Abstract
The enzyme telomerase plays an important role in sustaining the capacity of T lymphocytes for homeostatic replication. Recent data have suggested that gonadal steroids might modulate telomerase expression or activity within these cells. We used quantitative assay techniques for both telomerase mRNA expression and telomerase enzymatic activity to systematically examine the effects of physiologic concentrations of estradiol on human peripheral blood mononuclear cells under basal conditions and under conditions that normally enhance telomerase activity in T lymphocytes. Cells from women tended to exhibit higher responsiveness of telomerase activity to induction by T cell receptor engagement. However, we found no evidence of a direct effect of physiologic concentrations of estradiol on human telomerase reverse transcriptase (hTERT) mRNA expression, hTERT protein expression, or telomerase enzymatic activity in cultured PBMCs. While estrogen might exert developmental effects on T cells to alter telomerase responsiveness to T cell receptor engagement, mature peripheral T cells do not respond to estradiol with changes in expression or function of telomerase.
Keywords: Estrogen, T Lymphocytes, Telomerase
1. INTRODUCTION
Clonal expansion of T lymphocytes in the peripheral immune system is required to generate sufficient numbers of effector cells for response and sufficient numbers of memory cells for use upon re-exposure to the antigen. Furthermore, the maintenance of normal total T cell numbers in the face of declining thymic output during aging also requires that the potential of these cells to replicate must somehow be maintained in the face of limits on cellular capacity to proliferate (Weng, Hathcock and Hodes, 1998).
The telomere, a molecular complex at the ends of chromosomes that is comprised of hexameric repeating units of DNA (TTAGGG) with associated proteins is thought to be the molecular “counter” that sets limits on cell division (Greider, 1996). These telomeres shorten with each cellular division as a consequence of loss of terminal bases during DNA polymerase action (Weng et al., 1998). At some critical telomere length most normal cells are no longer capable of further division—a state called “clonal replicative senescence” (Harley, Futcher and Greider, 1990). Some cells, however, exhibit an apparently unlimited capacity for proliferation: normal human germ cells and neoplastic cells derived from a variety of tissues have such capability (Kim, Piatyszek, Prowse et al., 1994; Shay and Wright, 1996; Wright, Piatyszek, Rainey et al., 1996). The compensatory mechanism by which these cells escape proliferative limitation involves the enzyme system called telomerase, a complex ribonucleoprotein that functions to replace lost telomeric repeats at the ends of chromosomes (Blackburn, 2000). Expression of telomerase activity has been found to be developmentally regulated in the T lymphocyte lineage and to confer expanded replicative capacity on these cells (Weng et al., 1998). Thymocytes express high levels of telomerase activity—comparable to those observed in transformed cell lines (Weng, Levine, June et al., 1996). T cells in secondary lymphoid tissue express intermediate levels of telomerase activity and low levels are seen in circulating peripheral T cells. When the peripheral T cells are activated, for example by CD3/CD28 stimulation, the activity of telomerase rises to levels comparable to those seen in thymocytes (Weng et al., 1996).
A role for gonadal steroid hormones in the regulation of telomerase expression and function has been identified in a number of systems. Estrogens are known to induce telomerase activity in breast cancer cells (Kyo, Takakura, Kanaya et al., 1999) as well as in endometrium (Kyo, Takakura, Kohama et al., 1997), prostate (Meeker, Sommerfeld and Coffey, 1996), and ovarian epithelial cells (Misiti, Nanni, Fontemaggi et al., 2000) and a functional estrogen response element has been identified in the proximal promoter of the hTERT gene-- so that direct estrogen effects on the gene have been considered at least part of the induction mechanism (Misiti et al., 2000). Several recent reports have examined the effects of estrogen action on telomerase expression and activity in human lymphocytes (Effros, Dagarag, Spaulding et al., 2005; Choi, Fauce and Effros, 2008; Calado, Yewdell, Wilkerson et al., 2009). A number of aspects of these reports raised our curiosity about the phenomenon and we sought to use more quantitative assay techniques to systematically examine the effects of physiologic concentrations of estradiol on human peripheral blood mononuclear cells under basal conditions and under conditions that normally enhance telomerase activity.
2. MATERIALS AND METHODS
2.1 Subjects and Samples
Normal volunteers (6 females of ages 27–62 years and 8 males of ages 23–61 years) were recruited for the study. After informed consent, peripheral blood samples (20–30 ml) were obtained by phlebotomy. These studies were reviewed and approved by the Penn State Milton S. Hershey Institutional Review Board.
2.2 Isolation and Culture of PBMCs
Mononuclear cells were isolated from human peripheral blood samples by density gradient centrifugation on Histopaque 1077 (MP Biomedicals). Cells were washed with PBS twice, with medium once and then resuspended in medium [RPMI 1640 without phenol red (GIBCO 11835) supplemented with 10% charcoal stripped fetal bovine serum (GIBCO), 1% GlutaMAX-1 (GIBCO) and 100 I.U. penicillin / 100 μg/ml streptomycin (Cellgro)] at a density of 1×106 cells / ml for use in experiments.
For treatment with PHA, a 1 mg/ml stock was prepared in RPMI 1640 (Cellgro 10–040-CV), 10% fetal bovine serum, 1% GlutaMAX-1 and 100 I.U. penicillin / 100 μg/ml streptomycin. Aliquots of this stock were added to cell culture medium to yield a final concentration of 10 g/ml.
Dynabeads Human T-Activator CD3 / CD28 (Invitrogen) were used as the source of anti-CD3 / anti-CD28. The Dynabeads were washed following the manufacturer’s instructions, resuspended in a volume of growth media equal to the original aliquot amount and added to the cells at a bead: cell ratio of 1:1.
Estradiol stocks (17- estradiol; SIGMA) were prepared in ethanol at 1000X concentrations. Final concentrations of estradiol in cell cultures were: 0 nM (ethanol only at a final concentration of 0.1%), 0.1 nM, 1.0 nM, 10 nM, 100 nM and 1000 nM.
Cells were incubated for 48 hr at 37°C in an atmosphere of 5% CO2 in air. Parallel samples were harvested for mRNA isolation and for preparation of protein extracts for determination of telomerase activity.
2.3 hTERT mRNA quantitation
RNA (50–100 ng) isolated from PBMCs using a commercial kit (RNEasy; QIAGEN) was subjected to reverse transcription using the High Capacity RNA-to-cDNA kit (Applied Biosystems). RNA was brought up in 9 μl of nuclease free water. 10 μl of 2X RT Buffer Mix and 1 μl of 20X RT Enzyme Mix were added to each RNA sample. Samples were incubated for 1 h at 37°C then at 95°C for 5 min to inactivate the enzyme.
One-eighth of the products of each reverse transcription reaction was used as template for the amplification of hTERT and GAPDH (normalization control) cDNAs with 1X TaqMan Gene Expression Assays for hTERT (Hs00972656_m1) and GAPDH (Hs03929097_g1), respectively, and 1X TaqMan Gene Expression Master Mix (Applied Biosystems) in a 96 well plate. Samples were subjected to the following cycling parameters in an Applied Biosystems 7300 Real Time PCR System: 50°C for 2 min, 95°C for 10 min and 40 cycles of 95°C for 15 s and 60°C for 1 min. The Ct method was used to calculate the change in gene expression of hTERT in each treated sample relative to the untreated baseline sample.
2.4 Immunoblots for hTERT protein expression
Cellular protein extracts were prepared by lysis of PBMCs in RIPA buffer (50 mM Tris, pH 8.0 with 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, and 0.1% SDS) with added protease inhibitors and phosphatase inhibitors (Halt; Thermo Scientific). Samples were prepared by heating in LDS sample buffer with reducing agent, run on pre-cast NuPage 4–12% gradient gels in a MOPS buffer system (Life Technologies) and transferred to PVDF membrane. These membranes were blocked with 5% nonfat dry milk dissolved in Phosphate Buffered Saline with Tween and probed overnight at 4C with anti-hTERT antibody (rabbit monoclonal ab32020; Abcam) at a 1:1000 dilution. Blots were washed and incubated for 1 hour with 1:5000 dilution of goat anti-rabbit IgG conjugated to horseradish peroxidase (Santa Cruz Biotechnology, sc2004) and developed using commercial chemiluminescence reagents (Super Signal West Pico Chemiluminescence Kit; Thermo Scientific). Imaging and analysis was carried out using an Alpha Innotech Fluorchem SP imaging system.
2.5 Telomerase Activity Assay
Telomerase activity in cell extracts was measured using the TRAPeze RT Telomerase Detection Kit (Millipore S7710). Each assay mixture included 5 μl of 5X TRAPeze RT Reaction Mix, 17.6 μl PCR Grade Water, 0.4 μl of 50X TITANIUM Taq DNA Polymerase (Clontech) and 2 μl cell extract or control template. A dilution series of TSR8 control template was prepared in CHAPS Lysis buffer to serve as a standard curve. Reactions were set up in triplicate. Samples were subjected to the following cycling parameters using an Applied Biosystems 7300 Real-Time PCR system: 30 min at 30°C (extension of telomerase substrate); 2 min at 95°C then 45 cycles of 15 s at 94°C, 1 min at 59°C and 30 s at 45°C (PCR amplification of extended telomerase substrate).
The quantity (amoles) of extended telomerase substrate produced in each well from the telomerase activity of 2 μl of cell extract within 30 min was determined from a linear plot of the log10 of the quantities (amoles) of TSR8 control template standards versus the Ct values for their wells. The mean value of these quantities for the three replicate wells for each sample was calculated. This number was divided by the amount of protein (mg) contained in each assay (2 μl of extract) and then divided by 30 to determine the amount of extended telomerase substrate (amoles) produced per mg of protein per minute for each sample cell extract.
2.6 Data Analysis
GraphPad Prism software (GraphPad Software, La Jolla, CA) was used for data analysis and graphics. Values are expressed as mean and standard errors of the mean (SEM). Comparisons between groups were made by unpaired t test. Dose response curves were analyzed by ANOVA. Correlations between continuous variables were analyzed using Pearson’s r. P values less than 0.05 were considered significant.
3. RESULTS
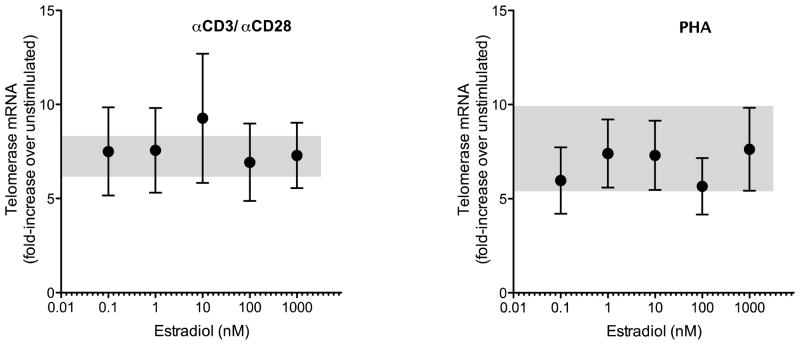
Telomerase reverse transcriptase (hTERT) mRNA was up-regulated in cultured human PBMCs by treatment with either PHA or anti-CD3/CD28-coated microspheres. Using a relative quantitation method ( Ct) based on real-time polymerase chain reaction amplification data for hTERT and GAPDH mRNA in each well we observed over seven-fold increase in the level of hTERT mRNA after treatment with anti-CD3/CD28; similar levels of induction were noted with PHA treatment (Table I). No significant differences were noted between females and males. Dose-response studies of the effects of estradiol on hTERT mRNA levels in human PBMCs cultured under nonstimulated conditions and after stimulation with either anti-CD3/CD28 or PHA revealed no apparent effect of the hormone on hTERT mRNA expression in cultured human PBMCs (Figure 1).
TABLE 1.
Induction of hTERT mRNA expression in PBMCs from males and females under stimulation with anti CD3/CD28 or PHA.
| T Cell Activation | Fold-Induction of hTERT mRNA (mean ± SEM) | ||
|---|---|---|---|
| Males (n = 3) | Females (n = 3) | All | |
| Anti CD3/CD28 | 6.99 ± 1.34 | 7.52 ± 2.28 | 7.26 ± 1.19 |
| PHA | 9.22 ± 4.13 | 6.24 ± 2.20 | 7.73 ± 2.20 |
Figure 1.
Estradiol effect on hTERT mRNA expression in cultured human PBMCs. Cells were cultured in phenol red-free RPMI 1640 with 10% charcoal-stripped serum (basal conditions) or with added anti-CD3/CD28 microspheres (left panel) or PHA (right panel) to drive T cell activation. Estradiol was added to cultures in concentrations ranging from 0.1 to 1000 nM. Relative expression of hTERT mRNA (compared to unstimulated cells) was determined using simultaneous quantitation of GAPDH mRNA and calculation by the Ct method. Shaded areas of each panel show mean ± SEM for hTERT induction by anti CD3/CD28 or PHA without added estradiol.
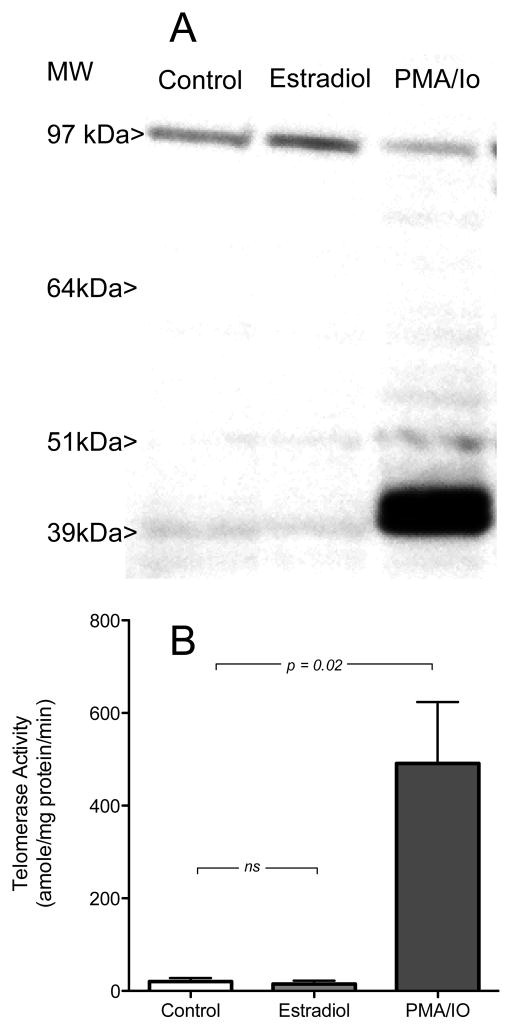
Levels of telomerase reverse transcriptase protein were assessed in estradiol-treated PBMCs by immunoblot. No significant difference in the amount of TERT protein was noted between cell extracts prepared from control and estradiol-treated cells (Figure 2A; two left lanes). Treatment of PBMCs with the combination of PMA/Io (which resulted in an apparent diminution in the high molecular weight band corresponding to TERT immunoreactivity, and the appearance of low molecular weight species (about 40 kDa) which were not seen in estradiol-treated cells (Figure 2A; right lane).
Figure 2.
(A) Immunoblot of telomerase protein in cultured human PBMCs treated with ethanol vehicle (control), with 1 M estradiol, or with a combination (PMA/Io) of PMA (phorbol myristate acetate; final concentration 50 ng/ml) and ionomycin (final concentration 1 g/ml). Molecular weight standards are shown at the left. (B) Quantitation of telomerase activity in human PBMCs under control conditions, treated with estradiol (1 M), or stimulated with PMA/Io as above. Telomerase activity is shown as amole of product/min/mg protein.
Since induction of telomerase activity in lymphocytes has been reported to occur independent of changes in mRNA or protein expression (Liu, Hodes and Weng, 2001) we measured telomerase activity in a PCR-based assay that permitted precise quantitation of enzymatic activity with normalization for the amounts of protein in each prepared cell extract. Control experiments (data not shown) using MCF-7 human breast cancer cells (Kyo et al., 1999) confirmed that our telomerase activity assay was capable of detecting a 66 ± 40% (mean ± SEM) increase in telomerase activity after hormone treatment. Estradiol treatment of PBMCs (at 1 M concentration) did not alter telomerase activity while the PMA/Io treatment associated with the change on immunoblot resulted in over 20-fold increase above the levels observed in control or estradiol-treated cells (Figure 2B).
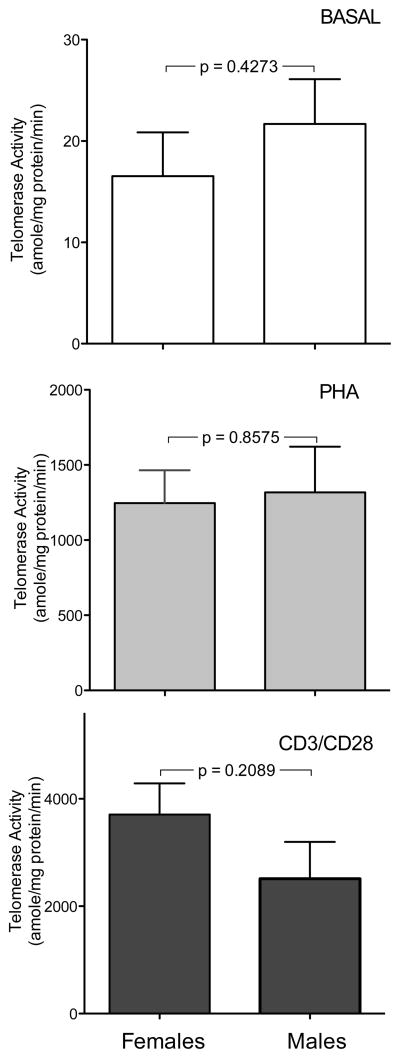
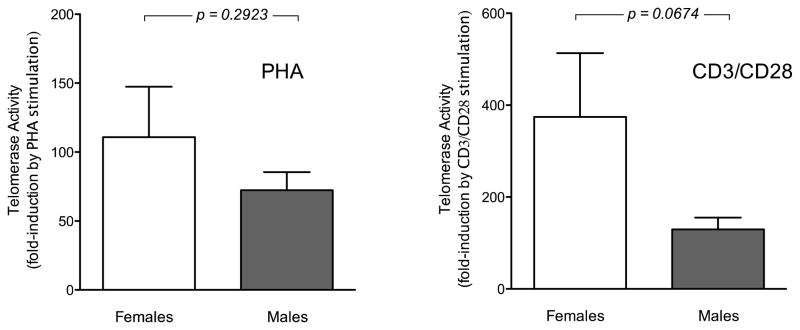
Telomerase activity under basal conditions or after cellular activation with either PHA or anti-CD3/CD28 did not show significant correlation with age of the subjects for either males or females (data not shown). PBMCs from females and males showed similar levels of telomerase activity under basal conditions and after treatment of cells with PHA (Figure 3A and 3B). After anti-CD3/CD28 treatment cells from females tended to show higher levels of telomerase activity than those from males, although the difference was not statistically significant (Figure 3C). When data were analyzed as relative induction compared to baseline females tended to show more pronounced responses (Figure 4), although this difference also did not reach statistical significance.
Figure 3.
Quantitation of telomerase activity in cultured PBMCs from females (left columns; n = 7) and males (right columns; n = 9). Cells were cultured as described under basal conditions (top panels) or stimulated with PHA (middle panels) or anti CD3/CD28 (bottom panels). Telomerase activity is shown as amole of product/min/mg protein. Note differences in scales between various treatments.
Figure 4.
Relative telomerase activity (fold induction compared to basal conditions) for PHA-treated PBMCs (left panel) and for anti-CD3/CD28-treated PBMCs (right panel). Data for women (n = 7) are shown in open bars; data for men (n = 9) are shown in filled bars.
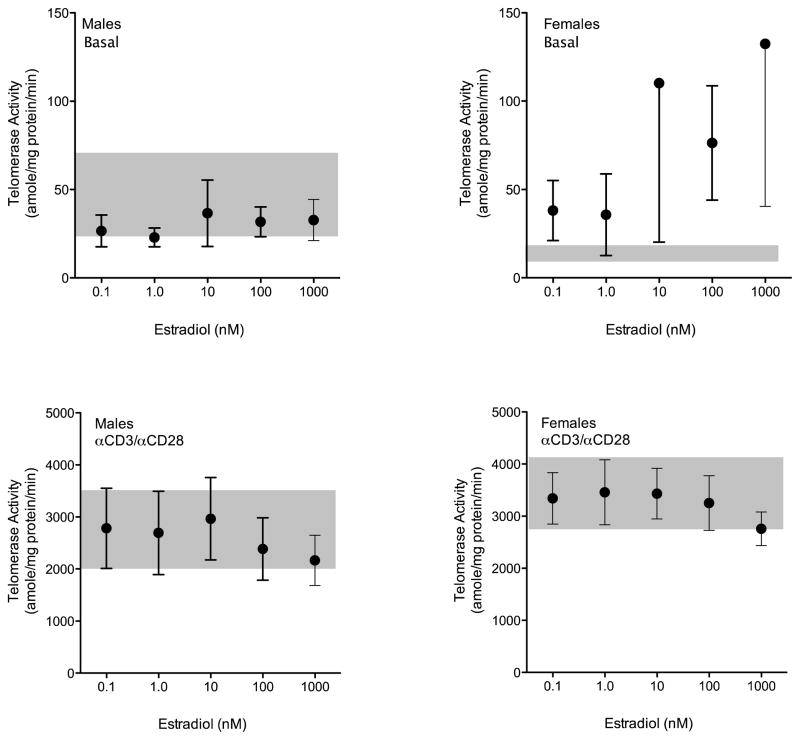
The effects of in vitro treatment with estradiol on PBMC telomerase activity were assessed separately in male and female cells under both basal and anti-CD3/CD28-stimulated conditions (Figure 5). Under basal conditions we found no effect of estradiol on telomerase activity in cultured cells over a four log concentration range. In females we observed greater variability in the data, particularly at estradiol concentrations well above physiologic, but no statistically significant effect could be demonstrated (Figure 5, top panels). In cells activated by T cell receptor engagement (Figure 5, bottom panels) we also found no effect of estradiol on cells from either males or females.
Figure 5.
Effects of estradiol on absolute telomerase activity in cultured human PBMCs from males (left panels; n = 9) and females (right panels; n = 7). PBMCs were cultured under basal conditions or under stimulation with anti-CD3/CD28 with varying concentrations of estradiol from 0.1 to 1000 nM. Shaded areas in each graph show mean ± SEM for cells cultured under identical conditions without added estradiol.
4. DISCUSSION
The question of whether gonadal steroids might regulate hTERT mRNA or protein expression or modulate telomerase activity in human T cells is of importance both for normal immune physiology and for pathophysiologic processes of autoimmunity. The immunosenescence of normal T cells occurs in the physiologic milieu of declining gonadal steroid hormone levels in both sexes and this immunosenescence is associated with the emergence of autoreactive clones of T cells and the emergence of some forms of autoimmunity (Hohensinner, Goronzy and Weyand, 2011). In females the hormonal changes are more dramatic at menopause, while in males the levels of gonadal steroid hormones decline more gradually (Harman, Metter, Tobin et al., 2001; Feldman, Longcope, Derby et al., 2002). Whether the immunologic phenomenon of T cell senescence is accelerated by the alterations in gonadal steroid levels that occur with aging in both sexes is unknown, but prior observations of apparent telomerase regulation by gonadal steroids in classical androgen and estrogen target cells has suggested that such telomerase-mediated hormonal influence on cellular senescence might also be operative in T cells.
We found no evidence of either direct hormonal regulation of hTERT mRNA or protein expression or of modulation of telomerase activity by estradiol in cultured PBMCs. These data differ from some previous reports. Two papers (Effros et al., 2005; Choi et al., 2008) have presented some preliminary data on estradiol effect on telomerase activity, but without replicate experiments to assess the intrinsic variability of the data no definitive conclusions were drawn. We were able to use quantitative assays for both hTERT mRNA and telomerase activity assays and our data do not support a significant effect of estradiol on human lymphoid cells in vitro. It has also been reported that supraphysiologic concentrations of a synthetic androgen receptor ligand (methyltrienolone) induce telomerase activity in PBMCs, and that the effects can be prevented by estrogen receptor (ER) blockade (with tamoxifen) or by addition of the aromatase inhibitor letrozole (Calado et al., 2009). Since methyltrienonlone is neither a ligand for ER nor a substrate for aromatization, these observations have been difficult to interpret. Our experiments revealed no estrogen effect in the range of concentrations of the hormone that approximate normal physiology. We did note apparently higher responses in telomerase activity in unstimulated cells at estradiol concentrations above 10nM (Figure 5), but because of the greater variability of the data at these concentrations, the differences were not significantly different than those observed in cells cultured without hormone. The magnitude of these effects was tiny in comparison to the induction of telomerase observed after T cell receptor engagement by anti-CD3 and no estrogen effect was discernible under those conditions.
While our study was designed to examine the acute regulatory effects of estradiol on hTERT expression and telomerase activity in peripheral T cells, it does not exclude the possibility of a developmental effect of estrogen or androgen exposure on the capacity of mature T cells to respond to activating stimuli by increases in telomerase activity. Such in vivo effects of estrogens on telomerase expression or function might involve mechanisms quite different from those underlying any direct regulatory effects that would be observed in vitro. In fact, we noted a greater proportional increase in telomerase activity in cells from females compared to males in response to CD3/CD28 stimulation, although these differences did not reach statistical significance. Future studies with larger numbers of age-matched subjects could address this specific point. Such developmental effects of gonadal steroid hormones on the properties of T lymphocytes have been demonstrated to be exerted at the level of the thymus (Kovacs and Olsen, 1987; Olsen, Watson, Henderson et al., 1991; Olsen and Kovacs, 2011), and mechanisms of action include indirect effects on thymocytes mediated through hormonal action on thymic stromal and epithelial components (Olsen, Olson, Viselli et al., 2001).
Highlights.
We evaluated a postulated role for estrogens in regulation of telomerase in T lymphocytes
T cells from females showed slightly greater telomerase induction with T cell receptor engagement
Estradiol did not directly change telomerase activity in resting or activated T cells
Our experiments exclude a direct effect of estradiol on telomerase induction in peripheral T cells
Acknowledgments
These studies were supported by NIH grant AG 029999 (to WJK)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blackburn EH. Telomere States and Cell Fates. Nature. 2000;408:53–6. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- Calado RT, Yewdell WT, Wilkerson KL, Regal JA, Kajigaya S, Stratakis CA, Young NS. Sex Hormones, Acting on the Tert Gene, Increase Telomerase Activity in Human Primary Hematopoietic Cells. Blood. 2009;114:2236–43. doi: 10.1182/blood-2008-09-178871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Fauce SR, Effros RB. Reduced Telomerase Activity in Human T Lymphocytes Exposed to Cortisol. Brain Behav Immun. 2008;22:600–5. doi: 10.1016/j.bbi.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB, Dagarag M, Spaulding C, Man J. The Role of Cd8+ T-Cell Replicative Senescence in Human Aging. Immunol Rev. 2005;205:147–57. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age Trends in the Level of Serum Testosterone and Other Hormones in Middle-Aged Men: Longitudinal Results from the Massachusetts Male Aging Study. The Journal of clinical endocrinology and metabolism. 2002;87:589–98. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- Greider CW. Telomere Length Regulation. Annu Rev Biochem. 1996;65:337–65. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, Greider CW. Telomeres Shorten During Ageing of Human Fibroblasts. Nature. 1990;345:458–60. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal Effects of Aging on Serum Total and Free Testosterone Levels in Healthy Men. Baltimore Longitudinal Study of Aging. The Journal of clinical endocrinology and metabolism. 2001;86:724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Hohensinner PJ, Goronzy JJ, Weyand CM. Telomere Dysfunction, Autoimmunity and Aging. Aging Dis. 2011;2:524–37. [PMC free article] [PubMed] [Google Scholar]
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific Association of Human Telomerase Activity with Immortal Cells and Cancer. Science. 1994;266:2011–5. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Kovacs WJ, Olsen NJ. Androgen Receptors in Human Thymocytes. J Immunol. 1987;139:490–3. [PubMed] [Google Scholar]
- Kyo S, Takakura M, Kanaya T, Zhuo W, Fujimoto K, Nishio Y, Orimo A, Inoue M. Estrogen Activates Telomerase. Cancer Res. 1999;59:5917–21. [PubMed] [Google Scholar]
- Kyo S, Takakura M, Kohama T, Inoue M. Telomerase Activity in Human Endometrium. Cancer research. 1997;57:610–4. [PubMed] [Google Scholar]
- Liu K, Hodes RJ, Weng N. Cutting Edge: Telomerase Activation in Human T Lymphocytes Does Not Require Increase in Telomerase Reverse Transcriptase (Htert) Protein but Is Associated with Htert Phosphorylation and Nuclear Translocation. J Immunol. 2001;166:4826–30. doi: 10.4049/jimmunol.166.8.4826. [DOI] [PubMed] [Google Scholar]
- Meeker AK, Sommerfeld HJ, Coffey DS. Telomerase Is Activated in the Prostate and Seminal Vesicles of the Castrated Rat. Endocrinology. 1996;137:5743–6. doi: 10.1210/endo.137.12.8940411. [DOI] [PubMed] [Google Scholar]
- Misiti S, Nanni S, Fontemaggi G, Cong YS, Wen J, Hirte HW, Piaggio G, Sacchi A, Pontecorvi A, Bacchetti S, Farsetti A. Induction of Htert Expression and Telomerase Activity by Estrogens in Human Ovary Epithelium Cells. Mol Cell Biol. 2000;20:3764–71. doi: 10.1128/mcb.20.11.3764-3771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen NJ, Kovacs WJ. Evidence That Androgens Modulate Human Thymic T Cell Output. J Investig Med. 2011;59:32–5. doi: 10.2310/jim.0b013e318200dc98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen NJ, Olson G, Viselli SM, Gu X, Kovacs WJ. Androgen Receptors in Thymic Epithelium Modulate Thymus Size and Thymocyte Development. Endocrinology. 2001;142:1278–83. doi: 10.1210/endo.142.3.8032. [DOI] [PubMed] [Google Scholar]
- Olsen NJ, Watson MB, Henderson GS, Kovacs WJ. Androgen Deprivation Induces Phenotypic and Functional Changes in the Thymus of Adult Male Mice. Endocrinology. 1991;129:2471–6. doi: 10.1210/endo-129-5-2471. [DOI] [PubMed] [Google Scholar]
- Shay JW, Wright WE. Telomerase Activity in Human Cancer. Current opinion in oncology. 1996;8:66–71. doi: 10.1097/00001622-199601000-00012. [DOI] [PubMed] [Google Scholar]
- Weng NP, Hathcock KS, Hodes RJ. Regulation of Telomere Length and Telomerase in T and B Cells: A Mechanism for Maintaining Replicative Potential. Immunity. 1998;9:151–7. doi: 10.1016/s1074-7613(00)80597-x. [DOI] [PubMed] [Google Scholar]
- Weng NP, Levine BL, June CH, Hodes RJ. Regulated Expression of Telomerase Activity in Human T Lymphocyte Development and Activation. J Exp Med. 1996;183:2471–9. doi: 10.1084/jem.183.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase Activity in Human Germline and Embryonic Tissues and Cells. Dev Genet. 1996;18:173–9. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]