Abstract
Objective
The mechanisms that contribute to the persistent activation of macrophages in rheumatoid arthritis (RA) are incompletely understood. This study was performed to determine the contribution of endogenous gp96 in TLR-mediated macrophage activation in RA.
Methods
RA synovial fluids (SFs) were employed to activate macrophages and HEK-TLR2 and HEK-TLR4 cells. Neutralizing antibodies to TLR2, TLR4 and gp96 were employed to inhibit activation. RA SF macrophages were isolated by CD14 negative selection. Cell activation was measured by the expression of TNFα or IL-8 mRNA. Arthritis was induced employing the K/BxN serum transfer model, gp96 expression determined by Immunoblot analysis, ELISA and immunohistochemistry. The arthritis was treated with neutralizing anit-gp96 or control serum.
Results
RA SF induced the activation of macrophages and HEK-TLR2 and HEK-TLR4 cells. RA SF-induced macrophage and HEK-TLR2 activation was suppressed by neutralizing anti-gp96 antibody only when high (>800 ng/ml), but not low (<400 ng/ml), concentrations of gp96 were present. Neutralization of RA SF macrophage cell surface gp96 inhibited the constitutive expression of TNFα. Supporting its role in RA, joint tissue gp96 expression was induced in the K/BxN serum transfer model of RA, and neutralizing antibodies to gp96, ameliorated joint inflammation on clinical and histologic examination.
Conclusions
These observations support the role of gp96 as an endogenous TLR2 ligand in RA and identify the TLR2 pathway as a therapeutic target.
INTRODUCTION
Rheumatoid Arthritis (RA) is a chronic inflammatory disease that, if not successfully treated, leads to cartilage and bone destruction (1–3). Recent observations suggest that RA is initiated in genetically predisposed individuals who possess HLA-DRβ1 alleles that contain the shared epitope following environmental exposure, such as cigarette smoke or periodontal disease (4–6). The environmental exposure results in protein citrullination and these modified proteins are selectively presented by shared epitope positive antigen presenting cells, resulting in anti-citrullinated peptide antibodies (ACPA), which are characteristic of RA (3, 5). Recent studies have demonstrated that immune complexes containing ACPA are capable of inducing inflammation, by activating macrophages through cell surface Fc receptors (7, 8).
Once inflammation is initiated, a number of regulatory and structural molecules are up regulated locally within the joint (9). Accumulating data suggests that some of these molecules may contribute to the persistence and destruction observed in RA by serving as endogenous Toll Like Receptor (TLR) ligands (9). However, a functional candidate has not been identified directly from RA synovial fluid (SF). TLRs include cell surface (eg TLR2 and TLR4) and endosomal (eg TLR3, 7, and 9) receptors, originally identified in mammals for their ability to bind microbial ligands. TLR ligation results in the activation of transcription factors such as NF-κB, JNK, ERK and p38, which promote the expression of proinflammatory chemokines, cytokines, and matrix metalloproteinases (10, 11). Prior studies have demonstrated the increased expression of TLR2 and TLR4 by RA synovial macrophages and an increased response to TLR2 or TLR4 microbial ligands (12).
However, the contribution of endogenous SF ligands to TLR2 or TLR4 activation has not been directly shown, although a number of potential endogenous TLR ligands have been identified in the joints of patients with RA, including heat shock protein (HSP) 60, HSP70, high mobility group box 1 protein (HMGB), tenacin C, and fibrinogen (13–18). However, none of these potential TLR ligands present in RA SFs has been shown to bind and activate through the TLR signaling pathway. While recombinant HSP60 and HSP70 activated TLR4 (13, 17), subsequent studies employing ultrapure recombinant proteins failed to detect TLR4 activation (19, 20), underscoring the risk of microbial TLR ligand contamination when employing recombinant proteins expressed in E.coli as TLR agonists, further supporting the importance of employing SFs.
We recently demonstrated that the endoplasmic reticulum associated stress response protein gp96 (gp96) is highly expressed in the synovial tissue and fluids of patients with RA (21). Both macrophage-expressed and recombinant N-terminal domain of gp96 (gp96-NTD) were capable of binding to TLR2 in pull-down experiments. Further, highly purified gp96-NTD activated macrophages mediated through TLR2, and induced the expression of TLR2, TNFα, and IL-8 by RA SF macrophages. However, no prior studies have demonstrated the ability of a specific potential endogenous TLR ligands present in RA SF to activate macrophages and HEK293 cells through TLR2 or TLR4.
In the current study, we demonstrate that elevated gp96 levels present in RA SFs promote TLR2-dependent macrophage activation. We further show that gp96 is also increased in an experimental mouse model of RA and that neutralizing gp96 in vivo ameliorates the arthritis. These observations identify gp96 as a clinically relevant endogenous TLR2 ligand in RA and suggest that the TLR signaling pathway is a viable target in RA.
MATERIALS AND METHODS
Patients and specimens
SFs were obtained from the inflamed joints of 12 patients with RA, diagnosed according to the American College of Rheumatology classification criteria (22). The SFs were obtained during routine clinical care, as part of ongoing treatment for an arthritis flare. Ten of the 12 patients were woman, the mean age was 59 (30–85) years, and the disease duration was 13 (0.5–25) years at the time the SF was obtained. Rheumatoid factor was tested on 7 patients and 2 were positive, while anti-CCP was examined on only 2 patients, and 1 was positive. The medications included prednisone ≤ 10 mg per day in 6 patients, methotrexate alone in 1, methotrexate plus plaquenil in 1, methotrexate plus a TNF inhibitor in 5 (plus sulfasalazine in 1), leflunomide alone in 1, and 2 patients were on no medication at the time of the SF aspiration. Two patients had stopped methotrexate or methotrexate plus a TNF inhibitor 2 or 4 months prior to the joint aspiration. The mean swollen joint count was 2.9 when the SF was obtained.
SFs were first centrifuged at 800g for 10 min to obtain the cells and cell-free SF. The SF cells were then fractionated by Histopaque-1077 density gradient to collect mononuclear cells followed by further purification to obtain CD14+ macrophages by negative selection (StemCell Technologies, Vancouver, Canada) as described (21). Peripheral blood mononuclear cells and monocytes from normal controls and patients with RA were isolated by the same procedures. Primary human macrophages (control macrophages) were obtained from normal peripheral blood monocytes, isolated by elutriation, followed by in vitro differentiation for 7 days, as previously described (12, 23–28). All patients were recruited from the Northwestern Medical Faculty Foundation or the Rehabilitation Institute of Chicago after obtaining informed consent. These studies have been reviewed and approved by the Northwestern Institutional Review Board.
Cell activation and detection
Macrophages were incubated with 25 % RA SF in RPMI-1640 for 4 hours. To identify the presence of TLR2 or TLR4 ligands in the SFs, rat monoclonal anti-TLR2 or anti-TLR4 antibodies (InvivoGen) or control rat IgG (10 µg/ml) were incubated with macrophages for 30 minutes prior to the addition of the SFs. To determine if gp96 in the SFs contributed to the macrophage activation, the SFs were pre-incubated with 1:50 control rabbit serum or neutralizing rabbit anti-gp96 antiserum (21) for 30 minutes prior to the addition to macrophages. Macrophage activation was determined by TNFα and IL-8 mRNA expression employing qRT-PCR (21). Known microbial TLR2 and TLR4 ligands were employed as positive controls in each experiment (data not shown).
HEK-2 or HEK-4 cells were incubated with 50% SF in DMEM selection medium for 20 hours. To identify gp96 mediated activation, the SFs were pre-incubated with 1:50 control rabbit serum or rabbit anti-gp96 antiserum for 30 minutes prior to incubation with HEK cells. Activation of HEK-TLR2 or HEK-TLR4 cells was determined by qRT-PCR for IL-8. Microbial TLR ligands were employed as positive controls in each experiment (data not shown).
Cell surface gp96
Cell surface gp96 was examined by two-color flow cytometry (12). Mononuclear cells were isolated from peripheral blood of healthy controls and patients with RA, and from RA SF. Monocytes and macrophages were identified by FITC-labeled anti-CD14. Cell surface gp96 was detected with a rat anti-gp96 monoclonal antibody (Lab Vision, Fremont CA) or an isoform matched rat IgG control followed by PE-labeled anti-rat IgG. Data was acquired on a BD LSR II flow cytometer (BD FACSDIVA software) and analyzed by Flowjo (TreeStar, Inc.). The level of macrophage surface gp96 expression was determined as the mean fluorescence intensity (MFI) of PE on the CD14+ or CD14− cell population (12).
K/BxN serum transfer arthritis model
K/BxN mice were generated and anti-GPI serum collected at 8–9 week of age as described (29, 30). Arthritis was induced by anti-GPI serum injected intraperitoneally 150 µl on days 0 and 2 or 100 µl on day 0 only. The development of arthritis was assessed by measuring the hind ankle thickness by a caliper and by grading the clinical index of all 4 ankles/paws on a scale of 0–3/each ankle/paw (maximum =12) as described (29, 30). At the time of harvesting, ankles were quickly dissected and skin removed, stored either in 10% neutral buffered formalin for histologic analysis or at −80°C for ELISA or Immunoblot analysis.
Histopathology
After 10% neutral buffered formalin, the ankles were incubated in EDTA/formalin decalcification buffer for 2 weeks, embedded in paraffin, and then 4 µm sections were stained with hematoxylin and eosin. Ankle sections were evaluated by a blinded pathologist, scoring 0–5 for joint and extra articular inflammation, pannus formation, bone erosion, median synovial line thickness, and cartilage destruction as previously described (29, 30). Photographs were obtained by an Olympus BX41 microscope (Olympus, Tokyo, Japan) and a DP2-BSW camera.
Detection of gp96 and IL-1β
Ankles were homogenized in PBS supplemented with a protease inhibitor cocktail (Sigma). Supernatants were collected by centrifugation 12,000× g for 5 min at 4 °C and protein concentration determined employing BCA protein assay reagents (Thermo scientific, Rockford IL). Gp96 concentrations was quantified by ELISA, as previously described (21), modified to recognize mouse gp96 by replacing the capture antibody with 100 µl of rat monoclonal anti-gp96 antibody (Lab Vision, Fremont, CA) at 2 µg/ml. The IL-1β in the same ankle homogenate was quantified by ELISA (DuoSets, R&D, Minneapolis, MN). The concentrations of gp96 (ng/ml) and IL-1β (pg/ml) were adjusted to mg of the total ankle protein. Gp96 in RA SF was detected by immunoprecipitation with a protein G sepharose-immobilized polyclonal rabbit anti-gp96 antiserum or control rabbit serum, followed by Immunoblot analysis with a goat anti-gp96 antibody, as described (21). Gp96 expression in murine ankle joints was also detected by immunohistochemistry (21).
Statistical Analysis
One way ANOVA followed by the Tukey-Kramer test was employed for comparison among multiple groups. The two-sided t-test was employed to analyze differences between two groups. For samples that failed the normality test, Mann-Whitney rank test was performed. Correlations were determined by Spearman’s non-parametric correlation since the data analyzed had a non-Gaussian distribution. Significance levels were set at 0.05.
RESULTS
Rheumatoid arthritis SF activates macrophages through TLR2 and TLR4
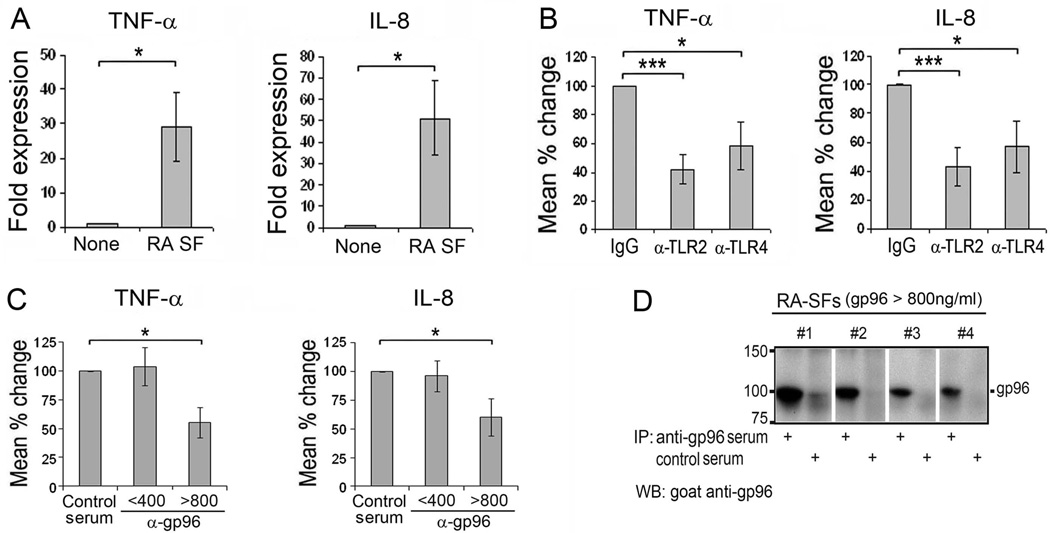
We previously identified gp96 as a potential endogenous TLR2 ligand present in RA SF and tissue (21). Therefore, primary human macrophages were employed to directly determine if RA SFs are capable of activating macrophages through TLR2 or TLR4, and if gp96 present in the SFs is responsible for TLR activation. We screened RA SFs containing >100 ng/ml of gp96 for their ability to activate macrophages, defined by a ≥2 fold induction of TNFα and IL-8, assessed by qRT-PCR (Figure 1A). Activation of macrophages by the RA SFs was suppressed following incubation with neutralizing antibodies to TLR2 or TLR4, demonstrating that triggering through these TLR receptors contributes to macrophage activation (Figure 1B).
Figure 1. Macrophage activation by RA SFs is suppressed by neutralizing antibodies to TLR2, TLR4 and gp96.
(A). Macrophages were incubated with RA SF (SF), selected for their ability to activate macrophages, diluted 1:4 in RPMI-1640 medium plus IgG control antibody for 4 hours. The mRNA expression of TNFα and IL-8 was determined employing qRT-PCR, presented as fold expression compared to medium alone (None). (B). Suppression of macrophage activation by neutralizing antibodies to TLR2 or TLR4 (10 µg/ml) is presented as the percentage of TNFα and IL-8 in the presence of anti-TLR antibodies compared to IgG controls (100%). n=7 RA SFs. (C). RA SFs were pre-incubated with control rabbit serum or rabbit anti-gp96 antiserum for 30 minutes prior to incubation with macrophages. The suppression of TNFα and IL-8 was presented as the percentage in the presence of anti-gp96 compared to control rabbit serum (100%). The fluids were grouped according to the gp96 concentration determined by ELISA as low gp96 (< 400 ng/ml) (mean 136±49, n=7) or high gp96 (> 800 ng/ml) (mean 1350±256, n=4). All experiments were repeated >2 times, and the mean of the results was employed for analysis. All values present are mean ± SEM. * represents p < 0.05 and *** p< 0.001 between the indicated groups. (D). Gp96 from the 4 RA SFs with >800ng/ml gp96 were immunoprecipitated by anti-gp96 antiserum or control rabbit serum. The immunoprecipitates were analyzed employing Immunoblot analysis probing with a second anti-gp96 antibody.
We next determined whether gp96 in the RA SFs contributed to macrophage activation. We demonstrated earlier that recombinant gp96-NTD was capable of activating TLR2 at 1 µg/ml, and that the mean level of gp96 in RA SFs was 817 ng/ml (21) . Therefore the SFs were divided into those with high (>800 ng/ml) or low (<400 ng/ml) gp96. SFs were pre-incubated with neutralizing anti-gp96 or control rabbit sera prior to incubation with human macrophages. While there was no difference in macrophage activation by the fluids with high or low concentrations of gp96, neutralizing anti-gp96 antibodies only suppressed the induction of TNFα and IL-8 (Figure 1C) by the RA SFs that contained high levels of gp96, but not those with the concentrations <400 ng/ml. We confirmed the specific interaction between the anti-gp96 antibodies and gp96 in the SFs by immunoprecipitation (Figure 1D). These observations demonstrate that RA SFs activate macrophages through TLR2 or TLR4, and by gp96 when present >800 ng/ml, however, they do not discern whether or not the activation by gp96 is mediated through TLR2 or TLR4.
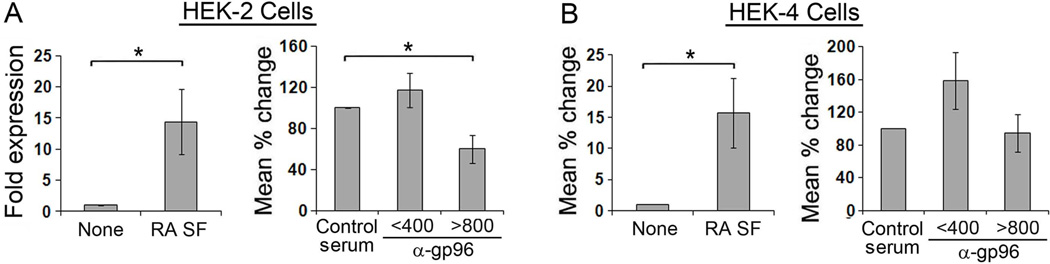
To directly determine if gp96 present in the SFs activates through TLR2 or TLR4, we incubated SFs containing >800 or <400 ng/ml of gp96 with HEK-TLR2 and HEK-TLR4 cell lines (Figure 2). There was no difference in the intensity of TLR2 or TLR4 activation by the SFs that contained high or low concentrations of gp96 (15.3±10.3 fold vs. 13.3±4.1 for HEK-TLR2 and 16.4±9.0 vs.17.8±7.2 for HEK-TLR4). Nevertheless, activation of the HEK-TLR2 cells induced by the SFs containing high, but not low, concentrations of gp96 was significantly suppressed by the neutralizing anti-gp96 antiserum compared to control rabbit serum (Figure 2A). In contrast, the anti-gp96 did not suppress activation of HEK-TLR4 cells by RA SF (Figure 2B), indicating that gp96 present in RA SF activates through TLR2 but not TLR4 or an alternative mechanism.
Figure 2. HEK-TLR2 activation by RA SF is suppressed by anti-gp96.
HEK-TLR2 or HEK -TLR4 cells were incubated with RA SF diluted 1:2 for 20 hours and activation was determined by the expression of IL-8 employing qRT-PCR (left, panels A, B). To determine the ability of gp96 to activated the HEK cells, SFs with gp96 <400 and > 800 ng/ml were pre-incubated with control rabbit serum or rabbit anti-gp96 antiserum for 30 min prior to incubation with HEK-TLR2 or HEK-TLR4 cells (right, panels A, B). The percentage of inhibition (right panel) by anti-gp96 was determined by comparing with the normal rabbit serum control (100%). All values represent the mean ± SEM from 2–3 repeated experiments for each individual fluid. * p represents < 0.05.
Gp96 present on the cell surface of RA SF macrophages promotes macrophage activation
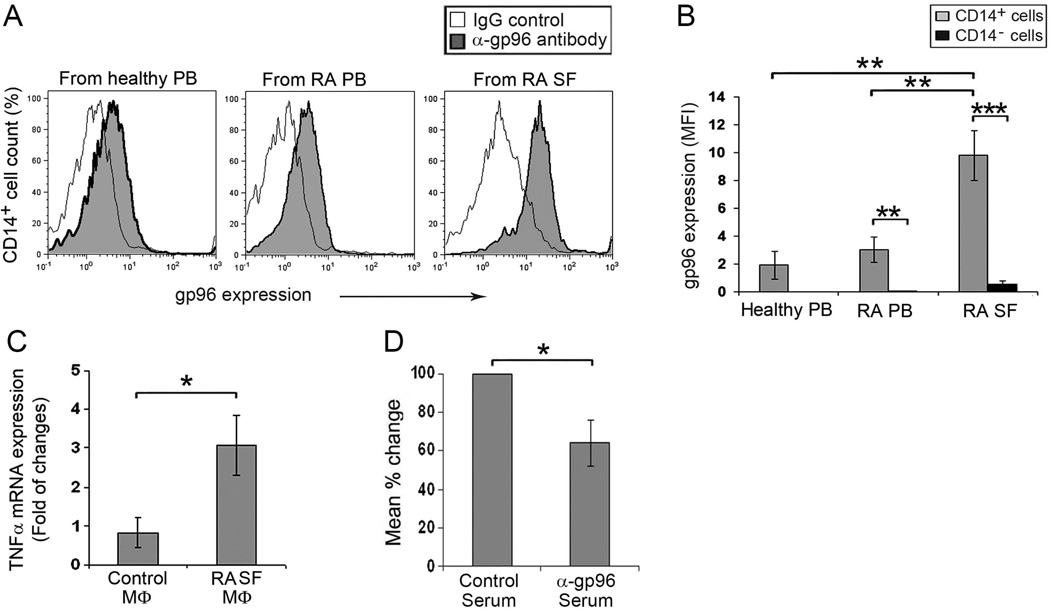
Gp96 is an endoplasmic reticulum resident protein in homeostatic situations, and our prior studies demonstrated that macrophages are one of the sources of gp96 (21). Therefore, we examined macrophages from RA SF for cell surface expressed gp96 by flow cytometry. Gp96 is present on normal and RA peripheral blood CD14+ monocytes, as well as RA SF macrophages (Figure 3A). Quantitative analysis of mean fluorescence intensity demonstrates that cell surface gp96 is significantly greater on RA SF macrophages compared with RA or control monocytes, while it was very low on CD14-negative mononuclear cells regardless of the source (Figure 3B).
Figure 3. Blocking cell surface gp96 on RA SF macrophages suppresses cell activation.
(A). Representative flow cytometry histograms examining gp96 on the cell surface of mononuclear cells isolated from peripheral blood (PB) of healthy controls and patients with RA, and RA SF s (SF). Cells were incubated with a rat anti-gp96 monoclonal antibody or a rat monoclonal IgG control followed by PE-labeled anti-rat IgG. Monocytes/macrophages were defined employing FITC-labeled anti-CD14 (B). The intensity of gp96 expression (MFI) on the surface of CD14+ or CD14− mononuclear cells from peripheral blood of healthy controls (n = 7), patients with RA (n=10), and RA SF macrophages (MФs) (n = 9). The values represent the mean ± SEM. (C). CD14+ macrophages isolated from RA SF are spontaneously activated compared to control in vitro differentiated macrophage (n=4). Cell activation was determined by fold induction of TNFα mRNA by qRT-PCR, compared to control macrophages. (D). The activation of CD14+ macrophages from RA SF was suppressed by pre-incubation of cells with neutralizing anti-gp96 antiserum (n=4), as determined by qRT-PCR compared to cells incubated with control normal rabbit serum (100%). * represents p < 0.05, ** p < 0.01 and *** p < 0.001.
We previously demonstrated by flow cytometry that TNFα is constitutively expressed by RA SF macrophages, but it was negligible in control in vitro differentiated macrophages (12). Consistent with these observations, the constitutive expression of TNFα which was 3 fold higher in the RA SF macrophages compared with the control in vitro differentiated macrophages, determined by qRT-PCR (Figure 3C). To determine if cell surface expressed gp96 contributed in the constitutive expression of TNFα, we pre-incubated RA SF macrophages with control or neutralizing anti-gp96 serum prior to incubation. The anti-gp96 antibodies suppressed the constitutive expression of TNFα by RA SF macrophages, compared to the control serum (Figure 3D) (12). These observations suggest that cell surface gp96 on RA SF macrophages is capable of promoting macrophage activation within the RA joint.
Synergistic RA SF macrophage activation
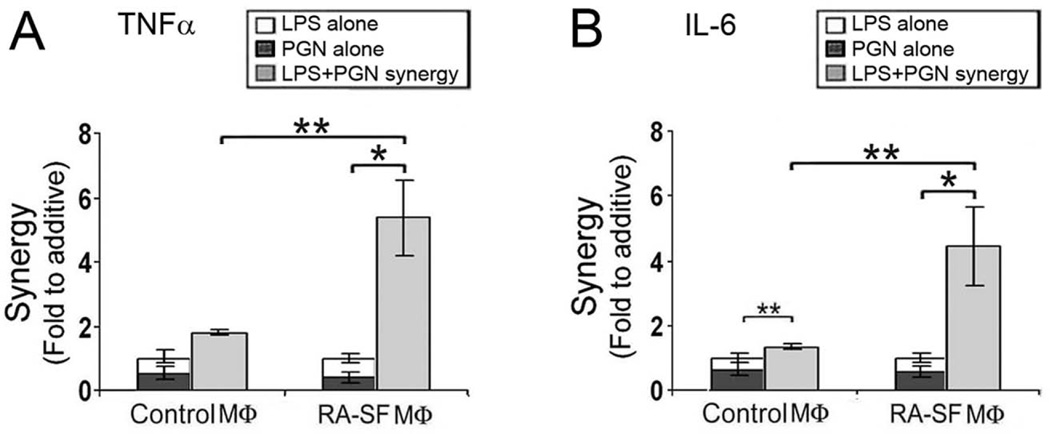
Our data suggest that endogenous TLR2 and TLR4 ligands are present in RA SFs. We demonstrated earlier that RA SF macrophages exhibit an enhanced response to microbial TLR2 or TLR4 ligands compared to control macrophages or those from the joints of patients with psoriatic arthritis or ankylosing spondylitis (12). We therefore investigated the potential synergistic activation of control and RA SF macrophages utilizing suboptimal concentrations of the TLR2 ligand peptidoglycan (PGN) and the TLR4 ligand lipopolysaccharide (LPS). The induction of IL-6 was slightly greater than additive, and not significantly different for TNFα employing the control in vitro differentiated macrophages (Figure 4). In contrast, the combination of suboptimal PGN and LPS synergistically activates RA SF macrophages as demonstrated by the induction of TNFα and IL-6, which were significantly greater than observed for control macrophages (Figure 4). These observations suggest that relatively low levels of endogenous TLR2 and TLR4 ligands present in the RA joint, including gp96, may synergize to promote local macrophage activation.
Figure 4. Synergistic activation of RA SF macrophages by TLR2 and TLR4 activation.
In vitro differentiated control macrophages or CD14+ macrophages isolated from RA SFs were incubated with a suboptimal concentration of LPS (0.1ng/ml) or PGN (0.2 µg/ml) individually or in combination for 4 hours. The TNFα (panel A) and IL-6 (panel B) in culture medium was determined by ELISA. n= 5 control and 4 RA SF macrophages (mean ± SEM). The synergistic effect is presented as fold increase of the combination compared to the sum of the response to the individual ligands. The values represent the mean ± SEM. * represents p < 0.05 and ** p< 0.01 between the indicated groups.
Gp96 correlates with disease activity in anti-GPI induced arthritis
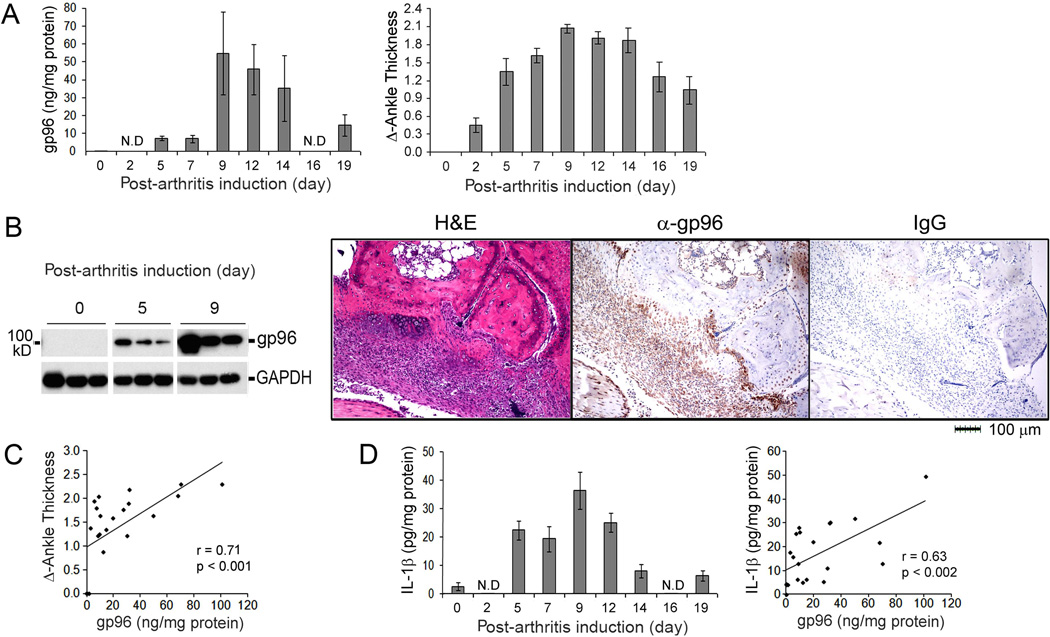
To further elucidate the role of gp96 in disease pathogenesis, we examined the expression of gp96 in the joints of mice following the induction of K/BxN anti-GPI serum transfer-induced arthritis. All the mice injected with anti-GPI serum developed arthritis. While gp96 was not detected by ELISA prior to the induction of arthritis, it was minimally detected on days 5 and 7, as the arthritis was increasing as determined by ankle thickness, and highly expressed between days 9 and 14, the peak of the arthritis, decreasing by day 19 (Figure 5A). The expression of gp96 in the joints was confirmed by immunoblot analysis and immunohistochemistry (Figure 5B), and the expression of gp96 in the joints was highly correlated (r=0.72, p<0.001) with the clinical arthritis (Figure 5C). Further, the anti-GPI serum transfer arthritis is dependent on the expression of IL-1β, which was increased in the inflamed joints and correlated (r=0.63, p<0.002) with the expression of gp96 (Figure 5D). These observations demonstrate that the local expression of gp96 is highly correlated with inflammation documented by clinical exam, and with the presence of IL-1β.
Figure 5. Gp96 expression in the inflamed ankle of anti-GPI induced arthritis correlates with disease activity.
(A) The serum transfer model of arthritis was induced by intraperitoneal injection of anti-GPI serum (150 µl) on days 0 and 2 and joint swelling measured over time as ankle thickness. Nineteen mice were injected with anti-GPI serum, and 3 mice were sacrificed at each time point, except day 19 when 4 mice were sacrificed. One ankle from each mouse was collected at the indicated time points and homogenized to determine the concentration of gp96 by ELISA and by immunoblot analysis (B). The other ankle was employed for immunohistochemistry employing anti-gp96 antibody or control IgG (B). Serial sections were also stained with hematoxylin and eosin (H&E). (C) The concentration of gp96 correlated with ankle thickness. (D) The concentration of IL-1β determined by ELISA was increased and correlated with the expression of gp96. The values in panels A, C and D represent the mean ± SEM. N.D, not determined.
Neutralizing anti-gp96 antibody ameliorates the progression of anti-GPI induced arthritis
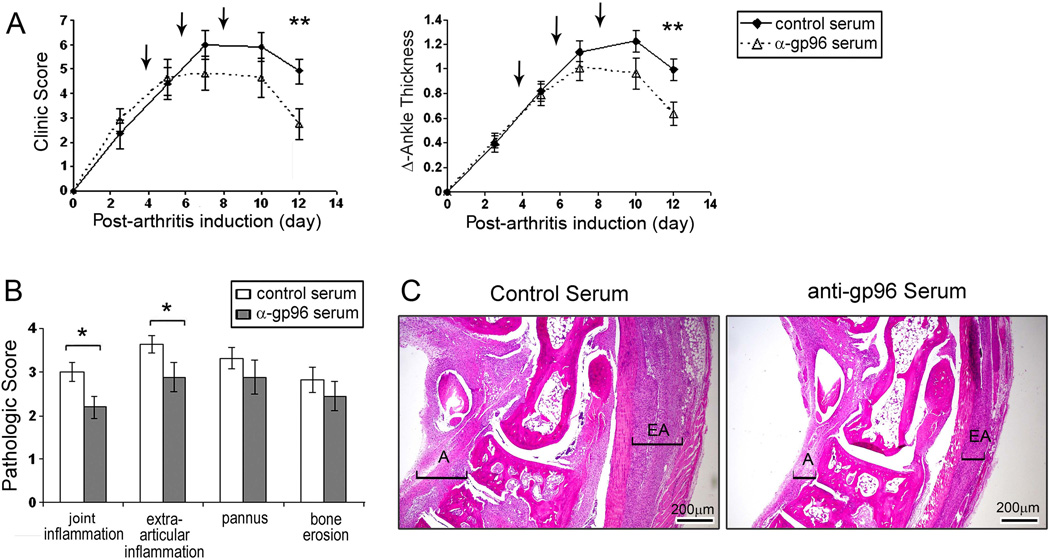
To directly determine the potential role of gp96 in arthritis progression, we investigated whether neutralizing anti-gp96 can ameliorate the disease. We treated mice with neutralizing anti-gp96 or control serum on days 4, 6 and 8. All the mice developed arthritis. Both the clinical score and the ankle thickness were significantly reduced (p<0.01) in mice treated with the anti-gp96 antibodies, compared with mice treated with the control serum. Improvement was noticeable within four days of gp96 neutralization, and lasted throughout the course of the disease (Figure 6A). Histologic examination of the joints obtained on day 12 demonstrated a significant (p<0.05) reduction of joint inflammation and extra-articular inflammation in mice treated with the anti-gp96 antibodies (Figure 6B, C). However, as expected, there was no reduction in pannus formation or bone erosion examined on day 12, since the joint damage cannot be repaired during this short time period. These observations support the role of gp96 in the progression and persistence of the arthritis observed in the anti-GPI serum transfer model of RA.
Figure 6. Neutralizing anti-gp96 antiserum ameliorates the progression of anti-GPI induced arthritis.
(A). The serum transfer model of arthritis was induced with a single intraperitoneal injection of 100 µl anti-GPI serum and the course of the arthritis was determined by the clinical activity score (maximum 12) and the swelling of the 2 hind ankles (ankle thickness). The arrows indicate the time of intraperitoneal treatment with either the control or the anti-gp96 antiserum. There were 12 mice in each group and the results represent the mean of two independent experiments. (B) Pathologic analysis of inflammation, pannus, and bone erosion on ankles collected at day12 post arthritis in 8 mice. The values represent the mean ± SEM. (C) Representative H&E staining sections from mice that received control rabbit serum or anti-gp96 antiserum, harvested at day 12 post-arthritis induction. The brackets identify articular (A) inflammation and extra-articular (EA) inflammation. * represents p<0.05 and ** p<0.01 between the two groups.
DISCUSSION
Our study documents for the first time that gp96, when present in RA SFs at concentrations > 800 ng/ml, is an endogenous TLR2 ligand, capable of activating macrophages. The activation of macrophages by RA SFs was inhibited by neutralizing antibodies to TLR2 and TLR4, as well as by neutralizing antibodies to gp96. Additionally, neutralizing antibodies to gp96 suppressed the RA SF induced activation of HEK-TLR2 cells, but not HEK-TLR4 cells, demonstrating specificity for TLR2. Further supporting the relevance of these observations, neutralization of cell surface gp96 on RA SF macrophages suppressed the constitutive expression of TNFα. Importantly, the gp96 that is present in the RA SFs was free of the potential endotoxin contamination that might affect the results when recombinant proteins are utilized, directly demonstrating the pathogenic potential of this endogenous TLR ligand in RA.
We previously demonstrated that gp96 was detected in RA SFs with a mean concentration of 817 ± 362 ng/ml (21). SFs from patients with other forms of inflammatory arthritis including psoriatic arthritis and ankylosing spondylitis demonstrated significantly less gp96 (mean 206±46 ng/ml), while gp96 was even lower in osteoarthritis SFs (mean 71±20). Therefore, although the gp96 is present in the SFs of patients with a variety of forms of arthritis, only RA SFs possessed concentrations capable of activating TLR2. However, it is possible that within the synovial tissue, where gp96 is being released to the extracellular space, the concentrations are sufficient to activate TLR2 in diseases other than RA. Our studies demonstrate that RA SF macrophages are significantly more responsive to microbial TLR2 and TLR4 ligands compared to macrophages isolated from the joints of patients with other forms of inflammatory arthritis or control peripheral blood monocytes or in vitro differentiated macrophages (12). Expanding on these observations, the ability of microbial TLR2 and TLR4 ligands to synergistically activate RA SF macrophages was significantly greater than control macrophages. This increased sensitivity may be due to decreased production of IL-10 or to the increased expression of interferon-γ by RA SF macrophages (31, 32). Together these observations suggest that RA macrophages present at the site of joint inflammation may respond to concentrations of endogenous TLR ligands even lower than those documented in this study.
The activation of macrophages by RA SF was suppressed by neutralizing TLR2 and TLR4 antibodies and both HEK-TLR2 and -TLR4 cells were activated by RA SFs, suggesting that endogenous TLR2 and TLR4 ligands are both present in RA SF. While other factors such as cytokines might contribute to HEK-TLR activation, the suppression of the RA SF-mediated activation of HEK-TLR2, but not HEK-TLR4, cells by neutralizing anti-gp96 antibodies, demonstrates that gp96 in the RA SFs was activating through TLR2. The observation that functional TLR2 and TLR4 ligands are present in the RA joint is supported by earlier studies. Others have shown that RA SF is capable of activating HEK-TLR4 cells and that neutralizing antibodies to TLR2 or to TLR4 suppressed the constitutive expression of proinflammatory TNFα expressed by RA synovial tissue culture explants (33, 34). Further supporting the role of gp96 in RA, neutralizing anti-g96 antibodies suppressed the constitutive expression of TNFα by isolated RA SF macrophages. We are aware of no data identifying gp96 as binding to other endogenous TLR ligands or cell-expressed molecules such as myeloid differentiation protein 2 in the process of activating through TLR2. Together these studies document the ability of endogenous TLR ligands, in particular gp96, to promote inflammation in RA.
The mechanism by which gp96 is released from the endoplasmic reticulum in RA is not clear. Cell surface gp96 has been described on a variety of tumors (35). Further, photodynamic therapy induced the expression of cell surface gp96, which was capable of inducing the expression of TNFα by macrophages (36). Under homeostatic conditions gp96 binds to KDEL receptors in the golgi and is returned to the endoplasmic reticulum. Aminoacyl-tRNA synthetase-interacting multifunctional protein 1 (AIMP1) promotes the retention of gp96 in the endoplasmic reticulum and AIMP-1 deficient cells demonstrate increased cell surface gp96 (37). Recently, TLR4-mediated activation of a macrophage cell line resulted in increased cell surface gp96 expression which was mediated by JNK-induced phosphorylation of AMP1 which resulted in disruption of the interaction between gp96 and AIMP1 (38). However, gp96 has been shown to bind to monocytes (39), and it is possible that the cell surface gp96 present on RA SF macrophages may be secondary to released gp96 bound to scavenger receptor class-A (40, 41) or to TLR2 itself. Although gp96 is released from necrotic cells (42), necrosis is not a common feature in RA. Therefore, the mechanism responsible for the release of gp96 to the cell surface and into the extracellular space and the SF remains to be determined.
Supporting its role in RA, gp96 was very low by ELISA or immunoblot analysis prior to the induction of experimental arthritis. Gp96 was weakly expressed early in the clinical course and by the time of maximal inflammation gp96 was highly expressed, and its expression strongly correlated with joint swelling on clinical exam and IL-1β. Consistent with the relevance of gp96 in the pathogenesis of joint inflammation, neutralizing antibodies to gp96 resulted in amelioration of arthritis and a decrease of joint and periarticular inflammation. Although the anti-gp96 was injected beginning on day 4, a significant clinical difference was not observed until day 12. This is most likely due to the fact that a marked increase of gp96 was not observed until day 9. Further no significant reduction of bone erosion was noted, most likely due to the rapid clinical course employing this model, in which erosions are already observed early in the disease (43). These observations support the role of gp96 in promoting chronic inflammation.
Our data suggest that TLR2 or gp96 may be a therapeutic target in RA. However, a concern for neutralizing TLR2 is the increased joint inflammation observed in the IL-1Ra−/−, TLR2−/− mice compared to the IL-1Ra−/−, TLR2+/− mice (33). In contrast, a neutralizing anti-TLR2 antibody suppressed zymosan-induced arthritis (44) and suppressed myocardial ischemia/reperfusion injury and inflammation in an experimental stroke model (45, 46). Additionally, neutralizing antibodies to TLR2 suppressed the activation of macrophages by RA SFs and the constitutive expression of TNFα by RA synovial tissue explants (34). Further supporting the role of endogenous TLR ligands in joint destruction, TLR2 is strongly expressed in the RA pannus, as it erodes into bone (47). Consistent with the role of TLR2 in disease progression, our unpublished observations demonstrate increased inflammation and joint destruction when low doses of microbial TLR2 ligands are injected into the ankles of mice with anti-GPI induced arthritis (data not shown). Together these observations suggest that inflammation promotes a positive feedback loop in RA by the induction of endogenous TLR ligands, which are released or expressed on the cell surface, promoting progressive, ongoing TLR-mediated inflammation resulting in further joint destruction and damage, identifying the TLR signaling pathway as a potential target in RA.
Acknowledgments
Support: Within Our Reach Grant from the American College of Rheumatology and NIH grant AR055240(RMP), AR050250 (HP), AR054796 (HP), AI092490 (HP), HL108795 (HP), AI082406 (CS), GM071723 (CS), and AR057532 (AD)
Footnotes
Conflict of Interest: No support from commercial sources, no conflict of interest
REFERENCES
- 1.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 2.Pope RM. Apoptosis as a therapeutic tool in rheumatoid arthritis. Nat Rev Immunol. 2002;2:527–535. doi: 10.1038/nri846. [DOI] [PubMed] [Google Scholar]
- 3.Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet. 2009;373:659–672. doi: 10.1016/S0140-6736(09)60008-8. [DOI] [PubMed] [Google Scholar]
- 4.Bowes J, Barton A. Recent advances in the genetics of RA susceptibility. Rheumatology (Oxford) 2008;47:399–402. doi: 10.1093/rheumatology/ken005. [DOI] [PubMed] [Google Scholar]
- 5.Lundstrom E, Kallberg H, Alfredsson L, Klareskog L, Padyukov L. Gene-environment interaction between the DRB1 shared epitope and smoking in the risk of anti-citrullinated protein antibody-positive rheumatoid arthritis: all alleles are important. Arthritis Rheum. 2009;60:1597–1603. doi: 10.1002/art.24572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikuls TR, Sayles H, Yu F, Levan T, Gould KA, Thiele GM, et al. Associations of cigarette smoking with rheumatoid arthritis in African Americans. Arthritis Rheum. 2010;62:3560–3568. doi: 10.1002/art.27716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clavel C, Nogueira L, Laurent L, Iobagiu C, Vincent C, Sebbag M, et al. Induction of macrophage secretion of tumor necrosis factor alpha through Fcgamma receptor IIa engagement by rheumatoid arthritis-specific autoantibodies to citrullinated proteins complexed with fibrinogen. Arthritis Rheum. 2008;58:678–688. doi: 10.1002/art.23284. [DOI] [PubMed] [Google Scholar]
- 8.Sokolove J, Zhao X, Chandra PE, Robinson WH. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcgamma receptor. Arthritis Rheum. 2011;63:53–62. doi: 10.1002/art.30081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang QQ, Pope RM. The role of toll-like receptors in rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:357–364. doi: 10.1007/s11926-009-0051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muzio M, Polentarutti N, Bosisio D, Prahladan MK, Mantovani A. Toll-like receptors: a growing family of immune receptors that are differentially expressed and regulated by different leukocytes. J Leukoc Biol. 2000;67:450–456. doi: 10.1002/jlb.67.4.450. [DOI] [PubMed] [Google Scholar]
- 11.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 12.Huang Q, Ma Y, Adebayo A, Pope RM. Increased macrophage activation mediated through toll-like receptors in rheumatoid arthritis. Arthritis Rheum. 2007;56:2192–2201. doi: 10.1002/art.22707. [DOI] [PubMed] [Google Scholar]
- 13.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 14.Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15:774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 15.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, et al. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 16.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 17.Vabulas RM, Ahmad-Nejad P, da Costa C, Miethke T, Kirschning CJ, Hacker H, et al. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276:31332–31339. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- 18.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao B, Tsan MF. Recombinant human heat shock protein 60 does not induce the release of tumor necrosis factor alpha from murine macrophages. J Biol Chem. 2003;278:22523–22529. doi: 10.1074/jbc.M303161200. [DOI] [PubMed] [Google Scholar]
- 20.Gao B, Tsan MF. Endotoxin contamination in recombinant human heat shock protein 70 (Hsp70) preparation is responsible for the induction of tumor necrosis factor alpha release by murine macrophages. J Biol Chem. 2003;278:174–179. doi: 10.1074/jbc.M208742200. [DOI] [PubMed] [Google Scholar]
- 21.Huang QQ, Sobkoviak R, Jockheck-Clark AR, Shi B, Mandelin AM, 2nd, Tak PP, et al. Heat shock protein 96 is elevated in rheumatoid arthritis and activates macrophages primarily via TLR2 signaling. J Immunol. 2009;182:4965–4973. doi: 10.4049/jimmunol.0801563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Eksarko P, Temkin V, Haines GK, 3rd, Perlman H, Koch AE, et al. Mcl-1 is essential for the survival of synovial fibroblasts in rheumatoid arthritis. J Immunol. 2005;175:8337–8345. doi: 10.4049/jimmunol.175.12.8337. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Ma Y, Cole SM, Zander C, Chen KH, Karras J, et al. Serine phosphorylation of STAT3 is essential for Mcl-1 expression and macrophage survival. Blood. 2003;102:344–352. doi: 10.1182/blood-2002-11-3396. [DOI] [PubMed] [Google Scholar]
- 25.Liu H, Perlman H, Pagliari LJ, Pope RM. Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages. Role of Mcl-1, independent of nuclear factor (NF)-kappaB, Bad, or caspase activation. J Exp Med. 2001;194:113–126. doi: 10.1084/jem.194.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma Y, Liu H, Tu-Rapp H, Thiesen HJ, Ibrahim SM, Cole SM, et al. Fas ligation on macrophages enhances IL-1R1-Toll-like receptor 4 signaling and promotes chronic inflammation. Nat Immunol. 2004;5:380–387. doi: 10.1038/ni1054. [DOI] [PubMed] [Google Scholar]
- 27.Pagliari LJ, Perlman H, Liu H, Pope RM. Macrophages require constitutive NF-kappaB activation to maintain A1 expression and mitochondrial homeostasis. Mol Cell Biol. 2000;20:8855–8865. doi: 10.1128/mcb.20.23.8855-8865.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perlman H, Pagliari LJ, Georganas C, Mano T, Walsh K, Pope RM. FLICE-inhibitory protein expression during macrophage differentiation confers resistance to fas-mediated apoptosis. J Exp Med. 1999;190:1679–1688. doi: 10.1084/jem.190.11.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scatizzi JC, Hutcheson J, Bickel E, Haines GK, 3rd, Perlman H. Pro-apoptotic Bid is required for the resolution of the effector phase of inflammatory arthritis. Arthritis Res Ther. 2007;9:R49. doi: 10.1186/ar2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scatizzi JC, Hutcheson J, Pope RM, Firestein GS, Koch AE, Mavers M, et al. Bim-Bcl-2 homology 3 mimetic therapy is effective at suppressing inflammatory arthritis through the activation of myeloid cell apoptosis. Arthritis Rheum. 2010;62:441–451. doi: 10.1002/art.27198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu X, Chakravarty SD, Ivashkiv LB. Regulation of interferon and Toll-like receptor signaling during macrophage activation by opposing feedforward and feedback inhibition mechanisms. Immunol Rev. 2008;226:41–56. doi: 10.1111/j.1600-065X.2008.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahrara S, Huang Q, Mandelin AM, 2nd, Pope RM. TH-17 cells in rheumatoid arthritis. Arthritis Res Ther. 2008;10:R93. doi: 10.1186/ar2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdollahi-Roodsaz S, Joosten LA, Koenders MI, Devesa I, Roelofs MF, Radstake TR, et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J Clin Invest. 2008;118:205–216. doi: 10.1172/JCI32639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ultaigh SN, Saber TP, McCormick J, Connolly M, Dellacasagrande J, Keogh B, et al. Blockade of Toll-like receptor 2 prevents spontaneous cytokine release from rheumatoid arthritis ex vivo synovial explant cultures. Arthritis Res Ther. 2011;13:R33. doi: 10.1186/ar3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melendez K, Wallen ES, Edwards BS, Mobarak CD, Bear DG, Moseley PL. Heat shock protein 70 and glycoprotein 96 are differentially expressed on the surface of malignant and nonmalignant breast cells. Cell Stress Chaperones. 2006;11:334–342. doi: 10.1379/CSC-187.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korbelik M, Sun J, Cecic I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res. 2005;65:1018–1026. [PubMed] [Google Scholar]
- 37.Han JM, Park SG, Liu B, Park BJ, Kim JY, Jin CH, et al. Aminoacyl-tRNA synthetase-interacting multifunctional protein 1/p43 controls endoplasmic reticulum retention of heat shock protein gp96: its pathological implications in lupus-like autoimmune diseases. Am J Pathol. 2007;170:2042–2054. doi: 10.2353/ajpath.2007.061266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim G, Han JM, Kim S. Toll-like receptor 4-mediated c-Jun N-terminal kinase activation induces gp96 cell surface expression via AIMP1 phosphorylation. Biochem Biophys Res Commun. 2010;397:100–105. doi: 10.1016/j.bbrc.2010.05.075. [DOI] [PubMed] [Google Scholar]
- 39.Radsak MP, Hilf N, Singh-Jasuja H, Braedel S, Brossart P, Rammensee HG, et al. The heat shock protein Gp96 binds to human neutrophils and monocytes and stimulates effector functions. Blood. 2003;101:2810–2815. doi: 10.1182/blood-2002-07-2261. [DOI] [PubMed] [Google Scholar]
- 40.Berwin B, Hart JP, Rice S, Gass C, Pizzo SV, Post SR, et al. Scavenger receptor-A mediates gp96/GRP94 and calreticulin internalization by antigen-presenting cells. Embo J. 2003;22:6127–6136. doi: 10.1093/emboj/cdg572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jockheck-Clark AR, Bowers EV, Totonchy MB, Neubauer J, Pizzo SV, Nicchitta CV. Re-examination of CD91 function in GRP94 (glycoprotein 96) surface binding, uptake, and peptide cross-presentation. J Immunol. 2010;185:6819–6830. doi: 10.4049/jimmunol.1000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 43.Mavers M, Cuda CM, Misharin AV, Gierut AK, Agrawal H, Weber E, et al. Cyclin-dependent kinase inhibitor p21, via its C-terminal domain, is essential for resolution of murine inflammatory arthritis. Arthritis Rheum. 2012;64:141–152. doi: 10.1002/art.33311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frasnelli ME, Tarussio D, Chobaz-Peclat V, Busso N, So A. TLR2 modulates inflammation in zymosan-induced arthritis in mice. Arthritis Res Ther. 2005;7:R370–R379. doi: 10.1186/ar1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ziegler G, Freyer D, Harhausen D, Khojasteh U, Nietfeld W, Trendelenburg G. Blocking TLR2 in vivo protects against accumulation of inflammatory cells and neuronal injury in experimental stroke. J Cereb Blood Flow Metab. 2011;31:757–766. doi: 10.1038/jcbfm.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arslan F, Smeets MB, O'Neill LA, Keogh B, McGuirk P, Timmers L, et al. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation. 2010;121:80–90. doi: 10.1161/CIRCULATIONAHA.109.880187. [DOI] [PubMed] [Google Scholar]
- 47.Seibl R, Birchler T, Loeliger S, Hossle JP, Gay RE, Saurenmann T, et al. Expression and regulation of Toll-like receptor 2 in rheumatoid arthritis synovium. Am J Pathol. 2003;162:1221–1227. doi: 10.1016/S0002-9440(10)63918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]