Abstract
Antibodies against adeno-associated viral (AAV) vectors are highly prevalent in humans. Both preclinical and clinical studies showed that antibodies against AAV block transduction even at low titers, particularly when the vector is introduced into the bloodstream. Here we measured the neutralizing antibody (NAb) titer against AAV serotypes 2, 5, 6 and 8 in the serum and matched synovial fluid (SF) from rheumatoid arthritis patients. The titer in the SF was lower than that in the matched plasma samples, indicating a difference in distribution of NAb to AAV depending on the body fluid compartment. This difference was more evident for AAV2, against which higher titers were measured. Of all serotypes, anti-AAV5 antibodies were the least prevalent in both the serum and SF. We next evaluated the impact of B-cell depletion on anti-AAV antibodies in rheumatoid arthritis patients who received one or two courses of the anti-CD20 antibody rituximab as part of their disease management. A drop of NAb titer was observed in a subset of those subjects carrying NAb titers ⩽1:1000; however, only in a minority of subjects titers dropped below 1:5. This work provides insights into strategies to overcome the limitation of pre-existing humoral immunity to AAV vectors.
Keywords: adeno-associated virus vectors, neutralizing antibodies, synovial tissue
Introduction
Rheumatoid arthritis (RA) is a chronic immune-mediated inflammatory disease of unknown etiology, affecting about 1% of the population worldwide.1 RA is characterized by inflammation of the synovium leading to progressive destruction of cartilage and bone.2 Although all joints can be affected, hands, feet and knees are the most commonly affected joints. The primary manifestations of RA are pain, swelling and limited motion of joints.
Over the past several years, remarkable progress has been made in the development of effective gene transfer strategies for the treatment of inherited and metabolic diseases, with some of the most exciting results of in vivo gene transfer in humans obtained using recombinant adeno-associated virus (AAV) vectors.3 For RA, most of the pre-clinical and clinical studies have focused on the delivery of therapeutic genes locally in the inflamed joint.4, 5, 6 First in human studies for the local delivery of a soluble tumor necrosis factor-α receptor to the synovial tissue of RA patients focused on the use of AAV2 vectors.7, 8 More recently, preclinical in vitro and in vivo studies identified AAV5 as one of the most efficient serotypes for the transduction of synovial tissue, especially fibroblast-like synoviocytes.9, 10 Currently, the use of an AAV5 vector expressing the anti-inflammatory cytokine interferon-β is under development for local gene therapy in patients with RA.6
Humoral immunity against AAV vectors represents an important barrier to intravascular gene transfer, resulting in clearance of the vector before it enters the target cell.11, 12 Antibodies directed against the AAV capsid are highly prevalent in humans, a natural host for this virus, and crossreact with a wide range of serotypes because of the degree of homology of capsid protein sequence.13 As a result, even relatively low titers of neutralizing antibodies (NAbs) can block AAV transduction when the vector is introduced into the bloodstream. Conversely, gene transfer to the eye, the brain or direct intramuscular delivery of AAV vectors seems to be less susceptible to neutralization by NAb.11, 12, 14, 15, 16, 17
NAbs to AAV are found in synovial fluid (SF) and have the potential to inhibit vector-mediated transduction in a serotype-dependent manner.18, 19 However, little is known about the NAb levels against different serotypes in the SF of RA patients (likely to have inflamed and potentially damaged joints) and the relationship between anti-AAV NAb titer in the serum vs SF. Finally, as NAb can efficiently block AAV-mediated transduction in vivo, strategies to overcome humoral immunity to the viral capsid are of great importance to achieve successful gene transfer.
Here we measured the neutralizing activity of matched serum and SF samples against a range of AAV serotypes using different antibody screening methods; anti-AAV immunoglobulin G (IgG) subclasses were also analyzed. Results from this screening suggest that NAb titers measured in the serum are generally higher than those of the SF. We also evaluated in RA patients the effect on the anti-AAV NAb titer of single or repeated courses of immunosuppression with the CD20+ B-cell-depleting antibody rituximab. Our results suggest that the use of AAV serotypes with low NAb prevalence, combined with the use of pharmacological modulation of B-cell responses, may help achieving effective gene transfer in subjects who would otherwise not be eligible for enrollment in AAV gene transfer trials.
Results
Prevalence of anti-AAV antibodies in matched sera and SF samples
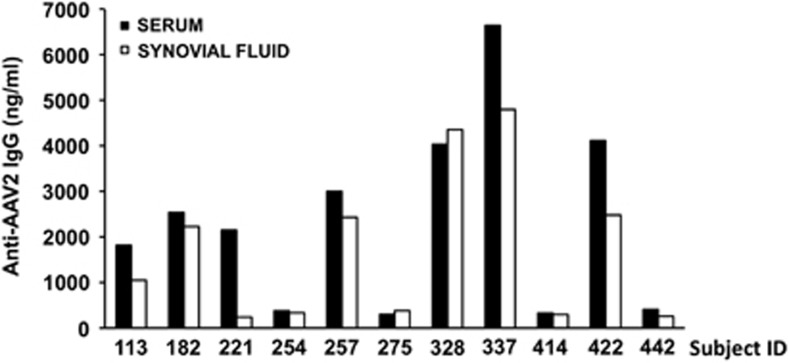
Previous reports18, 19 show that anti-AAV antibodies are present in SF. To test the relationship between the prevalence of antibodies in the serum and SF, matched samples were collected from 11 individuals affected by RA. Initial screening with a capture assay for the detection of AAV2-specific total IgG showed a statistically significant (P=0.0284, paired t-test) difference between systemic levels of IgG in the serum vs local anti-AAV IgG levels in the SF, with serum samples generally carrying a modestly higher titer (Figure 1).
Figure 1.
Anti-AAV2 total IgG titer in the serum and synovial fluid from subjects affected by rheumatoid arthritis. Total anti-AAV2 IgG were measured with a capture assay; results are expressed in ng ml−1. For each sample, the average of a duplicate reading is reported.
Antibody subclass analysis in the same set of samples confirmed initial findings for most of the subjects; median difference in anti-AAV IgG1 and IgG2 titers were higher in the serum vs SF in most subjects, whereas IgG4 antibody analysis showed an opposite trend (Table 1).
Table 1. Anti-AAV2 Ig subclass analysis in the serum and synovial fluid of RA patients.
| Subject ID |
IgG1 (ng ml−1) |
IgG2 (ng ml−1) |
IgG3 (ng ml−1) |
IgG4 (ng ml−1) |
IgM (ng ml−1) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Serum | Synovial fluid | Serum | Synovial fluid | Serum | Synovial fluid | Serum | Synovial fluid | Serum | Synovial fluid | |
| 113 | 9059 | 7401 | 4267 | 5620 | 711 | 608 | 1400 | 5038 | 773 | 658 |
| 182 | 36 039 | 15 886 | 4942 | 2027 | 1570 | 701 | 677 | 470 | 664 | 674 |
| 221 | 45 191 | 5351 | 20 916 | 1818 | 9854 | 437 | 2168 | 647 | 837 | 289 |
| 254 | 2679 | 2134 | 832 | 425 | 545 | 584 | 0 | 0 | 514 | 424 |
| 257 | 54 196 | 49 467 | 18 265 | 12 752 | 8389 | 9324 | 4120 | 5533 | 895 | 858 |
| 275 | 2242 | 2568 | 1074 | 817 | 1548 | 1987 | 1265 | 2013 | 427 | 374 |
| 328 | 118 664 | 114 876 | 30 582 | 10 127 | 12 653 | 12 684 | 10 073 | 13 704 | 1662 | 1520 |
| 337 | 108 415 | NT | 12 120 | NT | 5973 | NT | 719 | NT | 1147 | NT |
| 414 | 2542 | NT | 400 | NT | 664 | NT | 0 | NT | 415 | 390 |
| 422 | 67 016 | 38 844 | 6585 | 7621 | 1086 | 4868 | 1182 | 5421 | 1052 | 873 |
| 442 | 2424 | 2868 | 403 | 765 | 399 | 868 | 0 | 262 | 311 | 262 |
| Median difference | 3788 | 407 | −39 | −748 | 72 | |||||
Abbreviations: AAV2, adeno-associated virus 2; IgG, immunoglobulin G; NT, not tested; RA, rheumatoid arthritis.
Next, we measured the NAb titer of sera and matched SF samples towards AAV2, AAV5, AAV6 and AAV8. NAb titer was determined using an in vitro assay described previously,12 in which residual activity of the β-galactosidase reporter gene was measured as a surrogate for AAV vector neutralization by NAb.
NAb titers in the serum and SF confirmed the results of the capture assay used to measure total anti-AAV IgG, that is, anti-AAV NAb titers were higher in the serum than in SF (Table 2). Differences in anti-AAV NAbs were more obvious for AAV2, as NAb titers were generally higher than the other serotype tested and fell within the limit of detection of the assay. Anti-AAV NAb had the highest neutralizing titer against AAV2, with 0/11 and 1/11 samples showing titers <1:3.16 in the serum and SF, respectively. Anti-AAV5 NAb titers were the lowest, with 7/11 and 8/11 samples falling below 1:3.16 in the serum and SF, respectively. Anti-AAV6 NAb titers were also generally low, although higher than AAV5, with 1/11 and 2/10 samples falling below 1:3.16 in the serum and SF, respectively. Finally, Anti-AAV8 NAb titer profile was similar to AAV6, with 1/10 and 1/10 samples falling below 1:3.16 in the serum and SF, respectively.
Table 2. Anti-AAV NAb in the serum and synovial fluid of RA patients.
| Subject ID | Anti-AAV2 NAb | Anti-AAV5 NAb | Anti-AAV6 NAb | Anti-AAV8 NAb | ||||
|---|---|---|---|---|---|---|---|---|
| |
Serum |
Synovial fluid |
Serum |
Synovial fluid |
Serum |
Synovial fluid |
Serum |
Synovial fluid |
| 113 | 1:10 | 1:3.16 | <1:1 | <1:1 | 1:10 | 1:3.16 | 1:10 | 1:3.16 |
| 182 | 1:316 | 1:100 | 1:3.16 | <1:3.16 | 1:10 | 1:10 | 1:10 | 1:3.16 |
| 221 | 1:100 | 1:3.16 | <1:3.16 | <1:3.16 | 1:10 | <1:1 | NT | 1:3.16 |
| 254 | 1:10 | 1:3.16 | <1:1 | <1:1 | 1:3.16 | 1:3.16 | 1:3.16 | 1:3.16 |
| 257 | >1000 | 1:31.6 | <1:3.16 | 1:1 | 1:10 | 1:3.16 | 1:3.16 | 1:3.16 |
| 275 | 1:3.16 | <1:1 | <1:3.16 | <1:3.16 | <1:3.16 | <1:1 | <1:3.16 | <1:3.16 |
| 328 | >1:1000 | >1:1000 | 1:100 | 1:100 | 1:316 | 1:100 | 1:316 | 1:316 |
| 337 | >1:1000 | >1:1000 | 1:10 | 1:10 | 1:10 | NT | 1:100 | NT |
| 414 | 1:3.16 | 1:3.16 | <1:1 | <1:1 | 1:3.16 | 1:3.16 | 1:3.16 | 1:3.16 |
| 422 | >1:1000 | >1:1000 | 1:31.6 | 1:10 | 1:100 | 1:10 | 1:31.6 | 1:10 |
| 442 | 1:10 | 1:3.16 | 1:1 | <1:1 | 1:3.16 | 1:3.16 | 1:3.16 | 1:3.16 |
Abbreviations: AAV, adeno-associated virus; NAb, neutralizing antibody; NT, not tested; RA, rheumatoid arthritis.
Anti-AAV NAb titer determination is influenced by the efficiency of detection of the reporter gene used in the assay
One of the major limitations of in vitro NAb assays is that they measure cell transduction and residual reporter gene expression as a surrogate for the neutralization activity of a test sample. These assays work efficiently with AAV2, one of the few serotypes that transduces cells in vitro at high levels. Conversely, serotypes like AAV8, which is known to perform very poorly in vitro, require greater multiplicity of infection to deliver a detectable reporter transgene signal, resulting in a lower sensitivity of the assay, particularly at low NAb titers, when anti-AAV antibodies in the test serum are limiting.
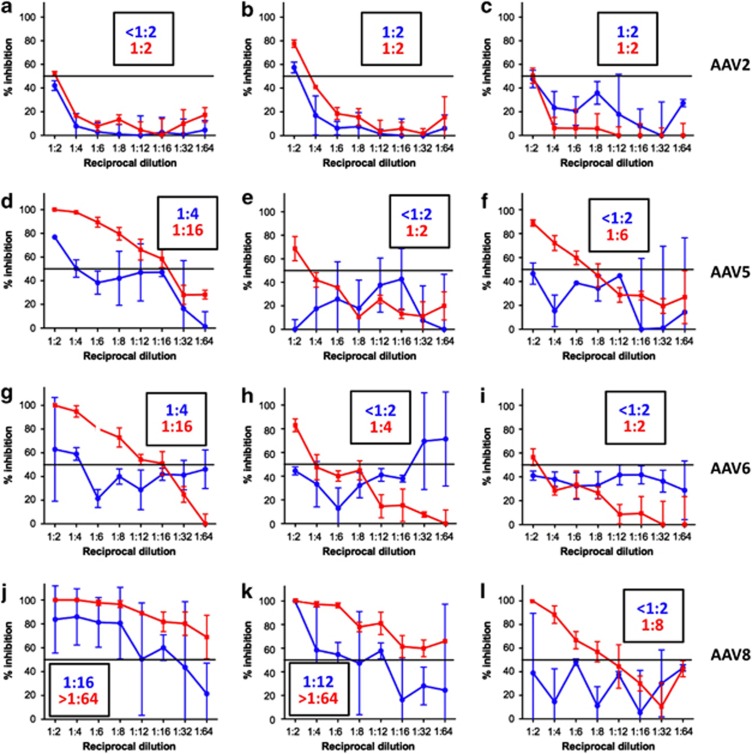
To improve the sensitivity of detection of NAb in vitro in a cell culture-based assay, we switched the AAV transgene expression cassette from a single-stranded genome expressing β-galactosidase (ssAAV-LacZ) to a self-complementary genome20, 21 expressing the luciferase reporter transgene (scAAV-Luc). The higher efficiency of transduction of self-complementary AAV in vitro compared with single-stranded AAV, and the higher sensitivity of detection of luciferase over β-galactosidase, allowed the use of multiplicity of infections 50- to 150-fold lower in the NAb assay. To test the efficiency of the improved assay, we rescreened various serum samples with known NAb titers (all <1:100) using the two assays (Figure 2). As expected, there was no significant difference in the anti-AAV-2 NAb titer resulting from the two assays, reflecting the high efficiency of cell transduction of this AAV serotype in vitro (Figures 2a–c). For AAV5, 6 and 8, much less efficient in transducing cells in vitro, the NAb titers in the serum measured with the ssAAV-LacZ vectors were lower than that with the scAAV-Luc vectors (Figures 2d–l). For these serotypes, the limited sensitivity and the high background noise measured in the LacZ-based assay resulted in a high variability of optical density readings for each dilution (Figure 2). NAb titers (a representative set of a larger number of experiments performed), determined as the serum dilution at which 50% of inhibition of reporter gene expression is measured, are reported in Figure 2 for both the β-galactosidase and the luciferase-based assays. These data indicate that for AAV serotype other than AAV2, the choice of the reporter gene system is a crucial determinant of the sensitivity of the NAb assay.
Figure 2.
Comparison of anti-AAV NAb titer determinations using ssAAV-LacZ or scAAV-Luc reporter vectors. The assays shown are a representation of a larger set. A different serum sample from an individual donor was used in each plot. Anti-AAV NAb titers were determined using an in vitro assay in which an ssAAV-LacZ (blue lines) or scAAV-Luc (red lines) reporter transgene vectors were used. (a–c) Anti-AAV2 NAb; (d–f) anti-AAV5; (g–i) anti-AAV6; and (j–l) anti-AAV8 NAb titer determinations. Results are reported as % inhibition of the reporter gene signal (y axis) after incubation of the reporter AAV vector with each given dilution of the serum (x axis). Horizontal lines represent an inhibition of reporter signal of 50%. Titers reported in each graph represent the serum dilution at which the inhibition of the reporter signal fell below 50% blue numbers, titers obtained with the ssAAV-LacZ vector; red numbers, titers obtained with the scAAV-Luc vector. Error bars represent the standard deviation of the average of triplicate readings.
B-cell depletion with rituximab results in partial reduction of anti-AAV NAb levels
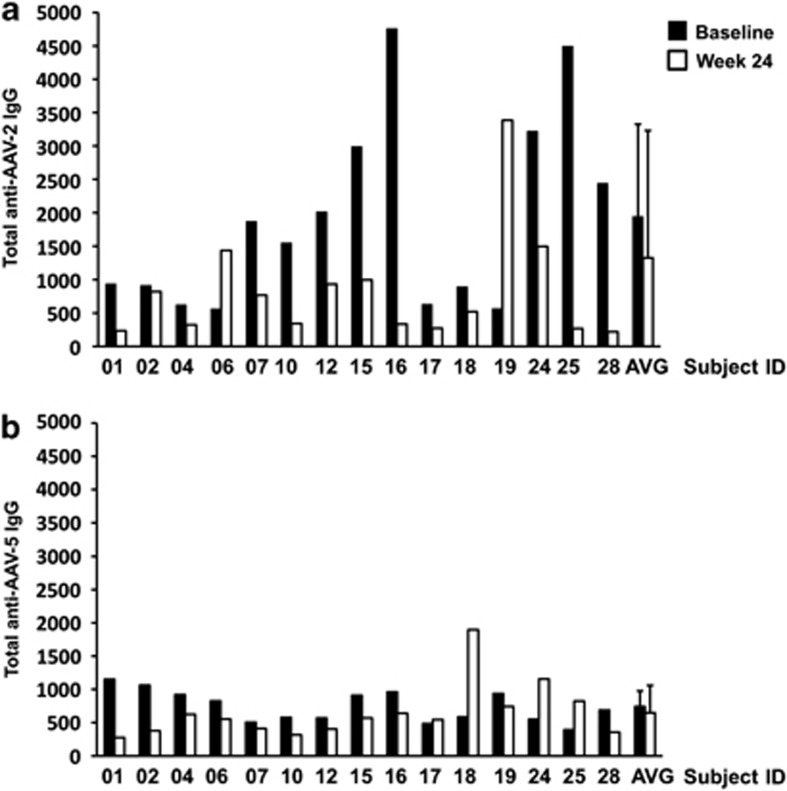
To test the efficacy of B-cell depletion with the anti-CD20 antibody rituximab, a series of 28 RA subjects receiving two intravenous infusions of 1000 mg of the drug 2 weeks apart were selected. All subjects were also stable on methothrexate, a cytotoxic drug with known antiproliferative effects on both B and T cells. Following rituximab administration, B cells (monitored in peripheral blood by staining for CD19) disappeared from the circulation for several weeks and began reappearing around week 24 post-rituximab infusion.22 Serum samples were analyzed for both non-NAb and NAb to AAV; no matched SF was available from these subjects. However, anti-AAV antibody titer determination in matched serum and SF samples suggests that NAb titer in the serum is representative of that in the SF (vide supra). Total anti-capsid IgG to both AAV2 and AAV5 were measured in a subset of subjects (n=15) before rituximab administration and 24 weeks after the course was given in these patients, a time point when maximum therapeutic benefit (that is, disease activity score reduction) was observed.22 For both serotypes, a drop in total IgG was observed in most of the subjects tested (Figures 3a and b), which was statistically significant in the case of anti-AAV-2 IgG (P=0.0371, paired t-test), but not for anti-AAV-5 IgG (P=0.5020, paired t-test). Both for AAV2 and AAV5 IgG, a drop in titer was not observed in all subjects, and the magnitude of the changes measured was variable. This is in agreement with the observed variability in the clinical response to rituximab administration in this cohort of subjects.22
Figure 3.
Anti-AAV IgG titers before and after a single course of rituximab. Anti-AAV IgGs were measured in the serum at baseline and 24 weeks after rituximab administration. (a) Anti-AAV2 IgG (ng ml−1) and (b) anti-AAV5 IgG (ng ml−1). For each sample, the average of a duplicate reading is reported. AVG, average±standard deviation of all individual measurements.
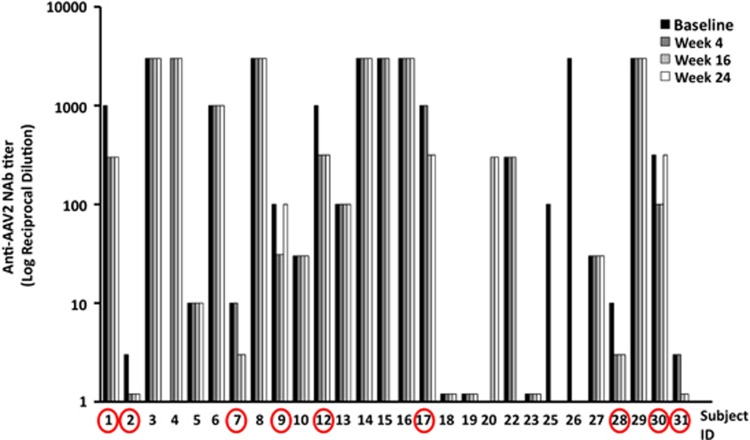
Despite the observed decrease in anti-AAV IgG in most of the subjects following rituximab administration, measurement of anti-AAV2 NAb in the cohort of 28 subjects at baseline, weeks 4, 16 and 24 showed that only subjects with starting NAb titers equal to or below 1:1000 achieved a lower serum-neutralizing activity following rituximab administration (Figure 4), while high-titer NAb did not change. Out of 28 subjects, 9 had a half-log drop in NAb titer, and in two subjects (2 and 31; Figure 4), the NAb titer decreased below 1:3.16 in the serum.
Figure 4.
Anti-AAV2 NAb titer measured over time in the serum of subjects receiving a single course of rituximab. Serum samples were collected at baseline (pre-rituximab administration), and 4, 16 and 24 weeks thereafter. Y axis, log of the reciprocal dilution at which the neutralizing activity of the test serum is lower than 50%. The circles indicate subjects that show a drop in NAb titer after rituximab administration compared to baseline NAb titer. Baseline samples from subjects 4 and 20 and week 5 sample from subject 20 were not tested. For each time point, the NAb titer was obtained by testing each serum dilution in triplicate.
We next measured the anti-AAV5 NAb titer. We focused on this serotype because of its superior ability to transduce synovial cells9 and its relatively low seroprevalence in humans.23 Anti-AAV5 NAb titers were measured in a subset of subjects who received one cycle of rituximab, who in turn received a second round of rituximab administration (n=24). In addition, NAbs were also measured following a second round of B-cell depletion with rituximab. A total of 16 out of the 24 subjects screened showed a drop of at least a half-log in the anti-AAV5 NAb titer following rituximab administration, of which 6 out of 16 had a reduction in NAb titer following two courses of B-cell depletion (Table 3 and Supplementary Figure 1). The subjects who experienced a drop in the anti-AAV5 NAbs were mostly the same who also had a decrease in the anti-AAV2 NAb with some exceptions.
Table 3. Effect of repeated rituximab administrations on anti-AAV5 NAb titers.
| Subject ID | First rituximab cycle | Second rituximab cycle | ||||
|---|---|---|---|---|---|---|
| |
Baseline NAb titer |
Lowest NAb titer |
Change in NAb titer |
Baseline NAb titer |
Lowest NAb titer |
Change in NAb titer |
| 1 | 1:31.6 | 1:10 | ↓ | N/A | N/A | N/A |
| 4 | 1:316 | 1:316 | = | N/A | N/A | N/A |
| 5 | 1:10 | 1:3.16 | ↓ | N/A | N/A | N/A |
| 6 | 1:316 | 1:100 | ↓ | 1:316 | 1:316 | = |
| 7 | 1:1 | 1:1 | = | 1:1 | 1:1 | = |
| 8 | 1:3160 | 1:316 | ↓ | N/A | N/A | N/A |
| 9 | N/A | N/A | N/A | 1:10 | 1:3.16 | ↓ |
| 10 | 1:3.16 | 1:3.16 | = | N/A | N/A | N/A |
| 12 | N/A | N/A | N/A | 1:1000 | 1:316 | ↓ |
| 13 | 1:1000 | 1:316 | ↓ | 1:1000 | 1:316 | ↓ |
| 14 | 1:3160 | 1:3160 | = | 1:3160 | 1:3160 | = |
| 17 | 1:316 | 1:100 | ↓ | 1:100 | 1:100 | = |
| 19 | 1:3.16 | 1:3.16 | = | N/A | N/A | N/A |
| 20 | 1:316 | 1:100 | ↓ | N/A | N/A | N/A |
| 23 | N/A | N/A | N/A | 1:1000 | 1:10 | ↓ |
| 24 | 1:100 | 1:1 | ↓ | 1:10 | 1:10 | = |
| 25 | 1:10 | 1:10 | = | 1:10 | 1:10 | = |
| 26 | 1:3160 | 1:100 | ↓ | N/A | N/A | N/A |
| 27 | 1:31.6 | 1:3.16 | ↓ | 1:10 | 1:3.16 | ↓ |
| 28 | 1:3.16 | 1:3.16 | = | 1:100 | 1:3.16 | ↓ |
| 29 | 1:31.6 | 1:31.6 | = | 1:1000 | 1:1000 | = |
| 30 | 1:10 | 1:10 | = | 1:10 | 1:10 | = |
| 31 | 1:10 | 1:3.16 | ↓ | 1:1 | 1:1 | = |
| 32 | 1:3160 | N/A | N/A | 1:3160 | 1:3160 | = |
Abbreviations: AAV5, adeno-associated virus 5; NAb, neutralizing antibody; NA, sample not applicable.
↓, NAb titer decreased following rituximab treatment; =, NAb titer unchanged following rituximab treatment.
Also for AAV5, only one subject (5) had a reduction in NAb titer below 1:5 following rituximab administration.
These data suggest that rituximab, in combination with methothrexate, is at least partially effective in reducing anti-AAV NAb titers in humans, in some cases to levels compatible to AAV vector administration.
Discussion
Humans are naturally exposed to wild-type AAV early in life, and consequently the frequency of subjects positive for anti-AAV antibodies increases steadily starting from 2 years of age.24 Humoral immunity to AAV can also be found in newborns, as a consequence of vertical transmission of maternal anti-AAV antibodies.24, 25 In adults, anti-AAV2 antibodies are the most prevalent (up to 70% of healthy humans), followed by serotypes like AAV5, AAV9 and AAV8, which are much less prevalent.23, 24, 25, 26
Studies in which AAV vectors were introduced to the systemic circulation of human subjects demonstrated that pre-existing humoral immunity to AAV vectors profoundly reduces the efficiency of transduction of a target tissue.12 Results from the first clinical trial for liver gene transfer for hemophilia B showed that relatively low NAb titers, 1:17, can completely neutralize large doses of vector, resulting in no detection of transgene expression.12 Similarly, studies conducted in non-human primates11 suggest that NAb titers as low as 1:5 can block vector transduction. With few exceptions, like direct intramuscular injection,14, 17, 27 subretinal delivery16, 28, 29 and intracranial delivery,15 vector administration to any body compartment in which immunoglobulins are present faces the hurdle of anti-AAV antibodies.
Intra-articular vector delivery has been explored for the treatment of arthritis7, 8 and hemophilic arthropathy.30 Although the approach is promising and holds several advantages compared to the systemic administration of a therapeutic AAV vector,7, 8 little is known on the prevalence of anti-AAV NAbs in the SF. Here we evaluated the seroprevalence of various AAV serotypes in matched serum and SF samples from RA-affected subjects. Anti-AAV antibodies were present in both compartments; however, the average titer in the serum was significantly higher than that in the SF, and differences in anti-AAV IgG subclasses were also noted. The significance of the higher prevalence of IgG4 in the SF is unclear and may be related to the diseased state of the joints of the subjects enrolled in this study; it may be worth noting that high levels of IgG4 have been associated with autoimmune disease.31 These results suggest that NAb titer in serum samples are representative of those in the SF; thus, serum screening for NAb to AAV could be used to select subjects to be enrolled in gene transfer studies. Another important aspect of this finding is that high-titer NAb to AAV vector can be found in the SF, requiring the prescreening of prospective subjects and the choice of AAV serotypes, like AAV5, with very low seroprevalence in humans and high tropism for synoviocytes9 to achieve efficient gene transfer at the joint level.19 Finally, the finding that NAbs in the SF are to some degree in equilibrium with the serum may pose a challenge to efficient vector readminstration, as humans develop high-titer anti-AAV NAb following intra-articular vector administration8 and anti-AAV antibodies in AAV-infused subject persist for years after gene transfer (Mingozzi and High, unpublished observation).
Given the impact of anti-AAV NAb on the efficiency of vector transduction, the development of sensitive, reliable methods to measure NAb titer is critical to the success of most gene transfer strategies. Cell-based in vitro NAb assays are among the most commonly used screening assays for NAbs.23, 24, 25, 26, 32, 33, 34 However, one major limitation of these assays is the poor efficiency of infection in vitro of AAV serotypes other than AAV2, which results in the underestimation of the NAb titer due to a need for higher multiplicity of infections to achieve a detectable reporter gene signal. Our data show that this obstacle can be at least partially overcome by using AAV vectors with higher transduction efficiency in vitro and expressing reporter genes such as luciferase that can be detected with high sensitivity.
The issue of pre-existing (and AAV vector-induced) humoral immunity to AAV could be overcome by switching to serotypes that are less prevalent;23, 26 however, the high degree of conservation of amino-acid sequence and structure of capsid makes the task difficult because of anti-AAV antibody crossreactivity.23 More recently, it has been shown that repeated cycles of plasmapheresis are at least partially effective in lowering anti-AAV NAb.32 The approach is effective in lowering NAb titers that are relatively low (⩽1:20), whereas it is not as effective with higher titers.
We investigated the use of a pharmacological approach to the modulation of humoral immunity to AAV by measuring anti-AAV antibody titer in serum samples from subjects receiving one or two cycles of the B-cell-depleting antibody rituximab, which, due to its safety and efficacy profile, has gained wide acceptance in the clinic for the treatment of autoimmune diseases like RA.22, 35 Our data suggest that this strategy results in lowering of anti-AAV NAb titer in small proportion of the subjects treated. Importantly, in some subjects anti-AAV NAb titer dropped below 1:5, to titers compatible with AAV vector administration. Although this result is not completely surprising, given the fact that rituximab efficiently depletes naïve and memory B cells,36 but fails to target plasma cells directly, which do not express the CD20 antigen,37 it is not clear at this point why some subjects had a response and some not. Two points could be relevant to this finding: the first is that a drop in NAb titer was observed when the baseline-neutralizing activity of the serum was <1:1000. This may reflect that the drug at these doses is only partially effective at taming B-cell responses, or else could simply reflect a lack of sensitivity of the assay, that is, a minor reduction in the NAb titer when the starting value is high does not translate into a change in the measured titer. The second is that the clinical response in RA patients treated with rituximab is not uniform,38 which may reflect different traits of the underlying disease or simply a variable response to the drug. However, in this study the subset of subjects who had clinical response to rituximab (that is, improvement in the disease activity score22) did not fully overlap with the subset of subjects who experienced a drop in anti-AAV NAb.
The design of this study does not allow any conclusion on whether several repeated administrations of rituximab would result in more effective reduction in anti-AAV NAb titers or whether rituximab would be more or less effective in lowering anti-AAV NAb in healthy subjects, or subjects with diseases other than RA. Future studies will be necessary to address these points and to test the efficacy of combining pharmacological modulation of B-cell responses with physical methods to remove antibodies from the plasma.32
Finally, these results seem to be consistent with a study testing the use of a single course of rituximab in RA patients to eradicate anti-infliximab antibodies, which showed an only partial (38%) reduction in antibody levels,39 and with results in non-human primates showing that a course of rituximab and cyclosporin A was at least partially effective in lowering anti-AAV antibodies.40
In conclusion, the data presented here suggest that NAb to AAV vectors present in the SF may constitute an obstacle to efficient synoviocyte transduction18 and highlight the importance of sensitive anti-AAV NAb screening in subjects undergoing intra-articular gene transfer. The use of serotypes that are less prevalent in humans may be a valuable strategy to overcome the limitation of anti-AAV antibodies. Similarly, pharmacological modulation of anti-AAV antibody responses is partially effective in lowering antibody titers, and the efficacy of this maneuver may be improved by combining it with physical methods, such as plasmapheresis,32 to lower levels of circulating anti-AAV antibodies, thus allowing for vector administration in subjects with pre-existing humoral immunity to AAV vectors.
Materials and methods
Serum and SF samples
Human samples were collected at the Academic Medical Center in Amsterdam, The Netherlands, under a protocol approved by the local Ethical Committee. All subjects were consented before sample collection according to the Declaration of Helsinki protocols. Samples were coded and de-identified. Serum and matched SF samples were collected from 11 RA patients positive for IgM rheumatoid factor (range 37–200 kU ml−1) with active disease. Except one patient, all patients were treated with non-steroidal anti-inflammatory drugs, whereas patients under disease-modifying anti-rheumatic drugs were excluded from the analysis. For the rituximab study, the serum was collected before treatment and 4, 16 and 24 weeks after one (n=28 subjects) or two (n=22 subjects) cycles of rituximab treatment, each consisting of two infusions. No SF was collected from these subjects. All subjects had active arthritis at baseline and were all on stable doses of metothrexate (5–30 mg per week) for at least 28 days before enrollment. Subjects on stable prednisone therapy (10 mg per day) and non-steroidal anti-inflammatory drug treatments were also included in the study. Each treatment cycle consisted of two infusions of 1000 mg of rituximab (Roche, Woerden, The Netherlands) on days 1 and 15 after premedication with 2 mg clemastine fumarate (anti-histamine) given intravenously and 1000 mg acetaminophen orally. This patient cohort was described previously.22
Anti-AAV antibody determination
Anti-AAV2, 5, 6 and 8 NAb titer was determined as described previously12 using an in vitro neutralization assay. Two AAV vector constructs were used in the assay, a single-stranded vector expressing β-galactosidase under the control of the cytomegalovirus promoter (ssAAV-LacZ), or a self-complementary vector20, 21 expressing the Renilla luciferase reporter gene under the control of the chicken β-actin promoter (scAAV-Luc). To increase the efficiency of transduction of AAV vectors in vitro, 2V6.11 cells (ATCC, Manassas, VA, USA) were used, which expressed the adenoviral gene E4 under the control of an inducible promoter. Cells were seeded in a 96-well plate at a density of 1.25 × 104 cells per well and a 1:1000 dilution of ponasterone A (Invitrogen, Grand Island, NY, USA) was added to the medium to induce E4 expression. On the day of the assay, serial half-log dilutions of heat-inactivated test serum were mixed with medium containing virus. For the ssAAV-LacZ vector, virus concentration used in the assay was ∼1 × 1010 vg ml−1 for AAV2 and ∼5.5 × 1010 vg ml−1 for AAV5, 6 or 8. For the scAAV-Luc vector, virus concentration in the assay was between ∼50- and 150-fold lower. Residual activity of the reporter transgene was measured using either a colorimetric assay (ssAAV-LacZ) or a luminometer (scAAV-Luc).
Anti-AAV capsid total IgG or Ig subclasses were measured with a capture assay; enzyme-linked immunosorbent assay plates were coated with 5 × 1010 capsid particles per ml of AAV empty capsids. Plates were blocked with 2% bovine serum albumin, 0.05% Tween-20 in phosphate-buffered saline for 2 h at room temperature and serial dilutions of samples were loaded onto the wells and incubated overnight at 4 °C. Biotin-conjugated anti-human IgG1, IgG2, IgG3, IgG4 or IgM antibody (Sigma, St Louis, MO, USA) were used as detecting antibodies; streptavidin-horse radish peroxidase was added for substrate detection. Ig concentration was determined against standard curves made with serial dilution of human purified IgG1, IgG2, IgG3, IgG4 or IgM (Sigma). Note that the differences in absolute values between total anti-AAV IgG and anti-AAV IgG subclasses are likely due to differences in affinity of the secondary antibodies used in the capture assays.
Virus production
The β-galactosidase cassette (ssAAV-LacZ) flanked by AAV2 inverted terminal repeats, and the self-complementary cassette for the expression of Renilla luciferase (scAAV-Luc) were packaged into capsids from either AAV2, 5, 6 or 8 serotypes. Vectors were produced by the Research Vector Core of the Center for Cellular and Molecular Therapeutics at the Children's Hospital of Philadelphia with an adenovirus-free system in HEK-293 cells using a triple transfection method.41 AAV vectors were purified by density gradient centrifugation, and vector titers were determined by dot blot.
Statistical analysis
Statistical analysis of results was performed using GraphPad InStat version 3.0a (GraphPad Software, San Diego, CA, USA). P-values <0.05 were considered statistically significant.
Acknowledgments
This work was supported by the Howard Hughes Medical Institute and by the National Heart, Lung and Blood Institute (Grant P01 HL078810).
PPT and MJV are affiliated with Arthrogen BV, a company developing AAV vector-based gene therapy for RA. FM and KAH are inventors on patents related to AAV gene therapy and have consulted for companies developing gene therapeutics. The remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Gene Therapy website (http://www.nature.com/gt)
Author contributions
YC and SCE performed the in vitro assays on human specimens. SZ prepared the vectors used in this study. RMT assisted with the identification subjects and collection of specimens. FM, KAH, PPT and MJV designed experiments, analyzed results and wrote the manuscript.
Supplementary Material
References
- Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol. 2002;2:364–371. doi: 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- Tak PP, Bresnihan B. The pathogenesis and prevention of joint damage in rheumatoid arthritis: advances from synovial biopsy and tissue analysis. Arthritis Rheum. 2000;43:2619–2633. doi: 10.1002/1529-0131(200012)43:12<2619::AID-ANR1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- Adriaansen J, Vervoordeldonk MJ, Tak PP. Gene therapy as a therapeutic approach for the treatment of rheumatoid arthritis: innovative vectors and therapeutic genes. Rheumatology (Oxford) 2006;45:656–668. doi: 10.1093/rheumatology/kel047. [DOI] [PubMed] [Google Scholar]
- Jorgensen C, Apparailly F. Prospects for gene therapy in inflammatory arthritis. Best Pract Res Clin Rheumatol. 2010;24:541–552. doi: 10.1016/j.berh.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Aalbers CJ, Tak PP, Vervoordeldonk MJ. Advancements in adeno-associated viral gene therapy approaches: exploring a new horizon. F1000 Med Rep. 2011;3:17. doi: 10.3410/M3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease PJ, Hobbs K, Chalmers A, El-Gabalawy H, Bookman A, Keystone E, et al. Local delivery of a recombinant adenoassociated vector containing a tumour necrosis factor alpha antagonist gene in inflammatory arthritis: a phase 1 dose-escalation safety and tolerability study. Ann Rheum Dis. 2009;68:1247–1254. doi: 10.1136/ard.2008.089375. [DOI] [PubMed] [Google Scholar]
- Mease PJ, Wei N, Fudman EJ, Kivitz AJ, Schechtman J, Trapp RG, et al. Safety, tolerability, and clinical outcomes after intraarticular injection of a recombinant adeno-associated vector containing a tumor necrosis factor antagonist gene: results of a phase 1/2 study. J Rheumatol. 2010;37:692–703. doi: 10.3899/jrheum.090817. [DOI] [PubMed] [Google Scholar]
- Adriaansen J, Tas SW, Klarenbeek PL, Bakker AC, Apparailly F, Firestein GS, et al. Enhanced gene transfer to arthritic joints using adeno-associated virus type 5: implications for intra-articular gene therapy. Ann Rheum Dis. 2005;64:1677–1684. doi: 10.1136/ard.2004.035063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apparailly F, Khoury M, Vervoordeldonk MJ, Adriaansen J, Gicquel E, Perez N, et al. Adeno-associated virus pseudotype 5 vector improves gene transfer in arthritic joints. Hum Gene Ther. 2005;16:426–434. doi: 10.1089/hum.2005.16.426. [DOI] [PubMed] [Google Scholar]
- Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA, et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108:3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantly ML, Chulay JD, Wang L, Mueller C, Humphries M, Spencer LT, et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci USA. 2009;106:16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- Boissier MC, Lemeiter D, Clavel C, Valvason C, Laroche L, Begue T, et al. Synoviocyte infection with adeno-associated virus (AAV) is neutralized by human synovial fluid from arthritis patients and depends on AAV serotype. Hum Gene Ther. 2007;18:525–535. doi: 10.1089/hum.2006.174. [DOI] [PubMed] [Google Scholar]
- Cottard V, Valvason C, Falgarone G, Lutomski D, Boissier MC, Bessis N. Immune response against gene therapy vectors: influence of synovial fluid on adeno-associated virus mediated gene transfer to chondrocytes. J Clin Immunol. 2004;24:162–169. doi: 10.1023/B:JOCI.0000019781.64421.5c. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ma HI, Li J, Sun L, Zhang J, Xiao X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Therapy. 2003;10:2105–2111. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Therapy. 2003;10:2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- Thurlings RM, Vos K, Wijbrandts CA, Zwinderman AH, Gerlag DM, Tak PP. Synovial tissue response to rituximab: mechanism of action and identification of biomarkers of response. Ann Rheum Dis. 2008;67:917–925. doi: 10.1136/ard.2007.080960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- Li C, Narkbunnam N, Samulski RJ, Asokan A, Hu G, Jacobson LJ, et al. Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Therapy. 2012;19:288–294. doi: 10.1038/gt.2011.90. [DOI] [PubMed] [Google Scholar]
- Calcedo R, Morizono H, Wang L, McCarter R, He J, Jones D, et al. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin Vaccine Immunol. 2011;18:1586–1588. doi: 10.1128/CVI.05107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroes ES, Nierman MC, Meulenberg JJ, Franssen R, Twisk J, Henny CP, et al. Intramuscular administration of AAV1-lipoprotein lipase S447X lowers triglycerides in lipoprotein lipase-deficient patients. Arterioscler Thromb Vasc Biol. 2008;28:2303–2304. doi: 10.1161/ATVBAHA.108.175620. [DOI] [PubMed] [Google Scholar]
- Amado D, Mingozzi F, Hui D, Bennicelli JL, Wei Z, Chen Y, et al. Safety and efficacy of subretinal readministration of a viral vector in large animals to treat congenital blindness. Sci Transl Med. 2010;2:21ra16. doi: 10.1126/scitranslmed.3000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Hakobyan N, Valentino LA, Feldman BL, Samulski RJ, Monahan PE. Intraarticular factor IX protein or gene replacement protects against development of hemophilic synovitis in the absence of circulating factor IX. Blood. 2008;112:4532–4541. doi: 10.1182/blood-2008-01-131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Yamamoto M, Suzuki C, Naishiro Y, Shinomura Y, Imai K. The birthday of a new syndrome: IgG4-related diseases constitute a clinical entity. Autoimmun Rev. 2010;9:591–594. doi: 10.1016/j.autrev.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Monteilhet V, Saheb S, Boutin S, Leborgne C, Veron P, Montus MF, et al. A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8. Mol Ther. 2011;19:2084–2091. doi: 10.1038/mt.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalenko M, Chen L, van Roey M, Donahue BA, Snyder RO, McArthur JG, et al. Epitope mapping of human anti-adeno-associated virus type 2 neutralizing antibodies: implications for gene therapy and virus structure. J Virol. 2000;74:1761–1766. doi: 10.1128/jvi.74.4.1761-1766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert CL, Miller AD, McNamara S, Emerson J, Gibson RL, Ramsey B, et al. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: Implications for gene therapy using AAV vectors. Hum Gene Ther. 2006;17:440–447. doi: 10.1089/hum.2006.17.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumans MJ, Tak PP. Rituximab treatment in rheumatoid arthritis: how does it work. Arthritis Res Ther. 2009;11:134. doi: 10.1186/ar2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata S, Saito K, Tokunaga M, Yamaoka K, Nawata M, Yukawa S, et al. Phenotypic changes of lymphocytes in patients with systemic lupus erythematosus who are in longterm remission after B cell depletion therapy with rituximab. J Rheumatol. 2011;38:633–641. doi: 10.3899/jrheum.100729. [DOI] [PubMed] [Google Scholar]
- Everly MJ, Terasaki PI. The state of therapy for removal of alloantibody producing plasma cells in transplantation. Semin Immunol. 2012;24:143–147. doi: 10.1016/j.smim.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Soliman MM, Hyrich KL, Lunt M, Watson KD, Symmons DP, Ashcroft DM. Effectiveness of rituximab in patients with rheumatoid arthritis: observational study from the British Society for Rheumatology Biologics Register. J Rheumatol. 2012;39:240–246. doi: 10.3899/jrheum.110610. [DOI] [PubMed] [Google Scholar]
- van den Bemt BJ, Vos K, den Broeder AA, Blom M, Thurlings RM, Bartelds GM, et al. A single course of rituximab does not abrogate anti-infliximab antibodies in patients with rheumatoid arthritis. Ann Rheum Dis. 2009;68:1368–1369. doi: 10.1136/ard.2008.095448. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Chen Y, Zhou S, Murphy S, Metzger M, Donahue R, et al. Pharmacological modulation of humoral immunity in a non-human primate model of AAV gene transfer for hemophilia B Mol Ther 2012. e-pub ahead of print 8 May 2012; doi: 10.1038/mt.2012.84 [DOI] [PMC free article] [PubMed]
- Matsushita T, Elliger S, Elliger C, Podsakoff G, Villarreal L, Kurtzman GJ, et al. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Therapy. 1998;5:938–945. doi: 10.1038/sj.gt.3300680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.