Abstract
Auditory-based communication skills are developed at a young age and are maintained throughout our lives. However, some individuals – both young and old – encounter difficulties in achieving or maintaining communication proficiency. Biological signals arising from hearing sounds relate to real-life communication skills such as listening to speech in noisy environments and reading, pointing to an intersection between hearing and cognition. Musical experience, amplification, and software-based training can improve these biological signals. These findings of biological plasticity, in a variety of subject populations, relate to attention and auditory memory, and represent an integrated auditory system influenced by both sensation and cognition.
Learning outcomes
The reader will (1) understand that the auditory system is malleable to experience and training, (2) learn the ingredients necessary for auditory learning to successfully be applied to communication, (3) learn that the auditory brainstem response to complex sounds (cABR) is a window into the integrated auditory system, and (4) see examples of how cABR can be used to track the outcome of experience and training.
Keywords: Learning, Training, Brainstem, Communication
1. Introduction
A variety of communication skills are employed in our everyday lives: reading and listening in noise among them. Enhancing and maintaining these skills leads to a more fulfilling existence as a communicating human being. Long-term activities such as musical training, short-term training – especially software-based auditory-skills training regimens, and a clear amplified signal are capable of increasing communication skills. An open question is how this is accomplished from a biological standpoint. The auditory system is highly integrated and interactive – no part of it operates in isolation. So, to answer this question is at present beyond the scope of what can be accomplished in humans. However, we have access, in humans, to a physiological measure that reflects the biological processing of sound in the integrated system. This measure, the auditory brainstem response to complex stimulation (cABR), responds to training and experience and tracks well with cognitive abilities such as attention and auditory working memory. This review discusses cABR and its relationship with communication-based skills, offers a model that explains the nature of training and experience’s role in shaping communication skills, and points toward training strategies that might best profit the auditory system in its responsibility as the center of human communication.
The cABR, unlike the click ABR or the various cortical evoked responses, has the advantage of having a morphology that mimics – with great fidelity – that of the stimulus. In both the time and frequency domains, this similarity lends itself to a quantification of processing precision. The inferior colliculus, a major contributor to cABR, is a convergence center in the integrated auditory system. Viewed as the auditory analogue to primary visual cortex (Nelken, 2008), it functions not only as an afferent route from periphery to higher processing centers, but also receives an abundance of downward corticofugal projections and is critical to learning (Bajo, Nodal, Moore, & King, 2010; Suga & Ma, 2003). Thus, while the cABR adheres closely to the stimulus, it is also modified slightly by factors unrelated to strict afferent processing. Specifically, and relevant to the topic of this paper, an individual’s communication ability is revealed in these slight response modifications.
Two highly complex auditory phenomena that change the brain are language and music. There has been much research on how speakers of different languages have different processing patterns in the brain (Bidelman, Gandour, & Krishnan, 2011; Chandrasekaran, Krishnan, & Gandour, 2009; Näätänen, et al., 1997); likewise for people with language difficulties such as dyslexia (Backes, et al., 2002; Brunswick & Rippon, 1994; Galaburda, 1993; Habib, 2000; Shaywitz, et al., 2002). Musicians’ brains show differences compared to nonmusicians’ brains both structurally (Gaser & Schlaug, 2003; Hyde, et al., 2009; Pantev, et al., 1998; Schlaug, 2001; Schneider, et al., 2002) and functionally (Fujioka, Trainor, Ross, Kakigi, & Pantev, 2004; Kraus & Chandrasekaran, 2010; Parbery-Clark, Strait, & Kraus, 2011; Shahin, Bosnyak, Trainor, & Roberts, 2003; Strait, Chan, Ashley, & Kraus, 2011; Trainor, Shahin, & Roberts, 2009); and communication abilities are enhanced in musicians (Marques, Moreno, Castro, & Besson, 2007; Moreno, et al., 2009; Schön, Magne, & Besson, 2004).
Some of these (positive) changes brought about by music experience and training are negatively mirrored by aging. As we age, there are declines in cognitive skills such as attention and memory (Andrés, Parmentier, & Escera, 2006; Bloss, et al., 2011; Craik, Luo, & Sakuta, 2010; Nyberg, et al., 2010; Wang, et al., 2011; Zacks, Hasher, & Li, 2000), hearing in noise (Hargus & Gordon Salant, 1995; Souza, Boike, Witherell, & Tremblay, 2007), perceptual timing skills (Parbery-Clark, Strait, Anderson, Hittner, & Kraus, 2011; Zendel & Alain, 2011), and neural timing (Caspary, Ling, Turner, & Hughes, 2008; Humes, Kewley-Port, Fogerty, & Kinney, 2010; Lister, Maxfield, Pitt, & Gonzalez, 2011; Tremblay, Piskosz, & Souza, 2003).
We have a series of experiments that look at musicianship’s effect on listening skills and biological processing in participants of all ages. We have explored the relationships between cABR and cognitive abilities such as attention and memory, with an eye toward illuminating how music confers a route to maintain these skills as we age. We also have investigated the use of assistive listening devices in the classroom to determine how a better, clearer signal impacts learning, communication, and neural processing. Coupled with these experiments are lines of research into software-based auditory training activities to determine whether short-term training can provide some of the benefits that long-term music training can provide in the three realms of perception, cognition, and physiology.
2. Long term experience (music)
Music affects language. For example, a musician’s ability to hear speech in noise is generally superior to a nonmusician’s (Parbery-Clark, Skoe, Lam, & Kraus, 2009; Parbery-Clark, Strait, Anderson, et al., 2011; Zendel & Alain, 2011). Musicians also excel at other verbal-based skills including reading (Chan, Ho, & Cheung, 1998; Forgeard, Winner, Norton, & Schlaug, 2008; Ho, Cheung, & Chan, 2003; Moreno, et al., 2011 ; Strait, Hornickel, & Kraus, 2011). The OPERA hypothesis (Patel, 2011), with the letters of its acronym, posits some of the reasons why musicianship is linked to language ability. The common levels of auditory biological processing (i.e. overlap) that music and speech share is one of the biggest. Also, the precision that learning and playing music demands exercises the auditory system more than speech does. The often strong positive emotions involved in performing music involve limbic areas of the brain which are known to be a precursor to auditory learning in animals (Kilgard & Merzenich, 1998; Weinberger, 2007). Task repetition and attention round out the forces invoked by music which initiate cortical plasticity. In turn, the entire integrated auditory system is thereby strengthened.
However, the influence of music training on cortical structure and function, and on perceptual and communicative skills, is not the whole story. In the last several years, cABR has uncovered subcortical effects of musical training across the life span as well. For example, the similarity of the cABR to its evoking stimulus – in this case speech – is greater in child (Strait et al., in preparation) and young-adult (Parbery-Clark, et al., 2009) musicians (Fig. 1, top). This measure of fidelity is also well correlated with both hearing-in-noise ability and auditory working memory, but not visual working memory (Fig 1, bottom). A second cABR measure, the difference in phase in responses to two different stop consonants (ba, ga), also is enhanced in musicians. Again, this musician enhancement parallels that seen in good listeners in noise (Skoe, Nicol, & Kraus, 2011) (Fig. 2).
Fig. 1.
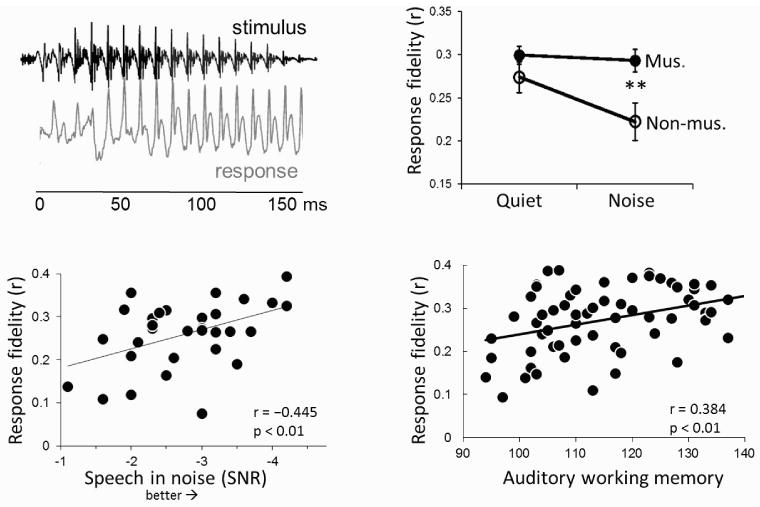
Top left: The stimulus (black) and resultant cABR (gray) are similar in their features. This similarity enables the application of conventional cross-correlation techniques in order to assess response fidelity. Top right: When “da” is presented in a noise-free background, musicians and nonmusicians have about the same response fidelity. However, the addition of background noise reduces the fidelity of the nonmusician response. Bottom: Response-in-noise correlations with the stimulus are related to hearing in noise ability (left) and auditory working memory (right).
Fig. 2.
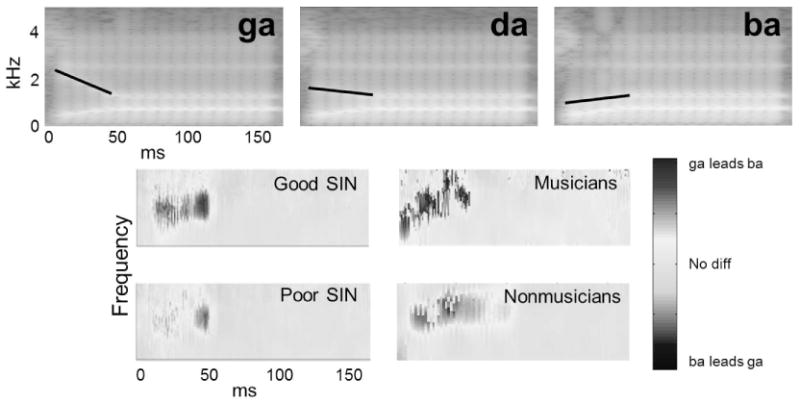
Top: Spectrograms of synthesized stop-consonant-vowel syllables. The black lines trace the second formant trajectory that distinguishes ga, da and ba. Bottom: Cross-phaseograms depict, in color, the timing differences between pairs of responses (Skoe, et al., 2011). Here, when comparing responses to ga and ba, warm colors signify portions of the response that are earlier to ga, a result of the higher-frequency second formant content in the first 50 ms. The extent to which this timing shift occurs is greater in good speech-in-noise (SIN) perceivers (left) and experienced musicians (right).
We believe that years of musical training, with its emphasis on memorization of sound, visual patterns, and auditory-motor sequences, leads to a strengthening of auditory memory – the ability to remember, manipulate (e.g., reorder), and recite lists of words, numbers or sentences. The improved memory affects realms outside of music by transferring to other communication skills such as hearing in noise. Strengthened cognitive skills improve auditory centers in the cortex, which, in turn, trickle down via the corticofugal efferent system to a more finely-tuned auditory brainstem. But can this chain of events be initiated in a shorter time frame?
3. Classroom amplification devices
In collaboration with a Chicago school for learning-disabled (LD) children, we have performed communication skills and neurophysiological testing on LD children prior to and after one year of school. Half of the cohort of children wore FM assistive listening devices (Phonak EduLink). These devices amplified the teacher’s voice so that ambient classroom noises were less distracting. Such devices have been shown to improve reading and communication skills (Blake, Field, Foster, Platt, & Wertz, 1991; DiSarno, Schowalter, & Grassa, 2002; Flexer, Kemp Biley, Hinkley, Harkema, & Holcomb, 2002; Johnston, John, Kreisman, Hall, & Crandell, 2009; Purdy, Smart, Baily, & Sharma, 2009; Rosenberg, et al., 1999), teacher perception of student learning (Purdy, et al., 2009), and neural measures of attention (Friederichs & Friederichs, 2005). Here, unlike the non-FM-device-wearing LD controls in the same classroom, the FM-device wearers significantly improved on measures of reading and phonological awareness. Consistent with the observed variability that characterizes performance in people with LD, a cABR measure of response consistency – intra-session response replicability – was also improved in FM-device wearers, and the improvement on this measure was positively correlated with the extent of phonological-awareness improvement. Interestingly, the children in the experimental group who had the least consistent cABRs prior to the year of FM device usage showed the largest improvements in reading measures (Hornickel, Zecker, & Kraus, 2011). The changes seen in the cABR and their relationship with objective measures of reading skills serve as evidence that corticofugal-driven plasticity in the integrated auditory system is responsive to clear speech signals in the course of just one year. In the classroom, students wore the devices all day long while learning was occurring. Are similar communication gains possible in a focused training setting?
4. Short term training
Given the effect of long-term musical experience on the cABR, medium-term classroom amplification, and the effects that training is known to have on projections to the inferior colliculus (Bajo, et al., 2010), we asked whether shorter-term training might also affect this measure of auditory system integration. We looked at this in three subject populations – children on the autism spectrum, normal young adults, and older adults – using three different software-based training programs. All designs followed the same basic procedure: Participants underwent cognitive- and communication-skill testing and cABR testing. Then, they underwent their respective training programs for the prescribed number of weeks. Finally, after training, the pre-training battery was repeated. Each experimental group was paired with a non-trained control group and the two adult studies had a follow-up test session to gauge the persistence of training effects.
In children with autism spectrum disorder (ASD), we wondered whether the typical attribute of poor voice pitch production and perception would have a cABR correlate. Two “ya” syllables with rising and falling pitch contours, resembling the tones of voice used for questions and statements, respectively, were used as stimuli. Previous work had revealed that ASD children have cABRs with poor pitch tracking: That is, the fundamental periodicity of the response does not adhere closely to the pitch of the evoking stimulus (Russo, et al., 2008). A small cohort of ASD children participated in five to ten weeks of “Fast ForWord” training (Scientific Learning Corp.). A marked reduction in pitch error – the deviation of the response from the stimulus – was observed in one of the participants. Another aspect of the cABR, specifically timing to the syllable “da”, was improved in three participants relative to the non-trained controls (Russo, Hornickel, Nicol, Zecker, & Kraus, 2010).
In a much larger group of college-age subjects, we likewise looked for training-induced changes in hearing-in-noise abilities and subcortical function. LACE training (Neurotone), which is specifically targeted toward increasing participants’ ability to hear speech in noise, was administered to 28 young adults. Compared to their pre-training ability, LACE-trained participants increased their ability to understand speech in noise, as measured by QuickSIN (Killion, Niquette, Gudmundsen, Revit, & Banerjee, 2004) and HINT (Nilsson, Soli, & Sullivan, 1994), relative to a matched untrained control group (n=32) (Fig. 3, top). These improvements coincided with an increase in cABR encoding of the fundamental frequency (F0) and second harmonic (H2) of a syllable “da” when it was presented in multi-talker background noise (Fig. 3, bottom left) (Song, Skoe, Banai, & Kraus, 2011). There was a significant correlation between hearing-in-noise improvement and cABR F0 and H2 encoding enhancement (Fig. 3, bottom right).
Fig. 3.
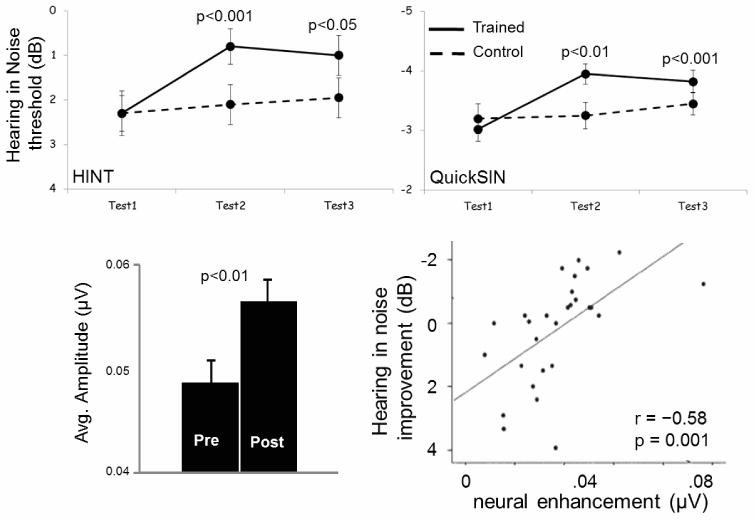
Top: A short-term training program (LACE) resulted in gains in speech-in-noise perception ability both immediately after concluding the program (test 2) and six months later (test 3). P values are test 2 vs. test 1 and test 3 vs. test 1 for trained group. Control group did not significantly improve. Bottom: Magnitude of spectral encoding of speech fundamental frequency and second harmonic was greater post-training for the experimental group (left) and the extent of the increase correlated with the improvement in a speech-in-noise measure (right).
In ongoing work in older adults, we likewise are investigating communication skills and brainstem encoding following directed training. In this case, the training program was Brain Fitness (Posit Science), which purports to improve communication, hearing, and memory skills. This program, which is a variant of Fast ForWord, was administered over an eight-week period to an experimental group, while quizzes on educational programming, viewed for the same duration, were administered to a control group. Cognitive and perceptual abilities, including auditory memory, hearing in noise ability, and backward masking performance, were improved in the experimental group only. A cABR measure of timing in noise likewise improved in the majority of Brain Fitness recipients; controls did not improve (Anderson & Kraus, 2011; Anderson, Parbery-Clark, Chio, & Kraus, 2012).
Taken together, training programs in three different populations have provided evidence that directed training on the order of weeks can not only benefit perceptual and behavioral communication outcomes but also rewire biological processing in the integrated auditory system. As speculated above in the discussion of music-training’s role in influencing cABR, we believe that the impetus of the change lay in the cognitive demands that the training required. The exercising of attention and memory skills required for performing the tasks strengthened these cortical processes and, in turn, focused the auditory processing of the brainstem structures involved in cABR generation. The brainstem, in turn, with its improved acuity sends a more robust signal “up” to cortical processing structures, completing a positive feedback loop.
5. Conclusion
The learning and maintenance of communication skills involves a highly-interactive and integrated auditory system. Much as it describes the overlap between music and language, the OPERA hypothesis also can serve as an enumeration of the ingredients required for effective training and successful learning. Effective learning requires both sensory and cognitive components (Bavelier, Achtman, Mani, & Föcker, 2011; Berry, et al., 2011); focusing on just the former risks the ‘curse of specialization.’ Learning takes place on multiple time frames: Both life-long endeavors, such as playing a musical instrument, and shorter-term amplification and focused listening training tune the auditory system. In each, sound-to-meaning relationships are formed, driven by cognitive processes such as attention and memory. Along with emotion, cognition affects basic response properties of the auditory system which can be accessed by cABR. Future longitudinal studies combining cortical physiology and imaging could be designed to solidify the supposition that cortical reorganization precedes subcortical tuning changes.
cABR, like other auditory brainstem response paradigms, is passively elicited. Yet, despite this, it is not a measure of passive processing. The cABR, as a nervous system index of sound processing, both reflects the encoding of the acoustics of sound and relates to executive function and everyday communication skills. Although inferential, this dual nature of cABR is consistent with the notion that “hearing” consists of the combination of multiple sensory and executive processes. cABR is a snapshot of the integrated auditory system – a system with strong afferent and efferent connections joining subcortical and cortical structures – revealing an auditory system molded by life experience (Fig. 4). These experiences enable auditory learning that promotes successful communication. The cABR, because it is so much more than a measure of one-way afferent encoding in the brainstem, has the potential for wide-spread adoption into schools, clinics, and other venues where there is an interest in assessing the biological basis of listening and monitoring the underlying changes wrought by education and training. With more research, it is anticipated that it might serve a predictive role. Intriguing preliminary findings hint at pre-training cABR “profiles” forecasting future communicative successes with directed auditory training.
Fig. 4.
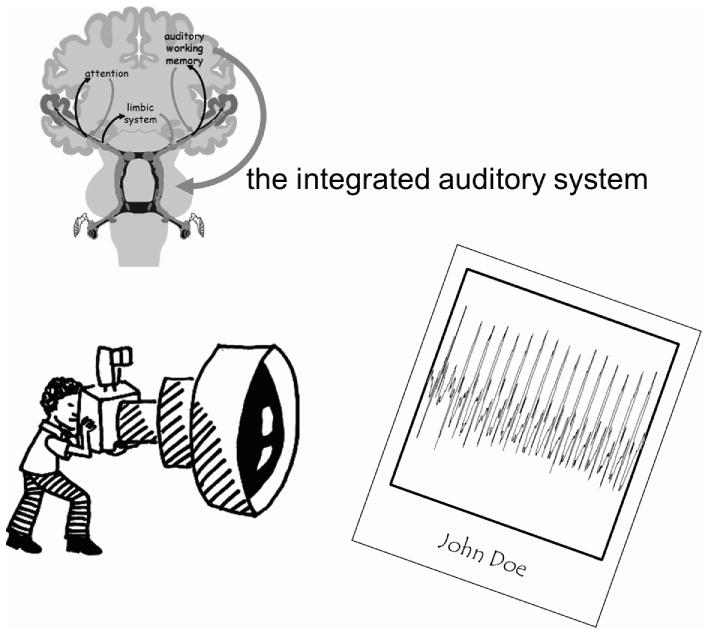
Top: The integrated auditory system’s afferent (black) pathways are accompanied by downward-descending efferent (red) pathways that enable cognitive and emotional centers to exert influence on biological processing of sound. Bottom: cABR is a snapshot of auditory function, consisting of a combination of multiple sensory and executive processes which, together, impact everyday communication skills.
Acknowledgments
This work was supported by grants from the National Science Foundation (BCS-0921275, BCS-1057556), the National Institutes of Health (R01-DC010016), and the Phonak Corporation.
Appendix A. Continuing education
-
Performance on speech in noise tasks is
mediated by attention and working memory
reflected in brainstem function
better in musicians compared to non-musicians
mainly related to audiometric thresholds
a, b, and c
-
Brainstem response to complex sounds can be used for evaluation of auditory processes because
the response waveform resembles the stimulus waveform
the brainstem response is a snapshot of the integrated auditory system
latency differences of at least a millisecond are clinically significant
a and b
all of the above
-
Which of the following is true about auditory training?
adults are unable to materially improve their listening in noise
training for only a short period of time (~1–2 months) can change neural encoding in the brainstem
training has the same effect on all participants
training related changes are seen in the cortex but not the brainstem
c and d
-
Musical training has been shown to yield benefits in
working memory
speech-in-noise perception
neural encoding of speech
a and b
all of the above
-
The effects of noise on the brainstem response
include delays in peak timing
are particularly pronounced in the steady state region of the response
are less pronounced in children with poor speech-in-noise perception
result in increased amplitudes in children with good speech-in-noise perception
all of the above
Answers: 1-e, 2-d, 3-b, 4-e, 5-a
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson S, Kraus N. Training improves neural timing in older adults. Conference on Aging and Speech Communication; Bloomington, IN. 2011. [Google Scholar]
- Anderson S, Parbery-Clark A, Chio HJ, Kraus N. Increased neural precision with auditory training in older adults. Conference of the Association for Research in Otolaryngology.2012. [Google Scholar]
- Andrés P, Parmentier FB, Escera C. The effect of age on involuntary capture of attention by irrelevant sounds: A test of the frontal hypothesis of aging. Neuropsychologia. 2006;44:2564–2568. doi: 10.1016/j.neuropsychologia.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Backes W, Vuurman E, Wennekes R, Spronk P, Wuisman M, van Engelshoven J, Jolles J. Atypical brain activation of reading processes in children with developmental dyslexia. Journal of Child Neurology. 2002;17:867–871. doi: 10.1177/08830738020170121601. [DOI] [PubMed] [Google Scholar]
- Bajo VM, Nodal FR, Moore DR, King AJ. The descending corticocollicular pathway mediates learning-induced auditory plasticity. Nature Neuroscience. 2010;13:253–260. doi: 10.1038/nn.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Achtman RL, Mani M, Föcker J. Neural bases of selective attention in action video game players. Vision Research. 2011 doi: 10.1016/j.visres.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Zanto TP, Clapp WC, Hardy JL, Delahunt PB, Mahncke HW, Gazzaley A. The influence of perceptual training on working memory in older adults. PLoS ONE. 2011;5:e11537. doi: 10.1371/journal.pone.0011537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidelman GM, Gandour JT, Krishnan A. Cross-domain effects of music and language experience on the representation of pitch in the human auditory brainstem. Journal of Cognitive Neuroscience. 2011;23:425–434. doi: 10.1162/jocn.2009.21362. [DOI] [PubMed] [Google Scholar]
- Blake R, Field B, Foster C, Platt F, Wertz P. Effect of FM auditory trainers on attending behaviors of learning-disabled children. Language, Speech, and Hearing Services in Schools. 1991;22:111–114. [Google Scholar]
- Bloss EB, Janssen WG, Ohm DT, Yuk FJ, Wadsworth S, Saardi KM, Morrison JH. Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. Journal of Neuroscience. 2011;31:7831–7839. doi: 10.1523/JNEUROSCI.0839-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunswick N, Rippon G. Auditory event-related potentials, dichotic listening performance and handedness as indices of lateralisation in dyslexic and normal readers. International Journal of Psychophysiology. 1994;18:265–275. doi: 10.1016/0167-8760(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. Journal of Experimental Biology. 2008;211:1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AS, Ho YC, Cheung MC. Music training improves verbal memory. Nature. 1998;396:128–128. doi: 10.1038/24075. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran B, Krishnan A, Gandour JT. Sensory processing of linguistic pitch as reflected by the mismatch negativity. Ear and Hearing. 2009;30:552–558. doi: 10.1097/AUD.0b013e3181a7e1c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FI, Luo L, Sakuta Y. Effects of aging and divided attention on memory for items and their contexts. Psychology and Aging. 2010;25:968–979. doi: 10.1037/a0020276. [DOI] [PubMed] [Google Scholar]
- DiSarno NJ, Schowalter M, Grassa P. Classroom amplification to enhance student performance. Teaching Exceptional Children. 2002;34:20–26. [Google Scholar]
- Flexer C, Kemp Biley K, Hinkley A, Harkema C, Holcomb J. Using sound-field systems to teach phonemic awareness to pre-schoolers. The Hearing Journal. 2002;55:38. [Google Scholar]
- Forgeard M, Winner E, Norton A, Schlaug G. Practicing a musical instrument in childhood is associated with enhanced verbal ability and nonverbal reasoning. PLoS ONE. 2008;3:e3566. doi: 10.1371/journal.pone.0003566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederichs E, Friederichs P. Electrophysiologic and psycho-acoustic findings following one-year application of a personal ear-level FM device in children with attention defict and suspected central auditory processing disorder. Journal of Educational Audiology. 2005;12:31–36. [Google Scholar]
- Fujioka T, Trainor LJ, Ross B, Kakigi R, Pantev C. Musical training enhances automatic encoding of melodic contour and interval structure. Journal of Cognitive Neuroscience. 2004;16:1010–1021. doi: 10.1162/0898929041502706. [DOI] [PubMed] [Google Scholar]
- Galaburda AM. Neuroanatomic basis of developmental dyslexia. Neurologic Clinics. 1993;11:161–173. [PubMed] [Google Scholar]
- Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. Journal of Neuroscience. 2003;23:9240–9245. doi: 10.1523/JNEUROSCI.23-27-09240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib M. The neurological basis of developmental dyslexia - An overview and working hypothesis. Brain. 2000;123:2373–2399. doi: 10.1093/brain/123.12.2373. [DOI] [PubMed] [Google Scholar]
- Hargus SE, Gordon Salant S. Accuracy of speech-intelligibility index predictions for noise-masked young listeners with normal-hearing and for elderly listeners with hearing impairment. Journal of Speech and Hearing Disorders. 1995;38:234–243. doi: 10.1044/jshr.3801.234. [DOI] [PubMed] [Google Scholar]
- Ho YC, Cheung MC, Chan AS. Music training improves verbal but not visual memory: Cross-sectional and longitudinal explorations in children. Neuropsychology. 2003;17:439–450. doi: 10.1037/0894-4105.17.3.439. [DOI] [PubMed] [Google Scholar]
- Hornickel J, Zecker S, Kraus N. Classroom FM system use and reading disorders: Biological and learning outcomes. 8th Annual Meeting of the Society for the Scientific Study of Reading.2011. [Google Scholar]
- Humes LE, Kewley-Port D, Fogerty D, Kinney D. Measures of hearing threshold and temporal processing across the adult lifespan. Hearing Research. 2010;264:30–40. doi: 10.1016/j.heares.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde KL, Lerch J, Norton A, Forgeard M, Winner E, Evans AC, Schlaug G. Musical training shapes structural brain development. Journal of Neuroscience. 2009;29:3019–3025. doi: 10.1523/JNEUROSCI.5118-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston KN, John AB, Kreisman NV, Hall JW, 3rd, Crandell CC. Multiple benefits of personal FM system use by children with auditory processing disorder (APD) International Journal of Audiology. 2009;48:371–383. doi: 10.1080/14992020802687516. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Killion MC, Niquette PA, Gudmundsen GI, Revit LJ, Banerjee S. Development of a quick speech-in-noise test for measuring signal-to-noise ratio loss in normal-hearing and hearing-impaired listeners. Journal of the Acoustical Society of America. 2004;116:2395–2405. doi: 10.1121/1.1784440. [DOI] [PubMed] [Google Scholar]
- Kraus N, Chandrasekaran B. Music training for the development of auditory skills. Nature Reviews Neuroscience. 2010;11:599–605. doi: 10.1038/nrn2882. [DOI] [PubMed] [Google Scholar]
- Lister JJ, Maxfield ND, Pitt GJ, Gonzalez VB. Auditory evoked response to gaps in noise: older adults. International Journal of Audiology. 2011;50:211–225. doi: 10.3109/14992027.2010.526967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques C, Moreno S, Castro SL, Besson M. Musicians detect pitch violation in a foreign language better than nonmusicians: behavioral and electrophysiological evidence. Journal of Cognitive Neuroscience. 2007;19:1453–1463. doi: 10.1162/jocn.2007.19.9.1453. [DOI] [PubMed] [Google Scholar]
- Moreno S, Bialystok E, Barac R, Schellenberg EG, Cepeda NJ, Chau T. Short-term music training enhances verbal intelligence and executive function. Psychological Science. 2011;22:1425–1433. doi: 10.1177/0956797611416999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Marques C, Santos A, Santos M, Castro SL, Besson M. Musical training influences linguistic abilities in 8-year-old children: more evidence for brain plasticity. Cerebral Cortex. 2009;19:712–723. doi: 10.1093/cercor/bhn120. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Lehtokoski A, Lennes M, Cheour M, Huotilainen M, Iivonen A, Alho K. Language-specific phoneme representations revealed by electric and magnetic brain responses. Nature. 1997;385:432–434. doi: 10.1038/385432a0. [DOI] [PubMed] [Google Scholar]
- Nelken I. Processing of complex sounds in the auditory system. Current Opinion in Neurobiology. 2008;18:413–417. doi: 10.1016/j.conb.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Soli SD, Sullivan JA. Development of the Hearing in Noise Test for the measurement of speech reception thresholds in quiet and in noise. Journal of the Acoustical Society of America. 1994;95:1085–1099. doi: 10.1121/1.408469. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Salami A, Andersson M, Eriksson J, Kalpouzos G, Kauppi K, Nilsson LG. Longitudinal evidence for diminished frontal cortex function in aging. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22682–22686. doi: 10.1073/pnas.1012651108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantev C, Oostenveld R, Engelien A, Ross B, Roberts LE, Hoke M. Increased auditory cortical representation in musicians. Nature. 1998;392:811–814. doi: 10.1038/33918. [DOI] [PubMed] [Google Scholar]
- Parbery-Clark A, Skoe E, Kraus N. Musical experience limits the degradative effects of background noise on the neural processing of sound. Journal of Neuroscience. 2009;29:14100–14107. doi: 10.1523/JNEUROSCI.3256-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parbery-Clark A, Skoe E, Lam C, Kraus N. Musician enhancement for speech-in-noise. Ear and Hearing. 2009;30:653–661. doi: 10.1097/AUD.0b013e3181b412e9. [DOI] [PubMed] [Google Scholar]
- Parbery-Clark A, Strait DL, Anderson S, Hittner E, Kraus N. Musical experience and the aging auditory system: implications for cognitive abilities and hearing speech in noise. PLoS ONE. 2011;6:e18082. doi: 10.1371/journal.pone.0018082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parbery-Clark A, Strait DL, Kraus N. Context-dependent encoding in the auditory brainstem subserves enhanced speech-in-noise perception in musicians. Neuropsychologia. 2011;49:3338–3345. doi: 10.1016/j.neuropsychologia.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AD. Why would musical training benefit the neural encoding of speech? The OPERA hypothesis. Frontiers in Psychology. 2011;2:142. doi: 10.3389/fpsyg.2011.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy SC, Smart JL, Baily M, Sharma M. Do children with reading delay benefit from the use of personal FM systems in the classroom? International Journal of Audiology. 2009;48:843–852. doi: 10.3109/14992020903140910. [DOI] [PubMed] [Google Scholar]
- Rosenberg GG, Blake-Rahter P, Heavner J, Allen L, Redmond BM, Phillips J, Stigers K. Improving classroom acoustics (ICA): a three-year FM sound field classroom amplification study. Journal of Educational Audiology. 1999;7:8–28. [Google Scholar]
- Russo NM, Hornickel J, Nicol T, Zecker S, Kraus N. Biological changes in auditory function following training in children with autism spectrum disorders. Behavioral and Brain Functions. 2010;6:60. doi: 10.1186/1744-9081-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo NM, Skoe E, Trommer B, Nicol T, Zecker S, Bradlow A, Kraus N. Deficient brainstem encoding of pitch in children with autism spectrum disorders. Clinical Neurophysiology. 2008;119:1720–1731. doi: 10.1016/j.clinph.2008.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G. The brain of musicians. A model for functional and structural adaptation. Annals of the New York Academy of Sciences. 2001;930:281–299. [PubMed] [Google Scholar]
- Schneider P, Scherg M, Dosch HG, Specht HJ, Gutschalk A, Rupp A. Morphology of Heschl's gyrus reflects enhanced activation in the auditory cortex of musicians. Nature Neuroscience. 2002;5:688–694. doi: 10.1038/nn871. [DOI] [PubMed] [Google Scholar]
- Schön D, Magne C, Besson M. The music of speech: Music training facilitates pitch processing in both music and language. Psychophysiology. 2004;41:341–349. doi: 10.1111/1469-8986.00172.x. [DOI] [PubMed] [Google Scholar]
- Shahin A, Bosnyak DJ, Trainor LJ, Roberts LE. Enhancement of neuroplastic P2 and N1c auditory evoked potentials in musicians. Journal of Neuroscience. 2003;23:5545–5552. doi: 10.1523/JNEUROSCI.23-13-05545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Menci WE, Fulbright RK, Skudlarski P, Gore JC. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Skoe E, Nicol T, Kraus N. Cross-phaseogram: Objective neural index of speech sound differentiation. Journal of Neuroscience Methods. 2011;196:308–317. doi: 10.1016/j.jneumeth.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Skoe E, Banai K, Kraus N. Training to improve hearing speech in noise: biological mechanisms. Cerebral Cortex. 2011;122:1890–1898. doi: 10.1093/cercor/bhr196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza PE, Boike KT, Witherell K, Tremblay K. Prediction of speech recognition from audibility in older listeners with hearing loss: Effects of age, amplification, and background noise. Journal of the American Academy of Audiology. 2007;18:54–65. doi: 10.3766/jaaa.18.1.5. [DOI] [PubMed] [Google Scholar]
- Strait DL, Chan K, Ashley R, Kraus N. Specialization among the specialized: Auditory brainstem function is tuned in to timbre. Cortex. 2011 doi: 10.1016/j.cortex.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Strait DL, Hornickel J, Kraus N. Subcortical processing of speech regularities underlies reading and music aptitude in children. Behavioral and Brain Functions. 2011;7:44. doi: 10.1186/1744-9081-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga N, Ma XF. Multiparametric corticofugal modulation and plasticity in the auditory system. Nature Reviews Neuroscience. 2003;4:783–794. doi: 10.1038/nrn1222. [DOI] [PubMed] [Google Scholar]
- Trainor LJ, Shahin AJ, Roberts LE. Understanding the benefits of musical training effects on oscillatory brain activity. Neurosciences and Music III: Disorders and Plasticity. 2009;1169:133–142. doi: 10.1111/j.1749-6632.2009.04589.x. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Piskosz M, Souza P. Effects of age and age-related hearing loss on the neural representation of speech cues. Clinical Neurophysiology. 2003;114:1332–1343. doi: 10.1016/s1388-2457(03)00114-7. [DOI] [PubMed] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, Arnsten AF. Neuronal basis of age-related working memory decline. Nature. 2011;476:210–213. doi: 10.1038/nature10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Auditory associative memory and representational plasticity in the primary auditory cortex. Hearing Research. 2007;229:54–68. doi: 10.1016/j.heares.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks R, Hasher L, Li K. Human memory. In: Craik F, Salthouse T, editors. Handbook of aging and cognition. Mahwah NJ: Erlbaum; 2000. pp. 293–358. [Google Scholar]
- Zendel BR, Alain C. Musicians experience less age-related decline in central auditory processing. Psychology and Aging. 2011 doi: 10.1037/a0024816. [DOI] [PubMed] [Google Scholar]


