Abstract
At attended locations emotion and attention interact to benefit contrast sensitivity, a basic visual dimension. Whether there are associated costs at unattended locations is unknown. Furthermore, emotion and attention affect response time, and anxiety modulates these effects. We investigated how trait-anxiety influences the interaction of emotion and attention on contrast sensitivity. On each trial, non-predictive precues (neutral or fearful faces) directed exogenous attention to four contrast-varying, tilted stimuli (Gabor patches). Attention was cued toward the target (valid), a distracter (invalid), or distributed over all locations. Observers discriminated target orientation, and completed self-report measures of anxiety. Effects of fearful expressions were mediated by trait-anxiety. Only high-trait anxious individuals showed decreased target contrast sensitivity after attention was diverted to a distracter by a fearful cue, and anxiety score correlated with degree of impairment across participants. This indicates that increasing anxiety exacerbates threat-related attentional costs to visual perception, hampering processing at non-threat-related locations.
Keywords: emotion, attention, contrast sensitivity, fear expression, anxiety
Emotion influences many cognitive processes, and has at least two distinct effects on visual attention and low-level visual perception. Emotion can enhance attention and improve perception under certain circumstances, but it can also impair them under others. These two effects stem from the preferential processing of emotional stimuli, in terms of speed and depth, compared to neutrally-valenced stimuli (for a review: Compton 2003). Preferential processing occurs especially for stimuli endowed with negatively arousing and potentially threatening emotions, such as fear. When threat-related stimuli attract attention to a given location, performance there typically improves; however, when threat-related stimuli distract attention away from that location, performance there typically worsens. These benefits and costs due to emotion and attention’s interaction have been shown in studies measuring speed of processing (reaction time, RT; e.g., Koster, Crombez, Van Damme, Verschuere & De Houwer, 2004), and spatial and temporal resolution (Bocanegra & Zeelenberg, 2011), whereas only the benefits have been demonstrated on contrast sensitivity (Phelps, Ling & Carrasco, 2006). In addition, self-reported anxiety level correlates with RT effects in tasks with emotional and attentional components, such as the dot probe (e.g., Macleod & Mathews, 1988) and spatial cueing (e.g., Fox, Russo, Bowles & Dutton, 2001) tasks. Here we ask (1) whether emotion potentiates both the benefit and cost of attention on contrast sensitivity at the attended and unattended locations, respectively, and (2) whether anxiety modulates the magnitude of these effects.
Fast, exhaustive processing of threat is crucial for survival (LeDoux, 1996), but this threat-advantage is manifested differently in our attentional and perceptual abilities. In the absence of emotion, covert exogenous attention (i.e., attending reflexively with fixed gaze) is a finite cognitive resource that improves early visual processes at attended locations but impairs them at unattended locations (for a review: Carrasco 2011). Attention researchers use peripheral cues (dots or bars) to direct exogenous attention, which is driven involuntarily by a transient change in the visual field. The effect peaks at ~100 ms post-cue onset, and decays shortly thereafter (e.g., Nakayama & Mackeben, 1989). When exogenous attention is cued to a spatial location, performance on visual tasks is improved there (e.g., Carrasco, Penpeci-Talgar & Eckstein, 2000), but is impaired at uncued locations (e.g., Pestilli & Carrasco, 2005). These performance changes occur even though the cues are uninformative (unpredictive of target location). Most relevant for the present study are the benefits and costs of exogenous covert attention on contrast sensitivity (Pestilli & Carrasco, 2005; Pestilli, Ling & Carrasco, 2009).
The conjoint effect of an emotional cue with attention results in greater benefits and costs in speed of processing (RT) compared to a neutral cue. The outcome of this interaction critically depends on the relevance of the emotional cue to the task. The spatial location of a cue relative to a target determines its relevance. Using Posner and colleagues’ (Posner & Petersen, 1990) three components of spatial attention (“shift-engage-disengage”) as a simple model, researchers have investigated the effects emotion has on the shifting, engagement, and disengagement of attention (e.g., Mogg, Holmes, Garner & Bradley, 2008). An emotional cue (e.g., a picture or word), may improve target processing in its vicinity due to the beneficial effects of attentional shifting to, and engagement at, target locations (valid cue). Enhanced attentional engagement with emotional stimuli, compared to a neutral control, results in faster RT on detection tasks (e.g., Koster et al., 2004). If that same emotional stimulus is at a non-target location, however, it can impair target processing due to costs of attentional disengagement and shifting from the task-irrelevant back to the relevant location (invalid cue). This impaired attentional disengagement from emotional versus neutral stimuli results in slower RT on detection tasks (e.g., Fox et al., 2001; Koster et al., 2004). Thus, tasks with emotional stimuli are typically completed faster at attended locations, but are slower at unattended locations, compared to neutral stimuli.
In addition to effects on RT, research has recently focused on psychophysical investigations of the interaction of emotion and attention on fundamental dimensions of visual perception (Bocanegra & Zeelenberg, 2009, 2011; Ferneyhough, Stanley, Phelps & Carrasco, 2010; Phelps et al., 2006). Contrast is a visual dimension that underlies stimulus visibility. Perception of contrast occurs at the earliest levels of the cortical visual hierarchy. Thus, contrast sensitivity, unlike RT, carries important information about the strength of the initial perceptual signal as it enters primary visual cortex (e.g., Boynton, Demb, Glover & Heeger, 1999). As a consequence of its basic nature, improvement or impairment of this signal by emotion and/or attention may influence a vast array of perceptual and cognitive processes downstream.
Phelps et al. (2006) used visual psychophysics methodology to investigate how emotion and attention interact to affect contrast sensitivity. They briefly presented a fearful face precue, reflexively drawing exogenous, covert attention to its location. When this precue appeared just prior to the onset of a tilted target Gabor patch (a sinusoidal grating convolved with a Gaussian), participants’ orientation discrimination improved, compared to the presentation of a neutral face precue. This improvement in performance was more pronounced when the fearful face appeared adjacent to the target location (valid cue) than when it appeared at all possible locations (distributed cue). Given that increased performance on orientation discrimination tasks depends on increased contrast sensitivity (e.g., Carrasco et al., 2000; Pestilli et al., 2009), these results showed that emotion improved contrast sensitivity, and potentiated the beneficial effect of attention in valid trials. There were no invalid cues, however, leaving open the question of how emotion influences the effect of attention on contrast sensitivity at the unattended locations.
The evidence described so far indicates that emotion and attention interact to produce benefits and costs for RTs (Koster et al., 2004) and only benefits for contrast sensitivity (Phelps et al., 2006). Thus, manipulating cue emotionality in an attention paradigm can have measurable consequences on the experimental outcome. At the same time, observers’ mental state or personality characteristics should be taken into consideration. There exists a wide range of emotional dispositions in the general ‘normal’ population. While investigating the interaction of emotion with attention, it is therefore important to look at individual variability in factors known to modulate emotional effects on attention. Anxiety is one such critical factor.
Non-clinical trait-anxiety correlates positively with both the RT benefits and costs in visual attention tasks. High trait anxious participants, for example, were faster to detect a probe that replaced threatening (valid trial) vs. neutral words (Macleod & Mathews, 1988) or faces (Mogg et al., 2008). Conversely, other researchers have found that high trait anxious participants were slower to detect probes appearing at non-threat locations (invalid trial; Fox et al., 2001; Koster et al., 2006). Methodological differences across studies make it difficult to explain why anxiety either speeds responses in valid threat (vs. valid non-threat) trials or slows responses in invalid threat (vs. invalid non-threat) trials, but never both. Note, however, that the cue-target asynchronies in all of these studies were considerably longer (250–524 and 500 ms, respectively) than the temporal limitations of exogenous attention (~100 ms; Carrrasco, 2011; Nakayama & Mackeben, 1989), so voluntary, endogenous attentional processes likely contribute to these effects.
Regardless of the particular directions, meta-analyses have proposed two routes through which anxiety has its effects on RT. They are (1) heightened attention via an enhanced threat detection system, and (2) prolonged maintenance of attention to potential threat due to impaired attentional control (e.g., Cisler & Koster, 2010). Together, heightened attention to threat and difficulty disengaging from threat are thought to contribute to anxiety sufferers’ propensity to dwell on negative thoughts and feelings in the absence of a trigger. Given the prevalence of anxiety’s effects across a range of attention tasks, we hypothesized that one possible mechanism underlying its modulation of RT is perceptual in nature. Detection and discrimination may be slowed, for example, if a target is harder to see – whether the target itself is lower in contrast, or the observers’ contrast sensitivity is reduced. It remains unknown, however, whether anxiety modulates the effects of exogenous attention on basic visual dimensions such as contrast sensitivity.
The goal of the present study was to investigate whether using fearful faces as attentional cues would exaggerate both the benefits and the costs of attention on perception, e.g., increase contrast sensitivity with fear-valid relative to neutral-valid, and decrease contrast sensitivity with fear-invalid, relative to neutral-invalid, cueing. We hypothesized trait-anxiety would modulate the strength of these benefits and costs. To investigate the possible role normal variation in anxiety may have on emotion’s effects on attention, we recruited participants whose scores on self-report measures of anxiety ranged within accepted norms (e.g., trait-anxiety: M=36, SD=10; Spielberger et al., 1983).
Methods
Participants
Forty-seven observers were recruited (32 female; age M=22, SD=4, range=18–34). All observers had normal or corrected-to-normal vision and were right-handed (M=81, SD=22) according to the Edinburgh Handedness Inventory; possible score range: −100 (completely left-handed) to +100 (completely right-handed; Oldfield, 1971). All observers completed the 40-item State-Trait Anxiety Inventory (STAI: Spielberger et al., 1983), which assesses degree of anxiety at the present moment (state) and in general (trait); possible score range: 20–80 (state-anxiety: M=38, SD=10; trait-anxiety: M=39, SD=10).
Apparatus and Stimuli
Stimuli were presented on a 21″ CRT monitor (1600x1200 pixels; 75 Hz) connected to an Intel IMac computer. Background luminance was set to 57 candelas/m2. During the experiment, participants’ heads were stabilized using a chin rest 57 cm from the monitor.
Face stimuli consisted of 22 contrast- and luminance-equated grayscale pictures of fearful and neutral faces from the Pictures of Facial Affect series (Ekman & Friesen, 1976; same as used in Phelps et al., 2006 and Ferneyhough et al., 2010). 2-cpd Gabor patches (SD=1°) were created using MATLAB and the Psychophysics Toolbox (Brainard, 1997). The face cues subtended 5x6.7°. The Gabor patches subtended 7.9°. The Gabors were tilted 5° counterclockwise or clockwise from vertical. Seven Gabor patch contrasts were chosen individually per observer to obtain performance levels that ranged from chance to asymptotic performance.
Procedure
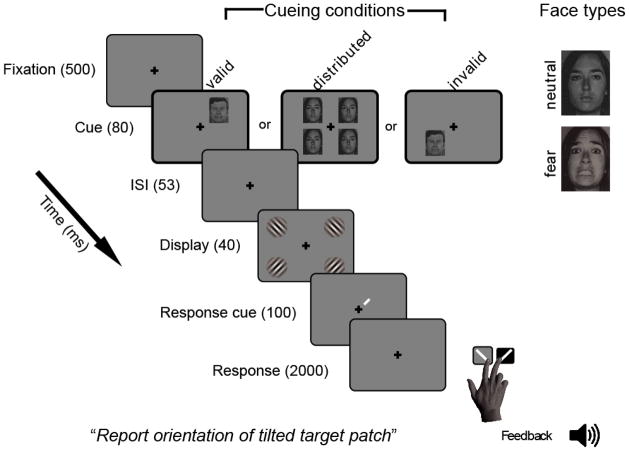
Observers were seated in a darkened room and completed an orientation discrimination task. Performance on orientation discrimination tasks is used to assess contrast sensitivity (e.g., Carrasco et al., 2000; Pestilli & Carrasco, 2005; Pestilli et al., 2009). On each trial (Figure 1), observers fixated a central cross for 500 ms; then a face precue (fearful or neutral) was presented for 80 ms to either one (valid or invalid) or four (distributed) locations along the intercardinal meridians (5° eccentricity) to manipulate exogenous attention; following a 53 ms ISI, four randomly tilted Gabor patches were each presented for 40 ms at one of four intercardinal locations (11° eccentricity). A response cue then appeared for 100 ms at Gabor offset indicating the location of the target Gabor. Participants were instructed to indicate the target orientation (counterclockwise or clockwise) with a button press within a 2000 ms response window. Feedback was given after each trial by a high tone for correct and a low tone for incorrect responses. Valid cues appeared adjacent to targets, invalid cues appeared adjacent to one of the three distracters with equal probability, and distributed cues appeared at all four possible locations. Each cue type appeared in 1/3 of the trials.
Figure 1.
Trial sequence. Images not to scale; contrast, target Gabor tilt and spatial frequency emphasized for clarity; spatial position of cues and targets not to scale.
On day 1 each observer completed a half-hour training session with black dot cues (0.3° diameter, 5° eccentricity) to avoid habituation to facial expression. Participants who performed ≥ 70% accuracy (about halfway between chance, 50%, and perfect performance) on average throughout training continued on to the first 4 blocks of the main experiment in which fearful or neutral faces were used as cues (facial expression was intermixed within blocks). On day 2, observers completed the other 8 blocks of the experiment and filled out the self-report surveys. In total, observers completed 1,344 trials (112 trials per 12 blocks).
Analysis
For each of the six conditions (fear-valid, fear-distributed, fear-invalid, neutral-valid, neutral-distributed, neutral-invalid), we calculated percent correct as a function of the seven contrast levels. Unique psychometric Weibull functions were then fit to the accuracy data for each condition for each observer using psignifit 2.5.6 (http://bootstrap-software.org/psignifit/; Wichmann & Hill, 2001). Contrast threshold was defined as the estimated stimulus intensity at which observers were correct 75% of the time. The dependent variable was contrast sensitivity, the inverse of contrast threshold. Observers’ six contrast sensitivity scores (one per condition) were individually normalized by dividing each condition mean by the average of all conditions; such normalized scores reduced variability introduced by differences in baseline contrast sensitivity across observers (e.g., Ferneyhough et al., 2010)i.
Results
Overall Contrast Sensitivity
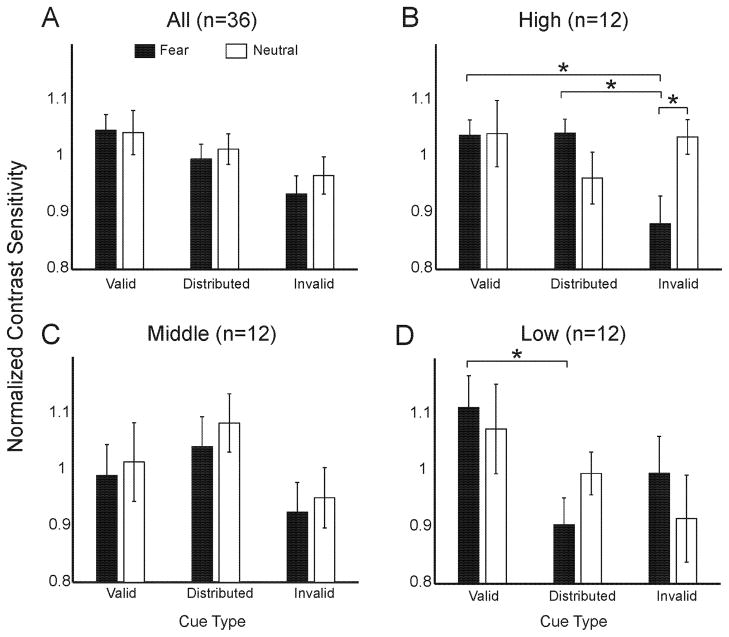
To investigate the effects of facial expression and cue validity on contrast sensitivity, a 2x3 repeated-measures ANOVA was conducted on normalized contrast sensitivity scores. Facial expression (fearful, neutral) and cue (valid, distributed, invalid) served as within-subjects factors. This resulted in a significant main effect of cue condition (F(2,68)=2.69, p<.05, one-tailed), in which contrast sensitivity was highest with valid than distributed cues, and lower with invalid cues (Figure 2A), consistent with previous research (Ferneyhough et al., 2010; Pestilli & Carrasco, 2005). Neither the main effect of facial expression, nor the interaction of facial expression and cue, were significant (F’s<1, p’s>.1).
Figure 2.
A–D: Cueing effects: all observers and by anxiety. The Y-axis is normalized contrast sensitivity. The X-axis is spatial cueing condition. Black bars indicate fear face cues and white bars indicate neutral face cues. (*) indicates a significant two-tailed t-test. Error bars are ± 1 SE of mean. A) All observers; B) high trait anxious observers; C) middle trait anxious observers; and D) low trait anxious observers.
Anxiety Differences
Given our hypothesis that trait anxiety would modulate contrast sensitivity benefits and costs, we included trait anxiety as a covariate in a 2x3 ANCOVA. This resulted in a significant three-way interaction of facial expression, cue and trait anxiety (F(2,68)=3.37, p<.05), and a significant two-way interaction of facial expression and cue (F(2,68)=3.13, p=.05).
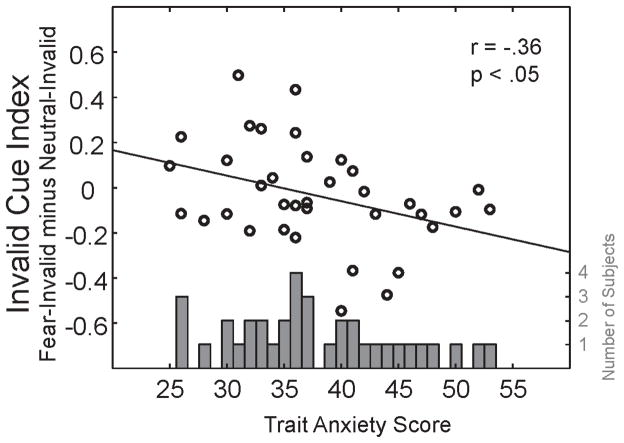
To clarify the role of anxiety in the significant ANCOVA interactions with facial expression and cueing condition, we first examined a histogram of the distribution of anxiety scores, observing a slight weighting towards the low-to-mid level anxiety range (Figure 3 gray bars). Based on this histogram, participants were then split into three equal-sized groups. The 12 participants with the lowest trait anxiety scores (25 to 34; M=30, SD=3) were in the low-anxiety group, the 12 intermediate scoring participants (35 to 40; M=37, SD=2) were in the middle-anxiety group, and the 12 highest scoring participants (41 to 53; M=46, SD=4) were in the high-anxiety group (see Peers & Lawrence, 2009 for similar methods). Had we instead performed a median-split (median trait anxiety = 36.5), proportionally more middle-anxious participants would have been grouped with the high-anxious participants, effectively diluting the effects of high anxiety compared to low anxiety. Dividing the participants into three groups enabled a more nuanced examination of emotion and attention interactions, helping to clarify how the interactions change as a function of anxiety. Furthermore, it allowed a complete separation between the low and high groups for easier comparison of the extremes. Given the 3-way interaction, separate 2x3 ANOVAs were conducted for each anxiety group with facial expression and cue validity as within-subjects factors.
Figure 3.
Gray bars Distribution of anxiety scores across the sample. The Y-axis is the number of participants as a function of trait anxiety score (X-axis). Black circles: Correlation of anxiety score and invalid cue index. The Y-axis is the invalid cue index (fear-invalid minus neutral-invalid normalized contrast sensitivity). A positive number indicates fear-invalid cues benefited contrast sensitivity whereas a negative number indicates they incurred a cost to contrast sensitivity.
For the high-anxiety group, there was a significant interaction of facial expression and cue (F(2,22)=5.01, p<.05; Figure 2B). This interaction was not significant in the middle-anxiety group (F(2,22)=0.01, p=.98; Figure 2C), and was a trend in the low-anxiety group (F(2,22)=2.52, p=.1; Figure 2D). Planned paired t-tests (all two-tailed) revealed that the high trait anxious group showed a significant decrease in contrast sensitivity in the fear-invalid relative to the neutral-invalid (t(11)=3.22, p<.01), the fear-distributed (t(11)=2.77, p<.05), and the fear-valid condition (t(11)=2.81, p<.05; Figure 2B). The low trait anxious group showed a significant increase in contrast sensitivity in the fear-valid relative to the fear-distributed condition (t(11)=3.05, p<.05; Figure 2D).
To understand how changes in anxiety are related to contrast sensitivity benefits and costs, we calculated a contrast sensitivity index for both valid and invalid cue conditions across all observers, and correlated participants’ trait-anxiety scores with these indices. The valid cue index was fear-valid minus neutral-valid contrast sensitivity, and the invalid cue index was fear-invalid minus neutral-invalid contrast sensitivity. For both indices, positive scores signified that fearful expressions resulted in higher contrast sensitivity (benefit), and negative scores signified that fearful expressions resulted in lower contrast sensitivity (cost), relative to neutral expressions. Trait-anxiety and the valid cue index did not correlate (r(36)=−.04, p>.1), whereas trait-anxiety and the invalid cue index were significantly correlated (r(36)=−.36, p<.05). As hypothesized, increased trait-anxiety was accompanied by increased contrast sensitivity cost with fear-invalid relative to neutral-invalid cues (Figure 3, black circles). That is, the cost in perception due to the fear-invalid cue was most pronounced for high trait anxious observers.
Discussion
We asked whether fear faces used as attention cues would produce more pronounced benefits and costs to contrast sensitivity than neutral faces, and whether anxiety would modulate these effects. We showed that trait-anxiety modulates the attention and emotion effects on perception.
First, we replicated previous findings that showed benefits to contrast sensitivity at attended, and costs at unattended, locations regardless of facial expression (Ferneyhough et al., 2010; Pestilli & Carrasco, 2005). Second, the low trait-anxiety group showed greater contrast sensitivity in the fear-valid relative to fear-distributed cue condition, indicating an effect of attention with fear face cues. This result is consistent with the effect of emotion on the benefit of attention to contrast sensitivity (see Phelps et al., 2006, p. 297, Figure 4a vs. 4c). Third, in high-trait anxious individuals, contrast sensitivity was impaired at the target location when a fear-invalid cue directed exogenous attention to a non-target location, relative to the neutral-invalid cue condition. This was demonstrated by the finding that high trait anxious individuals have decreased contrast sensitivity with fear-invalid cues relative to the neutral-invalid, fear-distributed, and fear-valid cue conditions. Indeed, we found trait-anxiety was significantly correlated with the invalid cue index (magnitude of the disengagement cost with emotion: fear-invalid minus neutral-invalid contrast sensitivity) providing more evidence in support of our hypothesis that anxiety increases attention disengagement costs with emotion.
Exactly how anxiety alters the allocation of spatial attention has been debated. Some research indicates that anxious individuals will be more strongly drawn to threatening stimuli such as faces with angry or fearful facial expressions, experiencing RT benefits at these attended locations (Macleod & Mathews, 1988; Mogg et al., 2008). Other research indicates they will instead be slower to disengage from threatening stimuli, experiencing costs at unattended locations (Fox et al., 2001; Koster et al., 2006; for a review: Cisler & Koster, 2010). The present results suggest high trait anxious observers had greater difficulty disengaging from threat, resulting in impaired contrast sensitivity following fear-invalid cues relative to neutral.
In our task, the rapid presentation of face cues directed exogenous, bottom-up attention towards or away from target stimuli. We used fearful faces, commonly used to recruit the amygdala (e.g., Bishop, Duncan & Lawrence, 2004; Dickie & Armony, 2008; Morris et al., 1998; Vuilleumier et al., 2004). Fearful faces can enhance bottom-up attention allocation by strengthening cue representation via amygdala feedback connections throughout the ventral visual pathway (Freese & Amaral, 2005). Numerous studies have investigated this possible link between amygdala activity and enhanced signal in visual cortex (e.g., Freese & Amaral, 2005; Anderson & Phelps, 2001; Morris et al., 1998; Vuilleumier et al., 2004). However, no single study has investigated how anxiety might modulate perception.
Neurocognitive theories of anxiety and attention have suggested amygdala activity is heightened in anxiety in response to sources of potential threat. This hyperactivity could in turn bias bottom-up attention allocation more strongly towards locations of threat, resulting in both enhanced perception at cued locations and impaired perception at uncued locations. Studies investigating the role of frontal brain regions in the top-down control of emotion have shown that anxious individuals may have, not only increased amygdala activity (Bishop et al., 2004; Dickie & Armony, 2008), but also decreased recruitment of frontal control regions (Bishop, 2008). This imbalance between bottom-up emotional response and top-down attention could underlie the difficulty anxious individuals have in disengaging attention from threat. Consistent with this imbalance, diffusion tensor imaging reveals that connections between amygdala and ventral medial prefrontal cortex are weakened in anxiety (Kim & Whalen, 2009). Furthermore, increased trait-anxiety is associated with decreased cortical volume in brain regions implicated in anxiety disorders, such as the amygdala, ventromedial and dorsolateral prefrontal cortex (Spampinato et al., 2009). These studies provide support for the idea that the expression of anxiety is closely linked to impaired amygdala-frontal cortex interactions, which could yield increased bottom-up response to threat. In tasks such as ours, in which both exogenous attention and emotion are manipulated, feedback from the amygdala and brain regions involved in exogenous attention shifts may interactively modulate V1 activity. With increased anxiety, stronger feedback from the amygdala may result in greater costs.
Although we tested the possibility that anxiety also increases the benefit of valid fear cues, our results showed no differences between fear-valid and neutral-valid cueing in the high trait anxious group, consistent with RT findings (e.g., Fox et al., 2001) showing that high trait anxious people show delayed disengagement rather than enhanced capture. One possible explanation for this is that for high anxious participants, neutral expressions could be interpreted as being emotionally ambiguous (e.g., Cooney et al., 2006). That is, the initial bottom-up response (within 100 ms) to fearful and neutral faces may be equivalent. Once the facial expression has been perceived, however, these participants may be able to disengage attention more quickly from neutral faces compared to fearful faces. The result is that valid cues, whether they are fearful or neutral, affect contrast sensitivity in a similar way, whereas invalid cues affect contrast sensitivity differently depending on facial expression. Fear-invalid cues hold attention more strongly, leading to costs in contrast sensitivity at unattended locations, and neutral-invalid cues do not, at least in the high-anxiety group.
For low-trait anxious observers, fear-valid cues increased contrast sensitivity more than fear-distributed cues. For these observers, however, fear-valid cues did not significantly increase contrast sensitivity above that of neutral-valid cues. A possible explanation for this discrepancy with the study of Phelps et al. (2006) stems from methodological considerations. First, the previous study tested fewer, more experienced observers and, second, collected significantly more data from each person (n=6, >11,000 trials, >6 hours), than the present study (n=36, 1344 trials, 2 hours). In the present study more observers were needed in order to investigate the effects of anxietyii. Third, whereas Phelps et al. used a staircase procedure, which is designed to iteratively sample contrasts closer and closer to the actual contrast threshold, in the present study we used the method of constant stimuli, sampling each of 7 predefined contrast levels equally; the former resulting in a more refined measurementiii. Fourth, in the present study three distracters appeared simultaneously with the target, and a postcue indicated the target location, increasing task difficulty.
The fact that anxiety can modulate contrast sensitivity suggests a prioritization of attentional resources that enhances and prolongs early visual, low-level processing of possibly threatening stimuli in the environment. This finding extends previous research showing anxiety modulates processing speed, and offers a possible perceptual mechanism underlying RT effects, i.e., if something is harder to see, identification would take longer. Greater sensitivity to differences between light and dark enhances the perception of borders and outlines of objects, which provides an advantage in efficiently parsing any stimulus, including those designating threat from non-threat. Higher anxiety may impart an even greater advantage in this process. As we show here, however, this threat-advantage can come at a cost of performing visual tasks unrelated to threat, such as discriminating orientation at other spatial locations. Evolutionarily, this is often an acceptable cost in comparison to potential costs from real threats. Be that as it may, life-threatening situations are rare in the modern world, and attentional biases due to anxiety can impair our ability to focus on some relevant, but mundane, aspects of everyday life.
In conclusion, emotion modulates the effects of attention on contrast sensitivity in a manner dependent on trait-anxiety. For high-anxious individuals there is a greater cost disengaging from fear faces at the attended location. Given that contrast sensitivity is a basic visual dimension carried out in primary visual cortex, these findings suggest that anxiety modulates the way in which emotion and attention alter the signal representing a stimulus in early vision, helping to bridge the perception and anxiety literatures.
Acknowledgments
We thank Damian Stanley, Tobias Brosch and David Carmel for helpful discussions, as well as other Phelps and Carrasco Lab members for comments on earlier versions of this manuscript. NIH R01-EY016200 to MC and NIH R01-MH062104 to EAP.
Footnotes
Data from seven observers could not be reliably fit with a Weibull function. Deviance scores, which assess goodness of fit, exceeded χ2.05(7)=14.1 (.05 refers to p-value; 7 refers to number of contrast levels). This was likely due to our participant population being inexperienced psychophysical observers and (1) making inconsistent responses and/or (2) having more “finger mistakes” in which the incorrect button is pressed even though the stimulus was correctly perceived. Four additional observers had contrast sensitivity >3 SDs from the group mean after normalization. Data from the 36 remaining observers (23 females) were included in the statistical tests. The mean deviance score across these participants and conditions was 4.43 (SD=2.76).
Although with similar numbers of total trials others showed that emotion can improve perception (Bocanegra & Zeelenberg, 2009), we had 32 trials per data point (2 facial expressions x 3 cue validities x 7 contrast levels) whereas they had 88 trials per data point (2 facial expressions x 5 spatial frequencies) - over twice the amount of data per data point.
Both methods have been used extensively in psychophysical testing. On the one hand, the downside with using a staircase procedure is that it can be time-consuming and notoriously sensitive to inconsistent responses (a disadvantage of inexperienced observers), which could throw the contrast threshold estimate drastically off course. To get a reliable contrast threshold estimate, the results of many staircases are pooled (25 per condition in the Phelps et al. study), and if an estimate is not acceptable, the whole staircase has to be run again. Given that we tested 6 times the number of participants in order to investigate individual differences in anxiety, we opted to save time in data collection by not using staircase procedures. On the other hand, the downside with method of constant stimuli is that, although all stimulus intensities are sampled, the contrast threshold may not be sampled enough in a 2 hour experiment.
References
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411(6835):305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nat Neurosci. 2008;12(1):92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J Neurosci. 2004;24(46):10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocanegra BR, Zeelenberg R. Emotion improves and impairs early vision. Psych Science. 2009;20(6):707–713. doi: 10.1111/j.1467-9280.2009.02354.x. [DOI] [PubMed] [Google Scholar]
- Bocanegra BR, Zeelenberg R. Emotional cues enhance the attentional effects on spatial and temporal resolution. Psychon Bull Rev. 2011;18(6):1071–1076. doi: 10.3758/s13423-011-0156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton GM, Demb JB, Glover GH, Heeger DJ. Neuronal basis of contrast discrimination. Vision Res. 1999;39:257–269. doi: 10.1016/s0042-6989(98)00113-8. [DOI] [PubMed] [Google Scholar]
- Brainard D. The psychophysics toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Carrasco M. Visual attention: The past 25 years. Vision Res. 2011;51:1484–1525. doi: 10.1016/j.visres.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Penpeci-Talgar C, Eckstein M. Spatial covert attention increases contrast sensitivity across the CSF: support for signal enhancement. Vision Res. 2000;40(10–12):1203–1215. doi: 10.1016/s0042-6989(00)00024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Koster EHW. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clin Psych Rev. 2010;30(2):203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton R. The interface between emotion and attention: A review of evidence from psychology and neuroscience. Beh Cog Neuro Reviews. 2003;2(2):115–129. doi: 10.1177/1534582303255278. [DOI] [PubMed] [Google Scholar]
- Cooney RE, Atlas LY, Joormann J, Eugene F, Gotlib IH. Amygdala activation in the processing of neutral faces in social anxiety disorder: Is neutral really neutral? Psychiatry Res: Neuroimaging. 2006;148:55–59. doi: 10.1016/j.pscychresns.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Dickie EW, Armony JL. Amygdala responses to unattended fearful faces: Interaction between sex and trait anxiety. Psychiatry Res. 2008;162(1):51–57. doi: 10.1016/j.pscychresns.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen W. Pictures of facial affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Ferneyhough E, Stanley DA, Phelps EA, Carrasco M. Cuing effects of faces are dependent on handedness and visual field. Psych Bulletin & Rev. 2010;17(4):529–535. doi: 10.3758/PBR.17.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E, Russo R, Bowles RJ, Dutton K. Do threatening stimuli draw or hold visual attention in subclinical anxiety? J Exp Psychol Gen. 2001;130(4):681–700. [PMC free article] [PubMed] [Google Scholar]
- Freese JL, Amaral DG. The organization of projections from the amygdala to visual cortical areas TE and V1 in the macaque monkey. J Comp Neurology. 2005:1–23. doi: 10.1002/cne.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ. The Structural Integrity of an Amygdala-Prefrontal Pathway Predicts Trait Anxiety. J Neuro. 2009;29(37):11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster EHW, Crombez G, Van Damme S, Verschuere B, De Houwer J. Does imminent threat capture and hold attention? Emotion. 2004;4(3):312–317. doi: 10.1037/1528-3542.4.3.312. [DOI] [PubMed] [Google Scholar]
- Koster EHW, Crombez G, Verschuere B, De Houwer J. Attention to threat in anxiety-prone individuals: Mechanisms underlying attentional bias. Cogn Ther Res. 2006;30(5):635–643. [Google Scholar]
- LeDoux JE. The Emotional Brain: The Mysterious Underpinnings of Emotional Life. Simon & Schuster; New York: 1996. [Google Scholar]
- MacLeod C, Mathews A. Anxiety and the allocation of attention to threat. Q J Exp Psychol A. 1988;40(4):653–670. doi: 10.1080/14640748808402292. [DOI] [PubMed] [Google Scholar]
- Mogg K, Holmes A, Garner M, Bradley BP. Effects of threat cues on attentional shifting, disengagement and response slowing in anxious individuals. Behav Res Ther. 2008;46(5):656–667. doi: 10.1016/j.brat.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith C, Young AW, Calder AJ, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121(Pt 1):47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Mackeben M. Sustained and transient components of focal visual attention. Vision Res. 1989;29(11):1631–1647. doi: 10.1016/0042-6989(89)90144-2. [DOI] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Peers PV, Lawrence AD. Attentional control of emotional distraction in rapid serial visual presentation. Emotion. 2009;9(1):140–145. doi: 10.1037/a0014507. [DOI] [PubMed] [Google Scholar]
- Pestilli F, Carrasco M. Attention enhances contrast sensitivity at cued and impairs it at uncued locations. Vision Res. 2005;45(14):1867–1875. doi: 10.1016/j.visres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Pestilli F, Ling S, Carrasco M. A population–coding model of attention’s influence on contrast response: Estimating neural effects from psychophysical data. Vision Res. 2009;49(10):1144–1153. doi: 10.1016/j.visres.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Ling S, Carrasco M. Emotion facilitates perception and potentiates the perceptual benefits of attention. Psych Sci. 2006;17(4):292. doi: 10.1111/j.1467-9280.2006.01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Petersen S. The attention system of the human brain. Ann Rev Neuro. 1990;13(1):25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Spampinato MV, Wood JN, De Simone V, Grafman J. Neural correlates of anxiety in healthy volunteers: a voxel-based morphometry study. J Neuropsychiatry Clin Neurosci. 2009;21(2):199–205. doi: 10.1176/jnp.2009.21.2.199. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto: 1983. [Google Scholar]
- Vuilleumier P, Richardson M, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci. 2004;7(11):1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Wichmann F, Hill N. The psychometric function: I. Fitting, sampling, and goodness of fit. Perception & Psychophysics. 2001;63(8):1293. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]