01* |
| Divided Attention | 1.4(±0.5) | 1.4(0.5) | 1.5(±0.5) | 0.12 |
| MFQ | ||||
| GenFF | 5.4(±0.8) | 5.5(±0.8) | 5.2(±0.7) | 0.01* |
| SF | 4.5 (±1.4) | 4.6(±1.5) | 4.3(±1.2) | 0.29 |
| RF | 3.3(±0.8) | 3.3(±0.9) | 3.2(±0.7) | 0.98 |
| MU | 2.8(±1.2) | 2.8(±1.3) | 2.6(±1.0) | 0.33 |
| 7 Question Sum | 1.1(±1.1) | 0.9(±1.0) | 1.5(±1.3) | 0.01* |
| SRT: Total Recall | 44.2(±8.4) | 44.6(±8.3) | 43.1(±8.8) | 0.14 |
| SRT: Short Delayed Recall | 5.7(±3.0) | 5.9(±2.9) | 5.3(±3.1) | 0.13 |
| SRT: Long Delayed Recall | 6.4(±3.4) | 6.6(±3.4) | 6.0(±3.5) | 0.33 |
| BNT-30 | 28.1(±1.9) | 28.3(±2.0) | 27.8(±1.6) | 0.02* |
| VFDT | 30.5(±2.0) | 30.5(±2.0) | 30.5(1.8) | 0.96 |
| L-#S | 9.9(±2.5) | 10.1(±2.5) | 9.2(±2.3) | 0.06 |
| TMTB | 83.9(±42.4) | 83.9(±45.9) | 84.1(±31.4) | 0.35 |
All values (except subjects, sex, SMC composite) represent means ± SD. SMC composite is reported as a z-score, with a higher z-scores indicated higher endorsement of complaints. Significant differences between PiB groups:
p < 0.05. Higher score on the E-Cog refers to greater complaints. Higher score on & Questions refers to greater complaints. Lower score on the MFQ refers to greater complaints. Trails B- higher score refers to worse performance.
3.2 Associations between the subjective measures only
The eleven subscales used in this study were correlated with each other to assess collinearity using Spearman’s rank correlations. Higher scores on the E-Cog and 7 Questions indicate greater SCC, whereas lower scores on the MFQ indicated greater SCC. Thus, we expected the MFQ to be inversely correlated with the E-Cog and 7 Questions. All of the subscales of the E-Cog were significantly correlated with each other and with the 7 Question sum. All the MFQ subscales were associated with each other, with the exception of the MU subscale, which was not associated with any of the other 10 SCC subscales. The MFQ GenFF and RF subscales were related to all E-Cog subscales and the 7 Question sum. The MFQ SF subscale was associated with E-Cog MEM, LANG, and ATT. See Table 2.
Table 2.
Spearman’s rank correlations between all the SCC subscales from the Everyday Cognition Scale, Memory Functioning Questionnaire, and the 7 Questions.
| MEM | LANG | VIS | PLAN | ORG | ATT | GenFF | SF | RF | MU | 7Qs | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MEM |
0.58 0.00 |
0.32 0.00 |
0.40 0.00 |
0.44 0.00 |
0.53 0.00 |
−0.45 0.00 |
−0.24 0.00 |
−0.51 0.00 |
0.04 0.59 |
0.60 0.00 |
|
| LANG |
0.41 0.00 |
0.46 0.00 |
0.40 0.00 |
0.52 0.00 |
−0.52 0.00 |
−0.52 0.00 |
−0.22 0.01 |
−0.03 0.74 |
0.46 0.00 |
||
| VIS |
0.33 0.00 |
0.38 0.00 |
0.26 0.00 |
−0.35 0.00 |
−0.14 0.09 |
−0.19 0.02 |
−0.04 0.60 |
0.24 0.00 |
|||
| PLAN |
0.48 0.00 |
0.36 0.00 |
−0.26 0.00 |
−0.08 0.31 |
−0.17 0.04 |
−0.06 0.43 |
0.34 0.00 |
||||
| ORG |
0.42 0.00 |
−0.38 0.00 |
−0.10 0.21 |
−0.24 0.00 |
0.05 0.58 |
0.37 0.00 |
|||||
| ATT |
−0.40 0.00 |
−0.24 0.00 |
−0.38 0.00 |
−0.06 0.49 |
0.34 0.00 |
||||||
| GenFF |
0.26 0.00 |
0.32 0.00 |
−0.02 0.82 |
−0.42 0.00 |
|||||||
| SF |
0.32 0.00 |
0.07 0.36 |
−0.02 0.83 |
||||||||
| RF | 0.13 0.09 |
−0.37 0.00 |
|||||||||
| MU | 0.04 0.61 |
Top row indicates ρ correlations and bottom row indicates p-value. Bolded numbers are significant p < 0.05.
3.3 Association between objective cognitive measures and subjective measures
We correlated the SMC composite with an objective memory measure (SRT: delayed recall) that has been shown to be sensitive in our previous work, and the SRT delayed recall measure was significant (r = −0.23, 0.004). The SMC composite was also significantly correlated with Letter-Number Sequencing (r = −0.19, p = 0.02). MFQ and EF measures? Additionally, we correlated specific NP measures to SCC subscales matched by cognitive domain. For example, the BNT was correlated with the E-Cog LANG subscale, and Letter-Number Sequencing was correlated with E-Cog ATT (see Table 3).
Table 3.
Correlations between SCC subscales and corresponding neuropsychological tests by cognitive domain.
| TR | DR | DR30 | BNT | VFDT | L#S | TMTB | |
|---|---|---|---|---|---|---|---|
| SMC Composite* | −0.17 0.03 |
−0.23 0.00 |
−0.18 0.03 |
−0.19 0.02 |
0.06 0.46 |
||
| E-Cog | |||||||
| MEM | −0.15 0.06 |
−0.18 0.03 |
−0.17 0.04 |
||||
| LANG | −0.16 0.05 |
||||||
| VIS | −0.1 0.91 |
||||||
| PLAN | −0.01 0.92 |
0.05 0.53 |
|||||
| ORG | −0.09 0.27 |
0.01 0.90 |
|||||
| ATT | −0.01 0.92 |
−0.06 0.49 |
|||||
| MFQ | |||||||
| GenFF* | 0.10 0.19 |
0.18 0.02 |
0.11 0.17 |
||||
| SF* | 0.00 0.96 |
0.03 0.67 |
0.05 0.54 |
||||
| MU* | −0.06 0.45 |
0.02 0.79 |
−0.3 0.71 |
||||
| RF* | −0.09 0.25 |
−0.04 0.59 |
−0.07 0.35 |
||||
| 7 Questions | −0.16 0.04 |
−0.14 0.08 |
−0.15 0.05 |
Top row indicates correlations and bottom row indicates p-value. Pearson’s correlations were used for subscales that were normally distributed (r), indicated by *. Spearman’s rank correlations were used for scores that were not normally distributed (ρ). Significance level set at p ≤ 0.005.
3.4 Association between cognitive measures and amyloid deposition
There were no associations between PiB retention and objective cognitive measures in our sample using Spearman’s rank correlations. However, when the group was divided by PiB positive and PiB negative groups, a significant difference was observed on the BNT (p = 0.02) and the MMSE (p = 0.001), but not any other cognitive measures using a Mann-Whitney U test.
3.5 Association between SCC and amyloid deposition
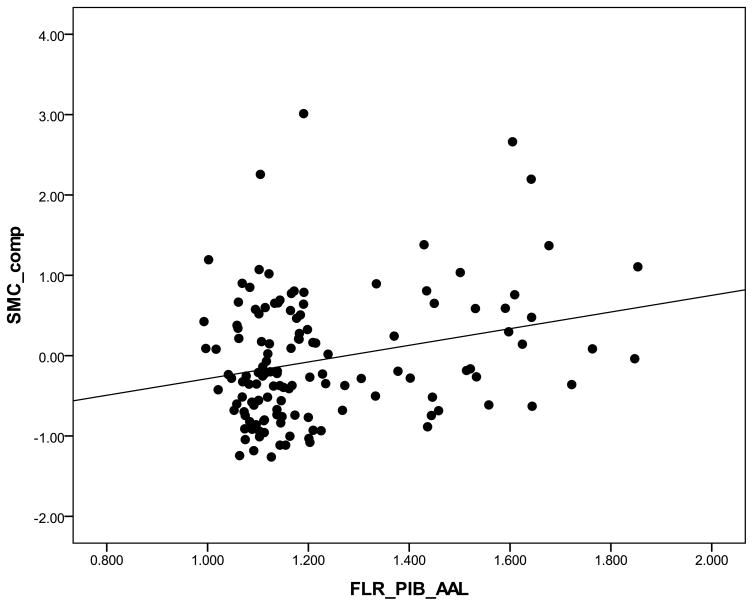
We ran a multiple regression model to identify the relationship between PiB retention and memory-related SCC (SMC composite) which was found to be significant (β= 1.03 p= 0.001; see Figure 1). The Geriatric Depression Scale was used as a covariate because it was significantly related to the dependent factor. Age, years of education, and gender were not used as covariates because they were not correlated with either independent or dependent variables using Pearson’s correlations. Post-hoc analyses that explored relationships across multiple subscales revealed higher PiB retention was associated with subjective complaints related to memory (MFQ: GenFF β= −0.91 p= 0.004;) and executive functioning (E-Cog ORG: ρ = 0.2, p = 0.02). By contrast, no significant relationships were found for the other subscales.
Figure 1.
Scatterplot of PiB retention by subjective memory complaints composite (SMC composite). Higher scores on SMC composite indicate higher SCC.
When the sample was divided into PiB positive and PiB negative groups, significant differences using a Mann-Whitney U test were found for the SMC composite (p = 0.02), the MFQ: GenFF subscale (p = 0.02), E-Cog: ORG subscale (p = 0.01), and the 7 Questions (p = 0.03). None of the other subscales from the E-Cog or MFQ showed a significant relationship with PiB retention (i.e., E-Cog: LANG, VIS, PLAN; MFQ: SF, RF, MU).
4. Discussion
In this study, we report an association between amyloid burden and SCC in older CN adults. Our primary analysis focused specifically on memory-related SCC, as episodic memory is thought to be one of the earliest cognitive domains affected in preclinical AD (Collie & Maruff, 2000). A subjective memory complaint composite score (SMC composite) was created by combining subscales from three subjective questionnaires. Our findings are in support of our primary hypothesis that subjective memory complaints are related to amyloid deposition in cognitively normal older individuals. Furthermore, a relationship between the SMC composite and an objective measure of episodic memory was found. By contrast, we did not find an association between an objective memory measure and amyloid burden.
Our findings suggest that SCC may offer unique information above and beyond traditional cognitive measures in a CN older sample that is at risk for AD. Consistent with several other studies, we did not find a relationship between standardized neuropsychological measures and amyloid in CN individuals cross-sectionally (Aizenstein et al., 2008; Jack et al., 2010; Mormino et al., 2009; Storandt, Mintun, Head, & Morris, 2009), though there have been several notable exceptions (Pike et al., 2007; Rodrigue et al., 2012). Taken together, neuropsychological measures may not reliably detect variability in performance given that these instruments have been designed to capture clinical impairment. SCC have the advantage of capturing cognitive changes that affect individuals on a day-to-day basis unlike traditional objective cognitive measures, which often bear little resemblance to tasks required for everyday functioning. Thus, it may be the case that at the earliest stages of cognitive decline, individuals are more likely to notice subtle changes in their every day functioning that are difficult to quantify in the laboratory.
In addition, we found the subjective executive complaints (ORG) are also associated with amyloid deposition. In retrospect, this finding is not entirely surprising given that complaints falling into this category (e.g., “Prioritizing tasks by importance,” Keeping living and work space organized”) generally require the coordination of multiple high-level cognitive processes that are facilitated by memory which may be vulnerable early in the disease process. This is supported by the finding in our study that SCC were associated with an EF measure (Letter-Number Sequencing). Moreover, it is possible that executive difficulties, in addition to memory, are also susceptible early in the disease process, which has been supported by several studies which found early executive deficits on specific neuropsychological tests predict future cognitive decline in individuals with prodromal AD (Albert, Moss, Tanzi, & Jones, 2001; Dickerson, Sperling, Hyman, Albert, & Blacker, 2007).
Our findings add to a growing body of evidence that suggests that SCC are related to early AD-related brain changes in CN individuals. A variety of studies have demonstrated a relationship between SCC and putative AD biomarker evidence, such as gray matter volume loss (Jessen, et al., 2006; Saykin, et al., 2006; van Norden, et al., 2008), cerebral hypometabolism (Mosconi, et al., 2008), amyloid accumulation (Perrotin, et al., 2012; Rowe, et al., 2010), brain activation on functional imaging (Hohman, et al., 2011; Rodda, Dannhauser, Cutinha, Shergill, & Walker, 2011), and genetic risk for AD (Small et al., 1999). While these positive findings with SCC and biomarkers are modest we observed them in CN individuals, a group that by definition is unlikely to demonstrate significant variability on neuropsychological or subjective measures as would be expected in a clinically impaired sample.
It is well-recognized that the development of sensitive tools to identify subjects who are at high-risk for AD will be critical for future secondary prevention trials (Sperling, Jack, & Aisen, 2011). While the relationship between SCC and AD biomarkers is modest, any opportunity to improve our predictive ability preclinically is worth investigating. Furthermore, gathering information about SCC is simple and cost-effective compared to neuroimaging and extensive cognitive testing. Thus, SCC may serve as a straightforward approach in identifying older patients who may require additional follow-up to examine the possibility of an incipient neurological process (Amariglio, et al., 2011).
To fully appreciate the dynamic interplay between SCC, objective cognitive performance, and AD pathological changes along the disease continuum, more extensive longitudinal studies are needed. While current findings suggest a relationship between early AD pathological markers and SCC, it will be important to follow our sample longitudinally to validate the predictive value of SCC. By the stage of MCI, the relationship between SCC and disease progression is inherently less clear, as evidenced by some studies demonstrating increased self-awareness and others demonstrating reduced self-awareness of cognitive deficits similar to that observed in AD dementia. These seemingly paradoxical findings appear to depend on subject sampling, whereby increased SCC are likely characteristic of early-MCI, but wane in late-MCI (Roberts, et al., 2009). However, additional longitudinal studies that track the evolution of SCC along the disease trajectory and consider the impact of modifying factors, such as personality traits (Reid & Maclullich, 2006) and cognitive reserve (van Oijen, de Jong, Hofman, Koudstaal, & Breteler, 2007) will be necessary to confirm the utility of SCC as an indicator of AD.
Taken together, our findings suggest that relationships between early AD pathology and SCC can be observed in CN individuals. Recent studies have started to employ sophisticated psychometric techniques to derive SCC items that are most useful in identifying diagnostic categories (i.e., AD and MCI) (S.T. Farias, 2011; Snitz et al., 2011). We also plan on using these psychometric techniques in a large CN population to determine whether we can detect the specific items that would be most sensitive at predicting imaging evidence of preclinical AD and subsequent cognitive decline. In a similar vein, the development of sensitive objective cognitive measures is being realized, which has shown promise in detecting early pathological changes in AD (Parra et al., 2010; Rentz et al., 2011; Rentz et al., 2010). Moving forward, it will likely be the case that a combination of variables including carefully selected SCC, sensitive objective cognitive measures, and AD biomarkers will best predict risk for progression from normal to AD dementia.
Acknowledgments
Funding
This work was supported by an Alzheimer Association grant IIRG-08-90934 (K.J.) and by National Institute on Aging grants P01-AG036694-01 (R.S.); P50- AG00513428 (R.S.).
The authors wish to thank the investigators and staff of the Massachusetts Alzheimer’s Disease Research Center and the individual research participants.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. S1552-5260(11)00104-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Klunk WE. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65(11):1509–1517. doi: 10.1001/archneur.65.11.1509. 65/11/1509 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc. 2001;7(5):631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- Amariglio RE, Townsend MK, Grodstein F, Sperling RA, Rentz DM. Specific subjective memory complaints in older persons may indicate poor cognitive function. J Am Geriatr Soc. 2011;59(9):1612–1617. doi: 10.1111/j.1532-5415.2011.03543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer HA, Edison P, Brooks DJ, Barnes J, Frost C, Yeatman T, Rossor MN. Amyloid load and cerebral atrophy in Alzheimer’s disease: an 11C-PIB positron emission tomography study. Ann Neurol. 2006;60(1):145–147. doi: 10.1002/ana.20889. [DOI] [PubMed] [Google Scholar]
- Bassett SS, Folstein MF. Memory complaint, memory performance, and psychiatric diagnosis: a community study. J Geriatr Psychiatry Neurol. 1993;6(2):105–111. doi: 10.1177/089198879300600207. [DOI] [PubMed] [Google Scholar]
- Benton A, Varney N, Hamsher K, Spreen O. Contributions to neuropsychological assessment. Oxford, UK: Oxford University Press; 1983. [Google Scholar]
- Chetelat G, Villemagne VL, Bourgeat P, Pike KE, Jones G, Ames D, Rowe CC. Relationship between atrophy and beta-amyloid deposition in Alzheimer disease. Ann Neurol. 2010;67(3):317–324. doi: 10.1002/ana.21955. [DOI] [PubMed] [Google Scholar]
- Collie A, Maruff P. The neuropsychology of preclinical Alzheimer’s disease and mild cognitive impairment. Neurosci Biobehav Rev. 2000;24(3):365–374. doi: 10.1016/s0149-7634(00)00012-9. [DOI] [PubMed] [Google Scholar]
- Comijs HC, Deeg DJ, Dik MG, Twisk JW, Jonker C. Memory complaints; the association with psycho-affective and health problems and the role of personality characteristics. A 6-year follow-up study. J Affect Disord. 2002;72(2):157–165. doi: 10.1016/s0165-0327(01)00453-0. S0165032701004530 [pii] [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Sperling RA, Hyman BT, Albert MS, Blacker D. Clinical prediction of Alzheimer disease dementia across the spectrum of mild cognitive impairment. Arch Gen Psychiatry. 2007;64(12):1443–1450. doi: 10.1001/archpsyc.64.12.1443. 64/12/1443 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dik MG, Jonker C, Comijs HC, Bouter LM, Twisk JW, van Kamp GJ, Deeg DJ. Memory complaints and APOE-epsilon4 accelerate cognitive decline in cognitively normal elderly. Neurology. 2001;57(12):2217–2222. doi: 10.1212/wnl.57.12.2217. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, Holtzman DM. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59(3):512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- Farias ST, Mungas D, Reed BR, Cahn-Weiner D, Jagust W, Baynes K, Decarli C. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology. 2008;22(4):531–544. doi: 10.1037/0894-4105.22.4.531. 2008-08178-012 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST. The measurement of everyday cognition: Development and validation of a short form of the Everyday Cognition scales. Alzheimer’s & Dementia. 2011;7:593–601. doi: 10.1016/j.jalz.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flicker C, Ferris SH, Reisberg B. A longitudinal study of cognitive function in elderly persons with subjective memory complaints. J Am Geriatr Soc. 1993;41(10):1029–1032. doi: 10.1111/j.1532-5415.1993.tb06448.x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. 0022-3956(75)90026-6 [pii] [DOI] [PubMed] [Google Scholar]
- Gagnon M, Dartigues JF, Mazaux JM, Dequae L, Letenneur L, Giroire JM, Barberger-Gateau P. Self-reported memory complaints and memory performance in elderly French community residents: results of the PAQUID Research Program. Neuroepidemiology. 1994;13(4):145–154. doi: 10.1159/000110373. [DOI] [PubMed] [Google Scholar]
- Geerlings MI, Deeg DJ, Penninx BW, Schmand B, Jonker C, Bouter LM, van Tilburg W. Cognitive reserve and mortality in dementia: the role of cognition, functional ability and depression. Psychol Med. 1999;29(5):1219–1226. doi: 10.1017/s0033291799008867. [DOI] [PubMed] [Google Scholar]
- Gilewski MJ, Zelinski EM, Schaie KW. The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychol Aging. 1990;5(4):482–490. doi: 10.1037//0882-7974.5.4.482. [DOI] [PubMed] [Google Scholar]
- Go RC, Duke LW, Harrell LE, Cody H, Bassett SS, Folstein MF, Blacker D. Development and validation of a Structured Telephone Interview for Dementia Assessment (STIDA): the NIMH Genetics Initiative. J Geriatr Psychiatry Neurol. 1997;10(4):161–167. doi: 10.1177/089198879701000407. [DOI] [PubMed] [Google Scholar]
- Gomperts SN, Rentz DM, Moran E, Becker JA, Locascio JJ, Klunk WE, Johnson KA. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71(12):903–910. doi: 10.1212/01.wnl.0000326146.60732.d6. 71/12/903 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohman TJ, Beason-Held LL, Lamar M, Resnick SM. Subjective cognitive complaints and longitudinal changes in memory and brain function. Neuropsychology. 2011;25(1):125–130. doi: 10.1037/a0020859. 2010-20717-001 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Wiste HJ, Vemuri P, Weigand SD, Senjem ML, Zeng G, Knopman DS. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain. 2010;133(11):3336–3348. doi: 10.1093/brain/awq277. awq277 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Feyen L, Freymann K, Tepest R, Maier W, Heun R, Scheef L. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging. 2006;27(12):1751–1756. doi: 10.1016/j.neurobiolaging.2005.10.010. S0197-4580(05)00341-6 [pii] [DOI] [PubMed] [Google Scholar]
- Johnson KA, Gregas M, Becker JA, Kinnecom C, Salat DH, Moran EK, Greenberg SM. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62(3):229–234. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Christensen H, Korten AE, Henderson AS, Jacomb PA, Mackinnon A. Do cognitive complaints either predict future cognitive decline or reflect past cognitive decline? A longitudinal study of an elderly community sample. Psychol Med. 1997;27(1):91–98. doi: 10.1017/s0033291796003923. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Christensen H, Korten AE, Jacomb PA, Henderson AS. Memory complaints as a precursor of memory impairment in older people: a longitudinal analysis over 7–8 years. Psychol Med. 2001;31(3):441–449. [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test: assessment of aphasia and related disorders. 2. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- Lam LC, Lui VW, Tam CW, Chiu HF. Subjective memory complaints in Chinese subjects with mild cognitive impairment and early Alzheimer’s disease. Int J Geriatr Psychiatry. 2005;20(9):876–882. doi: 10.1002/gps.1370. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16(5):834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Lopresti BJ, Klunk WE, Mathis CA, Hoge JA, Ziolko SK, Lu X, Price JC. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med. 2005;46(12):1959–1972. 46/12/1959 [pii] [PubMed] [Google Scholar]
- Masur DM, Fuld PA, Blau AD, Thal LJ, Levin HS, Aronson MK. Distinguishing normal and demented elderly with the selective reminding test. J Clin Exp Neuropsychol. 1989;11(5):615–630. doi: 10.1080/01688638908400920. [DOI] [PubMed] [Google Scholar]
- Minett TS, Da Silva RV, Ortiz KZ, Bertolucci PH. Subjective memory complaints in an elderly sample: a cross-sectional study. Int J Geriatr Psychiatry. 2008;23(1):49–54. doi: 10.1002/gps.1836. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, Jagust WJ. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132(Pt 5):1310–1323. doi: 10.1093/brain/awn320. awn320 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Mosconi L, De Santi S, Brys M, Tsui WH, Pirraglia E, Glodzik-Sobanska L, de Leon MJ. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol Psychiatry. 2008;63(6):609–618. doi: 10.1016/j.biopsych.2007.05.030. S0006-3223(07)00564-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra MA, Abrahams S, Logie RH, Mendez LG, Lopera F, Della Sala S. Visual short-term memory binding deficits in familial Alzheimer’s disease. Brain. 2010;133(9):2702–2713. doi: 10.1093/brain/awq148. awq148 [pii] [DOI] [PubMed] [Google Scholar]
- Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ. Subjective cognition and amyloid deposition imaging: a pittsburgh compound B positron emission tomography study in normal elderly individuals. Arch Neurol. 2012;69(2):223–229. doi: 10.1001/archneurol.2011.666. 69/2/223 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, Rowe CC. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130(Pt 11):2837–2844. doi: 10.1093/brain/awm238. awm238 [pii] [DOI] [PubMed] [Google Scholar]
- Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, Mathis CA. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25(11):1528–1547. doi: 10.1038/sj.jcbfm.9600146. 9600146 [pii] [DOI] [PubMed] [Google Scholar]
- Reid LM, Maclullich AM. Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord. 2006;22(5–6):471–485. doi: 10.1159/000096295. 96295 [pii] [DOI] [PubMed] [Google Scholar]
- Reisberg B, Prichep L, Mosconi L, John ER, Glodzik-Sobanska L, Boksay I, de Leon MJ. The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer’s disease. Alzheimers Dement. 2008;4(1 Suppl 1):S98–S108. doi: 10.1016/j.jalz.2007.11.017. S1552-5260(07)00658-9 [pii] [DOI] [PubMed] [Google Scholar]
- Reitan R. Manual for administration of neuropsychological test batteries for adults and children. Tuscon, AZ: Reitan Neuropsychology Laboratories; 1979. [Google Scholar]
- Rentz DM, Amariglio RE, Becker JA, Frey M, Olson LE, Frishe K, Sperling RA. Face-name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia. 2011;49(9):2776–2783. doi: 10.1016/j.neuropsychologia.2011.06.006. S0028-3932(11)00280-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz DM, Locascio JJ, Becker JA, Moran EK, Eng E, Buckner RL, Johnson KA. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010;67(3):353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JL, Clare L, Woods RT. Subjective memory complaints and awareness of memory functioning in mild cognitive impairment: a systematic review. Dement Geriatr Cogn Disord. 2009;28(2):95–109. doi: 10.1159/000234911. 000234911 [pii] [DOI] [PubMed] [Google Scholar]
- Rodda J, Dannhauser T, Cutinha DJ, Shergill SS, Walker Z. Subjective cognitive impairment: functional MRI during a divided attention task. Eur Psychiatry. 2011;26(7):457–462. doi: 10.1016/j.eurpsy.2010.07.003. S0924-9338(10)00147-1 [pii] [DOI] [PubMed] [Google Scholar]
- Rodda J, Okello A, Edison P, Dannhauser T, Brooks DJ, Walker Z. (11)C-PIB PET in subjective cognitive impairment. Eur Psychiatry. 2010;25(2):123–125. doi: 10.1016/j.eurpsy.2009.07.011. S0924-9338(09)00136-9 [pii] [DOI] [PubMed] [Google Scholar]
- Rodrigue KM, Kennedy KM, Devous MD, Sr, Rieck JR, Hebrank AC, Diaz-Arrastia R, Park DC. beta-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology. 2012;78(6):387–395. doi: 10.1212/WNL.0b013e318245d295. WNL.0b013e318245d295 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Villemagne VL. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31(8):1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. S0197-4580(10)00164-8 [pii] [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, Mamourian AC. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67(5):834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. 67/5/834 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmand B, Jonker C, Hooijer C, Lindeboom J. Subjective memory complaints may announce dementia. Neurology. 1996;46(1):121–125. doi: 10.1212/wnl.46.1.121. [DOI] [PubMed] [Google Scholar]
- Schofield PW, Jacobs D, Marder K, Sano M, Stern Y. The validity of new memory complaints in the elderly. Arch Neurol. 1997;54(6):756–759. doi: 10.1001/archneur.1997.00550180064014. [DOI] [PubMed] [Google Scholar]
- Small GW, Chen ST, Komo S, Ercoli L, Bookheimer S, Miller K, Pericak-Vance MA. Memory self-appraisal in middle-aged and older adults with the apolipoprotein E-4 allele. Am J Psychiatry. 1999;156(7):1035–1038. doi: 10.1176/ajp.156.7.1035. [DOI] [PubMed] [Google Scholar]
- Smith GE, Petersen RC, Ivnik RJ, Malec JF, Tangalos EG. Subjective memory complaints, psychological distress, and longitudinal change in objective memory performance. Psychol Aging. 1996;11(2):272–279. doi: 10.1037//0882-7974.11.2.272. [DOI] [PubMed] [Google Scholar]
- Snitz BE, Morrow LA, Rodriguez EG, Huber KA, Saxton JA. Subjective memory complaints and concurrent memory performance in older patients of primary care providers. J Int Neuropsychol Soc. 2008;14(6):1004–1013. doi: 10.1017/S1355617708081332. S1355617708081332 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, Yu L, Crane PK, Chang CC, Hughes TF, Ganguli M. Subjective Cognitive Complaints of Older Adults at the Population Level: An Item Response Theory Analysis. Alzheimer Dis Assoc Disord. 2011 doi: 10.1097/WAD.0b013e3182420bdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Jack CR, Jr, Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med. 2011;3(111):111cm133. doi: 10.1126/scitranslmed.3002609. 3/111/111cm33 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburg compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66(12):1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Miller TP, Tinklenberg JR. Correlates of memory decline: a 4-year longitudinal study of older adults with memory complaints. Psychol Aging. 1992;7(2):185–193. doi: 10.1037//0882-7974.7.2.185. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. S1053811901909784 [pii] [DOI] [PubMed] [Google Scholar]
- van Norden AG, Fick WF, de Laat KF, van Uden IW, van Oudheusden LJ, Tendolkar I, de Leeuw FE. Subjective cognitive failures and hippocampal volume in elderly with white matter lesions. Neurology. 2008;71(15):1152–1159. doi: 10.1212/01.wnl.0000327564.44819.49. 71/15/1152 [pii] [DOI] [PubMed] [Google Scholar]
- van Oijen M, de Jong FJ, Hofman A, Koudstaal PJ, Breteler MM. Subjective memory complaints, education, and risk of Alzheimer’s disease. Alzheimers Dement. 2007;3(2):92–97. doi: 10.1016/j.jalz.2007.01.011. S1552-5260(07)00013-1 [pii] [DOI] [PubMed] [Google Scholar]
- Wang L, van Belle G, Crane PK, Kukull WA, Bowen JD, McCormick WC, Larson EB. Subjective memory deterioration and future dementia in people aged 65 and older. J Am Geriatr Soc. 2004;52(12):2045–2051. doi: 10.1111/j.1532-5415.2004.52568.x. JGS52568 [pii] [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III, Wechsler Adult Intelligence Scale-third edition, administration and scoring manual. New York, NY: The Psychological Corporation; 1997. [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale. Psychiatry Res. 1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]