Abstract
Mutant KRAS in lung cancers induce molecular pathways that regulate cellular proliferation, survival and inflammation, which enhance tumorigenesis. Inducible nitric oxide synthese (NOS2) up-regulation and sustained nitric oxide (NO•) generation are induced during the inflammatory response and correlate positively with lung tumorigenesis. To explore the mechanistic contribution of NOS2 to KRAS-induced lung tumorigenesis and inflammation, we used a genetic strategy of crossing NOS2 knockout (NOS2KO) C57BL6 inbred mice with a KRASG12D-driven mouse lung cancer model. KRASG12D;NOS2KO mice exhibited delayed lung tumorigenesis and a longer overall survival time compared with that of KRASG12D;NOS2WT (wild-type) controls. Correspondingly, tumors in KRASG12D;NOS2KO mice had reduced tumor cell proliferation in adenomas and carcinomas. NOS2-deficiency also led to dramatically suppressed inflammatory response by attenuation of macrophage recruitment into alveoli and within tumor foci. In contrast, FOXP3+ regulatory T cells were increased in tumors from KRASG12D;NOS2KO mice. We further analyzed the expression of microRNA-21 (miR-21), an oncogenic non-coding RNA involved in oncogenic Ras signaling, by quantitative reverse transcription PCR and in situ hybridization. Lung carcinomas dissected from KRASG12D;NOS2KO mice showed a significantly reduced miR-21 expression along with decreased tumor cell proliferation, suggesting that NOS2-deficiency could attenuate RAS signaling pathways that transactivate miR-21 expression. Therefore, deletion of NOS2 decreases lung tumor growth as well as inflammatory responses initiated by oncogenic KRAS, suggesting that both KRAS and NOS2 cooperate in driving lung tumorigenesis and inflammation. Inhibition of NOS2 may have a therapeutic value in lung cancers with oncogenic KRAS mutations.
Keywords: Lung cancer, KRAS, NOS2, miR-21, inflammation
INTRODUCTION
Lung cancer, the second most common type of cancer in the United States, is also the most common leading cause of cancer-related deaths in both the United States and around the world.1 Non-small-cell lung cancer (NSCLC) accounts for approximately 85% of lung cancers, with adenocarcinomas being the predominant subtype.2 Activating mutations in the KRAS proto-oncogene, found in 10–40% of human lung adenocarcinomas, are considered to be an initiating event for this type of cancer, especially for those with smoking history.2, 3 Activated KRAS regulates multiple downstream pathways, resulting in proliferation, survival, invasion and migration, which can contribute to tumorigenesis.3 Another consequence of this oncogenic Ras signaling is the up-regulation of various pro-inflammatory genes, generating a pro-tumorigenic tumor microenvironment. Oncogenic Ras-induced secretion of IL-8 (CXCL8) and/or IL-6 can promote tumor growth in vivo, and these cytokines are also associated with development of human lung cancer.4–6 Correspondingly, several lung cancer mouse models harboring KRAS mutations showed a robust inflammation in lung characterized by an abundant infiltration of inflammatory cells, along with tumor progression.7–9 In addition, human lung adenocarcinomas harboring KRAS mutations displayed increased tumor-associated inflammation as well as up-regulated IL-8 mRNA levels in tumor specimens.7, 10
MicroRNAs (miRNAs) are small, non-coding RNAs that regulate target gene expression.11, 12 Several miRNAs are aberrantly expressed in most human cancers, possessing tumor suppressive or oncogenic properties.13 Alternatively, oncogenes and tumor suppressor genes exert their activity in part by regulating the expression of specific miRNAs.11, 14 MiR-21 expression is enhanced by EGFR signaling as well as Ras signaling in NSCLC, in agreement with its tumor promoting and anti-apoptotic functions.15–17 Furthermore, inflammatory stimuli can lead to the expression of specific miRNAs, such as miR-21.12 In fact, overexpression of miR-21 was observed in several inflammatory diseases.12 Moreover, pro-inflammatory cytokines, including IL-6 and IFNs can induce miR-21 expression.12, 18 It is noteworthy that inflammatory stimuli can increase oncogenic miR-21 expression which may contribute to inflammation-related carcinogenesis.12, 19
Nitric oxide (NO•), a small and highly diffusible free-radical involved in several physiological functions, is essentially controlled by the three major isoforms of NO• synthase (NOS), including constitutive isoforms NOS1 (neuronal NOS), NOS3 (endothelial NOS) and an inducible isoform, NOS2 (inducible NOS). NOS2 is activated not only in tumor cells but also in tumor-infiltrating cells, leading to the major amounts of generated NO• at nano-micromoler range as a response to inflammatory stimuli, while NOS1 and NOS3 generate small amounts of NO• at pico-nanomoler range.20 Thus, NOS2 is responsible for a high and sustained level of NO• in the tumor microenvironment. The role of NO• is complex and NO• can influence tumor biology as well as inflammation sometimes either positively or negatively depending on the cell types and cellular conditions.20–24 Previously, we also reported that NOS2 deletion in p53 knockout mice can either suppress or enhance lymphoma development depending on the inflammatory microenvironment.25, 26 However, NOS2 up-regulation as well as increased NO• within tumor microenvironment occurs in many solid cancers, which is generally thought to contribute to promoting tumorigenesis.20, 22, 27–29 In fact, NOS2 deletion as well as NOS2 inhibitors can inhibit tumorigenesis in murine tumor models.30–34 In lung cancer, aberrant expression of NOS2 has been observed in tumor, and lung cancer patients exhale elevated NO• levels, along with increased NOS2 content in cancer cells and alveolar macrophages.35, 36 Consistent with those findings, genetic ablation of NOS2 in a urethane-induced lung cancer model inhibited tumor multiplicity and decreased VEGF content.30 By contrast, in their model, NOS2-deficiency did not modulate infiltration of alveolar macrophage when lung inflammation was induced by tumor promoting agent, BHT.30 In addition, several lines of in vivo cancer models, including colon, breast and stomach, indicated that NOS2-deficiency inhibited tumorigenesis, but did not significantly influence tumor-related inflammation.27, 31, 32 Therefore, the role of NOS2 in tumor-related inflammation remains largely unknown. Likewise, the effect of NOS2 deletion in KRAS-driven carcinogenesis also remains to be determined. It is worth noting that NO• is able to mediate oncogenic pathways, inflammatory response and angiogenesis, while oncogenic KRAS is a central regulator of all these pathways.3, 20, 22, 23, 27, 29 Therefore, NOS2-derived NO• may cooperate with Ras signaling in regulating tumor growth and inflammation. We have, for the first time, tested this hypothesis using a mouse model of lung adenocarcinoma in which an oncogenic allele of KRAS is conditionally activated by Cre-recombinase 37. By combining conditional KRAS activation in lung with global NOS2 deletion, we show that loss of NOS2 decreases changes in lung inflammation and tumor growth, resulting in an increased survival in this model. We also demonstrated that miR-21, the transcriptional target of KRAS signaling, was reduced in tumors obtained from KRASG12D;NOS2KO mice, suggesting a mechanistic linkage between KRAS, miR-21 and NOS2 in lung carcinogenesis.
MATERIALS AND METHODS
Mice
LSL-KRASG12D mice37 were crossed with NOS2WT or NOS2KO in the C57BL6 background25 to generate LSL-KRASG12D;NOS2WT and LSL-KRASG12D;NOS2KO mice. To genotype mice, genomic DNAs were extracted from tail clippings and analyzed by PCR using REDExtract-N-Amp Tissue PCR kit (Sigma). Mutant KRAS activation was achieved via intranasal administration of 5x106 pfu of adenoviral Cre-recombinase at 5 weeks after birth 37. Conditional KRASG12D activation and NOS2KO was further confirmed by genomic PCR analysis of tumor DNA macrodissected from paraffin sections using the RecoverAll Total Nucleic Acid Isolation Kit (Ambion) (Supplementary Figure 1A). Multiplex PCR primers for confirmation of KRASG12D were 5’-GTCGACAAGCTCATGCGGGTG-3’, 5’-CCTTTACAAGCGCACGCAGACTGTAGA-3’ and 5’-AGCTAGCCACCATGGCTTGAGTAAGTCTGCA-3’, for NOS2 were 5’-ACATGCAGAATGAGTACCGG-3’, 5’-TCAACATCTCCTGGTGGAAC-3’ and 5’-AATATGCGAAGTGGACCTCG-3’. Absence of NOS2 protein in NOS2KO mice was confirmed in our previous study.25 Also, NOS2 expression was further determined by immunohistochemistry with anti-NOS2 antibody (NeoMarkers) in the lung of KRASG12D;NOS2WT and KRASG12D;NOS2KO mice (Supplementary Figure 1B). Mice were maintained in a climate controlled facility under pathogen free condition at NCI, Frederick. Moribund mice showing weight loss or difficulties in moving or breathing were euthanized upon detection and then routine gross anatomical and histological examinations were performed as described previously.38 At the time of necropsy, the number and size of grossly visible nodules on the surface of the lungs were counted. For subsequent histological evaluation of lung, whole lungs were manually inflated with 10% formalin, and then embedded in paraffin blocks. Longitudinal sections (5μm thick) of lung, including all major lobes, were stained with H&E and evaluated by a board-certified veterinary pathologist. Tumors were categorized as hyperplasia, adenoma and carcinoma based on previously established criteria.39 Severity of inflammation, “pneumonia, acidophilic macrophage” (PAM), was graded into one of five levels, according to the amount and degree of lung involvement.40, 41
Immunohistochemistry and apoptosis assay
Serial tissue sections (5μm) were sliced from paraffin-embedded formalin-fixed lungs. Tumor cell proliferation was evaluated by immunohistochemical staining with anti-Ki-67 (Abcam). Apoptosis was evaluated using TUNEL system (Promega) as described previously.38 Ki-67-stained proliferating cells and TUNEL-stained apoptotic cells were counted in 3 high power fields (HPF) per each carcinoma or 1–3 HPF per each adenoma. We obtained 0–3 carcinomas and 3 adenomas per mouse. The number of stained cells was compared between KRASG12D;NOS2WT mice (n=16) and KRASG12D;NOS2KO mice (n=18) in adenoma and carcinoma, respectively. Antibodies were used according to the manufacturer's instructions and followed with a biotinylated secondary antibody, streptavidin-HRP and DAB visualization as described previously.38 Phospho-Histone H3 (Ser10) (Cell Signaling)-stained mitotic tumor cell nuclei were also counted as described above in lung sections from ten randomly selected KRASG12D;NOS2WT and KRASG12D;NOS2KO mice.
To evaluate leukocyte infiltrates within tumor, serial lung sections from five randomly selected KRASG12D;NOS2WT and KRASG12D;NOS2KO mice were subjected to immunohistochemical staining with anti-F4/80 (Caltag), anti-CD3 (Serotec), anti-Gr-1 (BD), anti-FoxP3 (eBioscience) or anti-CD31 (Santa Cruz), followed by computer-assisted quantification as described earlier.42 Three to four independent and randomly selected tumor fields from each mouse were analyzed and the immunoreactivity of each antibody was compared between KRASG12D;NOS2WT and KRASG12D;NOS2KO mice.
RNA isolation and quantitative reverse transcription (qRT)-PCR analysis
Five-μm FFPE sections obtained from 16 KRASG12D;NOS2WT and 16 KRASG12D;NOS2KO mice that had at least one carcinoma in a longitudinal lung section, were subjected to RNA isolation using the RecoverAll Total Nucleic Acid Isolation Kit (Ambion). By using a sequential section stained with H&E as reference, two to five unstained sections were marked for carcinoma area (1–3 carcinomas per mouse). Each marked area was macrodissected with scalpels for RNA isolation and 40 nanograms of total RNA was used for expression analysis of mature miR-21 and miR-155. qRT-PCR and quantification of δCt values of microRNAs was done as previously described43 using expression levels of small nuclear RNA, snoRNA202, as endogenous normalization control. All assays were performed in triplicate. No sample was omitted since an average Ct value of snoRNA202 was less than 25 in each of all samples.
In situ hybridization (ISH)
In situ hybridization of miR-21 expression was performed on 5-μm FFPE lung sections obtained from five KRASG12D;NOS2WT and six KRASG12D;NOS2KO mice, following the previously described methods with some modifications.43 In brief, deparaffinized slides were incubated in protease K solution (20 μg/ml) for 30 min, and were hybridized at 52°C overnight with 5'-biotin-labeled miR-21 miRCURY LNA detection probe or scramble control probe (Exiqon) in hybridization buffer (Enzo Life Sciences). After hybridization, slides were washed in SSC (invitrogen) and were then exposed to 3%H2O2 and blocked in Protein Block (Dako). The staining was carried out using the GenPoint Tyramide Signal Amplification System (Dako) and DAB as a substrate (brown). Slides were counterstained with hematoxylin (blue) before mounting.
Statistical analysis
Statistical difference between groups was performed using unpaired t test or Mann-Whitney U test (Prism; GraphPad Software, Inc.). P values of <0.05 were considered significant. All data are expressed as mean ± SD.
RESULTS
The absence of NOS2 prolongs survival and decreases KrasG12D-induced lung tumorigenesis
In order to investigate the role of NOS2 in lung cancer driven by the oncogenic KRASG12D allele, we crossed NOS2KO mice with Lox-Stop-Lox (LSL) -KRASG12D mice, in which the expression of oncogenic KRAS is controlled by a removable transcription termination STOP element (Supplementary Figure 1A). LSL-KRASG12D mice rapidly develop a multifocal hyperplasia, followed by adenoma and adenocarcinoma after induction of the KRASG12D allele in the lung epithelium by intranasal instillation of Adenoviral Cre.
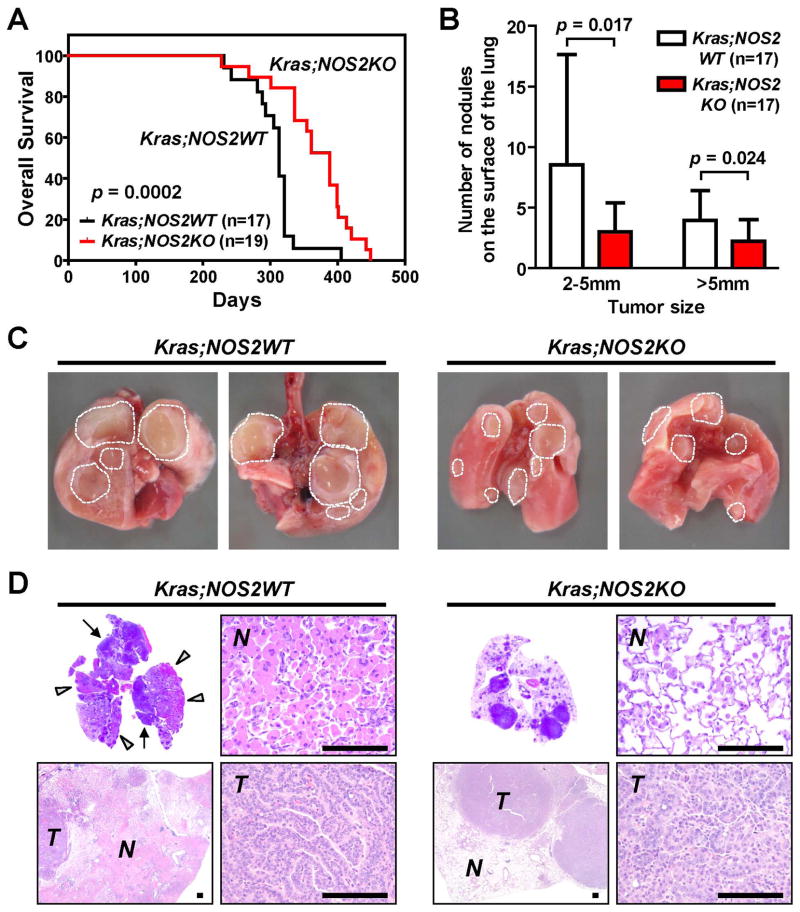
As shown in Fig. 1A, KRASG12D;NOS2KO mice displayed significantly increased survival compared with KRASG12D;NOS2WT mice (p=0.0002). All mice except for 2 KRASG12D;NOS2KO mice showed tumor-related death caused either by lung adenoma/carcinoma- or lung pneumonia-related respiratory failure, while these 2 mice died of skin ulcer (Supplementary Table 1). At the time of necropsy, grossly visible tumors on the surface of the lungs were counted (Figure 1B-C, Supplementary Table 1). Remarkably, the number of tumors of 2–5mm as well as >5mm in size was significantly reduced in KRASG12D;NOS2KO mice (p=0.017, p=0.024, respectively). Subsequent histological analysis revealed that all mice had multifocal lung proliferative lesions, including hyperplasia, adenoma and/or carcinoma. These lung lesions obtained from both groups were pathologically indistinguishable by standard H&E staining (Figure 1D). All KRASG12D;NOS2WT mice (100 %) developed at least one carcinoma in lung; while carcinoma penetrance was 89.5 % in KRASG12D;NOS2KO mice (Supplementary Table 1). These results suggested that NOS2KO might attenuate tumor growth and increase tumor latency, thus, leading to decreased tumor burden and prolonged survival time in KRASG12D;NOS2KO mice.
Figure 1.
Increased survival, delayed lung tumorigenesis and decreased inflammation in KRASG12D;NOS2KO mice. A, KRASG12D;NOS2KO mice showed significantly increased overall survival time compared with KRASG12D ;NOS2WT mice. P-value was evaluated by log-rank test. The median overall survival time was 313 days in KRASG12D;NOS2WT mice, while 388 days in KRASG12D;NOS2KO mice. B, The number of gross tumors on the surface of lung at the time of necropsy. The number of tumor was reduced in KRASG12D;NOS2KO mice (mean ± SD). P-values were evaluated by unpaired t-test. C, Gross finding of lungs isolated from KRASG12D;NOS2WT and KRASG12D;NOS2KO mice. D, Representative cross-sectional H&E histology of lungs from KRASG12D;NOS2WT and KRASG12D;NOS2KO mice. Non-tumor (N) and carcinoma (T) areas are indicated. In KRASG12D;NOS2WT lungs, there are tumor masses (arrows) and multiple scattered nodules adjacent to and/or within large eosinophilic consolidated areas (arrowheads), showing marked lung inflammation characterized by an abundant accumulation of alveolar macrophages. Lung inflammatory change was decreased in KRASG12D;NOS2KO mice (See also Table 1). Bar=200μm.
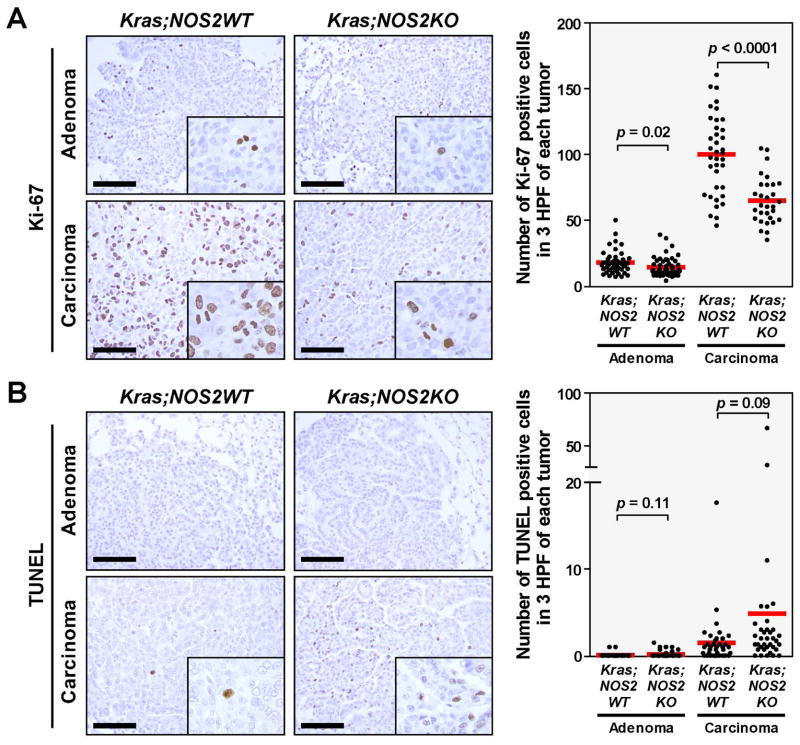
To further investigate the effects of NOS2 in KRAS-induced tumor growth, we examined whether NOS2-deficiency alters the proliferative and apoptotic status of KRAS-induced tumor (Figure 2A and 2B). Tumor cell proliferation in adenomas as well as carcinomas was determined by immunohistochemistry with anti-Ki-67 antibody. As the tumor progressed from adenoma to carcinoma, the number of Ki-67 stained cells markedly increased in both KRASG12D;NOS2WT and KRASG12D;NOS2KO mice (5.7-fold and 4.6-fold increase, respectively). Notably, NOS2-deficiency significantly reduced tumor cell proliferation in both adenomas and carcinomas, as compared to KRASG12D;NOS2WT mice. To confirm the finding of Ki-67 staining, mitotic activity was also assessed by mitotic marker phospho-Histone H3 (Ser10), showing decreased tendency of mitosis in carcinomas from KRASG12D;NOS2KO mice (Supplementary Figure 2). Apoptosis was examined by TUNEL assay. In general, stained cells were rarely observed in adenoma, and occasionally observed in carcinoma. Although a few carcinomas obtained from KRASG12D;NOS2KO mice showed a relatively large number of apoptotic cells, statistical significance was not reached when compared with those of KRASG12D;NOS2WT mice.
Figure 2.
Lung adenomas and carcinomas from KRASG12D;NOS2WT (n=16) and KRASG12D;NOS2KO (n=18) mice were stained with anti-Ki-67 antibody, and subjected to TUNEL assay for the evaluation of proliferation and apoptosis, respectively. Data represent the average number of stained cells in 3 randomly selected high-power fields (HPF) per each tumor. P-values were evaluated by unpaired t-test. Bar=100μm. A, Tumor cell proliferation was significantly decreased in KRASG12D;NOS2KO mice, in both adenoma and carcinoma. B, No significant difference was observed in apoptosis.
Decreased lung inflammation in NOS2 deficient KRASG12D mice
To determine if NOS2-deficiency modulates lung inflammation along with carcinogenesis, we first investigated the lung inflammation in KRASG12D;NOS2WT mice by standard histology on H&E sections. Similar to the previous reports of lung cancer models using CC10-Cre;LSL-KRASG12D mice7, KRASLA2 mice8 and CCSPCre;LSL-KRASG12D mice9, this model also showed striking lung inflammation characterized by an abundant accumulation of alveolar macrophages with variable degrees of granulocyte infiltration (Figure 1D and Supplementary Figure 3). Occasionally, elongated crystalline structures were present in alveolar and bronchiolar spaces. This type of pathological feature has been defined as “pneumonia, acidophilic macrophage (PAM)” and is by far the most common type of lung inflammation in laboratory mice.40, 41 To semi-quantify the extent of inflammatory changes in whole lung area, we evaluated the severity of inflammation by using PAM grade, which assessed the amount and the degree of such lung involvement. Mice were separated into five levels of severity grade as shown in Table 1 and Supplementary Table 1. KRASG12D;NOS2KO mice showed significantly decreased levels of PAM grade compared to KRASG12D;NOS2WT mice (p<0.01) (Figure 1D and Table 1). In addition, the tumor count on the surface of the lungs was positively correlated with PAM grade (Supplementary Figure 4), indicating a cooperative interaction between KRAS-induced tumorigenesis and inflammation. Notably, increased tumor number coinciding with higher PAM grade was more prominently observed in 2–5mm tumors as compared to >5mm tumors (p=0.005 and p=0.024, respectively). Thus, this finding implies that KRAS is responsible for the PAM and that PAM was not caused by large tumor mass-related major airway obstruction followed by infection. Collectively, these results demonstrate that KRAS-induced accumulation of alveolar macrophage is reduced by NOS2-deficiency.
Table 1.
Incidence and severity of pulmonary inflammation (PAMa)
| Group | No. of mice | PAM Grade
|
Averageb | Pc | ||||
|---|---|---|---|---|---|---|---|---|
| + | ++ | +++ | ++++ | +++++ | ||||
| 0.0031 | ||||||||
| Kras;NOS2WT | 16 | 1 | 3 | 9 | 3 | 3.88 | ||
| Kras;NOS2KO | 18 | 2 | 4 | 7 | 5 | 2.83 | ||
Pneumonia, acidophilic macrophage.
Average; (PAM Grade x Number of animals) / Number of animals in group.
P-value was estimated by Mann-Whitney U-test.
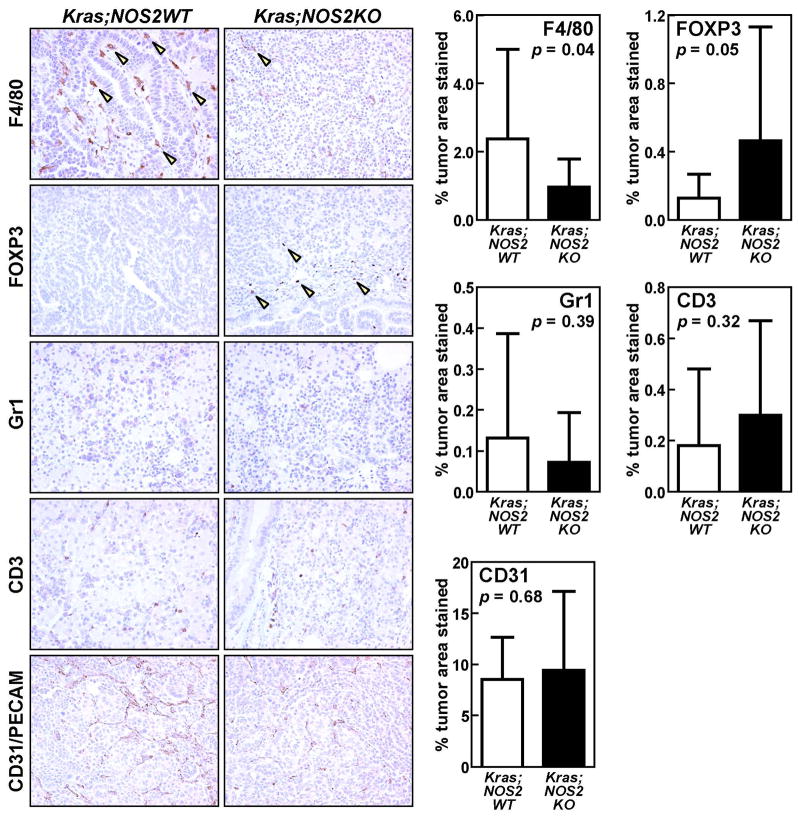
PAM occurred predominantly in the area adjacent to and within proliferative lesions (Supplementary Figure 3), which indicates that KRASG12D activation could mediate leukocyte recruitment into the tumor microenvironment. To better characterize the profile of tumor-infiltrating leukocytes betweenm KRASG12D;NOS2WT and KRASG12D;NOS2KO mice, we performed immunohistochemical analyses on tumors, including F4/80, FOXP3, Gr-1 and CD3 (Figure 3). Since leukocyte infiltration is known to be associated with angiogenesis44, we also performed CD31/PECAM staining to evaluate the tumor vasculature. By computer-assisted quantification of immunoreactive areas, staining of F4/80, a murine macrophage marker, was significantly decreased in tumors of KRASG12D;NOS2KO mice (p=0.04). Although a similar trend of decreased staining of Gr1+ granulocytes was observed in KRASG12D;NOS2KO mice, this did not reach statistical significance. In contrast, FOXP3+ regulatory T cells (Tregs) were moderately increased in tumors of KRASG12D;NOS2KO mice (p=0.05). However, no significant difference was observed between groups in the tumor vasculature assessed by CD31/PECAM staining and the infiltration of CD3+ pan-T cells. Taken together, NOS2-deficiency could suppress both the alveolar macrophage accumulation and the macrophage infiltration into the tumor microenvironment that are induced by oncogenic KRAS in the mouse lung.
Figure 3.
Immunohistochemistry of F4/80+ macrophage, FOXP3+ regulatory T cell (Treg), Gr1+ granulocyte and CD3+ pan T cell infiltration in lung tumors of KRASG12D;NOS2WT (n=5) and KRASG12D;NOS2KO (n=5) mice. Tumor vasculature was also assessed by CD31/PECAM staining. The ratio of immunostained area / total cell area was calculated by computer-assisted quantification in 3–4 independent and randomly selected tumor fields (mean ± SD). KRASG12D;NOS2KO tumors showed decreased infiltration of F4/80+ macrophages (arrowheads) and increased infiltration of FOXP3+ Tregs (arrowheads) compared with KRASG12D;NOS2WT tumors. P-values were evaluated by unpaired t-test.
MicroRNA-21 expression is reduced in lung tumors of KrasG12DNOS2KO mice
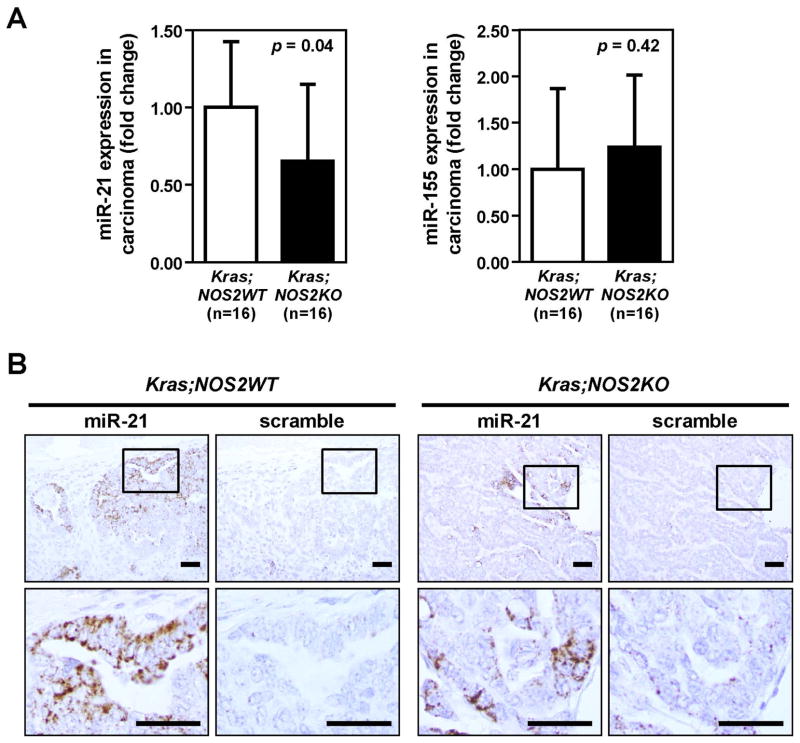
The results demonstrated above indicate that NOS2-deficiency suppresses KRAS-induced tumorigenesis and inflammation. Furthermore, given the recent reports that Ras signaling increases miR-21 expression in vitro and in vivo15–17, we tested the hypothesis that miR-21 expression might be altered in the NOS2KO mice. In addition, miR-21 and miR-155 are considered to be mediators of inflammation-associated carcinogenesis.12 To assess the direct effect of NOS2 deletion in lung, we first examined the expression of miR-21 and miR-155 by qRT-PCR in the whole lung without adenoviral Cre-induced activation of KRASG12D. No difference in miR-21 or miR-155 expression between unactivated-KRASG12D;NOS2WT and unactivated-KRASG12D;NOS2KO mice was found, indicating that NOS2-deficiency alone could not alter the expression of miR-21 or miR-155 in lung (Supplementary Figure 5). We then measured the expression of miR-21 and miR-155 in dissected lung carcinomas obtained from both KRASG12D;NOS2WT and KRASG12D;NOS2KO mice. Figure 4A shows that the expression of miR-21 was significantly lower in carcinomas from KRASG12D;NOS2KO mice than in those from KRASG12D;NOS2WT mice. However, no difference was observed in the expression of miR-155. Next, we performed in situ hybridization (ISH) on lung sections to determine the distribution of miR-21 in lung tumor as well as non-tumor tissue and to confirm the expression of miR-21 as assessed by the qRT-PCR analysis. ISH signals were carefully observed by microscopy in non-tumor, adenoma and carcinoma areas, respectively. Overall, specific miR-21 signals were hardly detectable in non-tumor as well as adenoma areas. In lung carcinoma, a significant amount of miR-21 staining was heterogeneously detected and was exclusively localized in the cytoplasm of cancer cells (Figure 4B and Supplementary Figure 6). These observations of in situ miR-21 staining were consistent with the qRT-PCR results, indicating that NOS2-deficiency decreases miR-21 expression in KRAS-induced lung cancer.
Figure 4.
Reduced miR-21 expression in lung carcinomas from KRASG12D;NOS2KO mice. A, MicroRNA expression in lung adenocarcinomas from KRASG12D;NOS2WT and KRASG12D;NOS2KO mice by quantitative RT-PCR analysis (mean ± SD). Carcinoma areas from KRASG12D;NOS2WT and KRASG12D;NOS2KO mice were macroscopically dissected from parafin-embedded lung sections for RNA isolation. P-values were evaluated by unpaired t-test. B, In situ hybridization of miR-21. Representative images of in situ miR-21 staining in carcinomas from KRASG12D;NOS2WT and KRASG12D;NOS2KO mice. miR-21 positive staining was present only in the cytoplasm of cancer cells. Relatively lower levels of miR-21 signals were found in KRASG12D;NOS2KO carcinoma. Bar=50μm.
DISCUSSION
A substantial proportion of NSCLC, especially in smokers, develops through activating mutations in the KRAS oncogene. Accordingly, KRAS mutations represent a biologically and clinically relevant molecular subset in patients with NSCLC. However, despite years of effort, KRAS-targeting therapies have been unsuccessful. Furthermore, KRAS mutations are known to correlate with poor outcome as well as therapeutic resistance.2, 3 Therefore, a better understanding of the biology associated with KRAS-induced lung cancer is needed. Here, we present evidence that NOS2, which is responsible for a high and sustained level of NO• , contributes not only to KRAS-dependent lung tumorigenesis but also to KRAS-induced lung inflammation in a mouse model of lung cancer. Our findings are consistent with the hypothesis that there exists a link between several key events of carcinogenesis, including oncogenic KRAS, NOS2, miR-21 and cancer-related inflammation.
Our results show that genetic deletion of NOS2 delays tumor growth and the tumors arising in KRASG12D;NOS2KO mice were significantly reduced in size and number coincident with decreased lung inflammation as compared to those in KRASG12D;NOS2WT mice. Since the major cause of death in this model is lung tumor- or pneumonia-induced respiratory failure, it is clear that NOS2-deficiency contributed to favorable survival outcome in KRASG12D;NOS2KO mice. Furthermore, KRASG12D;NOS2KO mice showed a decrease in tumor cell proliferation in adenoma and carcinoma as compared with KRASG12D;NOS2WT mice. Presumably, this is partly due to the fact that NO• posttranslationally regulates a wide range of biological functions, including the activation of oncogenic pathways.27 Indeed, both ERK and Akt, which are involved in the downstream pathways regulated by Ras activation, are also well-studied downstream targets of NO• .21, 22, 27, 28 These reports consistently demonstrated that NO•-mediated ERK and Akt phosphorylation events provide a tumor promoting phenotype. Moreover, a recent study has shown that physiologic concentrations of NO• activate PI3K/Akt and Raf/MEK/ERK signaling pathways mediated by activation of Ras in breast cancer cells.29 Accordingly, our findings may be explained by the fact that NO• can induce tumor cell proliferation through activation of Ras downstream signaling cascades. Since there is evidence that nonselective NOS inhibitors or selective NOS2 inhibitors decrease tumor growth in animal tumor models and human cancer xenograft33, 34, our findings may shed light on the potential of NOS2 as a therapeutic target in lung cancer.
It has also been shown that NO• induces VEGF overexpression and increases angiogenesis.22 However, we found no significant effect of NOS2-deficiency on angiogenesis evaluated by CD31 positive tumor microvasculature. In view of that, the role of NOS2 in angiogenesis in KRAS-induced lung tumorigenesis remains unclear in this animal model.
KRAS induces immune responses by up-regulating the production of chemokines and cytokines, which are key transcriptional targets of Ras and are required for Ras-driven tumorigenesis.4, 6, 45 Consistent with previously reported lung cancer mouse models of KRAS7–9, we observed an abundant accumulation of neutrophils and macrophages accompanied by KRAS-induced lung proliferative lesions in KRASG12D;NOS2WT mice. Notably, in this model, NOS2-deficiency strongly suppressed lung inflammation as characterized by accumulation of intra-alveolar macrophages. Furthermore, we also observed a significant decrease in tumor-infiltrating macrophages stained with F4/80 antibody in KRASG12D;NOS2KO mice. The infiltration of macrophages, which represents one of the hallmarks of tumor-associated inflammation, is predominantly mediated by chemokines and interacts with tumor cells to enhance proliferation, invasion and angiogenesis through a variety of mediators.44 Given that tumor-associated macrophages mostly serve pro-tumorigenic functions and are known to be required for KRAS-induced tumorigenesis, NOS2 may contribute to KRAS-induced inflammation and tumorigenesis at least in part by increasing macrophage recruitment into the tumor microenvironment. This is consistent with the compelling evidence that oncogenic Ras can transactivate certain chemokines that mediate leukocyte recruitment, while NO• is also able to enhance the production of such chemokines.4, 23, 45 Indeed, the high expression of these chemokines has been observed in KRAS-induced lung cancer models.7–9 Taken together, Ras activation and NOS2-derived NO• may cooperate in up-regulation of inflammatory mediators, resulting in enhancement of macrophage recruitment which is required for promoting KRAS-induced lung cancer. However, further mechanistic studies are required to understand the molecular mechanism by which NOS2 and NO• affect immune response associated with oncogenic KRAS signaling pathways.
Specialized subsets of T cells, known as regulatory T cells (Tregs), are essential for the maintenance of self-tolerance by suppressing a wide variety of immune responses, thus playing a critical role in autoimmune disorders as well as in tumor immunity.46 Among several markers that recognize several kinds of Treg subsets, a transcription factor, forkhead box p3 (FOXP3) is the most specific with regard to Treg activity. Recently, FOXP3 has been shown to be negatively regulated by the direct effect of NO• signaling in antigen-primed T cells.47 It is demonstrated that reduction of NO• as well as NOS2 inhibition led to increased FOXP3 expression, while increased NO• reduced the expression of FOXP3, which is consistent with our finding that FOXP3 positive Tregs were increased in NOS2KO mice. However, the mechanistic role of Tregs in our model remains unknown.
miR-21 is known to have oncogenic activities, including high proliferation, low apoptosis, and high invasion and metastasis potential, and has proven to be a useful prognostic indicator in many cancer types, including NSCLC.12, 13, 48, 49 Furthermore, increasing evidence has implicated miR-21 as a key player in Ras-dependent tumorigenesis. Talotta et al. showed that miR-21 is induced by the transcription factor AP-1 in response to Ras in in vitro.50 It has further revealed that miR-21 induction by Ras requires the activation of at least two different Ras downstream pathways, MAPK and PI3K pathways.16 Significantly, Hatley et al. demonstrated an in vivo autoregulatory loop between oncogenic Ras and miR-21 by using gain-of-function transgenic mice and loss-of-function knockout mice of miR-21 allele in combination with the KRASLA2 lung cancer mouse model.17 The authors showed that Ras activation increases the expression of miR-21, and in turn, miR-21 enhances tumor proliferation and survival by targeting both antagonists of Ras signaling pathways and proapoptotic genes. In the present study, NOS2-deficiency alone did not alter miR-21 expression in lung without KRAS activation, but resulted in marked reduction in both proliferative tumor cells and miR-21 expression in activated KRAS-induced tumor. This implies that NOS2 may alter miR-21 expression dependent on the activation of KRAS. Given that miR-21 as a downstream target of Ras, it is possible that NOS2-derived NO• regulates miR-21 expression as well as increases tumor growth at least in part by the activation of Ras signaling pathways. Further studies are required to elucidate the molecular mechanisms of NO•-mediated microRNAs regulation in relation to oncogenic pathways as well as inflammatory responses.
In conclusion, this is the first examination of KRAS-induced lung carcinogenesis specifically related to NOS2 in vivo by using a genetic strategy. Here we demonstrate that NOS2 significantly contributes to KRAS-induced lung cancer. In this lung cancer model, KRASG12D;NOS2KO mice showed delayed tumor growth, which is accompanied by reduced tumor cell proliferation and decreased expression of oncogenic microRNA, miR-21. Moreover, NOS2-deletion led to reduced inflammation as characterized by infiltration of macrophage into both alveoli and within tumors. Given that both oncogenic KRAS and NOS2 may cooperate in driving the lung tumorigenesis and inflammatory response and that NOS2 is aberrantly expressed in lung cancer, NOS2 could be a potential target for KRAS-induced lung cancer therapy.
Supplementary Material
Brief Description.
This is the first study demonstrating the contribution of NOS2 to KRAS-induced lung cancer in vivo by using a genetic strategy. NOS2-deficiency inhibited KRAS-induced tumorigenesis and decreased oncogenic miR-21 expression. Furthermore, KRAS-induced lung inflammation was also suppressed by NOS2-deficiency. Because NOS2 is frequently over-expressed in human lung cancer and NOS2-derived NO• plays a significant role in this animal model of lung cancer, NOS2 could be a potential target for KRAS-induced lung cancer.
Acknowledgments
We thank Drs. Tyler Jacks and Kim Mercer for providing mice and technical advice. We also thank Drs. Glenwood E. Trivers, Draginja Djurickovic, Aaron Schetter and Izumi Horikawa for technical advice, Eleazar Vega-Valle for nasal instillation and Terry Sweeney for maintaining the mouse colony, Dr. Diana Haines for histological diagnosis, Noralyn Dudt for editorial advice, and Leonidas Leondaridis for developing the database. This research was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
Abbreviations used
- NOS
nitric oxide synthese
- NO•
nitric oxide
- KO
knockout
- WT
wild-type
- NSCLC
non-small cell lung cancer
- PAM
pneumonia, acidophilic macrophage
- Treg
regulatory T cell
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–80. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 4.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–58. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng YL, Bowman ED, Engels EA, Caporaso NE, Harris CC. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J Natl Cancer Inst. 2011;103:1112–22. doi: 10.1093/jnci/djr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ancrile B, Lim KH, Counter CM. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 2007;21:1714–9. doi: 10.1101/gad.1549407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji H, Houghton AM, Mariani TJ, Perera S, Kim CB, Padera R, Tonon G, McNamara K, Marconcini LA, Hezel A, El-Bardeesy N, Bronson RT, et al. K-ras activation generates an inflammatory response in lung tumors. Oncogene. 2006;25:2105–12. doi: 10.1038/sj.onc.1209237. [DOI] [PubMed] [Google Scholar]
- 8.Wislez M, Spencer ML, Izzo JG, Juroske DM, Balhara K, Cody DD, Price RE, Hittelman WN, Wistuba, Kurie JM. Inhibition of mammalian target of rapamycin reverses alveolar epithelial neoplasia induced by oncogenic K-ras. Cancer Res. 2005;65:3226–35. doi: 10.1158/0008-5472.CAN-04-4420. [DOI] [PubMed] [Google Scholar]
- 9.Moghaddam SJ, Li H, Cho SN, Dishop MK, Wistuba, Ji L, Kurie JM, Dickey BF, Demayo FJ. Promotion of lung carcinogenesis by chronic obstructive pulmonary disease-like airway inflammation in a K-ras-induced mouse model. Am J Respir Cell Mol Biol. 2009;40:443–53. doi: 10.1165/rcmb.2008-0198OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sunaga N, Imai H, Shimizu K, Shames DS, Kakegawa S, Girard L, Sato M, Kaira K, Ishizuka T, Gazdar AF, Minna JD, Mori M. Oncogenic KRAS-induced interleukin-8 overexpression promotes cell growth and migration and contributes to aggressive phenotypes of non-small cell lung cancer. Int J Cancer. 2012;130:1733–44. doi: 10.1002/ijc.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 12.Schetter AJ, Heegaard NH, Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–61. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujita K, Mondal AM, Horikawa I, Nguyen GH, Kumamoto K, Sohn JJ, Bowman ED, Mathe EA, Schetter AJ, Pine SR, Ji H, Vojtesek B, et al. p53 isoforms Delta133p53 and p53beta are endogenous regulators of replicative cellular senescence. Nat Cell Biol. 2009;11:1135–42. doi: 10.1038/ncb1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seike M, Goto A, Okano T, Bowman ED, Schetter AJ, Horikawa I, Mathe EA, Jen J, Yang P, Sugimura H, Gemma A, Kudoh S, et al. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc Natl Acad Sci U S A. 2009;106:12085–90. doi: 10.1073/pnas.0905234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frezzetti D, De Menna M, Zoppoli P, Guerra C, Ferraro A, Bello AM, De Luca P, Calabrese C, Fusco A, Ceccarelli M, Zollo M, Barbacid M, et al. Upregulation of miR-21 by Ras in vivo and its role in tumor growth. Oncogene. 2011;30:275–86. doi: 10.1038/onc.2010.416. [DOI] [PubMed] [Google Scholar]
- 17.Hatley ME, Patrick DM, Garcia MR, Richardson JA, Bassel-Duby R, van Rooij E, Olson EN. Modulation of K-Ras-dependent lung tumorigenesis by MicroRNA-21. Cancer Cell. 2010;18:282–93. doi: 10.1016/j.ccr.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang CH, Yue J, Fan M, Pfeffer LM. IFN induces miR-21 through a signal transducer and activator of transcription 3-dependent pathway as a suppressive negative feedback on IFN-induced apoptosis. Cancer Res. 2010;70:8108–16. doi: 10.1158/0008-5472.CAN-10-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathe E, Nguyen GH, Funamizu N, He P, Moake M, Croce CM, Hussain SP. Inflammation regulates microRNA expression in cooperation with p53 and nitric oxide. Int J Cancer. 2011 doi: 10.1002/ijc.26403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanwar JR, Kanwar RK, Burrow H, Baratchi S. Recent advances on the roles of NO in cancer and chronic inflammatory disorders. Curr Med Chem. 2009;16:2373–94. doi: 10.2174/092986709788682155. [DOI] [PubMed] [Google Scholar]
- 21.Weiss JM, Ridnour LA, Back T, Hussain SP, He P, Maciag AE, Keefer LK, Murphy WJ, Harris CC, Wink DA, Wiltrout RH. Macrophage-dependent nitric oxide expression regulates tumor cell detachment and metastasis after IL-2/anti-CD40 immunotherapy. J Exp Med. 2010;207:2455–67. doi: 10.1084/jem.20100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas DD, Espey MG, Ridnour LA, Hofseth LJ, Mancardi D, Harris CC, Wink DA. Hypoxic inducible factor 1alpha, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide. Proc Natl Acad Sci U S A. 2004;101:8894–9. doi: 10.1073/pnas.0400453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi Y. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. J Leukoc Biol. 2010;88:1157–62. doi: 10.1189/jlb.0310149. [DOI] [PubMed] [Google Scholar]
- 24.Ambs S, Merriam WG, Ogunfusika MO, Bennett WP, Ishibe N, Hussain SP, Tzeng EE, Geller DA, Billiar TR, Harris CC. p53 and vascular endothelial growth factor regulate tumor growth of NOS2-expressing human carcinoma cells. Nat Med. 1998;4:1371–6. doi: 10.1038/3957. [DOI] [PubMed] [Google Scholar]
- 25.Hussain SP, Trivers GE, Hofseth LJ, He P, Shaikh I, Mechanic LE, Doja S, Jiang W, Subleski J, Shorts L, Haines D, Laubach VE, et al. Nitric oxide, a mediator of inflammation, suppresses tumorigenesis. Cancer Res. 2004;64:6849–53. doi: 10.1158/0008-5472.CAN-04-2201. [DOI] [PubMed] [Google Scholar]
- 26.Hussain SP, He P, Subleski J, Hofseth LJ, Trivers GE, Mechanic L, Hofseth AB, Bernard M, Schwank J, Nguyen G, Mathe E, Djurickovic D, et al. Nitric oxide is a key component in inflammation-accelerated tumorigenesis. Cancer Res. 2008;68:7130–6. doi: 10.1158/0008-5472.CAN-08-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ambs S, Glynn SA. Candidate pathways linking inducible nitric oxide synthase to a basal-like transcription pattern and tumor progression in human breast cancer. Cell Cycle. 2011;10:619–24. doi: 10.4161/cc.10.4.14864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prueitt RL, Boersma BJ, Howe TM, Goodman JE, Thomas DD, Ying L, Pfiester CM, Yfantis HG, Cottrell JR, Lee DH, Remaley AT, Hofseth LJ, et al. Inflammation and IGF-I activate the Akt pathway in breast cancer. Int J Cancer. 2007;120:796–805. doi: 10.1002/ijc.22336. [DOI] [PubMed] [Google Scholar]
- 29.Pervin S, Singh R, Hernandez E, Wu G, Chaudhuri G. Nitric oxide in physiologic concentrations targets the translational machinery to increase the proliferation of human breast cancer cells: involvement of mammalian target of rapamycin/eIF4E pathway. Cancer Res. 2007;67:289–99. doi: 10.1158/0008-5472.CAN-05-4623. [DOI] [PubMed] [Google Scholar]
- 30.Kisley LR, Barrett BS, Bauer AK, Dwyer-Nield LD, Barthel B, Meyer AM, Thompson DC, Malkinson AM. Genetic ablation of inducible nitric oxide synthase decreases mouse lung tumorigenesis. Cancer Res. 2002;62:6850–6. [PubMed] [Google Scholar]
- 31.Nam KT, Oh SY, Ahn B, Kim YB, Jang DD, Yang KH, Hahm KB, Kim DY. Decreased Helicobacter pylori associated gastric carcinogenesis in mice lacking inducible nitric oxide synthase. Gut. 2004;53:1250–5. doi: 10.1136/gut.2003.030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellies LG, Fishman M, Hardison J, Kleeman J, Maglione JE, Manner CK, Cardiff RD, MacLeod CL. Mammary tumor latency is increased in mice lacking the inducible nitric oxide synthase. Int J Cancer. 2003;106:1–7. doi: 10.1002/ijc.11178. [DOI] [PubMed] [Google Scholar]
- 33.Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer. 2006;6:521–34. doi: 10.1038/nrc1910. [DOI] [PubMed] [Google Scholar]
- 34.Hirst D, Robson T. Nitric oxide in cancer therapeutics: interaction with cytotoxic chemotherapy. Curr Pharm Des. 2010;16:411–20. doi: 10.2174/138161210790232185. [DOI] [PubMed] [Google Scholar]
- 35.Liu CY, Wang CH, Chen TC, Lin HC, Yu CT, Kuo HP. Increased level of exhaled nitric oxide and up-regulation of inducible nitric oxide synthase in patients with primary lung cancer. Br J Cancer. 1998;78:534–41. doi: 10.1038/bjc.1998.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marrogi AJ, Travis WD, Welsh JA, Khan MA, Rahim H, Tazelaar H, Pairolero P, Trastek V, Jett J, Caporaso NE, Liotta LA, Harris CC. Nitric oxide synthase, cyclooxygenase 2, and vascular endothelial growth factor in the angiogenesis of non-small cell lung carcinoma. Clin Cancer Res. 2000;6:4739–44. [PubMed] [Google Scholar]
- 37.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, Tuveson DA. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–8. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saito M, Kumamoto K, Robles AI, Horikawa I, Furusato B, Okamura S, Goto A, Yamashita T, Nagashima M, Lee TL, Baxendale VJ, Rennert OM, et al. Targeted disruption of Ing2 results in defective spermatogenesis and development of soft-tissue sarcomas. PLoS One. 2010;5:e15541. doi: 10.1371/journal.pone.0015541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nikitin AY, Alcaraz A, Anver MR, Bronson RT, Cardiff RD, Dixon D, Fraire AE, Gabrielson EW, Gunning WT, Haines DC, Kaufman MH, Linnoila RI, et al. Classification of proliferative pulmonary lesions of the mouse: recommendations of the mouse models of human cancers consortium. Cancer Res. 2004;64:2307–16. doi: 10.1158/0008-5472.can-03-3376. [DOI] [PubMed] [Google Scholar]
- 40.Murray AB, Luz A. Acidophilic macrophage pneumonia in laboratory mice. Vet Pathol. 1990;27:274–81. doi: 10.1177/030098589002700409. [DOI] [PubMed] [Google Scholar]
- 41.Hoenerhoff MJ, Starost MF, Ward JM. Eosinophilic crystalline pneumonia as a major cause of death in 129S4/SvJae mice. Vet Pathol. 2006;43:682–8. doi: 10.1354/vp.43-5-682. [DOI] [PubMed] [Google Scholar]
- 42.Weiss JM, Back TC, Scarzello AJ, Subleski JJ, Hall VL, Stauffer JK, Chen X, Micic D, Alderson K, Murphy WJ, Wiltrout RH. Successful immunotherapy with IL-2/anti-CD40 induces the chemokine-mediated mitigation of an immunosuppressive tumor microenvironment. Proc Natl Acad Sci U S A. 2009;106:19455–60. doi: 10.1073/pnas.0909474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voortman J, Goto A, Mendiboure J, Sohn JJ, Schetter AJ, Saito M, Dunant A, Pham TC, Petrini I, Lee A, Khan MA, Hainaut P, et al. MicroRNA expression and clinical outcomes in patients treated with adjuvant chemotherapy after complete resection of non-small cell lung carcinoma. Cancer Res. 2010;70:8288–98. doi: 10.1158/0008-5472.CAN-10-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 45.Yang G, Rosen DG, Zhang Z, Bast RC, Jr, Mills GB, Colacino JA, Mercado-Uribe I, Liu J. The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc Natl Acad Sci U S A. 2006;103:16472–7. doi: 10.1073/pnas.0605752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mougiakakos D, Choudhury A, Lladser A, Kiessling R, Johansson CC. Regulatory T cells in cancer. Adv Cancer Res. 2010;107:57–117. doi: 10.1016/S0065-230X(10)07003-X. [DOI] [PubMed] [Google Scholar]
- 47.Brahmachari S, Pahan K. Myelin basic protein priming reduces the expression of Foxp3 in T cells via nitric oxide. J Immunol. 2010;184:1799–809. doi: 10.4049/jimmunol.0804394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saito M, Schetter AJ, Mollerup S, Kohno T, Skaug V, Bowman ED, Mathe EA, Takenoshita S, Yokota J, Haugen A, Harris CC. The association of microRNA expression with prognosis and progression in early-stage, non-small cell lung adenocarcinoma: a retrospective analysis of three cohorts. Clin Cancer Res. 2011;17:1875–82. doi: 10.1158/1078-0432.CCR-10-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, Calin GA, Liu CG, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–98. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 50.Talotta F, Cimmino A, Matarazzo MR, Casalino L, De Vita G, D'Esposito M, Di Lauro R, Verde P. An autoregulatory loop mediated by miR-21 and PDCD4 controls the AP-1 activity in RAS transformation. Oncogene. 2009;28:73–84. doi: 10.1038/onc.2008.370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.