Abstract
Reports of abuse and toxic effects of synthetic cathinones, frequently sold as “bath salts” or “legal highs”, have increased dramatically in recent years. One of the most widely used synthetic cathinones is 3,4-methylenedioxypyrovalerone (MDPV). The current study evaluated the abuse potential of MDPV by assessing its ability to support intravenous self-administration and lower thresholds for intracranial self-stimulation (ICSS) in rats. In the first experiment, rats were trained to intravenously self-administer MDPV in daily 2 hr sessions for 10 days at doses of 0.05, 0.1, or 0.2 mg/kg/infusion. Rats were then allowed to self-administer MDPV under a progressive ratio (PR) schedule of reinforcement. Next, rats self-administered MDPV for an additional 10 days under short (2 hr/day, ShA) or long (6 hr/day, LgA) access conditions to assess escalation of intake. Aseparate group of rats underwent the same procedures with the exception of self-administering methamphetamine (0.05 mg/kg/infusion) instead of MDPV. In a second experiment, the effects of MDPV on ICSS thresholds following acute administration (0.1, 0.5, 1 and 2 mg/kg i.p.) were assessed. MDPV maintained self-administration across all doses tested. A positive relationship between MDPV dose and breakpoints for reinforcement under PR conditions was observed. LgA conditions led to escalation of drug intake at the 0.1 and 0.2 mg/kg doses, and rats self-administering methamphetamine showed similar patterns of escalation. Finally, MDPV significantly lowered ICSS thresholds at all doses tested. Together, these findings indicate that MDPV has reinforcing properties and activates brain reward circuitry, suggesting a potential for abuse and addiction in humans.
Keywords: synthetic cathinone; 3,4-methylenedioxypyrovalerone; intravenous self-administration; extended access; intracranial self-stimulation; bath salt; abuse liability
Introduction
In recent years, there has been a dramatic increase in the use of designer drugs known as synthetic cathinones in both Europe and the United States (Spiller et al., 2011; Drug Enforcement Administration, 2011a). Use of synthetic cathinones has emerged rapidly, fueled largely by online marketing and widespread availability over the internet and in smoke shops and convenience stores (Psychonaut WebMapping Research Group, 2009a,b; Kavanagh et al. 2010; Vardakou et al., 2011). These synthetic drugs are derivatives of cathinone, a naturally occurring beta-ketone amphetamine analogue found in khat (Catha edulus), a plant that is abused for its stimulant-like effects (Magdum, 2011). Typically, synthetic cathinones are sold as “bath salts”, “plant food”, and other misleading terms and are marketed as “legal highs” and alternatives to traditionally abused stimulants such cocaine and amphetamines (Drug Enforcement Administration, 2011a). While many synthetic cathinones exist and are predicted to emerge as abused substances in the future, the analogues most frequently used at present include mephedrone (4-methylmethcathinone, 4-MMC), 3,4-methylenedioxypyrovalerone (MDPV), and methylone (3,4-methylenedioxymethcathinone, MDMC) (Drug Enforcement Administration, 2011a). As of October 2011, mephedrone, MDPV, and methylone have been temporarily classified in the United States as Schedule I controlled substances (Drug Enforcement Administration, 2011b).
Despite the widespread increase in use of these compounds, very little scientific data exist regarding their reinforcing effects and abuse potential. Of the three most common synthetic cathinones mentioned above, most scientific investigations have focused on mephedrone, and recently it has been shown that rats will readily self-administer mephedrone at a dose of 0.24 mg per 10 μl infusion (Hadlock et al., 2011). While mephedrone has been the subject of most popular press coverage and recent scientific investigations, MDPV use is also common and has been marketed as a replacement mephedrone in places where it has previously been banned (Durham, 2011; Coppola & Mondola, 2012). MDPV is a methylenedioxy analogue of pyrovalerone (Yohannan and Bolenko, 2010), a drug with stimulant-like properties (Holliday et al., 1964) that was once prescribed for the treatment of chronic fatigue and lethargy (Goldberg et al., 1973) before being shown to possess abuse potential in drug addicts (Deniker et al., 1975). Although the precise molecular mechanisms of actions of MDPV are currently unknown, likely mechanisms are inhibition of monoamine uptake, as pyrovalerone has been shown to inhibit synaptosomal dopamine and norepinephrine reuptake transporters (DAT and NET, respectively), and to a lesser extent serotonin transporters (SERT) (Lancelot et al., 1992; Meltzer et al., 2006; Kelly, 2011; Coppola & Mondola, 2012). MDPV increases extracellular levels of DA in the striatum of mice after oral administration (Fuwa et al., 2009). Behaviorally, MDPV leads to dose-dependent increases in locomotor activity in mice to a greater extent than methamphetamine when using identical doses (Marusich et al., 2011). Together, these data provide early evidence that MDPV possesses stimulant-like properties and corroborates users reports describing subjective effects similar to those of methylphenidate, cocaine, and amphetamines (Psychonaut WebMapping Research Group 2009a,b).
To our knowledge, there have been no published studies directly examining the reinforcing and rewarding effects of MDPV. The present study addressed this issue by examining the ability of MDPV to support intravenous self-administration (IVSA) and to lower thresholds for intracranial self-stimulation (ICSS). In Experiment 1, the reinforcing effects MDPV during IVSA were assessed at three doses (0.05, 0.1, and 0.2 mg/kg per infusion) during three phases of experimentation: (1) 2 hr daily access sessions, (2) a progressive ratio (PR) schedule of reinforcement, and (3) short (2 hr daily, ShA) vs. long (6 hr daily, LgA) sessions. A separate group of animals underwent the same procedures but self-administered methamphetamine (0.05 mg/kg/infusion) as a positive control. In Experiment 2, MDPV (0.1, 0.5, 1, and 2 mg/kg, i.p.) was administered acutely to determine effects on thresholds for ICSS, a well-established measure of brain reward function (Kornetsky & Bain, 1992).
Methods and Materials
Subjects
All experimental procedures were conducted with the approval of the Institutional Animal Care and Use Committee at Arizona State University, and according to the Guide for Care and Use of Laboratory Animals as adopted by the National Institutes of Health (NIH). Forty-one male Sprague-Dawley rats (Harlan Laboratories, Livermore, CA), weighing approximately 250 g, were individually housed upon arrival. Forty-eight rats were implanted with jugular vein catheters and vascular access ports and underwent IVSA procedures for Experiment 1. Five non-catheterized rats underwent ICSS procedures for Experiment 2. Rats were housed according to NIH standards on a 12 hr light-dark cycle and given ad libitum access to food and water during all experimental procedures except during behavioral testing. All experimental sessions took place during the dark phase, with the exception of a 16 hr overnight lever-press training sessions and PR tests which began at 4:00 p.m. and ended the following morning at approximately 8:00 a.m. Throughout the course of experiments, 13 of the 48 rats in Experiment 1 were removed due to catheter patency failure and one of the 5 rats in Experiment 2 was removed due to health-related issues.
Drugs and Assessment of Purity
MDPV was obtained through an internet website www.researchchemz.com (Laboratory Supply USA, San Diego, CA). Ten mg samples of MDPV were analyzed by LC-MS for purity at Research Triangle Institute (Durham, NC). Samples were analyzed using a Waters Synapt HDMS quadrupole time of flight (Q-TOF) mass spectrometer interfaced to a Waters Acquity UPLC system. Data were acquired using a capillary voltage of 3 kV, source temperature of 150 C, desolvation temperature of 500 C, sampling cone at 30 V, and extraction cone at 4 V. The mass spectrometer was externally calibrated from 50 – 700 Da using a sodium formate solution, and mass shifts during acquisition were corrected for using leucine enkephalin as a lockmass. Liquid chromatography was performed using a BEH C18 column (2.1 × 50 mm, 1.7 μm particles) held at 40 C. Sample identity was confirmed based on exact mass, retention time, and fragmentation match to a certified reference standard from Cerilliant (Round Rock, TX). MDPV samples were determined to have an apparent purity of >95%. For all behavioral studies, MDPV and methamphetamine (Sigma-Aldrich, St. Louis, MO) were dissolved in sterile saline. For Experiment 2, MDPV was administered i.p. in a volume of 1 ml/kg.
Experiment 1: Intravenous self-administration (IVSA) procedure
Surgical Procedures
Prior to arrival, rats were implanted with intravenous catheters into the jugular vein at Harlan Laboratories. On the day following arrival, rats were anesthetized with isoflurane (2% v/v) vaporized oxygen at a flow rate of 2 L/min. A 2.5 cm longitudinal incision was made between the scapulae for implantation of a threaded vascular access port (Plastics One, Roanoke, VA, USA). Threaded vascular access ports were attached to be mesh collar sutured underneath the surrounding tissue within the incision. Access ports were sealed with a piece of Tygon tubing closed at one end and a protective cap. All rats were given allowed to recover from surgery for 5 days prior to the initiation of behavioral testing, and during this time animals received daily intravenous infusions of 70 U/ml heparin (0.2 ml volume) to maintain catheter patency and 100 mg/ml cefazolin (0.1 ml volume) to protect against infection. Meloxicam (2.5 mg/ml s.c.) was administered for the first 3 days following surgical procedures to provide additional relief post-surgical discomfort. In addition, rats were given ten 45 mg sucrose pellets in their homecage four days prior to IVSA procedures to eliminate neophobia to sucrose pellets that could delay acquisition of self-administration during 16 hr overnight training sessions.
Apparatus
Drug self-administration sessions were conducted in operant self-administration chambers (ENV-008, Med Associates, St. Albans, VT, USA). All self-administration chambers were located inside sound-attenuating cubicles equipped with a house light and exhaust fan designed to mask external noise and odors, and were interfaced to a PC computer. Chambers were equipped with two stainless steel response levers located on one wall with a 4.2 × 5 cm food pellet receptacle placed between levers. Each response lever was located approximately 7 cm above a stainless steel grid floor, and positioned above each lever was a 2.5 cm diameter white stimulus light. Located near the top of the self-administration chambers was a Sonalert speaker that provided an auditory stimulus during drug delivery. Outside each chamber was a syringe pump that was interfaced to the computer and delivered the drug solution via a single-channel liquid swivel mounted atop the chamber via polyethylene tubing.
Experimental Design: IVSA Procedures
Following recovery from surgical procedures, self-administration sessions commenced. During all self-administration sessions, except during progressive ratio training, each press on the active lever delivered the reinforcer on an FR1 schedule of reinforcement. Reinforcer delivery was accompanied by concurrent illumination of a stimulus light and presentation of an auditory stimulus for two seconds followed by a 20-sec timeout period during which additional lever presses were recorded but produced no programmed responses. Inactive lever presses were recorded but produced no programmed consequences. Self-administration procedures were initiated with a 16 hr overnight training session whereby active lever presses delivered a 45 mg sucrose pellet (TestDiet, Richmond, IN). Approximately 24 hr following sucrose training, rats were separated into one of four groups based upon MDPV dose (0.05, 0.1, or 0.2 mg/kg/infusion) or as a positive control, methamphetamine (0.05 mg/kg/infusion). Each drug infusion was delivered in a volume of 0.06 ml. Next, daily 2 hr self-administration sessions were commenced with intravenous MDPV or methamphetamine as the reinforcer. MDPV or methamphetamine was delivered to the vascular access port by polyethylene tubing housed in a stainless steel spring tether that was attached to the liquid swivel. Self-administration sessions were conducted 7 consecutive days per week, and each session was preceded and followed by an intravenous infusion of 0.1 ml of 70 U/ml heparin plus 100 mg/ml cefazolin to maintain catheter patency. Daily 2 hr self-administration sessions were conducted for a minimum of 10 days and until stability criterion was reached (<15% deviation in active lever pressing for each dose group for two consecutive days). All groups met stability on day 10.
Following ten days of 2 hr IVSA sessions, a 16 hr overnight progressive ratio (PR) schedule was conducted to assess the reinforcing efficacy of MDPV or methamphetamine. During PR tests, the number of lever presses required to obtain a single infusion of MDPV was determined by the following the equation: responses per reinforcer delivery = 5 × e(injection number -0.2) - 5 (i.e., 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, etc.) (Richardson and Roberts, 1996). Breakpoints were considered to be met when rats did not emit any lever presses for 2 hours. Following PR testing, each dose group of rats (0.05, 0.1, or 0.2 mg/kg/infusion of MDPV) was divided into two subgroups, such that half of the rats in each dose group continued with 2 hr daily self-administration sessions for ten days (short access, ShA), while the other half began 6 hr daily sessions (long access, LgA) for ten days. All aspects of the self-administration procedures were identical except for session length (2 vs. 6 hr per day). For rats self-administering methamphetamine, all rats were assigned to the LgA group to demonstrate escalation of drug intake.
Experiment 2: ICSS procedures
Surgical Procedures
Rats were anesthetized with isoflurane (2% v/v) vaporized oxygen at a flow rate of 2 L/min and placed into a stereotaxic frame. A stainless-steel bipolar electrode (PlasticsOne, Roanoke, VA, USA, 2 mm diameter, insulated except at the ventral tip) was implanted into the medial forebrain bundle (AP −0.05 mm; ML ± 1.7 mm, DV −8.3 mm from dura). Four skull screws and dental cement were used to permanently secure electrodes to the skull. To counterbalance for any hemispheric differences, half the animals received electrodes in the left hemisphere and the other in the right hemisphere. Following surgery, rats were given 7 days to recover before beginning ICSS procedures during which they received daily injections of 2.5 mg/ml meloxicam (0.15 ml volume) to minimize post-surgical discomfort.
Apparatus
All ICSS testing was conducted in operant chambers (ENV-007CT, Med Associates). Chambers were housed inside sound-attenuating cubicles equipped with an exhaust fan to mask external noise and odors. Chambers contained a house light on the back wall and a front wall mounted nose-poke aperture with LED stimulus lights located inside the access hole (ENV-114M, Med Associates). The nose-poke aperture was 2.5 cm in diameter, located 5 cm above the stainless steel grid floor, and contained an infrared detector placed 0.64 cm from the front edge of the panel for recording responses. Located outside chambers was a dual programmable ICSS stimulator (PHM-150B/2, Med Associates) that was interfaced to a computer to deliver electrical current to the electrode. Chambers were interfaced to a PC computer using Med-PC IV software that controlled all stimulation parameters, test functions, and data collection (Med Associates).
Experimental Design: ICSS procedures
The procedure for measuring reward thresholds was a modified version of the discrete trials current-threshold method (Kornetsky et al., 1979; Markou and Koob, 1992). During all ICSS phases, stimulation availability was signaled by illumination of the nose poke aperture by the LED stimulus light complex. Rats initiated training on a FR1 schedule of reinforcement where nose pokes resulted in the delivery of a 200 μsec square-wave cathodal pulses at 100 Hz at a current of 120 μA. After acquisition criteria were met (> 600 responses in 30 min for 2 sessions), rats began discrete-trials training procedures. Each discrete trial began with a free stimulation of 120 μA, followed by a 7.5 second period during which the first response (trial response) yielded an identical stimulation. Following the trial response, LED lights turned off and subsequent responses (inter-trial interval (ITI) responses) were recorded, but yielded no stimulation. Progression through discrete trials training required rats to meet criterion (>60% of total response were trial responses) at four ITI lengths (2, 5, 10, and 15 sec). Upon completing training, rats then began discrete-trials current-threshold determination procedures. Each current threshold determination session began with 120 μA of current and progressed through 4 cycles of ascending and descending current intensities. At a given current intensity, trial blocks began with a free stimulation, followed by 7.5 seconds during which the animal could emit a nose-poke response to receive an identical stimulation. Following a single trial response, LED stimulus lights turned off initiating an ITI period between 7.5 and 15 seconds (mean of 10 second) that separated trials. Responses during the ITI interval further lengthened the ITI by 12.5 seconds. When animals emitted appropriate responses on ≥3 of 5 trials, electrical stimulation decreased by 5 μA for the next 5-trial block. Block intensities continued to descend until rats responded ≤2 out of 5 trials during a given trial block, at which point the current intensities reversed into ascending mode, with increases in current intensities of 5 μA each for the subsequent block. Thus, the procedure determined the minimum amount of current (threshold) for which the rat was willing to respond. Thresholds were calculated by averaging the midpoint of current intensities between positive (responses on ≥3 of 5 trials) or negative (responses on ≤2 of 5) trial blocks. Rats received a minimum of 10 days of baseline threshold assessment and were required to meet stable baseline criteria prior to administration of MDPV, defined as when the average of thresholds for the last 4 days minus the first 4 days of an 8-day window was less than 10% of the average of the full 8 days. Rats continued to receive baseline testing throughout the course of the experiment 4 days per week. Rats received vehicle injections 20 min prior to placement in ICSS procedures. MDPV doses were assigned randomly and injections given 20 minutes prior to threshold determination procedures. All rats, with the exception of one that was removed halfway through MDPV testing due to loss of cranial implant, underwent 2 determinations of each dose of MDPV, and 5 determinations of vehicle.
Statistical Analysis
All statistical analyses were conducted using IBM SPSS Statistics version 19 (Armonk, New York, USA). All data points represent mean ± SEM. A significance criterion of p<0.05 was used for all analyses. For the first 10 IVSA sessions, the ability of MDPV to maintain responding was first analyzed separately for each dose of MDPV by a mixed analysis of variance (ANOVA) with lever (active vs. inactive) and session as factors. Post-hoc one-way ANOVAs were also conducted to determine the number of sessions required to obtain lever discrimination. The total number of MDPV infusions obtained per session was analyzed by a mixed ANOVA with MDPV dose and session as factors. Holm-Sidak post-hoc tests determined overall dose effects, and one-way ANOVAs followed by Holm-Sidak post-hoc tests further determined dose effects during each session. Analysis of the total number of infusions obtained during PR sessions at different doses of MDPV were analyzed by a one-way between subjects ANOVA followed by Holm-Sidak post-hoc tests. The 0.05 mg/kg doses of methamphetamine and MDPV were analyzed separately by an independent samples t-test. For ShA vs LgA IVSA sessions, the effects of session length (ShA vs. LgA) on total infusions obtained was analyzed by mixed ANOVA for each dose of MDPV or methamphetamine. Post-hoc one-way ANOVAs further explored differences in the number of infusions obtained across MDPV doses for each session. To determine escalation of drug intake, mixed ANOVAs for each dose of MDPV or methamphetamine were conducted with infusions obtained in ShA vs. LgA (first 2 hr only) and session as factors. Post-hoc tests compared each session separately. For the 0.1 and 0.2 mg/kg MDPV dose groups, repeated measures ANOVAs were conducted separately for ShA, LgA, and LgA (first 2 hr) to determine if drug intake escalated across time (session 1 – 10) as determined by significant increases over the first session of the ShA vs. LgA phase. For Experiment 2, raw ICSS current intensity thresholds (in μA) for all baseline sessions conducted after drug-administration tests began were first compared to vehicle sessions with a t-test to assess for potential injection effects. Next, ICSS current intensity thresholds were obtained following all doses, including vehicle, and converted to scores reflecting the percent change from thresholds obtained following vehicle administration for each rat. Threshold measures following vehicle treatment were calculated by averaging ICSS thresholds obtained across the 5 vehicle test days. Percentage change scores were analyzed by one-way repeated measures ANOVA.
Results
Experiment 1: Self-Administration of MDPV in 2 hr/day Sessions
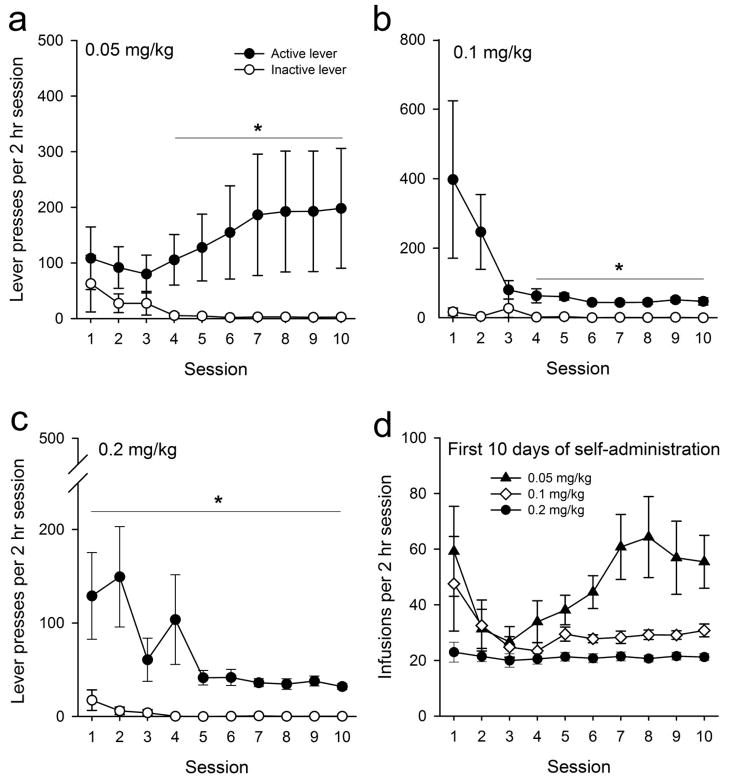
For the 0.05 mg/kg dose group, a significant main effect of lever (F1,13=8.67, p<0.01) and session (F9,117 =2.64, p<0.01) was observed, but a lever x session interaction was not found. Presses on the active lever were significantly greater than those on the inactive lever for sessions 4 through 10 (p<0.01), indicating that rats successfully learned to discriminate between active and inactive levers after 4 experimental sessions (Fig. 1a).
Figure 1.
Intravenous self-administration of MPDV. Data presented are active and inactive lever presses across the first ten days of IVSA procedures for the (a) 0.05, (b) 0.1, and (c) 0.2 mg/kg/infusion groups (n = 9 for each group). * indicates p <0.05 between active and inactive lever presses. (d) Total number of infusions during 2 hr daily access sessions across the first ten days of IVSA and for each dose of MDPV tested.
For the 0.1 mg/kg dose group, a significant main effect of lever (F1,13=6.06, p<0.05) was observed, but significant effects of session or a lever x session interaction were not observed. Presses on the active lever were significantly greater than those on the inactive lever for sessions 4 through 10 (p<0.01), indicating that rats successfully learned discriminate between the active and inactive levers after 4 experimental sessions (Fig. 1b).
For the 0.2 mg/kg dose group, a significant effect of lever (F1,16=14.06, p<0.01), session (F9,144=3.872, p<0.001), and a lever x session interaction (F9,144=2.731, p<0.01) were observed. Presses on the active lever were significantly greater than those on the inactive lever for all sessions (p<0.01), indicating that rats successfully discriminated between the active and inactive levers (Fig. 1c). Similar lever discrimination was observed in rats self-administering methamphetamine (data not shown).
When analyzing overall drug intake (number of drug infusions), significant main effects of MDPV dose (F2,24=6.96, p<0.01), session (F9,216=3.791, p<0.01), and a dose x session interaction (F18, 216=2.15, p<0.01) were observed. The overall number of infusion obtained per 2 hr session across all 10 sessions was significantly greater in the 0.05 mg/kg dose group as compared to 0.2 mg/kg dose groups (p<0.05) and approached significance compared to the 0.1 mg/kg dose group (p=0.07). Post-hoc comparisons revealed significant differences in the number of infusions obtained in the 0.05 vs. 0.1 mg/kg dose groups, and in the 0.05 vs. 0.2 mg/kg dose group for sessions 6 through 10 (p<0.05, Fig. 1d)
Progressive Ratio Responding
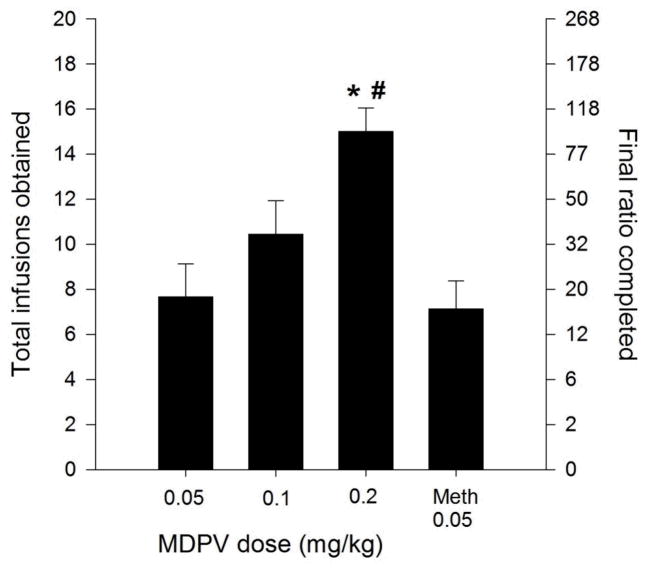
Under a PR schedule of reinforcement, a significant effect of MDPV dose (F2,24=7.472, p<0.01) was observed for the total number of infusions obtained prior to cessation of responding (i.e., breakpoints) (Fig. 2). Post-hoc tests revealed that the number of infusions obtained in the 0.2 mg/kg dose group were significantly greater than those in the 0.05 (p<0.001) and the 0.1 mg/kg (p<0.05) dose groups. Thus, there appeared to be positive relationship between MDPV dose and breakpoints for MDPV reinforcement. Rats self-administering methamphetamine exhibited breakpoints that were not significantly different that those of rats self-administering the 0.05 mg/kg dose of MDPV (p>0.05).
Figure 2.
Total number of infusions earned during PR responding for the 0.05, 0.1, and 0.2 mg/kg/infusion doses of MDPV (n = 9 for each group) as well as a separate group of rats self-administering methamphetamine at a dose of 0.05 mg/kg/infusion (n=9). The total number of infusions earned during the PR session is plotted along the left y-axis. As a reference, the total number of active lever presses completed during the test is plotted along the right y-axis. * indicates p<0.05 vs. the 0.05 mg/kg dose of MDPV. # indicates p <0.05 vs. the 0.1 mg/kg dose of MDPV.
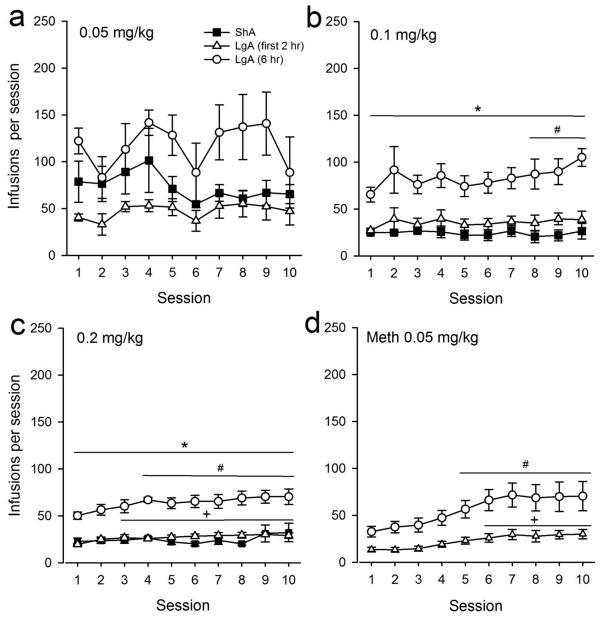
Self-Administration of MDPV during ShA vs. LgA
For the 0.05 mg/kg dose group, no significant effects of session or session length were observed (Fig. 3a). For the 0.1 mg/kg dose group, a significant effect of session length (F1,7=18.644, p<0.01) was observed, but no effect of session or a session length x session interaction were found. The number of infusions obtained was significantly greater in LgA vs. ShA groups for all experimental sessions (p<0.05). Additionally, a significant effect of session was observed for LgA rats (F9,27=2.285, p<0.05), but not for ShA, such that the number of infusions obtained during sessions 8, 9 and10 were significantly greater than those observed during session 1 (p <0.05, Fig 3b).
Figure 3.
Total number of infusions obtained during ShA, LgA, and the first 2 hr of LgA sessions across the final ten days of IVSA procedures for the (a) 0.05, (b) 0.1, and (c) 0.2 mg/kg/infusion MDPV groups (n = 5 for each LgA group), as well as rats self-administering methamphetamine at a dose of 0.05 mg/kg/infusion (d, n=9). * indicates p < 0.05 for sessions in which the number of total infusions obtained during LgA was significantly greater than total infusions obtained during ShA. # indicates p<0.05 for total number of infusions obtained during LgA sessions vs. Day 1 of LgA. + indicates p<0.05 for total number of infusions obtained during the first 2 hr of LgA sessions vs. Day 1 of LgA (first 2 hr).
For the 0.2 mg/kg dose group (Fig. 3c), a significant effect of session length (F1,7=50.209, p<0.001) was observed, but no effect of session nor a session length x session interaction was observed. The number of infusions obtained was significantly greater in LgA vs. ShA groups for all experimental sessions (p<0.01). A significant effect of session for LgA rats was observed (F9,27=2.288, p<0.05), and post-hoc tests revealed that the number of infusions obtained was significantly higher during sessions 4 through 10 as compared to session 1 (p<0.05). Taken together, these results revealed that rats self-administering either the 0.1 or 0.2 mg/kg/infusion dose of MDPV under LgA conditions displayed escalated drug intake across experimental sessions.
Additional analyses were conducted to determine if escalation of intake also occurred during the first 2 hr of 6 hr LgA sessions. No significant increases in the number of infusions during the first 2 hr of LgA sessions were evident in rats self-administering the 0.1 mg/kg dose of MDPV. However, in rats self-administering the 0.2 mg/kg dose, a significant effect of session (F9,36=3.924, p <0.005) was observed. Post-hoc tests revealed significant differences in the number of infusions obtained during the first 2 hr of LgA during sessions 3 through 10 as compared with session 1 (p<0.001). Thus, only rats self-administering the 0.2 mg/kg dose of MDPV displayed escalated drug intake during the first 2 hr of LgA.
In rats self-administering methamphetamine under LgA conditions, a significant effect of session was observed for the number of infusions obtained during the entire 6 hr LgA session (F9,72=7.413, p<0.001) as well as during the first 2 hrs of the LgA sessions (F9,72=6.359, p<0.001) Post-hoc tests revealed significant differences in the number of infusions obtained during the entire LgA during sessions 5 through 10 as compared with session 1 (p<0.001), as well as significant differences in number of infusions obtained in the first 2 hr of LgA during sessions 6 through 10 as compared with session 1 (p<0.001).
Experiment 2: Effects of MDPV on Thresholds for ICSS
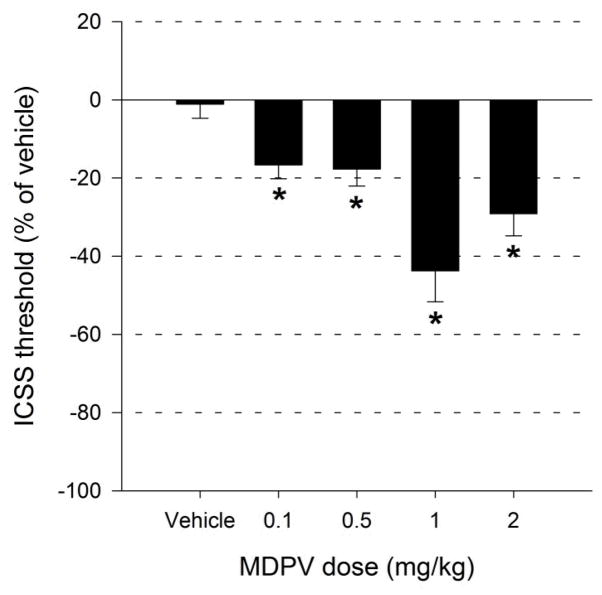
An independent samples t-test revealed no significant differences between baseline and vehicle scores t(58) = −1.39, p > 0.05. A significant effect of MDPV dose (F4,35=11.549, p<0.001) on thresholds for ICSS was observed (Fig. 4). When compared to vehicle, ICSS thresholds following MDPV administration were significantly lower at all doses tested (p<0.05).
Figure 4.
Effects of vehicle and MDPV (0.1, 0.5, 1 and 2 mg/kg i.p.) on thresholds for ICSS (n = 5). * indicates p < 0.05 vs. vehicle.
Discussion
To our knowledge, this is the first systematic verification of the reinforcing effects of MDPV in rats. The current study revealed that during daily 2 hr IVSA sessions, all doses of MDPV tested maintained active lever responding across experimental sessions, and rats successfully discriminated between active and inactive levers by the 4th day of self-administration. Furthermore, significant dose effects on MDPV intake were observed as measured by the total number of infusions obtained during experimental sessions. Following stable responding on IVSA procedures, a PR test revealed a positive relationship between MDPV dose and reinforcing efficacy, as measured by breakpoints for MDPV self-administration. Breakpoints for methamphetamine reinforcement at a dose of 0.05 mg/kg/infusion were similar to those obtained for the same dose of MDPV. Under extended access conditions (6 hr/day), an escalation of MDPV intake at the 0.1 and 0.2 mg/kg doses was observed for the entire extended access session, and this also occurred during the first 2 hr of LgA sessions for the 0.2 mg/kg dose, but not for other doses. Extended access to methamphetamine also produced escalation of drug intake. Finally, a reduction in ICSS thresholds across all doses of MDPV following acute administration was observed, indicating an increase in brain reward function.
The IVSA method was chosen for the present study given the high degree correspondence between drugs that can have addictive potential in humans and drugs that function as reinforcers in IVSA procedures in animals (Collins et al., 1983). In order to establish that a drug functions as a reinforcer in IVSA procedures, a number of criteria need to be met, including higher responding on the active vs. inactive lever, and responding must show orderly and differential effects across a range of drug doses (Meisch, 1987). The first criterion was verified across the first 10 days of IVSA procedures during which all MDPV doses maintained active lever pressing while inactive lever pressing progressively declined. These results suggest that responding occurred due to the reinforcing effects of MDPV and not as the result of any indirect locomotor or general response-enhancing effects of MDPV. The second criterion was also met when results revealed an orderly inverse dose-effect on total drug intake (i.e., number of infusions obtained) such that animals received the fewest infusions for the 0.2 mg/kg dose, followed sequentially by the 0.1 and 0.05 mg/kg doses. This inverse pattern between dose and drug intake replicates findings of abused stimulants under continuous schedules of reinforcement, and likely represents the upper end of the typical inverse U-shaped pattern typically seen across wider dose ranges (Panlilio, 2011). In addition, the results of the present study are strikingly similar to self-administration patterns for methamphetamine under nearly identical experimental conditions and doses (present study and Gass et al., 2009). This finding provides evidence of similar potencies between MDPV and methamphetamine. Together, these findings indicate that MDPV likely possess a potential for abuse similar to that of methamphetamine and other stimulants.
The progressive ratio schedule of reinforcement has been used extensively to evaluate the reinforcing efficacy of drugs of abuse, as it is an index of the motivation to obtain infusions of the drug in the face of increasing behavioral demand. PR schedules have consistently shown a positive relationship between dose and reinforcer efficacy, and this relationship has been consistently observed with other abused stimulants such cocaine (Roberts et al., 1989), d-amphetamine, and methamphetamine (Richardson and Roberts, 1996). The results from the present study also revealed this positive relationship between MDPV dose breakpoints for MDPV reinforcement. As with responding on the FR1 schedule above, under the same PR schedule with identical doses (0.1 and 0.2 mg/kg/infusion), breakpoints for MDPV self-administration were similar to those we and others have previously observed for methamphetamine (Gass et al., 2009; Richardson and Roberts, 1996) as well as D-amphetamine (Richardson and Roberts, 1996). In addition, breakpoints for MDPV self-administration under PR conditions at a dose of 0.05 mg/kg/infusion were similar to those observed in rats self-administering the same dose of methamphetamine, further demonstrating methamphetamine-like potency and reinforcing efficacy of MDPV.
While demonstrating that a drug functions as a reinforcer is an important first step in determining abuse liability, such observations do not unequivocally indicate the potential for addiction potential in humans (Ahmed, 2011). One of the defining characteristics of drug addiction is an escalation in drug use, often due to tolerance to the reinforcing effects of the drug (American Psychiatric Association, 2004). As a result, a common procedure for modeling human patterns in animals has been termed the “escalation model” (Ahmed and Koob, 1998). In this procedure, animals are given extended access to the drug (typically 6 – 12 hr/day access sessions) vs. traditionally employed shorter access (1 – 2 hr/day). As a result of extended access to the drug, animals display an escalation in drug intake that parallels intake patterns characteristic of compulsive drug-seeking and addiction in humans (Ahmed, 2011). The current study revealed that, during extended access to MDPV, rats responding for the two highest doses of MDPV displayed a significant escalation in overall drug intake across the final 10 experimental sessions. Furthermore, this escalation was also seen during the first 2 hrs of LgA sessions for the high dose of 0.2 mg/kg. These findings are similar to those reported for other addictive stimulants including cocaine (Ahmed and Koob, 1998), D-amphetamine (Gipson and Bardo, 2009) and methamphetamine (Kitamura et al., 2006), and the present study also demonstrated escalation of methamphetamine intake at a dose of 0.05 mg/kg/infusion. Unlike these studies, however, our results revealed escalation of MDPV intake at higher rather than lower doses. These data suggest that MDPV may possess some unique reinforcing properties that are not reflective of other prototypical stimulants such as methamphetamine. While additional comparative studies are needed to further corroborate these findings, the current results further strengthen the possibility that MDPV possesses the potential for compulsive use in humans.
Olds and Milner first discovered that rats would show a place preference for and perform an operant task to receive ICSS (Olds and Milner, 1954), and numerous studies have revealed that both ICSS and drug reinforcers likely active the same brain reward circuitry (Wise, 1996). Drug-induced lowering of ICSS thresholds is generally accepted to be due to the facilitation of brain reward functioning, providing a direct measure of the hedonic and rewarding properties of drugs of abuse (Panlilio, 2011), and nearly all abused stimulants including cocaine (Esposito et al., 1978), amphetamine (Horovitz et al., 1972), and methamphetamine (Sarkar and Kornetsky, 1995) lower ICSS thresholds. The current results reveal that, when using the discrete-trials current threshold procedure, MDPV lowers ICSS thresholds across a wide range of doses as compared to vehicle. Thus, these findings both parallel previous findings with other addictive stimulants and provide further evidence that MDPV possesses similar rewarding properties.
In summary, the current study demonstrates that the synthetic cathinone MDPV possesses potent reinforcing properties and suggests a high degree of abuse potential in humans. The results revealed that MDPV dose-dependently functions as a reinforcer on a continuous reinforcement schedule. A positive relationship between MDPV dose and reinforcer efficacy was demonstrated in during progressive ratio testing, and breakpoints for MDPV reinforcement at the lowest dose tested were similar to those for the same dose of methamphetamine. Extended access to MDPV produced escalated intake over time for the two higher doses, indicative of a compulsive pattern of intake characteristic of addiction in humans. Finally, the ability of MDPV to lower thresholds for ICSS provides further evidence of hedonic and rewarding effects of MDPV. Taken together, these results suggest that that MDPV possesses a strong potential for compulsive use and addiction in humans. These findings have important implications for future research on synthetic cathinone addiction, as well the development of appropriate drug policies and legislative measures regarding its status as a controlled substance.
Acknowledgments
This work was supported by NIH grant DA025606. The authors would also like to thank Kelly Wischerath, Emily Wininger, Megan Johnson, and Evan Armstrong for their assistance during experimental procedures.
Footnotes
Author contributions
LRW, PRK, and MFO were responsible for the study concept and design. NEN, KS, and SW contributed to the acquisition of data. MG, BFT, and JAM contributed to the mass spectrometry analysis of MDPV. LRW drafted the manuscript, and PRK and MFO provided critical revisions of the manuscript. All authors reviewed the content approved the final version for publication.
Financial Disclosures
The authors report no financial or other potential conflicts of interest.
References
- Ahmed SH. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed S. Escalation of drug use. In: Olmstead MC, editor. Animal Models of Drug Addiction. Humana Press; Totowa, NJ: 2011. pp. 267–292. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, DSM-IV-TR. American Psychiatric Press; Washington, DC: 2004. [Google Scholar]
- Collins RJ, Weeks JR, Cooper MM, Good PI, Russell RR. Prediction of abuse liability of drugs using IV self-administration by rats. Psychopharmacology. 1983;82:6–13. doi: 10.1007/BF00426372. [DOI] [PubMed] [Google Scholar]
- Coppola M, Mondola R. 3,4-Methylenedioxypyrovalerone (MDPV): Chemistry, pharmacology and toxicology of a new designer drug of abuse marketed online. Toxicol Lett. 2012;208:12–15. doi: 10.1016/j.toxlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Deniker P, Loo H, Cuche H, Roux JM. Abuse of pyrovalerone by drug addicts. Annales Médico-Psychol. 1975;2:745–748. [PubMed] [Google Scholar]
- Drug Enforcement Administration. Background, data and analysis: mephedrone (4-MMC), methylone (MDMC) and 3,4-methylenedioxypyrovalerone (MDPV) United States Department of Justice, Office of Drug Control, Drug and Chemical Evaluation Section; Washington, D.C.: 2011a. [Google Scholar]
- Drug Enforcement Administration. Federal Register, 21 CFR Part 1308, Docket No DEA-357. Unites States Department of Justice; Washington, D.C.: 2011b. Schedules of controlled substances: temporary placement of three synthetic cathinones into Schedule I. [Google Scholar]
- Durham M. Ivory wave: the next mephedrone. Emerg Med J. 2011;28:1059–1060. doi: 10.1136/emj.2011.112920. [DOI] [PubMed] [Google Scholar]
- Esposito R, Motola A, Kornetsky C. Cocaine: acute effects on reinforcement thresholds for self-stimulation behavior to the medial forebrain bundle. Pharmacol Biochem Behav. 1978;8:437–439. doi: 10.1016/0091-3057(78)90082-5. [DOI] [PubMed] [Google Scholar]
- Fuwa W, Kodama T, Honda Y, Tanaka Y, Kubo Y, Ohashi N, Nakae N, Ogat A. CNS effects of methylenedioxypyrovalerone using microdialysis. Integ Managem Biol Chem. 2009;9:62–72. [Google Scholar]
- Gass JT, Osborne M, Watson NL, Brown JL, Olive MF. mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology. 2009;34:820–833. doi: 10.1038/npp.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Bardo MT. Extended access to amphetamine self-administration increases impulsive choice in a delay discounting task in rats. Psychopharmacology (Berl) 2009;207:391–400. doi: 10.1007/s00213-009-1667-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J, Gardos G, Cole JO. A controlled evaluation of pyrovalerone in chronically fatigued volunteers. Int Pharmacopsychiatry. 1973;8:60–69. doi: 10.1159/000467975. [DOI] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, Hoonakker AJ, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday A, Morris R. Compound 84/F 1983 compared with D-amphetamine and placebo in regard to effects on human performance. Psychopharmacology. 1964;6:192–200. doi: 10.1007/BF00404009. [DOI] [PubMed] [Google Scholar]
- Horovitz ZP, Chow MI, Carlton PL. Effects of d-amphetamine, scopolamine, chlordiazepoxide and diphenylhydantoin on self-stimulation behavior and brain acetylcholine. Psychopharmacologia. 1972;3:1–16. doi: 10.1007/BF00414409. [DOI] [PubMed] [Google Scholar]
- Kavanagh P, McNamara S, Angelov D, McDermott S, Mullan D, Ryder S. [Accessed 21 February 2012];The characterization of “legal highs”. 2011 available from head shops in Dublin. Available at http://addictionireland.com/_fileupload/publications/Legal_Highs_Poster.pdf.
- Kelly JP. Cathinone derivatives: a review of their chemistry, pharmacology and toxicology. Drug Test Anal. 2011;3:439–453. doi: 10.1002/dta.313. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio S, Koob G. Escalation of methamphetamine self-administration in rats: a dose–effect function. Psychopharmacology (Berl) 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU, McLean S, Jacobson JO. Intracranial self-stimulation thresholds: a model for the hedonic effects of drugs of abuse. Arch Gen Psychiatry. 1979;36:289–292. doi: 10.1001/archpsyc.1979.01780030055004. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Bain G. Brain-stimulation reward: a model for the study of the rewarding effects of abused drugs. NIDA Res Monogr. 1992;124:73–93. [PubMed] [Google Scholar]
- Lancelot JC, Robba M, Bonnett JJ, Vaugeois JM, Costentin J. Synthetic and preliminary study of the activity of thiophene analogues of pyrovalerone on the neuronal uptake of the monoamines. Eur J Med Chem. 1992;27:297–300. [Google Scholar]
- Magdum SS. An overview of Khat. Addict Disord Treat. 2011;10:72–83. [Google Scholar]
- Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav. 1992;51:111–119. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Blough BE, Wiley JL. Effects of “bath salts” and other novel dopamine and serotonin agonist on overt behaviors in rodents. Program No. 571.02. 2011 Neuroscience Meeting Planner; 2011; Washington, DC: Society for Neuroscience; 2011. Online. [Google Scholar]
- Meisch RA. Factors controlling drug reinforced behavior. Pharmacol Biochem Behav. 1987;27:367–371. doi: 10.1016/0091-3057(87)90584-3. [DOI] [PubMed] [Google Scholar]
- Meltzer PC, Butler D, Deschamps JR, Madras BK. 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (pyrovalerone) analogues: a promising class of monoamine uptake inhibitors. J Med Chem. 2006;49:1420–1432. doi: 10.1021/jm050797a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Panlilio LV. Stimulant self-administration. In: Olmstead MC, editor. Animal Models of Drug Addiction. Humana Press; Totowa, NJ: 2010. pp. 57–82. [Google Scholar]
- Psychonaut WebMapping Research Group. Mephedrone report. Institute of Psychiatry, King’s College London; London, UK: 2009a. [Accessed 21 February 2012]. Available at http://www.psychonautproject.eu/documents/reports/Mephedrone.pdf. [Google Scholar]
- Psychonaut WebMapping Research Group. MDPV report. Institute of Psychiatry, King’s College London; London, UK: 2009b. [Accessed 21 February 2012]. Available at http://www.psychonautproject.eu/documents/reports/MDPV.pdf. [Google Scholar]
- Richardson N, Roberts D. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. J Neurosci Meth. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Loh EA, Vickers G. Self-administration of cocaine on a progressive ratio schedule in rats: dose-response relationship and effect of haloperidol pretreatment. Psychopharmacology (Berl) 1989;97:535–538. doi: 10.1007/BF00439560. [DOI] [PubMed] [Google Scholar]
- Sarkar M, Kornetsky C. Methamphetamine’s action on brain-stimulation reward threshold and stereotypy. Exp Clin Psychopharm. 1995;3:112–117. [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol. 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- Vardakou I, Pistos C, Spiliopoulou C. Drugs for youth via Internet and the example of mephedrone. Toxicol Lett. 2010;201:191–195. doi: 10.1016/j.toxlet.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Yohannan JC, Bozenko JS. The characterization of 3, 4-methylenedioxypyrovalerone (MDPV) Microgram J. 2010;7:5–15. [Google Scholar]