Abstract
Pruritus, the sensation of itch, which evokes reflex scratching behavior, has a diverse etiology. Because of its clinical significance, mechanisms of pruriception are an important topic. In the present work we describe and validate a paw motion detector (PMD) system. The system employs a small removable metal band placed on one hind paw that provides a signal indicative of paw movement through perturbation of an electromagnetic (EM) field. C57Bl/6 mice were fitted with a unilateral hind paw band and adapted to testing cylinders equipped with EM signal emission and detection. The following observations were made: 1) in mice, unilateral SQ injection of 48/80 into the dorsolateral aspect of the neck evoked periodic high frequency bursts of scratching at the injected site with the ipsilateral (banded) but not the contralateral (not banded) hind paw. 2) Cross correlation between PMD and human observer counts after SQ 48/80 using the specified computational algorithm revealed a highly significant correlation. 3) SQ histamine and 48/80 over a 1 hour interval produced dose dependent scratching, which diphenhydramine dose dependently reversed. Chloroquine scratching displayed an inverse u-shaped dose response curve, which was insensitive to diphenhydramine. 4) SQ 48/80 at intervals over 28 days showed no change in the scratching response within the same cohort of mice. 5) Power analysis showed 40% changes in scratching activity could be detected at the p<0.05 level with groups of 4 mice. These observations indicate that the system described can efficiently define the actions and pharmacology of pruritogenic agents.
Keywords: pruriception, pruritus, itching, histamine, 48/80, chloroquine
1. INTRODUCTION 1
The unpleasant cutaneous sensation that is associated with intermittent bursts of high frequency scratching directed at skin dermatomes in response to sensory input initiated by specific classes of irritants applied to that skin region reflects the condition of pruritus. Such pruriception may be initiated by a variety of agents including some which release histamine (e.g. 48/80) and are blocked by histamine antagonist, while other agents with which histamine antagonists are largely ineffective, are deemed to be histamine independent (e.g. chloroquine) (Ikoma, et al., 2006). In humans, pruritus represents a significant clinical problem, which may be caused by drug therapies (e.g. morphine), various normal states (e.g. pregnancy 3rd trimester), as well as pathologies including renal, liver, and serious skin diseases such as atopic dermatitis (Greaves, 2005; Paus et al., 2006; Ikoma, et al., 2006). Chronic pruritus can be manifested in up to 8% of the adult population (Dalgard et al., 2004). Management of pruritus is challenging, especially when an underlying etiology cannot be identified. Owing to the poorly understood pathophysiology, the development of effective and specific treatment modalities for pruritus has proven difficult. At present, there is no universally accepted therapy for itch.
An important issue in advancing pruritus research is development of a reliable method to assess the targeted homotopic hindpaw scratching behavior present in rodent models after application of a pruritic agent to the neck or shoulder. There are several published strategies. The most widely employed is manual counting by a trained observer blinded as to treatment. Given the high frequency and ongoing nature of the behavior, this often involves review of video files (Lamotte et al., 2011). While useful, this process is subject to inter-observer bias and observer fatigue. Several automated systems have been described to measure paw movement associated with the scratching behavior. In one model, paw movement is videotaped and the file processed using motion detection software (Inagaki et al., 2003; Orito et al., 2004; Ishii et al., 2008; Yuman et al., 2009). This process requires specialized camera equipment and a computer capable of running the software. Another model uses the acoustic waveforms produced by paw movement to detect scratching (Umeda et al., 2006). The main drawbacks of this system are its dependence on eliminating external noise and the lack of ability to determine the specific origin of the evoked sound. Other systems assess movement directly. In one, whole body movement of the mouse is measured using a strain gauge mounted cage (Brash et al., 2005). Again, it is whole body movement and not the site specific homo-lateral paw movement being measured. In a second model, paw movement is detected using an EM field with a subcutaneous magnet injected into the paw, posing the need to implant a foreign body (Oude Elferink et al., 2011). A similar system, using a magnetic field to detect scratches, utilizes a permanent ring attached to the lower leg, which as the authors note can lead to limb swelling (Elliott et al., 2000).
We have previously described a paw motion detector system involving a small and very lightweight rectangular metal band applied to the plantar surface of the paw of the mouse or rat without use of anesthesia. The band is noninvasive in that it does not produce any insult to the paw skin, poses no risk of limb or paw occlusion, and is removed at the end of the test period (Yaksh et al., 2001). Band movement is detected by perturbation of an electromagnetic standing wave. An important issue is the exclusion of non-scratching behaviors (e.g. ambulation, grooming, biting), and perhaps more significantly the removal of scratching behavior directed at areas other than the site of injection. Here we describe a paw motion detector (PMD) system which employs an algorithm that processes and detects a wave signal produced by the high frequency scratching burst, which we define as a microburst. These microbursts occur with the ipsilateral hind paw in response to pruritogen injection in the dorsolateral neck. The present work aimed to optimize the signal processing algorithms, to provide validating data on the system’s scratch detection, and to demonstrate the robustness of the system as a screening tool for anti-pruritogenic agents.
2. Material and methods
All studies were performed according to protocols approved by the Animal Care and Use Committee of the University of California San Diego.
2.1 Animal Model
C57Bl/6 mice (male, 25–30 gram, obtained from Harlan Sprague Dawley) were employed. Limited studies were also carried out in the rat (male Holtzman 250–300 grams, obtained from Harlan Sprague Dawley).
To initiate scratching behavior, animals are shaven on the dorsolateral aspect of the neck and upper shoulder. The detection band is placed around the hind paw ipsilateral to the shaven area. Animals are then adapted to the testing chambers for 1 hour. To initiate scratching behavior, a subcutaneous (SQ) or intradermal (ID) injection of the test article is made in the middle of the shaven area of skin using a 30 gauge needle. Data acquisition is then initiated.
2.2 Drugs
In the present studies, drugs were delivered in 0.1 mL volumes. Drugs employed were Histamine dihydrochloride (0.111, 1.110, 11.100 ug/mL), Chloroquine (0.1, 0.5, 2 mg/mL), 48/80 for the mouse (0.125, 0.25, 0.5, 1 mg/mL), 48/80 for the rat (1, 5, and 10 mg/mL) and Diphenhydramine given systemically (0.1, 0.3, 1 mg/kg). All drugs were prepared for delivery in saline (0.9%), and were obtained from Sigma-Aldrich.
2.3 Paw Motion Detector (PMD)
2.3.1 Physical Specifications
The PMD detects the movement of a non-ferrous metal band placed around one hind paw of the rodent (for the mouse the band weight = 0.1 gram while for the rat the band weight=0.5 gram). There are two distinct bands one for the rat and one for the mouse (see the band in Yaksh et al., 2001). The testing apparatus consists of cylindrical chambers (mouse: 8.5 cm diameter/22.5 cm tall; Rat: 15 cm diameter/ 30 cm tall). Under each cylinder is a pair of circular concentric electromagnetic coils, which serve respectively as antennae for transmission and reception. Outer coil diameters for the mouse and rat are 12 cm and 20 cm, respectively. The transmitter coil assembly is constructed to emit a 5–8 mW, 6–8 kHz, sinusoidal electromagnetic field (Blue Max 800 Precision scan search coil White's Electronics, Inc. 1011 Pleasant Valley Road Sweet Home, Oregon 97386). The detection principal is that Eddy currents created by the movements of the ferrous and nonferrous metals perturb the EM field. Such perturbations produce an output waveform and are subsequently detected (Yaksh et al., 2001).
2.3.2 Signal conditioning
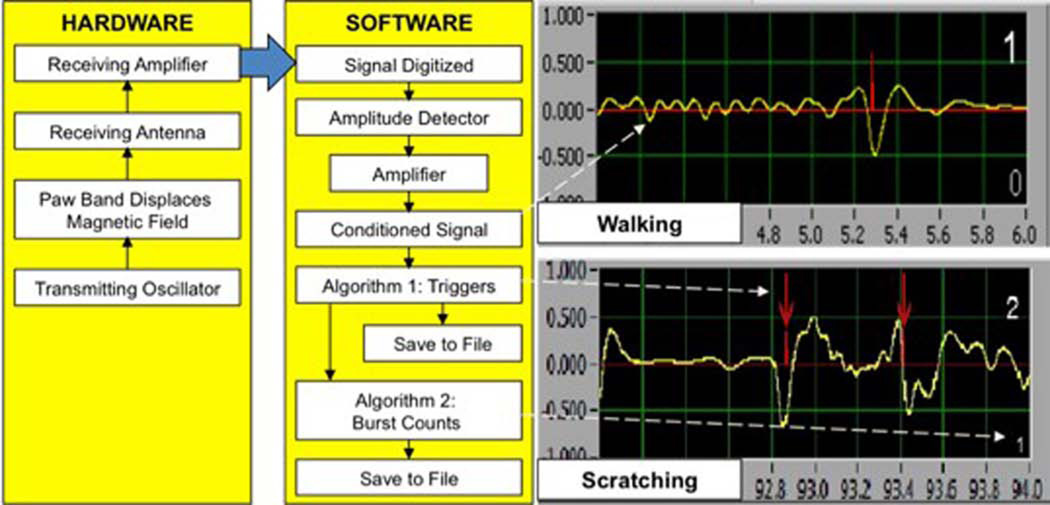
The analog signal from the sensing coil assembly is fed into a standard National Instruments based signal acquisition A-D converter system (I6030 or PCI-MI0-16Xe-10 National Instruments Corp.) driven into a standard LabView based software (Lab view v.5.1 National Instruments Corporation 11500 N Mopac Expwy Austin, TX 78759-3504). The signal is filtered, amplified, and digitized (sampling rate of 1,000 Hz, 12-bit resolution). This signal (yellow line on Fig. 2) is then entered and passed through a two component-triggering algorithm configured in the Lab view program. In the first phase, the processed signal is sent through an algorithm to establish a scratch movement trigger. In this, the processed signal is subjected to a “zero-crossing interval-peak height” analysis based on the signal amplitude in a sliding time window (see Yaksh et al., 2001). The range of voltages (1 volt to 0.01 volt) over a moving 128-ms interval is configured to produce a continuous output waveform. This secondary waveform contained jagged peaks that correlated well with the scratch movement associated voltage transients found in the acquired waveform. The signal was then smoothed by using a non-weighted moving average filter. The smoothed range waveform was examined in real time by a peak-detection algorithm set to pick out spikes of >500 ms and amplitudes of >0.3 V. Each peak provided a trigger signal, which is represented by a red line on the automated system's display, see Fig. 2. The signal was then processed through a second level algorithm to define bursting. A high frequency scratching burst, which we will now call a microburst, was defined as multiple trigger signals occurring within a specified moving window time frame (e.g. 2 trigger signals within a 1.15 second time frame) without double counting trigger signals. As will be noted, we systematically examined these parameters to define the optimal analytic configuration. We will show that these outputs correspond with the concurrent scratch counts provided by a trained human observer.
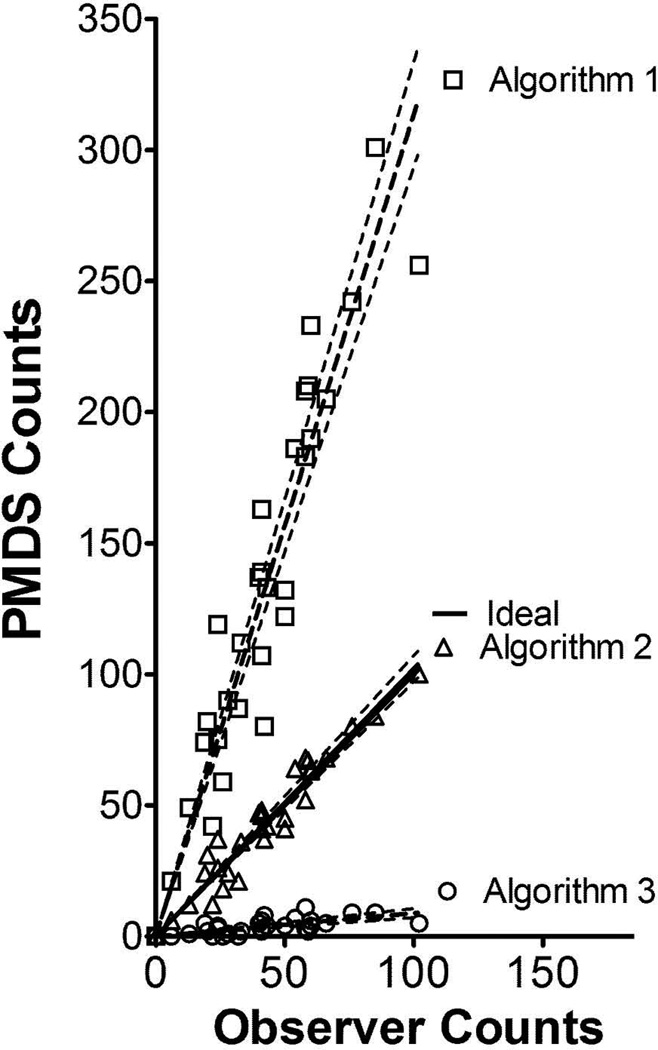
Figure 2.
Regression lines with 95% confidence intervals for cumulative microburst counts per 10 min. intervals counted by the paw movement detection system plotted vs. the corresponding human count per 10 min. intervals as a function of the algorithm used by the systems to count microbursts. Data for Regression line of Algorithm 2 for all 5 animals pooled: Slope (mean+/−SEM) = 1.022 +/− 0.024, CI (range) = 0.9716 to 1.072, Correlation Constant (R) = 0.964)
2.3.3 Data Collection
Paw movement events meeting the criteria of a scratch microburst are captured and summed at 1-min increments by default (and can be summed from 1 second to 60 minute increments). Summed data are stored in Microsoft Excel compatible spreadsheets. The Labview programming provides 4 independent input – output channels, and presents the data for four animals simultaneously on the screen. The front panel includes in the file for each animal: 1) study / animal identifiers, treatment codes, dates, and other information relevant to the study; 2) a window displaying the previous 2 s of digitized signal (as shown in figure 1) with previously described markers indicating any flinch detection activity and a count of the microbursts within that window; and 3) a line graph of each animal’s trigger count by sampling interval (1 min by default) since initiation of testing. The system saves in two separate files 1) the triggers produced by algorithm 1; and 2) the burst counts produced by algorithm 2. A typical view of the window displaying digitized signal is shown in figure 1.
Figure 1.
Left: Flow chart shows the signal processing data stream for the PMD schematically presenting the functions of the hardware and software. Right: Front panel view of the computer presentation for two channels. Each is a screen shot with a two sec. sequence showing a trigger generated by a walking step (top) and a scratch sequence (bottom). The smooth curve represents the conditioned signal, while the sharp vertical lines indicate the signal trigger provided by the first algorithm’s analysis.
2.4 Study protocol
2.4.1 Model optimization and validation
For validating studies, SQ injections in volumes of 0.1 mL of either vehicle (0.9% saline) or the itch-inducing agent 48/80 (0.5 mg/mL) were performed. A trained observer visually monitored the animal minute by minute for 60 minutes to provide a time locked assessment of scratching bursts. The visual observation of the scratching was defined as the application of the ipsilateral paw to the injection site followed by the appearance of high frequency movement of the paw across the injection site. Concurrently, the automated system recorded the unprocessed data for 60 minutes. Data was then analyzed by running the unprocessed trace through different algorithms. Observer counts and automated counts for each 10 min interval were plotted against each other. The best-fit regression lines were calculated.
2.4.2 Macroburst Analysis
We sought to define and quantify the itch-scratch-quiescence cycle commonly seen in pruritic animals (Ikoma et al., 2006). We observed clusters of microbursts (i.e. many distinct scratching movements over several minutes followed by several minutes of inactivity) in the histograms of individual animals. We defined these clusters of microbursts followed by a period of quiescence as a macroburst. The histograms of each individual animal’s microbursts were transformed and then normalized minute by minute using a FFT algorithm in order to differentiate peaks of scratching behavior from troughs of inactivity. (Oppenheim et al., 1999)
2.4.3 Homotopic detection of scratching behavior
To determine the ability of the system to show the homo-laterality and site specificity of the detected scratching behavior, mice received SQ 48/80 on the same side to the banded paw and the opposite side to the banded paw.
2.4.4 Repeatability
In order to assess the ability of the system to repeat results in the same animals over time we injected 4 mice with 48/80 and 4 mice with saline six times over a period of 28 days (day 0, 3, 7, 14, 21, 28).
2.4.5 Power Analysis
Determination of power and minimum group size was accomplished using standard methodologies (Statsoft, Inc.). Data were used to undertake a power analysis and to predict nominal group sizes for assessing statistically significant changes in scratching activity.
2.5 Statistical Analysis
For assessment of the covariance between observer and machine counting with different trigger processing algorithms, the best fit regression line constrained to pass through 0 was calculated with 95% confidence intervals. Also, a correlation constant (R) was found for each line. This analysis was done for each individual animal, as well as for five animals pooled. Scratching analysis was accomplished by summing the total scores for a 1 hour period after pruritogen injection. These data were used to calculate mean and SEM or SD. Cross treatment comparisons were made with 1 way ANOVA with post hoc comparisons made using Bonferroni or an unpaired two-tailed t-test when comparing only two groups. For the study involving repeated injections a 2-way ANOVA with repeated measures with a post hoc comparison using Bonferroni was undertaken. For pharmacological data presented as a percent of control the standard error was estimated using the Doulborg formula standard error of quotients (Doulborg, 1940). Data analyses were performed using Prism (v.5).
3. RESULTS
The following studies were undertaken to optimize and validate the model as well as to explain the system’s development and functionality.
3.1 Comparison of Human Observation and PMD Counts
The SQ delivery of 48/80, histamine, and chloroquine to the dorsolateral neck resulted in vigorous scratching over a 60 min interval by the ipsilateral paw. Visual inspection revealed that this scratching behavior was characterized by brief bouts of high frequency application of the paw to the injected site (e.g. scratching microbursts).
3.1.1 Microburst analysis
Counts of scratching microbursts were accumulated by an observer while concurrently acquiring the output from the paw motion detector for 5 mice. The output data was run through the PMD with counts being generated using three different microburst counting algorithms. The three described here were: i) 1 trigger / 1.15 sec, ii) 2 triggers / 1.15 sec, and iii) 3 triggers/ 1.15 sec. For example in algorithm ii, a microburst was counted if two triggers were observed within a 1.15 sec interval. We separated the PMD observed microbursts and human observed scratching bursts over the sixty minute period into six 10 min epochs. In figure 2, the microburst counts for each 10 min epoch as determined by the PMD for each algorithm were plotted vs. human observer counts for the corresponding 10 min epochs. The calculated best fit regression line of the pooled data revealed that algorithm 2 yielded a regression line not statistically different from 1 with an R value of 0.964. Subsequently, we also plotted individual regression lines for each of the 5 mice separately. Again, PMD counts and human observer counts were compared in ten minute epochs over the 60 minutes. The mean and SD of the 5 slopes of the linear regression lines calculated for algorithm 2 was 1.025 ± 0.026 with an average correlation coefficient (R) of .972 (see Table 1). Thus, the best correlation of automated scratching counts with human observer scratching counts for the three algorithms shown here was produced by algorithm 2.
Table 1.
Mean of the Slope (slope±SD), Confidence Intervals (CI range), and Mean of the Correlation Coefficient (R±SD) of the 5 separate linear regressions calculated for the 5 mice
| Line | Slope µ±σ (n=5) |
95% CI of Slopes |
R µ±σ (n=5) |
|---|---|---|---|
| Perfect Line | 1.000 ± 0.000 | 1.000 to 1.000 | 1.000 ± 0.000 |
| Algorithm 1 | 3.125±0.241 | 2.826 to 3.425 | 0.924±0.052 |
| Algorithm 2 | 1.025 ± 0.048 | 0.966 to 1.084 | 0.960 ±0.043 |
| Algorithm 3 | 0.091±0.038 | 0.043 to 0.139 | 0.681±0.296 |
Using the optimal algorithm as defined, the time course of the scratching microbursts observed after subcutaneous 48/80 (0.5 mg/mL) and subcutaneous saline are presented in Figure 4A for two representative mice. As indicated in the accompanying cumulative count histogram for a 1 hour period, 48/80 displayed a significantly greater count than did saline (i.e. background activity in the absence of scratching behavior) with a pruritogen to noise (control-no pruritogen) ratio of approximately 5 (Figure 4B).
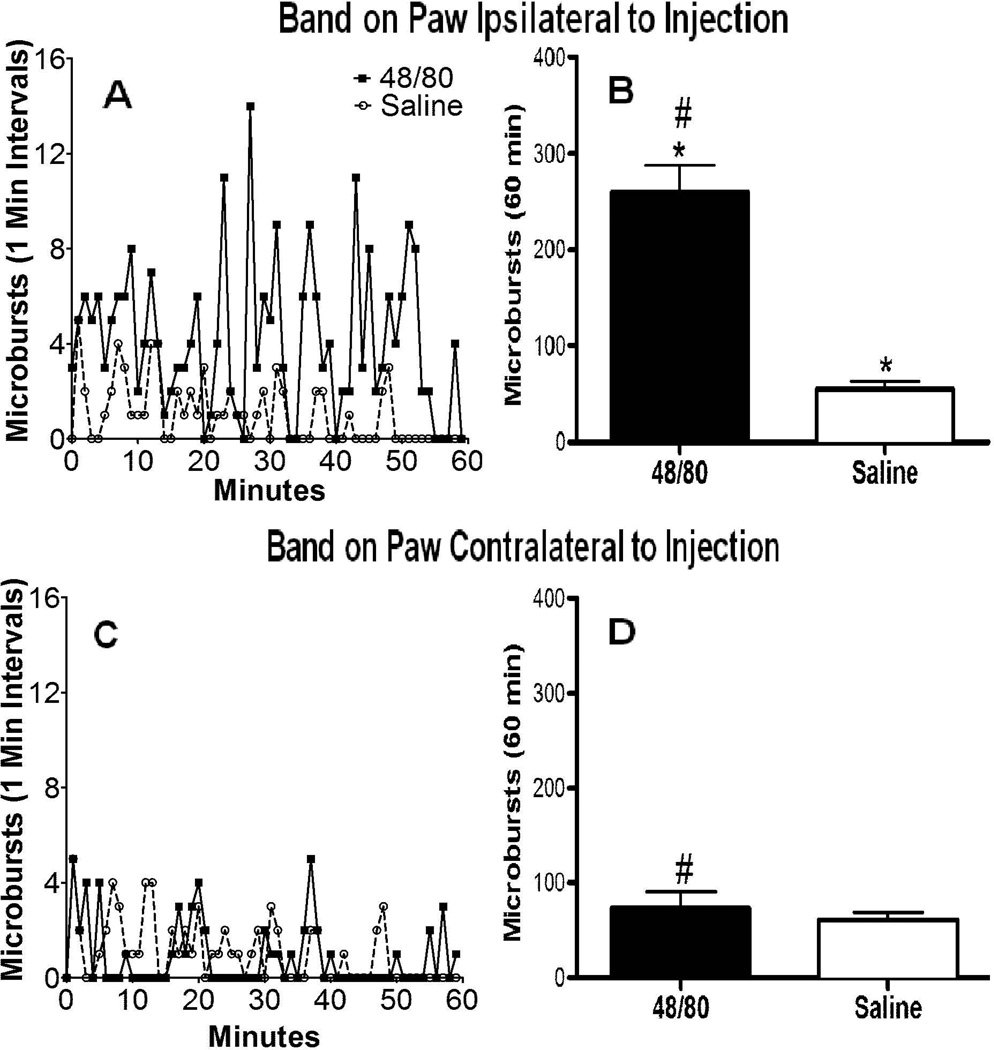
Figure 4.
A: PMD microburst counts plotted vs. time after injection of 48/80 (0.5 mg/mL) or saline into single mice with the detection band ipsilateral to the injection site. (N=1 for both as representatives of the mean) B: Cumulative microburst counts (mean ± SEM) over 1 hr after the SQ injection of 48/80 (N=5) or saline (N=5). C: Same as A except detection band was placed on contralateral hind paw. D: Same as B, except detection band was placed on contralateral hind paw (n=4) 2-tailed t-test: * p<0.01 48/80 ipsilateral vs. saline; 2-tailed t-test # p<0.001 48/80 ipsilateral vs. 48/80 contralateral. The difference between saline and 48/80 contralateral was not statistically significant p=0.51.
3.1.2 Macroburst analysis
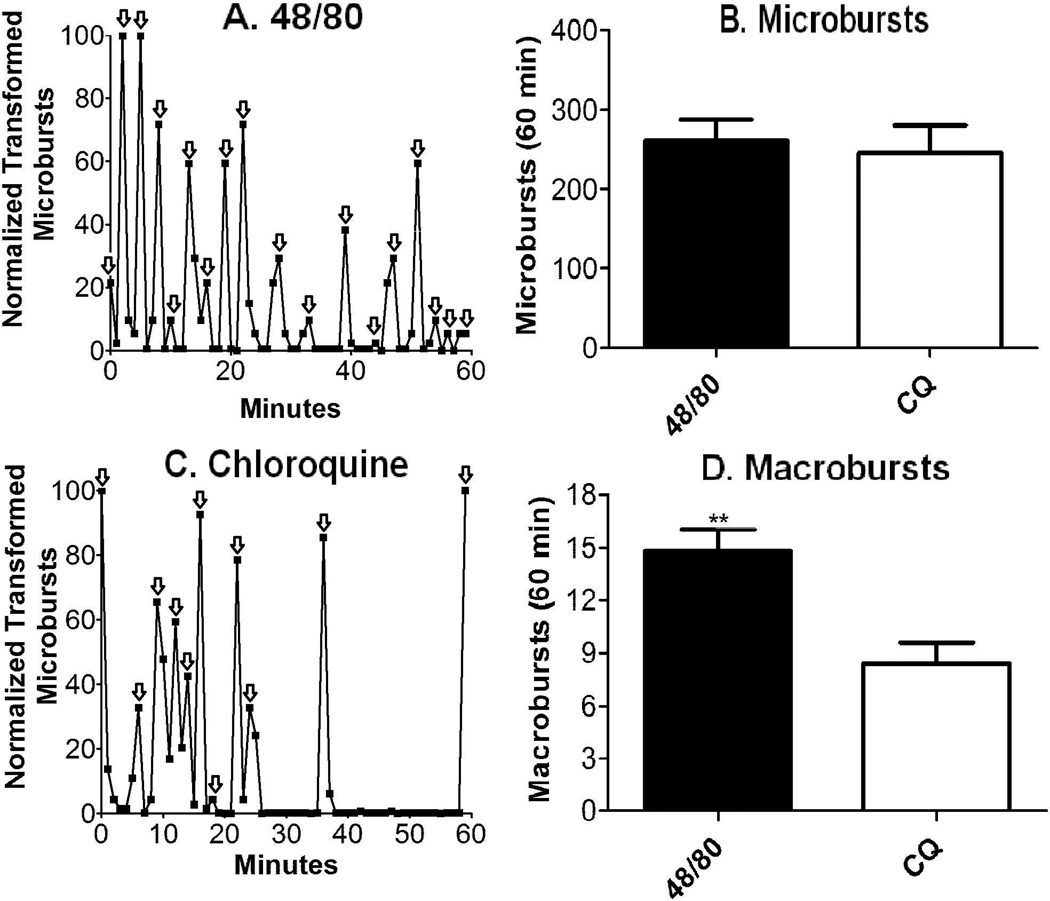
Periods of high intensity scratching followed by intervals of inactivity or quiescence were typically observed in animals injected with pruritic compounds. To quantify the clustering of scratching behavior we systematically defined a macroburst. Macrobursts are characteristic periods of intense scratching, which are quantified by an analysis of the clustering of microbursts. Each individual data point in a sixty minute time course (the number of microbursts per minute) is inserted into the given FFT algorithm which produces a transformed number for each corresponding time point. The numbers were then normalized. The algorithm changes the data by minimizing and maximizing the data points’ y values in order to make maximums farther from 0 and minimums closer to 0. This results graphically in major increases for periods where several minutes of contiguous high microburst counts appear, while several contiguous minutes of low or no microburst counts are decreased roughly to 0. These sixty minutes of new data points are then graphed using their corresponding unchanged × values. Each peak, (see arrows Figure 3A and 3C) again representing the maximized parts of the graph where microburst counts were high for several contiguous minutes, was counted as a macroburst. For comparison see figure 4A (48/80 normal 60 min time course of microbursts) to figure 3A (48/80 FFT transformed and normalized 60 min time course of macrobursts). The peaks were counted for each animal and averaged over five animals. While both agents at their respective SQ concentrations produced a similar overall microburst count (3B), the respective macroburst analysis revealed distinguishable macroburst frequencies for the two agents (3D). We observed for 48/80 an average of 14.8 macrobursts versus on average only 8.4 macrobursts for chloroquine.
Figure 3.
A: PMD Transformed normalized microburst counts plotted vs. time after injection of 48/80 (0.5 mg/mL, N=1 representative of the mean). Each arrow represents 1 macroburst counted. B: Cumulative microburst counts (mean ± SEM) over 1 hr. after the SQ injection of 48/80 (0.5 mg/mL, N=5) or chloroquine (1 mg/mL, N=5). Treatment groups were not significantly different. 2-tailed t-test p=0.73 C: Same as A except SQ chloroquine was injected (1 mg/mL, N=1 representative of the mean) D: Cumulative macroburst counts (mean ± SEM) over 1 hr. after SQ injection of 48/80 (0.5 mg/mL, N=5) or chloroquine (1 mg/mL, N=5). Treatment groups were significantly different. 2-tailed t-test ** p< 0.001 48/80 macrobursts vs. chloroquine macrobursts.
3.2 Homotopic detection of scratching behavior
To determine if the hind paw movement initiated by SQ 48/80 showed site specificity by being homo-lateral specific, the detection band was placed on the paw contralateral to the SQ injection site. As indicated in Figure 4D, cumulative paw movement counts of the contralateral paw after contralateral 48/80 were not different from those observed after saline. Also, contralateral counts were significantly less than those observed when the ipsilateral paw movement was measured after the same dose of SQ 48/80 (Figure 4B).
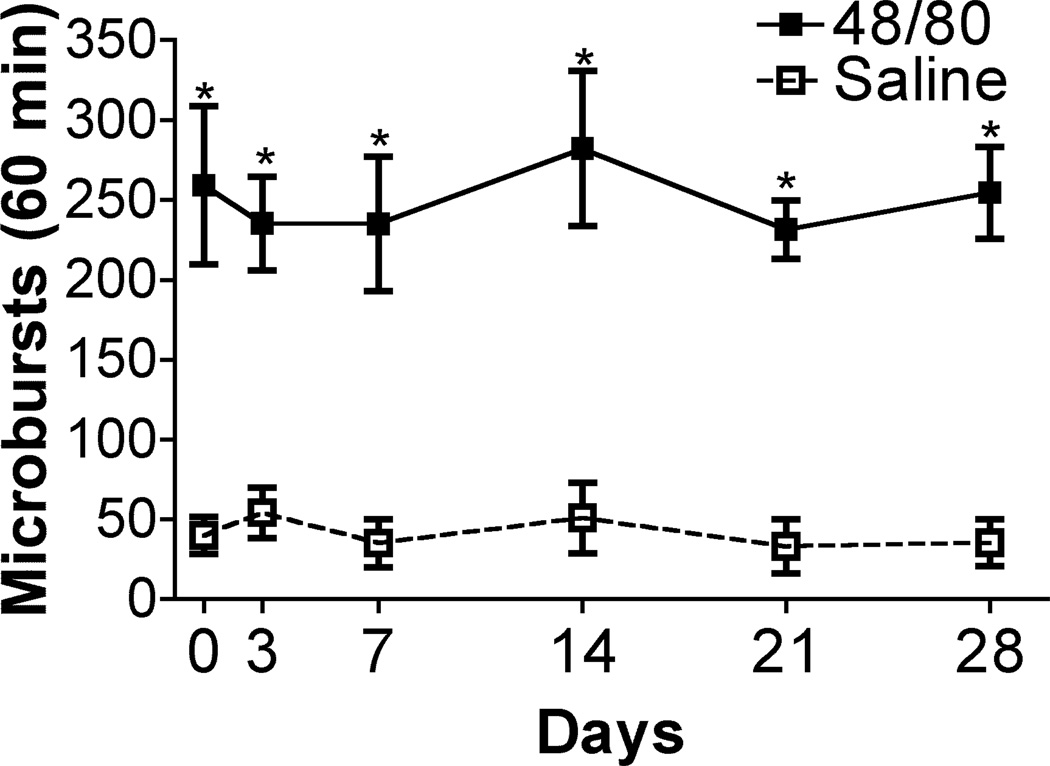
3.3 Repeatability
To determine response repeatability SQ 48/80 (0.5 mg/mL) or saline was given at intervals in the left dorsolateral neck periodically over 28 days and ipsilateral paw microbursts were counted. As indicated in Figure 5, 48/80 resulted in a reliable increase over saline counts at all time points when assessed at intervals out to 28 days, with 48/80 vs. saline F(1,6)=70.82, p< 0.001, and Bonferroni post hoc 48/80 vs. saline p<.001 for all time points. There was no difference, however, between the individual mice within their respective treatment group, with matching within groups statistically significant, F(6,30)=2.81, p<0.027. No time effect or interaction was statistically significant with the 2-way ANOVA repeated measures. Thus, the microburst counts at all time points for each respective treatment group were not significantly different from each other.
Figure 5.
Cumulative microburst counts (mean ± SEM) during the hr. after the SQ delivery of 48/80 (N=4) or SQ saline (N=4) given in the same animals periodically out to 28 days. 2-way ANOVA repeated measures found an effect between treatment groups F(1,6)=70.82, p< 0.001, indicating a significant difference between saline and 48/80 injected animals; an effective matching within treatment groups F(6,30)=2.81, p<0.027, indicating no significant difference between animals within each group respectively; no effect of time F(5,30)=0.46 p>0.80; no interaction between time and treatment groups F(5, 30)=0.26, p>0.90. Thus, time did not change the outcome of the results for either treatment group respectively, nor did time interact with one treatment group differently than the other. Bonferroni post hoc saline vs. 48/80 groups at all time points, * p<0.001
3.4 Pruritogen pharmacology
Using the algorithm defined above, we examined the effect of intradermal and subcutaneous pruritogens to assess the ability of the system to define scratching behavior in mouse and rat.
3.4.1 Histamine
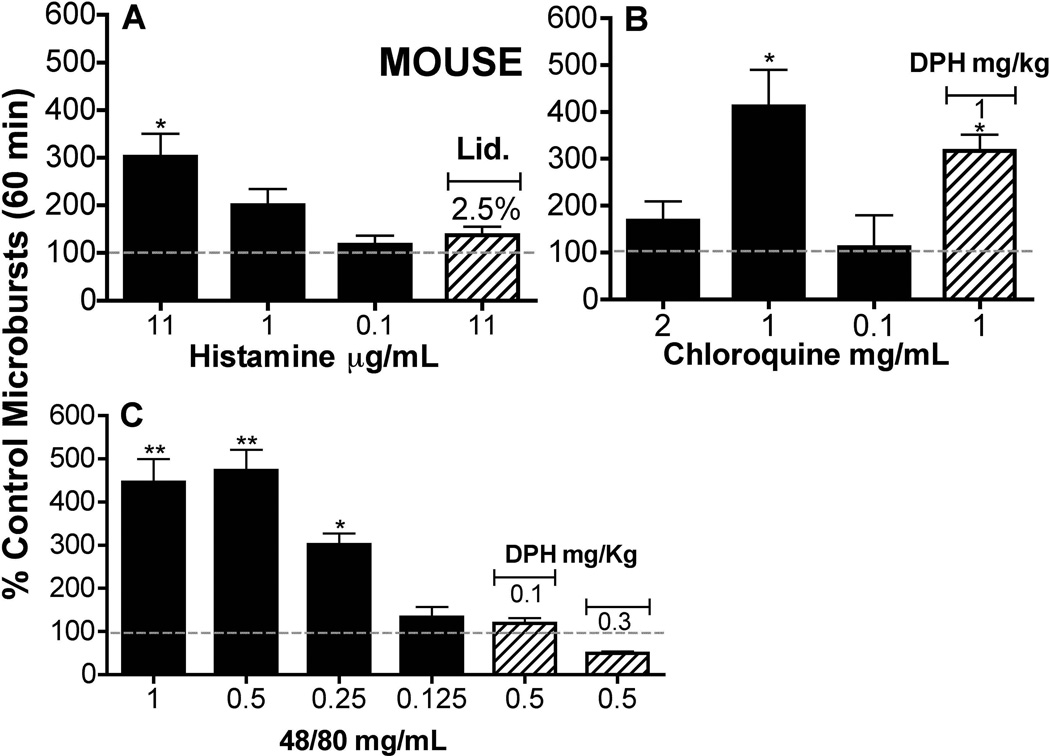
ID histamine resulted in a robust dose dependent scratching over a range of .111 to 11.1 µg/mL. In support for the assertion that the SQ injection of histamine reflected a local afferent activation, injection of the local anesthetic lidocaine blocked the scratching behavior (Figure 6A).
Figure 6.
Magnitude (Mean ± SEM) of cumulative scratching expressed as a percent of saline control at different doses of Histamine, 48/80, Chloroquine, DPH, or Lidocaine (n=4 or n=5 for all groups) A: SQ histamine alone or with SQ lidocaine (lid.) (2.5%) injected into the site 5 min prior to histamine. 1-way ANOVA, Bonferroni post hoc vs. saline. ** p<0.01. B: SQ Chloroquine. 1-way ANOVA, Bonferroni post hoc vs. saline. ** p<0.01. SQ chloroquine (1 mg/mL) with pretreatment (30 min) of diphenhydramine (1 mg/kg, IP) 1-way ANOVA, Bonferroni post hoc vs. saline ** p<0.01. C: SQ 48/80. 1-way ANOVA, Bonferroni post hoc vs. saline ** p<0.01 and * p<.05. SQ 48/80 (.5 mg/mL) with pretreatment (30 min) with increasing doses of IP diphenhydramine. 1-way ANOVA, Bonferroni post hoc vs. saline ** p<0.001.
3.4.2 Chloroquine
The SQ delivery of chloroquine (0.1 to 2 mg/mL) resulted in a significant scratching incidence which was similar to that produced by the highest doses of histamine and 48/80 (Figure 6A and Figure 6C). An inverse u shape dose response curve was observed (Figure 6B). Diphenhydramine at 1 mg/kg had no statistically significant effect upon chloroquine (1 mg/mL) evoked scratching.
3.4.3 48/80
SQ 48/80 produced a dose dependent scratching over a range of 0.125 to 0.5 mg/mL. The doses of 1 mg/mL and 0.5 mg/mL were not statistically different (Figure 6C). In contrast to chloroquine, diphenhydramine, the H1 antagonist, resulted in a complete reversal of scratching produced by the highest dose of 48/80 at 0.1 mg/kg. Higher doses of diphenhydramine resulted in a suppression of behavior as compared to saline treated animals.
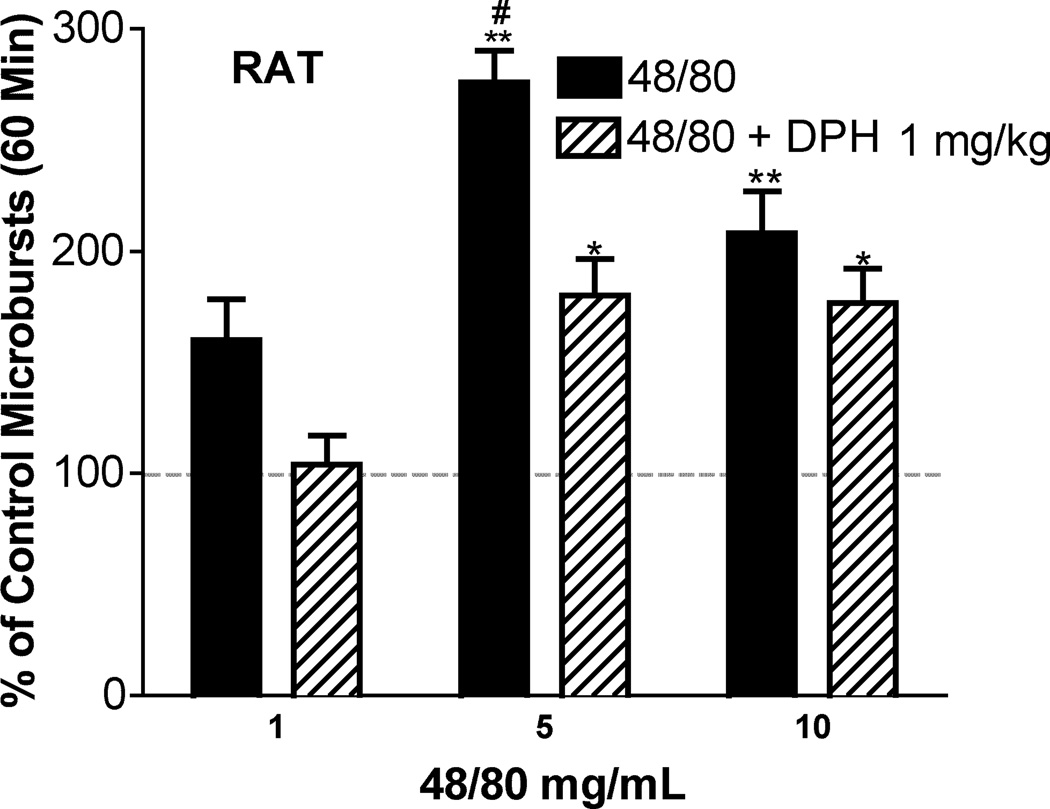
3.5 Rat
In parallel studies we examined the effects of 48/80 on homo-lateral scratching behavior in the rat. As indicated in Figure 12, SQ 48/80 resulted in an increase in scratching behavior at 5mg/mL, but this increase was not present at 10 mg/mL indicating disruption of behavior because of systemically active concentrations. As indicated, diphenhydramine resulted in a significant reduction in the observed magnitude of scratching.
3.6 Power analysis
Optimal group size based on a power analysis was calculated where the group mean and SEM for scratching in groups receiving a pruritogen (e.g 48/80) would nominally be 258.8 ± 24.8, with the minimum microburst score being 55.2± 8.3 (as observed after SQ saline) (see Figure 5B). In screening work, we would wish to show a 50% reversal of the peak scratching response (50% of the difference between peak and baseline) over the baseline as compared to 48/80. For example: ([258.8 − 55.2] × 0.7 + 55.2) vs. 258.8 ± 26.7. Thus, a score of 157 compared to 258.8, as calculated with a power=0.90 and an alpha =0.05 corresponds to a group size of 3. We would expect that with 3 animals, a 50% reduction in scratch counts using the PMD would be statistically significant at the p<0.05 level. Using similar calculations, it is indicated that 4 animals could show a 40% reduction, 8 animals a 30% reduction, and 16 animals a 20% reduction at the p<0.05 level.
4. DISCUSSION
The broad clinical relevance of pruritus has led to an increasing interest in the development of mechanistic insights into its manifestation. The pharmacology of the systems underlying pruritus has grown increasingly complex. Aside from histamine mediated events, a variety of histamine independent mechanisms have now been identified, including MrgprA3, (a Mas-related G-protein-coupled receptor), GRPR signaling (Gastrin-realeasing peptide receptor, a metabotrophic receptor for the mammalian homolog of amphibian bombesin), and PAR (protease activated receptors activated by a variety of protease such as mucunain in cowhage) (See Jeffry, et al 2011). All of these systems have been shown to activate populations of sensory axons and discrete populations of dorsal horn neurons which carry information believed to be relevant to pruriception and its motor expression (scratching) (Davidson and Giesler, 2011).
An important component to such work is the development of preclinical surrogate models to permit study of drug effects. Such models depend upon the ability to reliably quantify the scratching induced by pruritogens. In the present work, we characterized the scratching behavior using a minimally invasive approach to capture unilateral hind paw movements evoked by SQ pruritogens injected into the back of the neck in mouse. Issues pertinent to the interpretation of these data will be considered below.
4.1 Observer based capture of pruritic activity
The gold standard of scratching behavior analyses is the observation of the behavior of the animal by a trained observer. Given the high frequency nature of this event, the typical approach is the review of video. Limitations to this approach are several. i) Appropriate training of the observer requires time to teach the technique. ii) Each observer should be cross correlated with results of the other observers to allow pooling of results by different observers. iii) Because of the likelihood of systematic differences in observer scoring, in principle each observer should contribute data to all groups (to preclude a systematic data biasing secondary to such systematic difference between observers). iv) The real time observations, even with speeded up video are tedious and the likelihood of observer fatigue must be considered when large numbers of records are being analyzed. v) Finally, because the counts are subject to potential observer bias, all counting should be done without knowledge as to treatment. It should be noted that aside from blinding, we are aware of no published reports in the literature that systematically address these concerns regarding intraobserver reliability, interobserver correlation or temporal stability of the observer counting. Given these limitations of the observer based analysis of scratching, there has been an interest in developing and validating automated data collection and analysis systems. Validation of an indirect measurement system must include comparisons with the gold standard (i.e. real time human observer counting), assessment of the system’s sensitivity, and a demonstration of its reliability.
4.2 Principle of the PMD
The present system employs detection of the displacement of a metal band in an EM field. The raw data output reveals a characteristic time variant signal with paw movement. The detection algorithms for a positive displacement of the paw, which may be a step, flinch, or upward movement to produce a scratching motion are similar. Equally, the optimal parameters have been previously shown to define a trigger (Yaksh et al., 2001). Assessment of the scratching wave produced by the band displacement in the PMD’s EM field, however, has provided us with specific components that enable the development of an algorithm to identify scratching as a microburst while minimizing the contributions of grooming and walking behaviors.
4.3 Validation of the scratching detection by the PMD
4.3.1 Covariance of PMD counts with the human observer
The principal validation of the automated systems is based on the correlation of the machine counts with human observer counts assessed concurrently in mice displaying a pruritogen initiated scratching response. Here analysis on a mouse by mouse basis or analysis as a pooled group revealed a highly significant regression of the observer vs. PMD counts, producing a slope not significantly different from one with an R value of .964 (pooled) and .972 (mean of 5 R values from the 5 single mouse regressions). Cross validation between other automated data acquisition systems and observer counts have used similar statistical methods in order to confirm their systems’ accuracy (Brash et al., 2005; Umeda et al., 2006).
4.3.2 Pharmacological Assessment
Standard pruritogens, including histamine, 48/80, and chloroquine were observed in the present work to produce dose dependent increases in measured scratching in dose ranges that have been previously reported to be effective (Inagaki et al., 1999; Liu et al., 2012). 48/80 was seen to cause scratching in a dose dependent fashion, while scratching was attenuated in an equally dose dependent fashion with administration of diphenhydramine (Sugimoto et al., 1998). Chloroquine was seen to produce the widely reported inverse U shaped dose response curve (Green et al., 2006), and was shown to be diphenhydramine insensitive. Of importance and consistent with previous work, the pruritogenic effects of 48/80 but not chloroquine were shown to be effectively blocked in a dose dependent fashion by the antihistaminic diphenhydramine. Chloroquine treated animals given diphenhydramine had a lower mean microburst count than those treated with chloroquine alone, but this was not statistically significant. Chloroquine has been shown to degranulate mast cells, thus releasing some histamine, which would explain diphenhydramine’s statistically non-significant effect. (Q. Liu et al., 2009)
Our microburst counts closely mirror the observed scratch counts of other investigators using non-automated counting methods. Authors have found 48/80 produces a range of 135 to 480 scratch counts extrapolated to 60 min at similar doses to 1 mg/mL (Han et al, 2006; Silva et al, 2011; Liu et al, 2012). Our observed counts of 258.8 ± 26.7 fall within this range. Our saline counts, however, appear to be higher than several published results 40–60 vs. 20–30 extrapolated over 60 min (Han, 2006). Other data have shown more variability. Shim reports in the same paper two groups of controls that scratched 7.2 bouts in 20 min (C57Bl/6J) while the other scratched 25 bouts in 20 min (ICR), which extrapolate to 20 for inbred and 75 for outbred scratch bouts per 60 min (Shim et al 2007). Fukamachi reports untreated ICR mice scratching 40–50 times in 20 min (Fukamachi, 2012). Unfortunately, saline control results are not widely reported in most studies involving pruritus. The reported higher scratch counts were the specific controls used to compare against 48/80 only. The pooled saline data (n=28) had a mean of 39 ± 19 (Mean ± SD), which suggests less of a difference from published data perhaps on the order of 10–15 scratch bouts over an hour, with no statistically significant difference. Differences in procedure and the fact that saline injected animals may produce more confounding behaviors because they are not occupied with scratching for the entire 60 min are other possible explanations for the observation.
4.3.3 Repeatability
An important attribute of an automated test system is its reliability. Here we show that over a 28 day period the same mice maintain remarkable stability in their response to 48/80. This supports not only repeatability, of the system, but the ability to generate reliable data from the same animal with at least one pruritogen. Such a property is important for allowing the repeated use of the same animal such as a genetically modified mouse, which may have limited availability. For example, recent studies have shown using TLR knockout mice that the innate immune system plays an important role in the scratching behavior of mice (T. Liu et al., 2012). In the present study we showed that 48/80 administered multiple times in a week, and once a week after the initial injections showed no statistically significant difference between time points in the number of scratches evoked. 48/80, however, is a mast cell degranulator (Enerback et al., 1974; Befus et al., 1982; Irman-Florjanc et al., 1983). Nielsen described a decrease in the total number of granules in mast cells over an extended period of time in the rat (Nielsen et al., 1982). The present behavioral data, however, suggests that the behavioral result (scratching) of SQ injection of 48/80 remained consistent. This may be a result of differences in physiology of the rat versus the mouse, or simply the fact that despite lower granule counts 48/80 still provides a robust behavioral effect with repeated injections at 1, 3, and 7 day intervals.
4.4 Scratching behavior phenotype
Visual assessment of the locally evoked scratching behavior reveals two evident characteristics: a specific somatotopic response and a complex temporal profile.
4.4.1 Somatotopy
After the local delivery of a pruritogen such as histamine or chloroquine, a homotopic scratching is observed, which we define as the targeted scratching behavior at the site of the pruritogen injection. The homotopic specificity demonstrated here by the PMD in regards to paw band placement and the drug delivery site is an important attribute in quantifying the behavior of scratching. Placement of the band contralateral to the injection site revealed a response index no different from the saline injected animal.
It should be noted that this site directed measurement limits what models are applicable to the system. This system has been designed for a specific and widely used unilateral shoulder injection in mouse and rat. This means bilateral scratching behavior cannot be simultaneously detected. Two hindpaw bands cannot be used because the signal becomes overly saturated with waves so that the algorithm cannot detect the appropriate behavior. Theoretically, the system can be used for any unilateral location where the rodent may scratch with the hind paw exclusively. This means areas in the lower back or cheek where wiping or biting may represent part of the scratching behavior cannot be detected by the system. The scratching portion (versus wiping) of the cheek model may be accurately examined with our system, but we have not yet confirmed this. The intra-paw model may also be suitable for our system but has not yet been tested.
4.4.2 Temporal profile
Examination of the paw movement and the signal that it generates indicated that there are two components. Component one is a high amplitude component that corresponds to the rapid discrete movement of the paw being lifted to the pruritic site. This behavior is represented by a transient signal with a maximum 500 ms duration and amplitudes of >0.3 V (in this system). This characteristic constituted the basic trigger. Examining concurrent video records, however, revealed that the trigger properties were shared by motor components of walking and grooming. Accordingly, in the absence of a pruritogen, there is a high resting count of the trigger signal that reflects normal motor behavior in the rodent.
The second component of the scratching response is the appearance of several high frequency movements in a close sequence which correspond to the paw being rapidly moved across the pruritogenic site usually one, two, or three times followed by the paw’s return to a rest position. It is this composite of movements, lifting the leg up, moving the paw across the pruritic area, and putting the paw back down that correspond to the scratch count reported by the observer. All three segments of scratching are taken into account by the algorithms in order to identify a waveform as a scratch. Thus, the system counts a microburst as the entire movement of the “scratch” from the initial lift of the leg to the pruritic site, to the movement of the paw across the pruritic site, and finally to the removal of the paw from the site. The waveforms produced by the various temporal components of scratching of the hind paw to the dorsolateral neck identified by the first algorithm causes the system to trigger at a specific frequency. Using an algorithm that produced a count based on 2 triggers per 1.15 seconds, which we defined as a microburst, we matched microbursts to the scratch counts of the trained observer.
The components of pruritogen evoked scratching were revealed in the analysis of hour long epochs. It was found that microbursts were cyclically distributed in a lower frequency event that suggests that these microbursts after 48/80 or chloroquine occur in clusters, which we termed macrobursts. These slower frequency macrobursts likely correspond to the itch-scratch-quiescence cycle (Ayres, 1964; Aoki et al., 2003) for a histamine dependent pruritic agent (48/80) and a histamine independent pruritic agent (chloroquine). Importantly, the differences in total scratch counts (microbursts) do not indicate the different patterns of scratch behavior between these agents. The ability to bin scratch bursts down to the second allows for an analysis of scratching behavior over the course of the treatment in a way that has not previously been undertaken in a behavioral model.
These binary periodicities in scratching activity, i.e. the animal either scratches in clusters or remains inactive, are intriguing as they likely reflect the properties of the underlying systems generating the scratch response. Recording from primary afferents after histamine (48/80) or histamine independent stimulus (such as cowhage or chloroquine) typically reveals such bursting behavior in small afferents (see for example Johanek et al., 2008; Ringkamp et al., 2011). In addition, there is evidence that the integration of pruritic input at the level of the spinal dorsal horn is subject to considerable modulation. (Carstens, 2008; Akiyama et al., 2011) Properties of this periodicity in scratching as suggested by the present analysis may also reflect upon this central modulation (See for example Davidson et al., 2007; Davidson et al., 2009). A combination of behavioral analysis with electrophysiology data will be of great interest in determining the periodic properties produced by the circuitry of pruriception.
4.5 Conclusion
The present studies show that the paw motion detector system can use a lightweight metal band’s movement through an electromagnetic field to detect scratching behavior in the mouse. Placed on the paw, this metal band perturbs the electromagnetic field to produce a signal, which when processed through an optimized analysis algorithm will produce specific waveforms for walking, scratching, and grooming. The system has been shown to detect the waveform correlated closely with human observed scratching behavior produced by a locally delivered pruritogen into the dorsolateral neck of mice.
Figure 7.
Scratching in the rat. Magnitude (Mean ± SEM) of cumulative scratching expressed as a percent of saline control after SQ 48/80 at 3 doses either alone or with diphenhydramine. (1 mg/kg, IP, all groups N=3) 1 way ANOVA, Bonferroni post hoc 48/80 vs. saline ** p<0.01 and 48/80+DPH vs. saline * p<0.05; Bonferroni post hoc 48/80 5 mg/mL vs. 48/80 5 mg/mL + 1mg/mL DPH # p<.05
Paw Motion Detector validated vs. human observer for measuring scratching in mice
PMD system uses EM field perturbation by metal paw band to detect scratches
PMD validated pharmacologically with Histamine, 48/80, Chloroquine, and DPH
Showed homolaterality/homotopic site specificity in scratch detection
Power analysis: 40% changes in scratching detectable at alpha=.05 with 4 mice
ACKOWLEDGEMENTS
This work was funded by NS16541 and by NS077348. We would like to thank Jorge Noguera who prepared the burst counting algorithm. We also thank Dr. Graham Beaton, Dr. Fabio Tucci, and Dr. Steve Sands of EPIGEN Biosciences Inc, San Diego, for their useful discussions regarding pruritus. Lastly, we would like to thank Dr. Gert Cauwenberghs at UCSD for his suggestion of an appropriate FFT algorithm to analyze microburst data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: PMD: Paw Motion Detector; EM: Electromagnetic
Contributor Information
Marc Marino, Email: mjmarino@ucsd.edu.
Polly Huang, Email: p3huang@ucsd.edu.
Shelle Malkmus, Email: smalkmus@ucsd.edu.
Erin Robertshaw, Email: e5robert@ucsd.edu.
Elaine A. Mac, Email: emac@ucsd.edu.
Yuri Shatterman, Email: yshatterman@ucsd.edu.
Tony L. Yaksh, Email: tyaksh@ucsd.edu.
REFERENCES
- Akiyama T, Iodi Carstens M, Carstens E. Transmitters and pathways mediating inhibition of spinal itch-signaling neurons by scratching and other counterstimuli. PLoS One. 2011;6(7):e22665. doi: 10.1371/journal.pone.0022665. Epub 2011 Jul 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T. ‘Pleasure of scratch’ is a complex sensation of itch and pain. 2nd International Workshop for the Study of Itch. 2003 Oct;:23–25. [Google Scholar]
- Ayres SJ. The fine art of scratching. JAMA. 1964;189:1003–1007. doi: 10.1001/jama.1964.03070130023006. [DOI] [PubMed] [Google Scholar]
- Befus AD, Pearge FL, Jorewood P, Binenstock J. Mucosal mast cells. I. Isolation and functional characteristics of rat intestinal mast cells. J. Immunol. 1982;128:2475–2480. [PubMed] [Google Scholar]
- Brash HM, McQueen DS, Christie D, Bell JK, Bond SM, Rees JL. A repetitive movement detector used for automatic monitoring and quantification of scratching in mice. J. Neurosci. Methods. 2005;142(1):107–114. doi: 10.1016/j.jneumeth.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Carstens E. Scratching the brain to understand neuropathic itch. J. Pain. 2008;9(11):999–1005. doi: 10.1016/j.jpain.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Dalgard F, Svensson A, Holm JO, Sundby J. Self-reported skin morbidity among adults: associations with quality of life and general health in a Norwegian survey. J. Investig. Dermatol. Symp. Proc. 2004;9:120–125. doi: 10.1046/j.1087-0024.2003.09111.x. [DOI] [PubMed] [Google Scholar]
- Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33(12):550–558. doi: 10.1016/j.tins.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ., Jr The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J. Neurosci. 2007;27(37):10007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ., Jr Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nat. Neurosci. 2009;12(5):544–546. doi: 10.1038/nn.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doulborg, Geunier . Statistical Methods for medical and biological students. London: George Allen and Curwin Ltd; 1940. [Google Scholar]
- Elliott GR, Vanwersch RA, Bruijnzeel PL. An automated method for registering and quantifying scratching activity in mice: use for drug evaluation. J. Pharmacol. Toxicol. Methods. 2000;44(3):453–459. doi: 10.1016/s1056-8719(01)00111-3. [DOI] [PubMed] [Google Scholar]
- Enerback L, Lundin PM. Ultrastructure of mast cells in normal and compound 48/80-treated rats. Cell Tissue Res. 1974;150:95–105. doi: 10.1007/BF00220383. [DOI] [PubMed] [Google Scholar]
- Fukamachi S, Mori T, Sakabe J, Shiraishi N, Kuroda E, Kobayashi M, Bito T, Kabashima K, Nakamura M, Tokura Y. Topical cholecystokinin depresses itch-associated scratching behavior in mice. J Invest Dermatol. 2011 Apr;131(4):956–961. doi: 10.1038/jid.2010.413. [DOI] [PubMed] [Google Scholar]
- Greaves MW. Itch in systemic disease: therapeutic options. Dermatol. Ther. 2005;18:323–327. doi: 10.1111/j.1529-8019.2005.00036.x. [DOI] [PubMed] [Google Scholar]
- Green AD, Young KK, Lehto SG, Smith SB, Mogil JS. Influence of genotype, dose and sex on pruritogen-induced scratching behavior in the mouse. Pain. 2006;124:50–58. doi: 10.1016/j.pain.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006 Nov 22;52(4):691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nature Rev. Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Nakamura N, Nagao M, Musoh K, Kawasaki H, Nagai H. Participation of histamine H1 and H2 receptors in passive cutaneous anaphylaxis-induced scratching behavior in ICR mice. Eur. J. Pharmacol. 1999;367:361–371. doi: 10.1016/s0014-2999(98)00974-1. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Igeta K, Shiraishi N, Kim JF, Nagao M, Nakamura N, Nagai H. Evaluation and Characterization of Mouse Scratching Behavior by a New Apparatus. MicroAct. Skin Pharmacol. Appl. Skin Physiol. 2003;16:165–175. doi: 10.1159/000069755. [DOI] [PubMed] [Google Scholar]
- Irman-Florjanc T, Erjavec F. Compound 48/80 and substance P induced release of histamine and serotonin from peritoneal mast cells. Agents Actions. 1983;13:138–141. doi: 10.1007/BF01967317. [DOI] [PubMed] [Google Scholar]
- Ishii I, Kurozumi S, Orito K, Matsuda H. Automatic Scratching Pattern Detection for Laboratory Mice Using High-Speed Video Images. IEEE Transactions on Automation Science and Engineering. 2008;5(1):176–182. [Google Scholar]
- Jeffry J, Kim S, Chen ZF. Itch signaling in the nervous system. Physiology (Bethesda) 2011;26(4):286–292. doi: 10.1152/physiol.00007.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, Hartke T, LaMotte RH, Ringkamp M. A role for polymodal C-fiber afferents in nonhistaminergic itch. J. Neurosci. 2008;28(30):7659–7669. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Shimada SG, Sikand P. Mouse models of acute, chemical itch and pain in humans. Exp. Dermatol. 2011;20(10):778–782. doi: 10.1111/j.1600-0625.2011.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Berta T, Xu ZZ, Park CK, Zhang L, Lü N, Liu Q, Liu Y, Gao YJ, Liu YC, Ma Q, Dong X, Ji RR. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J. Clin. Invest. doi: 10.1172/JCI45414. [epublished ahead of print 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139(7):1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen EH, Clausen J. Electron microscopic study of the regeneration in vivo of rat peritoneal mast cells after histamine secretion. Cell Tissue Res. 1982;224(2):465–468. doi: 10.1007/BF00216889. [DOI] [PubMed] [Google Scholar]
- Oppenheim AV, Schafer R. Discrete-Time Signal Processing. second ed. New York: Prentice Hall; 1999. [Google Scholar]
- Orito K, Chida Y, Fujisawa C, Arkwright PD, Matsuda H. A new analytical system for quantification scratching behaviour in mice. Br J. Dermatol. 2004;150(1):33–38. doi: 10.1111/j.1365-2133.2004.05744.x. [DOI] [PubMed] [Google Scholar]
- Oude Elferink RP, Kremer AE, Martens JJ, Beuers UH. The molecular mechanism of cholestatic pruritus. Dig. Dis. 2011;29(1):66–71. doi: 10.1159/000324131. [DOI] [PubMed] [Google Scholar]
- Paus R, Schmelz M, Biro T, Steinhoff M. Frontiers in pruritus research: scratching the brain for more effective itch therapy. J. Clin. Invest. 2006;116:1174–1186. doi: 10.1172/JCI28553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringkamp M, Schepers RJ, Shimada SG, Johanek LM, Hartke TV, Borzan J, Shim B, LaMotte RH, Meyer RA. A role for nociceptive, myelinated nerve fibers in itch sensation. J. Neurosci. 2011;31(42):14841–14849. doi: 10.1523/JNEUROSCI.3005-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva LL, Gomes BS, Sousa-Neto BP, Oliveira JP, Ferreira EL, Chaves MH, Oliveira FA. Effects of Lecythis pisonis Camb. (Lecythidaceae) in a mouse model of pruritus. J Ethnopharmacol. 2012 Jan 6;139(1):90–97. doi: 10.1016/j.jep.2011.10.023. Epub 2011 Oct 28. [DOI] [PubMed] [Google Scholar]
- Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, Lee CH, Kim M, Oh U. TRPV1 Mediates Histamine-Induced Itching via the Activation of Phospholipase A2 and 12-Lipoxygenase. Neurosci. 2007;27(9):2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y, Umakoshi K, Nojiri N, Kamei C. Effects of histamine H1 receptor antagonists on compound 48/80-induced scratching behavior in mice. Eur. J. Pharmacol. 1998;351(1):1–5. doi: 10.1016/s0014-2999(98)00288-x. [DOI] [PubMed] [Google Scholar]
- Umeda K, Noro Y, Murakami T, Tokime K, Sugisaki H, Yamanaka K, Kurokawa I, Kuno K, Tsutsui H, Nakanishi K, Mizutani H. A novel acoustic evaluation system of scratching in mouse dermatitis: rapid and specific detection of invisibly rapid scratch in an atopic dermatitis model mouse. Life Sci. 2006;79(22):2144–2150. doi: 10.1016/j.lfs.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Ozaki G, McCumber D, Rathbun M, Svensson C, Malkmus S, Yaksh MC. An automated flinch detecting system for use in the formalin nociceptive bioassay. J. Appl. Physiol. 2001;90:2386–2402. doi: 10.1152/jappl.2001.90.6.2386. [DOI] [PubMed] [Google Scholar]
- Yuman N, Ishii I, Yamamoto K, Orito K, Matsuda H. Real-time scratching behavior quantification system for laboratory mice using high-speed vision. J. Real-Time Image Proc. 2009;4:181–190. [Google Scholar]
- StatSoft, Inc. Electronic Statistics Textbook. Tulsa, OK: StatSoft; 2012. WEB: http://www.statsoft.com/textbook/poweranalysis. [Google Scholar]