Abstract
Purpose
NUT midline carcinoma (NMC) is a poorly differentiated squamous cancer characterized by rearrangement of the NUT gene. Research advances have provided opportunities for targeted therapy in NMC, yet the clinical features of this rare disease have not been systematically characterized. We report on a large population of such patients to identify the disease characteristics and treatments, correlate them with outcome, and to consider clinical recommendations.
Experimental Design
A clinical database was established using retrospective demographic and outcomes data available on all known cases of NMC. Questionnaires were completed by treating physicians. Pathologic, demographic, and clinical variables were assessed for 63 patients, the largest cohort of NMC patients studied to date. Outcome data from 54 patients were available for survival analyses.
Results
The diagnosis of NMC has increased annually since 2007. Since 2009, there has been an observed increase in the age at diagnosis (p<0.05). Geographic distribution of NMC patients has been concentrated in the United States (n=41, 65%). The median overall survival for patients with NMC was 6.7 months. The 2-year progression-free survival (PFS) was 9% with a 95% CI of 1%–17% (1-year PFS 15% (5%–24%)) and 2-year overall survival (OS) was 19% with a 95% CI of 7%–31% (1-year OS: 30% (27%–34%). Multivariate analysis suggested that extent of surgical resection and initial radiotherapy were independent predictors of PFS and OS. Notably, no chemotherapeutic regimen was associated with improved outcome.
Conclusions
NMC portends a poor prognosis among all squamous cell neoplasms and appears to be frequently unrecognized. The finding that conventional chemotherapy has been inadequate indicates a pressing need for the development of targeted therapeutics. Intensive local therapies such as gross total resection and radiotherapy might be associated with enhanced survival.
Keywords: NUT midline carcinoma, outcomes, registry
INTRODUCTION
NUT midline carcinoma (NMC) is an aggressive, genetically-defined subset of human squamous cell carcinoma characterized by chromosomal rearrangement of the NUT gene (also known as Chr15orf55)(1). Somatic rearrangement of the NUT gene in cancer was identified in 2003 (2), in the context of the characteristic t(15;19) translocation which positions NUT in-frame with BRD4, a ubiquitously-expressed transcriptional co-activator. A subset of NMCs lack BRD4 rearrangement and are termed NUT-variants. Many of these tumors reveal NUT rearrangement to a gene family member, BRD3, while others possess as yet unresolved molecular biology (3). Histologically, NMC displays variable degrees of squamous differentiation, with a predominance of the poorly differentiated component.
Although initially described in children and adolescents, NMC has subsequently also been observed in adults (4). As the cytogenetic and molecular evaluation of carcinomas in adults is often limited, the actual incidence and clinical manifestations of NMC remain unclear. Isolated case reports and small case series reveal several common clinical features (2–23). NMC typically arises within midline head and neck structures or in the thorax and shows an aggressive behavior, with early locoregional invasion and distant metastases. Treatment approaches have been heterogeneous. Although a single case report demonstrated excellent outcome (15), other reported outcomes have been poor.
Since its recognition as a genetically-defined cancer (2, 18), many cases of NMC have come to our attention because of our unique ability to perform testing for the characteristic gene rearrangements and aberrant antigen expression. Over the past decade, we have served as the primary diagnostic center for NMC for many pathologists in North America and Europe. This concentration of cases provided a unique opportunity to develop an international registry, established in 2010, in which retrospective clinical and pathologic data served as the foundation for prospective data capture of clinical and therapeutic interventions, as well as analysis of outcomes. Here, we investigate the clinical features of 63 cases of NMC of which we are aware, either through histological review at our institution (n=59) or literature review (n=4). We compared progression-free and overall survival on 54 evaluable patients with respect to demographic, molecular, and clinical features, as well as treatment variables including extent of surgical resection, provision of radiotherapy and selected chemotherapeutic agents.
METHODS
Patients
From 1990–2010, we learned of 63 patients with NMC who were defined as those with NUT rearrangement demonstrated by FISH or RT-PCR, aberrant NUT expression demonstrated by immunohistochemistry, or characteristic t(15;19) in the setting of carcinoma. Fifty-nine of them came to our attention in the context of rendering the molecular and/or histologic diagnosis. The other four cases were found by literature review (9–11, 19). We have previously described 35 of these cases, albeit with limited annotation of clinical characteristics and outcomes (2–4, 6, 12, 13, 15, 17, 18, 22, 24). In this study, we collected previously unpublished clinical data for 28 of these 35 cases and updated outcome data for all 35 (Supplementary Table 1). In addition, we describe 24 previously unpublished cases. All cases known to us and diagnosed before August 2010 were included. Nineteen of the 63 cases were diagnosed by retrospective review of pathology material and 44 were diagnosed at the time of initial presentation.
For 56 cases, a questionnaire was sent to treating physicians inquiring about demographic, clinical, treatment, and outcome variables. Outcome data were submitted for 54 patients. Approval for the International NMC Registry (www.nmcregistry.org), including the retrospective and prospective analysis of NMC patient data, was obtained from the Institutional Review Board of the Dana-Farber Cancer Institute. Review of retrospective clinical data was approved with a waiver of patient consent.
Patient data were collected and analyzed in aggregate; some outcome data were analyzed in two groups defined by age (age<18 years versus age≥18 years). Pathology reports and actual histology were reviewed when available. For the purposes of this study, cases were classified into three histopathologic categories: carcinoma with squamous differentiation, carcinoma without squamous differentiation, and other histology. Clinical staging data (site of primary tumor, lymph node involvement, and location of metastasis), as well as the response to therapeutic interventions, were provided by the treating physician. Initial therapy was defined as treatment administered from initial diagnosis until first relapse or progression. Surgical extent was classified as gross total resection (no residual disease or microscopic residual disease) or less than gross total resection. Chemotherapy was categorized into regimens containing either cisplatin and carboplatin, or regimens containing anthracyclines and non-platinum alkylating agents. Many patients received combination therapy, thus there exists some overlap between these groups. Therapy initiated following relapse or progression was not analyzed. For patients who were alive at the time of analysis, date of last contact was no later than March, 2011, and no earlier than June, 2010.
For progression-free survival (PFS), time-to-event was measured as the time from initial diagnosis of cancer at the treating hospital until the time of first disease relapse, progression or death, or until last contact if none of these events occurred. For overall survival (OS), time was measured from diagnosis until the time of death or until last contact. Clinical responses to initial therapies were classified as complete or partial responses, stable disease, or progressive disease according to the clinical judgment of the treating physician.
Statistical Analysis
Kaplan-Meier plots and log-rank tests were performed to assess factors associated with PFS or OS. One- and 2-year PFS and OS point estimates are presented with a 95% confidence interval (CI). Two-sided Fisher’s exact tests were performed to assess factors associated with the presence of each NUT translocation. For these analyses, there was no correction for multiple testing. A Cox proportional hazards regression model was performed to identify variables independently predictive of PFS or OS. Only variables that were statistically significant in the log-rank test were tested in the Cox model. For all analyses, p-values < 0.05 were considered statistically significant. For illustrative purposes, all graphical representations of data have been truncated at three years.
RESULTS
Demographic Features
Geographic and demographic features are summarized in Tables 1 and 2, respectively. Forty-one of sixty-three cases presented in the United States (Table 1). Table 2 includes the 54 cases for which outcome data were available. Of the 44 cases of NMC diagnosed at the time of initial presentation (“real-time,” as opposed to at the time of relapse or posthumously), 59% were recognized since 2006 (Figure 1). Males and females were equally affected, and median age was 16 years (range 0.1–78 years, Table 2). As awareness of the disease has grown, there has been an apparent increase in the number of cases of NMC. The majority of tumors arose in the thorax (n=35, 56%), or head and neck (n=24, 21%), and presented with either lymph node involvement or distant metastases (n=32, 51%, Table 2).
Table 1.
Geographical distribution of NMC cases
| Location | n |
|---|---|
| United States | 41 |
| Massachusetts | 6 |
| Virginia | 5 |
| Minnesota | 4 |
| California | 3 |
| New York | 3 |
| Colorado | 2 |
| Connecticut | 2 |
| Georgia | 2 |
| Maryland | 2 |
| Pennsylvania | 2 |
| Washington | 2 |
| Florida | 1 |
| Idaho | 1 |
| Illinois | 1 |
| Kentucky | 1 |
| Maine | 1 |
| Michigan | 1 |
| New Mexico | 1 |
| Ohio | 1 |
| Italy | 5 |
| Australia | 4 |
| Sweden | 3 |
| Ireland | 2 |
| Japan | 2 |
| China | 1 |
| Croatia | 1 |
| Greece | 1 |
| Netherlands | 1 |
| New Zealand | 1 |
| Switzerland | 1 |
Abbreviation: NMC (NUT Midline Carcinoma)
Table 2.
Patient characteristics by Progression-Free Survival (PFS) and Overall Survival (OS)
| n | 2-year PFS (%) (95% CI) |
p-value | 2-year OS (%) (95% CI) |
p -value | ||
|---|---|---|---|---|---|---|
| Overall | 54 | 9 (1,17) | 19 (7,31) | |||
| Age | Age 17 years or younger | 29 | 14 (2,26) | 0.1 | 30 (12,48) | 0.07 |
| Age 18 years or older | 25 | 4 (0,12) | 5 (0,15) | |||
| Gender | Female | 26 | 4 (0,12) | 0.1 | 9 (0,21) | 0.1 |
| Male | 28 | 14 (0,28) | 29 (9,49) | |||
| Tumor Location | Thoracic | 31 | 3 (0,9) | 0.04 | 15 (1,29) | 0.1 |
| Head/Neck | 19 | 16 (0,32) | 27 (2,52) | |||
| Other Locations | 4 | 25 (0,68) | 25 (0,68) | |||
| Tumor Histology | Carcinoma with Squamous Differentiation | 21 | 14 (0,30) | 0.4 | 33 (9,57) | 0.2 |
| Carcinoma without Squamous Differentiation | 23 | 4 (0,12) | 6 (0,18) | |||
| Other Histology | 9 | 11 (0,33) | 22 (0,49) | |||
| NUT Translocation | BRD4-NUT | 38 | 8 (0,12) | 0.1 | 12 (0,24) | 0.4 |
| BRD3-NUT | 3 | 0 | 33 (0,86) | |||
| NUT-Variant | 12 | 17 (0,39) | 33 (6,60) | |||
| Metastases | No Metastases | 21 | 19 (1,37) | 0.04 | 25 (3,37) | 0.1 |
| Metastases | 32 | 3 (0,9) | 16 (2,26) | |||
| Lymph Node Involvement | No Involvement | 29 | 10 (0,22) | 0.3 | 20 (2,38) | 0.3 |
| Involvement | 25 | 8 (0,18) | 18 (2,34) | |||
| Extent of Surgical Resection | Less than Gross Total | 10 | 10 (0,30) | 0.01 | 44 (7,81) | 0.03 |
| Complete | 5 | 60 (17,100) | 80 (45,100) | |||
| Less than Gross Total or No Surgical Resection | 49 | 4 (0,10) | 0.001 | 12 (0,24) | 0.002 | |
| Complete | 5 | 60 (17,100) | 80 (45,100) | |||
| Initial Radiotherapy | No | 27 | 0 | <0.0001 | 5 (0,15) | 0.0003 |
| Yes | 26 | 19 (3,35) | 35 (13,57) | |||
| Chemotherapy Regimen | Platinum-based | 33 | 9 (0,19) | 0.6 | 22 (4,40) | 0.3 |
| Other | 20 | 10 (0,24) | 15 (0,31) | |||
| Anthracycline/Alkylator-based | 17 | 18 (0,36) | 0.6 | 28 (4,40) | 0.4 | |
| Other | 36 | 6 (0,14) | 15 (1,29) |
Abbreviation: CI (confidence interval)
Figure 1. Cases of NUT midline carcinoma in adult (n=18) and pediatric (n=22) patients per year of diagnosis.
Cases are limited to those diagnosed in “real-time”, that is, at time of initial presentation.
Outcomes
Outcome data were available for 54 of 63 cases. The overall 1-year and 2-year PFS was 15% (95% CI: 5%–24%) and 9% (95% CI: 1%–17%), respectively; and the 1-year and 2-year OS 30% (95% CI: 27%–34%) and 19% (95% CI: 7%–31%), respectively (n=54; Table 2). With a 31.8 month median follow-up time for living patients, the median overall survival (OS) was 6.7 months (range 0.7 months to 19+ years; Figure 2). OS and PFS were compared for patients less than or greater than 18 years old pediatric and adult groups); no significant differences were observed (Table 2). For 29 pediatric patients, the PFS was 24% (95% CI: 9%–40%) at 1-year and 14% (95% CI: 2%–26%) at 2-years; and the OS was 41% (24%–59%) at 1-year and 30% (95% CI: 12%–48%) at 2-years (Figure 2A). For 25 adult patients, the PFS was 4% (95% CI: 0–12%) at 1-year and 4 (95% CI: 0–12%) at 2-years; and the OS was 16% (95% CI: 2%–30%) at 1-year and 5% (95% CI: 0–15%) at 2-years (Figure 2B). There was statistically significantly lower PFS and OS associated with either a thoracic tumor location, or the presence of metastases (Table 2). There was no statistically significant difference in PFS or OS by tumor histology, sex, or lymph node involvement (Table 2). Pattern of treatment failure at first relapse or progression was evaluable for 50 cases: 22 of 50 (44%) demonstrated isolated locoregional disease; 11/50 (22%) had isolated distant disease; and 17/50 (34%) combined locoregional and distant disease.
Figure 2. Survival of patients with NMC.
The probability of overall survival (OS) and progression free survival (PFS) is presented for (A) pediatric (age<18; n=29) and (B) adult (age≥18; n=25) NUT midline carcinoma patients.
BRD4-NUT, BRD3-NUT, and NUT-variant translocations were not prognostic of OS or PFS. However, there was a statistically significant difference in the proportion of patients with BRD4-NUT translocation by tumor location, with the majority of head and neck NMC possessing the BRD4-NUT translocation (p=0.04; Table 2).
Impact of Therapeutic Interventions
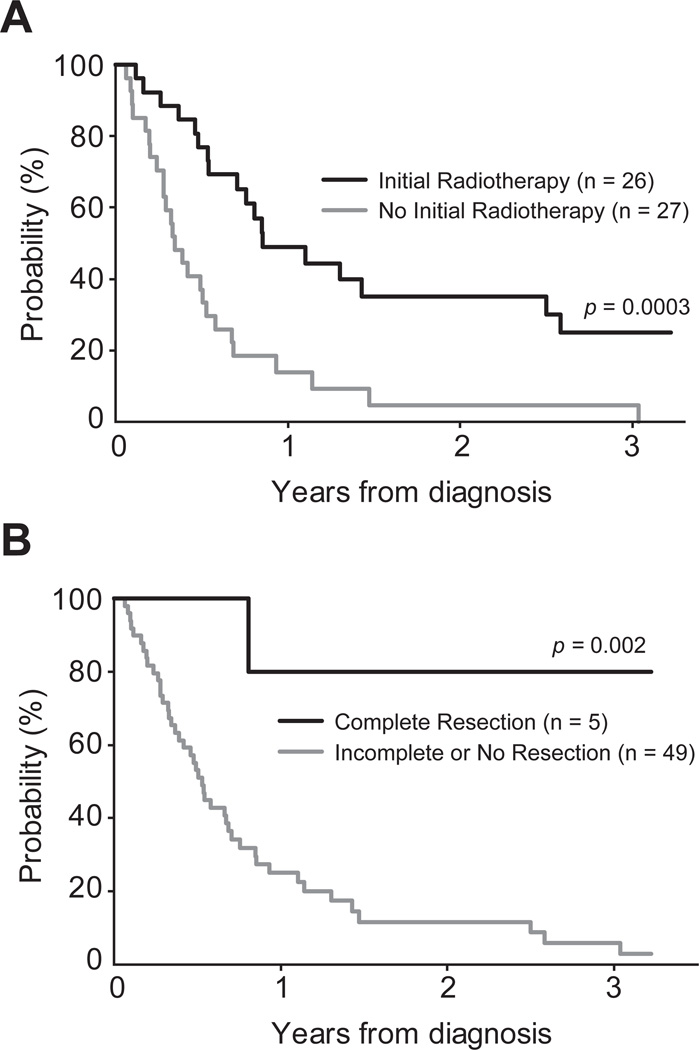
PFS and OS were statistically significantly higher who received early administration of radiotherapy (Table 2, Figure 3A). The total radiotherapy dose was evaluable for 18 of 26 patients. Seventeen received doses ranging from 30.0 to 66.6 Gy (median 52.2 Gy), and a single patient received only 5.5 Gy. There was no observed difference in PFS or OS between the lower radiation dose group (dose ≤52.2 Gy). and the higher radiation dose group (dose >52.2 Gy; p=0.8 and p=0.9, respectively). PFS and OS were statistically significantly higher with gross total resection compared to those who had less than gross total resection or no surgical resection (Table 2, Figure 3B). Of the five cases with gross total resection, two demonstrated microscopic residual disease and three had no residual disease. To identify factors independently prognostic of PFS and OS, extent of surgical resection, initial radiotherapy, tumor location (performed for PFS only), and presence of metastases were tested in the Cox model. The extent of surgical resection and initial radiotherapy were independently predictive of PFS and OS. Patients who received less than a gross total surgical resection or no surgical resection had a five-fold greater risk of progression (p=0.03) and eight-fold greater risk of death (p=0.05) compared with patients who underwent gross total surgical resection. Patients who did not receive initial radiotherapy had a 2.8 times greater risk of progression (p=0.002), and 2.2 times greater risk of death (p=0.01) compared with patients who received initial radiotherapy (Table 3).
Figure 3. Influence of therapeutic intervention on survival of patients with NMC.
The probability of overall survival is presented for NMC patients, as influenced by use of (A) radiotherapy; and (B) surgical resection.
Table 3.
Multivariable analysis of factors independently prognostic of PFS and OS
| PFS | ||
|---|---|---|
| Factors with Increased Risk of Progression* | Hazard Ratio (95% CI) | p-value |
| Incomplete or No Surgical Resection | 5.00 (1.12, 22.2) | 0.03 |
| No Initial Radiotherapy | 2.82 (1.48, 5.29) | 0.002 |
| OS | ||
| Factors with Increased Risk of Death* | Hazard Ratio (95% CI) | p-value |
| Incomplete or No Surgical Resection | 7.94 (1.03, 62.5) | 0.05 |
| No Initial Radiotherapy | 2.20 (1.18, 4.09) | 0.01 |
tested in the model and found to be not statistically significant were: tumor location [HR: 1.13 (0.72, 1.78), p = 0.6] and metastases [HR: 1.47 (0.80, 2.69), p = 0.2].
tested in the model and found to be not statistically significant was: tumor location [HR: 1.10 (0.58, 2.09), p = 0.8].
Abbreviations: PFS (progression-free survival); OS (overall survival); CI (confidence interval); HR (hazard ratio)
The clinical response to combined initial therapies (including chemotherapy, radiotherapy, and surgery) could be evaluated in 51 patients: 8 had a complete response and 18 a partial response. Of the 8 CRs, 4 progressed, and 2 ultimately died. Median time to progression was 9.3 months with a range of 5.0–16.3 months. At last contact, the other 4 had not progressed. The follow-up times (from diagnosis) for these 4 pts were 2.6, 2.9, 3.2, and 19 years. Only those patients treated with initial surgical resection or radiation, with or without chemotherapy, had a complete response. The remaining 25 patients had stable or progressive disease. Complete response was positively associated with initial radiotherapy (p=0.002), age less than 18 years (p=0.04), and absence of metastases (p=0.04). Clinical response to therapy was not significantly associated with NUT translocation subtype, tumor histology, sex, tumor location, or lymph node involvement. Cause of death (determined for 46 patients) was noted as tumor progression (n=44), tumor lysis syndrome (n=1), and septicemia (n=1). In this analysis, there was no evidence that chemotherapy improved outcomes among the 53 patients who received it (Table 2).
DISCUSSION
NMC is a genetically-defined subtype of squamous carcinoma, in particular of the mediastinum, head and neck. In an effort to better characterize the clinical outcomes of NMC patients, we performed a retrospective analysis of outcomes in all known cases. Until recently, testing for NUT rearrangement relied on a highly specialized fluorescent in situ hybridization (FISH) or RT-PCR assay, or cytogenetic analysis, which was unavailable in most laboratories. Since the description of NMC as an entity, we have served as the major diagnostic referral center for NMC. For this reason, this retrospective collection of all cases sent to us for review, as well as all published cases, represents the most comprehensive collection ever reported, and attempts to minimize selection bias. Despite inclusion of all cases known to us, the sample size remains small, and the results should be interpreted with caution. We will perform validation analysis of all significant findings in a prospective, inclusive cohort.
Although we found both the incidence of NMC diagnosis and age at diagnosis appeared to be rising, we recognize that this might be a reflection of reporting bias of this still rare and likely under-recognized disease. For example, we noted a statistically significant difference in age between patients diagnosed between 2007–2008 (median age: 14 years (range 0.1 years, 40 years)) and those diagnosed between 2009–2010 (median age: 29 years (range 2 years, 62 years); p=0.015). In 2010, the number of adult cases of NMC (n=7) outnumbered pediatric cases (n=1). Our findings suggest that NMC remains under-recognized, in particular amongst adult patients. Indeed, it is uncommon for tissue derived from adult patients to be assessed for cytogenetic abnormalities which historically established the diagnosis of NMC. Recently, a simple immunohistochemical stain for the NUT gene-product was developed, using a commercially-available clinical immunoglobulin, which in the context of evaluating patient-derived tumor specimens is highly sensitive and specific for NMC (17). With increased awareness of NMC, and with consideration of systematic use of this facile diagnostic assessment, it is likely the number of NMC cases will continue to increase.
Based on our findings and the near-term investigation of bromodomain-targeted investigational agents, we have established general recommendations for when it is appropriate to test for NMC. We recommend immunohistochemical testing for NUT expression in all poorly differentiated carcinomas without glandular differentiation arising in the chest, head and neck. Testing is appropriate with or without squamous differentiation, but is not required with prior confirmation of a specific etiology (such as presence of EBV or HPV infection of tumor cells). The diagnosis should be made by demonstrating >50% nuclear staining with the commercially-available NUT monoclonal antibody C52 (Cell Signaling), which has proven to be 100% specific and highly sensitive (87%) for the diagnosis of NMC (17). Characterization of the fusion gene (BRD4-NUT, BRD3-NUT) is not required for the diagnosis, but is recommended, either by FISH, cytogenetics, or RT-PCR.
In contrast to a previous report that suggested an improved outcome for NUT-variant NMC (18), our more comprehensive study failed to identify a significant association between translocation type (BRD4-NUT, BRD3-NUT, or NUT variant) and outcome. This might be due to a lack of sufficient power for detecting small differences, in spite of the fact that this is the largest cohort of NMC patients ever studied. Nonetheless, it was intriguing that 5 of the 7 longest survivors in our series had BRD3-NUT (n=1) or NUT variant (n=4) fusions. It is possible that improved identification of the fusion partner of NUT variant carcinomas and longer follow-up might identify specific molecular subtypes with unique prognostic features. We note that the vast majority of head and neck NMC (88%) harbored BRD4-NUT translocations. Given that the cell of origin of NMC remains unknown, this association establishes a hypothesis for experimental evaluation.
The most striking finding of this study is the extremely poor prognosis of patients diagnosed with NMC, who have an 6.7 month median survival and a greater than 80% likelihood of death within the first year after diagnosis for adult patients. Heterogeneous systemic therapies have been utilized to treat patients with NMC, including intensive chemotherapy regimens commonly used in the treatment of germ cell tumors, squamous cell carcinoma of the head and neck, non-small cell lung carcinoma, and sarcoma. Many of those regimens have utilized platinum agents, anthracyclines and non-platinum alkylating agents alone or in combination. Due to the fact that most patients received a combination of therapies, we were unable to perform an analysis that identified specific chemotherapeutic agents with improved outcomes. Many patients with NMC were observed to respond initially to combined modality treatment but the overall outcomes for these patients remained very poor. There remains only one known long-term survivor of NMC, who was treated with radiotherapy and chemotherapy (15). This solitary successful case possessed unique features, including histology without features of carcinoma and primary location within bone. Our overall findings suggest that intensive local control was associated with modest extension of survival.
NMC exemplifies the paradigmatic shift in clinical oncology brought by the molecular characterization of cancer, as a disease unified not anatomically but rather by a common genetic pathophysiology, which reprograms the tumor epigenome (24) and confers catastrophic clinical consequences. Recent biological advances in the laboratory study of NMC demonstrate that bromodomain-containing NUT fusion proteins result in aberrant histone acetylation (24) and blockade of differentiation (3). These key findings have established hyperacetylation therapy, using histone deacetylase inhibitors, as a mechanism-based investigational approach (24). Recently, our group developed first-in-class, direct-acting inhibitors of the BRD3 and BRD4 bromodomains, as targeted therapy for NMC (25). A prototype bromodomain inhibitor, JQ1, exhibits potent differentiating activity in vitro, and confers prolonged survival in patient-derived and primary xenograft models of NMC. The clinical translation of this research requires robust, historical outcome data and a coordinated, flexible network of patients and providers assembled around the study and eradication of this malignancy. This is a challenge shared with many rare malignancies; it is only through the development of integrated, multi-institutional, and international initiatives that advances in the understanding and treatment of these cancers can occur. We hope that the establishment of the International NMC Registry (www.nmcregistry.org) will serve as the first step towards this goal.
Supplementary Material
TRANSLATIONAL RELEVANCE.
NUT midline carcinoma is caused by a recurrent gene fusion event resulting in cellular epigenetic deregulation. We have drawn on our unique position as the major referral center for NUT midline carcinoma diagnosis to create an international registry and describe the clinicopathologic features and long-term outcomes of this disease. The rate of diagnosis continues to increase, especially in adults, suggesting this carcinoma remains underdiagnosed. We demonstrate the dismal response rate, progression-free survival, and overall survival characteristics of this disease. While intensive local control appears associated with modest extension of survival, no specific chemotherapy regimen is clearly associated with superior outcome. Given the specific molecular derangement caused by BRD-NUT gene fusion and poor outcomes to conventional therapies, we anticipate this disease will make an excellent candidate for rational investigation of targeted inhibitors. These results establish a robust baseline against which future therapeutic interventions may be compared.
Acknowledgments
Research Support:
This work was supported by the Adolescent and Young Adult Gap Fund (JEB and CAF), the Smith Family Foundation (JEB) and the National Institutes of Health grants 3K01CA124581 03S1 (CSL), 1R01CA124633 and 3R01CA124633-04S1 (CAF).
Footnotes
Statement of originality:
The analysis presented herein comprises an original publication, which has not been presented elsewhere in either oral or written format.
Disclosures:
The authors report no conflicts of interest.
REFERENCES
- 1.French CA. Pathogenesis of NUT Midline Carcinoma. Annual Reviews of Pathology: Mechanisms of Disease. 2012;7:247–265. doi: 10.1146/annurev-pathol-011811-132438. [DOI] [PubMed] [Google Scholar]
- 2.French CA, Miyoshi I, Kubonishi I, Grier HE, Perez-Atayde AR, Fletcher JA. BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer research. 2003;63(2):304–307. [PubMed] [Google Scholar]
- 3.French CA, Ramirez CL, Kolmakova J, Hickman TT, Cameron MJ, Thyne ME, et al. BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene. 2008;27(15):2237–2242. doi: 10.1038/sj.onc.1210852. [DOI] [PubMed] [Google Scholar]
- 4.Stelow EB, Bellizzi AM, Taneja K, Mills SE, Legallo RD, Kutok JL, et al. NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. The American journal of surgical pathology. 2008;32(6):828–834. doi: 10.1097/PAS.0b013e31815a3900. [DOI] [PubMed] [Google Scholar]
- 5.Dang TP, Gazdar AF, Virmani AK, Sepetavec T, Hande KR, Minna JD, et al. Chromosome 19 translocation, overexpression of Notch3, and human lung cancer. J Natl Cancer Inst. 2000;92(16):1355–1357. doi: 10.1093/jnci/92.16.1355. [DOI] [PubMed] [Google Scholar]
- 6.Teo M, Crotty P, O'Sullivan M, French CA, Walshe JM. NUT midline carcinoma in a young woman. J Clin Oncol. 29(12):e336–e339. doi: 10.1200/JCO.2010.32.7486. [DOI] [PubMed] [Google Scholar]
- 7.Rahbar R, Vargas SO, Miyamoto CR, Perez-Atayde AR, French CA, Robson CD, et al. The role of chromosomal translocation (15;19) in the carcinoma of the upper aerodigestive tract in children. Otolaryngol Head Neck Surg. 2003;129(6):698–704. doi: 10.1016/S0194-59980301451-7. [DOI] [PubMed] [Google Scholar]
- 8.Vargas SO, French CA, Faul PN, Fletcher JA, Davis IJ, Dal Cin P, et al. Upper respiratory tract carcinoma with chromosomal translocation 15;19: evidence for a distinct disease entity of young patients with a rapidly fatal course. Cancer. 2001;92(5):1195–1203. doi: 10.1002/1097-0142(20010901)92:5<1195::aid-cncr1438>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Lee AC, Kwong YI, Fu KH, Chan GC, Ma L, Lau YL. Disseminated mediastinal carcinoma with chromosomal translocation (15;19). A distinctive clinicopathologic syndrome. Cancer. 1993;72(7):2273–2276. doi: 10.1002/1097-0142(19931001)72:7<2273::aid-cncr2820720735>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 10.Kubonishi I, Takehara N, Iwata J, Sonobe H, Ohtsuki Y, Abe T, et al. Novel t(15;19)(q15;p13) chromosome abnormality in a thymic carcinoma. Cancer research. 1991;51(12):3327–3328. [PubMed] [Google Scholar]
- 11.Kees UR, Mulcahy MT, Willoughby ML. Intrathoracic carcinoma in an 11-year-old girl showing a translocation t(15;19) Am J Pediatr Hematol Oncol. 1991;13(4):459–464. doi: 10.1097/00043426-199124000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Toretsky JA, Jenson J, Sun CC, Eskenazi AE, Campbell A, Hunger SP, et al. Translocation (11;15;19): a highly specific chromosome rearrangement associated with poorly differentiated thymic carcinoma in young patients. Am J Clin Oncol. 2003;26(3):300–306. doi: 10.1097/01.COC.0000020960.98562.84. [DOI] [PubMed] [Google Scholar]
- 13.Shehata B, Steelman CK, Abramowsky CR, Olson T, French C, Saxe D, et al. NUT Midline Carcinoma in a Newborn with Multiorgan Disseminated Tumor and a Two-Year-Old with a Pancreatic/Hepatic Primary. Pediatr Dev Pathol. 2009 doi: 10.2350/09-10-0727-CR.1. [DOI] [PubMed] [Google Scholar]
- 14.Nelson BA, Lee EY, French CA, DE B, SO V. BRD4-NUT Carcinoma of the Mediastinum in aPediatric Patient: Multidetector CT Imaging Findings. Journal of Thoracic Imaging. 2009 doi: 10.1097/RTI.0b013e3181b5d84d. In press. [DOI] [PubMed] [Google Scholar]
- 15.Mertens F, Wiebe T, Adlercreutz C, Mandahl N, French CA. Successful treatment of a child with t(15;19)-positive tumor. Pediatr Blood Cancer. 2007;49(7):1015–1017. doi: 10.1002/pbc.20755. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh MS, French CA, Liang CW, Hsiao CH. NUT Midline Carcinoma: Case Report and Review of the Literature. Int J Surg Pathol. 2009 doi: 10.1177/1066896909353600. [DOI] [PubMed] [Google Scholar]
- 17.Haack H, Johnson LA, Fry CJ, Crosby K, Polakiewicz RD, Stelow EB, et al. Diagnosis of NUT Midline Carcinoma Using a NUT-specific Monoclonal Antibody. The American journal of surgical pathology. 2009 doi: 10.1097/PAS.0b013e318198d666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.French CA, Kutok JL, Faquin WC, Toretsky JA, Antonescu CR, Griffin CA, et al. Midline carcinoma of children and young adults with NUT rearrangement. J Clin Oncol. 2004;22(20):4135–4139. doi: 10.1200/JCO.2004.02.107. [DOI] [PubMed] [Google Scholar]
- 19.Engleson J, Soller M, Panagopoulos I, Dahlen A, Dictor M, Jerkeman M. Midline carcinoma with t(15;19) and BRD4-NUT fusion oncogene in a 30-year-old female with response to docetaxel and radiotherapy. BMC Cancer. 2006;6:69. doi: 10.1186/1471-2407-6-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellizzi AM, Bruzzi C, French CA, Stelow EB. The cytologic features of NUT midline carcinoma. Cancer Cytopathol. 2009 doi: 10.1002/cncy.20044. [DOI] [PubMed] [Google Scholar]
- 21.French CA, Miyoshi I, Aster JC, et al. BRD4 bromodomain gene rearrangement in aggressive carcinoma with translocation t (15;19) Am J Pathol. 2001;159(6):1987–1992. doi: 10.1016/S0002-9440(10)63049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ziai J, French CA, Zambrano E. NUT gene rearrangement in a poorlydifferentiated carcinoma of the submandibular gland. Head and neck pathology. 4(2):163–168. doi: 10.1007/s12105-010-0174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.den Bakker MA, Beverloo BH, van den Heuvel-Eibrink MM, Meeuwis CA, Tan LM, Johnson LA, et al. NUT midline carcinoma of the parotid gland with mesenchymal differentiation. The American journal of surgical pathology. 2009;33(8):1253–1258. doi: 10.1097/PAS.0b013e3181abe120. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz BE, Hofer MD, Lemieux ME, Bauer DE, Cameron MJ, West NH, et al. Differentiation of NUT Midline Carcinoma by Epigenomic Reprogramming. Cancer research. 71(7):2686–2696. doi: 10.1158/0008-5472.CAN-10-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, et al. Selective inhibition of BET bromodomains. Nature. 468(7327):1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.