Abstract
Background
Limited data are available in Honduras describing the etiology and seasonality of respiratory infections, especially in rural outpatient settings. Better data may lead to improved therapeutic and preventative strategies. The goal of our study was to determine the viral etiology and seasonality of acute respiratory infections in a rural Honduran population of children.
Methods
Prospective clinic surveillance was conducted to identify children <5 years of age presenting with respiratory symptoms <5 days duration. We obtained data on age, sex, medical history, breastfeeding history, symptoms, risk factors, house hold setting, temperature, respiratory rate, and chest exam findings. To assess the association between specific viruses and weather, regional meteorological data were collected. Nasopharyngeal samples were tested for 16 respiratory viruses using a multiplex PCR panel.
Results
From February 2010 through June 2011, 345 children <5 years of age were enrolled; 17%, 23%, 30%, and 31% were <6, 6–11, 12–23, and 24–60 months old, respectively. Including all clinics in the region, 44.5% of patients <5 years of age with documented respiratory diagnoses were enrolled. At least one virus was identified in 75.4% children, of which 7.5% were co-infections; 13.3% were positive for parainfluenza, 11.9% for influenza, 8.1% for human metapneumovirus (hMPV), and 7.5% for respiratory syncytial virus (RSV). Rainfall correlated with parainfluenza (p≤0.0001), influenza (p≤0.0001), hMPV (p= 0.0182), and RSV (p≤0.0001).
Conclusions
These results suggest that the spectrum of viruses in ill rural Honduran children is similar to that in North and Central America, though the seasonality is typical of some tropical regions.
Keywords: viral respiratory infections, influenza, seasonality, Honduras, child
INTRODUCTION
Acute respiratory infections (ARIs), including pneumonia, are the leading cause of death among children under five years of age in several regions with limited resources1,2. Recent data from the World Health Organization (WHO) suggest that 18% of global deaths in children under five are due to pneumonia alone1,2. Throughout the world, the etiologies of ARIs and pneumonia are largely unknown. It is estimated that 18% to 65% of global pediatric patients admitted to the hospital for ARIs and pneumonia are infected with viruses3–7. Even with the best diagnostic techniques, 25% to 33% of pneumonia cases do not have a clear etiology8.
In the United States, up to 9% of all infants younger than 6 months of age are hospitalized or seen in a clinic or emergency department secondary to influenza during some influenza seasons9. The burden of influenza is unknown in most resource-poor countries10, but some data from the tropics and subtropics demonstrate incidence and hospitalization rates for influenza that exceed those reported for temperate regions11–15. The seasonality of influenza virus in the tropics is variable16, with studies demonstrating year round disease10,17 or one or two clear annual peaks18–23. Several sites in sub-Saharan Africa, Latin America, and Asia have recently added influenza surveillance programs24–26; but new technologies, including RT-PCR (reverse transcription-polymerase chain reaction) for virus detection, are often unavailable in these settings.
Honduras is a resource-limited country in Central America with a population of approximately 7.5 million people and gross national income per capita of US$1869.827. The under-five mortality rate per 1000 births is 42.6, and acute respiratory infections are the leading cause of death in this age group28. Only two studies have examined the viral etiology of respiratory illness in Honduras29,30, and both of these studies were localized to urban regions and did not include surveillance in rural areas.
Because mortality rates for acute respiratory infections in resource-limited countries far exceed those of economically developed countries25,31,32, information about burden of these pathogens in the resource-limited countries is crucial for the development of effective prevention, surveillance, and treatment strategies. The objective of our study is to describe the etiologic agents, seasonality, and clinical characteristics of viral respiratory infections in rural Honduran children less than five years of age.
MATERIALS AND METHODS
Study Design
Prospective clinic surveillance was conducted to describe the spectrum of viral etiologies of acute respiratory infections in a rural outpatient Honduran population of children less than five years of age.
Surveillance Sites
We conducted surveillance in two villages, Santa Lucia and Magdalena, located in Intibucá, one of the poorest of the 18 departments of Honduras. Santa Luciais located in a remote, mountainousregionat 13° N and 88° W. The average monthly temperature ranges from 17° to 22° Celsius, and the rainy season extends from May through October with peaks in June and September. Santa Lucia is located approximately five kilometers from the El Salvador border, and Magdalena is approximately three kilometers north of Santa Lucia. The capital of Intibucá, La Esperanza, is approximately three hours by car from each town. The mountainous terrain and limited transportation hinder access to care for Hondurans in this region. Pneumococcal vaccines were recently introduced in Honduras and were not available to participants at the time of the study33. Influenza vaccine was introduced in 2003 in Honduras34 and is intermittently available to pregnant women in this region.
Surveillance was conducted at the Clinica Hombro a Hombro in Santa Lucia and Centro de Salud in Magdalena. The two clinics provide primary medical care, health education, and community resources for more than twenty thousand rural Hondurans. Both clinics are operated by Shoulder to Shoulder and Hombro a Hombro, two partnering, private, non-profit, non-governmental organizations (NGOs) formed in the United States and Honduras, respectively. No other access to primary care is available in this region of Honduras.
Subjects
All children less than 5 years of age who presented at the two clinics from February 2010 through June 2011 and met the study criteria were eligible for enrollment. Children were eligible for recruitment if they presented with an acute respiratory illness defined as a maternal report of illness, with any one respiratory symptom including runny nose, nasal congestion, cough, difficulty swallowing or difficulty breathing, occurring more than 7 days after a previous illness. For children greater than 1 year of age, fever was defined as a temperature> 37.8 degrees Celsius. This definition of respiratory illness with fever was modified for infants and young children from the Centers for Disease Control and Prevention (CDC) definition of influenza-like illness. Patients were excluded if they were ≥ 5 years of age, previously enrolled within 7 days, or symptomatic for greater than 5 days. All other children less than 5 years of age seen in the clinics were recorded in the clinics’ computer database to determine our capture rate within the community. Weekly queries identified all children seen in the clinics less than 5 years of age.
Study Procedures
If the child was eligible for the study and the parent agreed to participate, informed consent from the parent or guardian of the child was obtained. Following informed consent, there were four parts to data collection: a parent questionnaire, nasopharyngeal sampling, physician’s exam, and data abstraction from the child’s medical record by study personnel.
All demographic, clinical, and laboratory data were entered into a study computer database by study personnel. These data included parent questionnaire responses, clinical exam findings recorded by the physician, medical history recorded from the clinical chart, and multiplex PCR results when available. Denominator data were obtained from the clinic database, identifying all patient encounters in children less than 5 years with respiratory code diagnoses. Clinic staff manually enter clinical data from all patient encounters in the clinic databases on a daily basis. Meteorological data were obtained from the nearest weather station located at the airport in Santa Rosa de Copán, approximately 190 kilometers from Santa Lucia.
Laboratory Procedures
Two flexible nasopharyngeal nylon flocked swabs (FLOQSwabs™, Copan Diagnostics, Murrieta, California, USA) were used for specimen collection for each participant. The distance between the participant’s nares and ear lobe was measured to estimate the maximum depth of insertion, and the swabs were gently inserted towards the pharynx until resistance was felt and then rotated three times to obtain epithelial cells from the nasopharynx. Swabs were placed in universal transport medium (UTM). The UTM specimens were then separated into aliquots and stored in the laboratory freezer (−80 degrees Celsius). Specimens were shipped bimonthly by air on dry ice to Cincinnati Children’s Hospital Medical Center.
Specimens were tested for respiratory viruses using the commercially available respiratory viral panel (RVP ID-Tag™, Luminex Diagnostics, Austin, Texas, USA), a multiplex nucleic acid amplification test. This as saycan detect 16 different viruses and subtypes from a single respiratory specimen, including: parainfluenza 1, 2, 3, 4, influenza A, influenza B, human metapneumovirus (hMPV), respiratory syncytial virus (RSV), enterovirus/rhinovirus, adenovirus, and coronavirus. This method has a reported sensitivity of 97.8% and a specificity of 96.4% for detection of any respiratory virus14,35,36, and is a Federal Drug Administration certified diagnostic test.
Statistical analysis
The viral agents, demographic characteristics, and clinical characteristics of viral respiratory infections were described using Chi Square and Fisher’s Exact tests. We defined a fever as an axillary temperature > 37.8 degrees Celsius14, and we used the World Health Organization’s Integrated Management of Childhood Illness guidelines29 to define fast breathing in children less than 12 months old (≥50 breaths per minute) or 12 months of age and older (≥40 breaths per minute). The monthly distribution of respiratory viral infections and rainfall were graphed, and the relationship was assessed by correlation coefficient (R2). We used Poisson regression to model the number of influenza positives as a function of rainfall.
Ethical Considerations
The protocol was approved by the Institutional Review Board (IRB) of both Cincinnati Children’s Hospital Medical Center and the Hospital Regional del Occidente in Santa Rosa de Copan, Honduras.
RESULTS
From February 15, 2010 to June 14, 2011, 355 children were eligible for enrollment and 345 children (97.2%) were enrolled. Ten children (2.8%) refused enrollment. During this time period, the Clinica Hombro a Hombroand the Centro de Salud had 3298 patient encounters for children less than 5 years of age documented in the computerized clinic database. According to diagnostic codes assigned by the Honduran physicians, 776 of these children had respiratory symptoms during their clinic visits. Including both clinics, 44.5% of patients less than 5 years of age with documented respiratory diagnoses were enrolled in the study.
Demographic Characteristics
Of the 345 participants, 158 (45.8%) were female, and the average age of the children enrolled was 19 months (range 18 days – 58 months) (see Table, Supplemental Digital Content 1, http://links.lww.com/INF/B254). Fifty-nine (17.1%) were less than 6 months of age, 78 (22.6%) were 6–11 months of age, 102 (29.6%) were 12–23 months of age, and 106 (30.7%) were 24–59 months of age. Most children (84.3%) were born at home. Very few children slept in the room where food was prepared (5.5%), but 95.7% of subjects lived in homes where wood was used to prepare food. Of all participants, 14.8% were exposed to cigarette smoke at home. More than half of the subjects (53.3%) have at least one family member working in the United States. Children with family in the United States were more likely to have influenza, and children without family in the United States were more likely to have hMPV.
Clinical Characteristics
Overall, the most common symptoms reported by parents were cough (88.1%), fever (78.6%), and runny nose (78.8%)(see Table, Supplemental Digital Content 2, http://links.lww.com/INF/B255). Parents reported shortness of breath in 67.5% of children. The mean temperature recorded at the clinic was 37.1° Celsius, and 19.5% of children had a fever at the time of recruitment. Of the 269 children (78%) with an oxygen saturation measured, 49 (18.2%) had an oxygen saturation less than 92%. Of children less than 12 months old, 17.5% had a respiratory rate at or above 50 breaths per minute, while 20.2% of children 12 months of age or older had a respiratory rate at or above 40 breaths per minute. Per clinical chart abstraction, no participants required transfer or hospitalization.
The mean temperature recorded for children with RSV was significantly higher than the mean for parainfluenza (p=0.0179). Children with parainfluenza also had a significantly lower rate of fever compared to children with other viruses, both by chart review (p=0.0164) and parent history (p<0.001).
The most common physical exam findings documented by the physician were rhinorrhea (62.6%), followed by wheezing (25.8%), and pharyngitis (23.2%) (see Table, Supplemental Digital Content 3, http://links.lww.com/INF/B256). Children with hMPV and RSV were more likely to have rhinorrhea than children with parainfluenza or influenza (p<0.001). Presence of wheezing was similar between viruses (p=0.538). Otitis media was documented by physicians in 3.8% of participants.
Viral Agents Identified
At least one virus was detected in 75.4% of children. Coinfections were identified in 26 children (7.5%). Enterovirus/rhinovirus was most commonly identified in 33.4% of children. Parainfluenza was identified in 46 children (13.3%); 8 (2.3%) were type 1, 24 (7.0%) were type 3, and 14 (4.1%) were type 4. Influenza was identified in 41 children (11.9%); 21 (6.1%) were influenza A, H3; 12 (3.5%) were influenza A, non-H3; and 8 (2.3%) were influenza B. Human metapneumovirus was identified in 28 children(8.1%). RSV was seen in 26(7.5%) children; 10 (2.9%) were RSV A and 16 (4.6%) were RSV B. Eight specimens (2.3%) yielded adenovirus.
Seasonality (Figure 1)
Figure 1. Respiratory viruses by month (%) and mean monthly rainfall (mm).
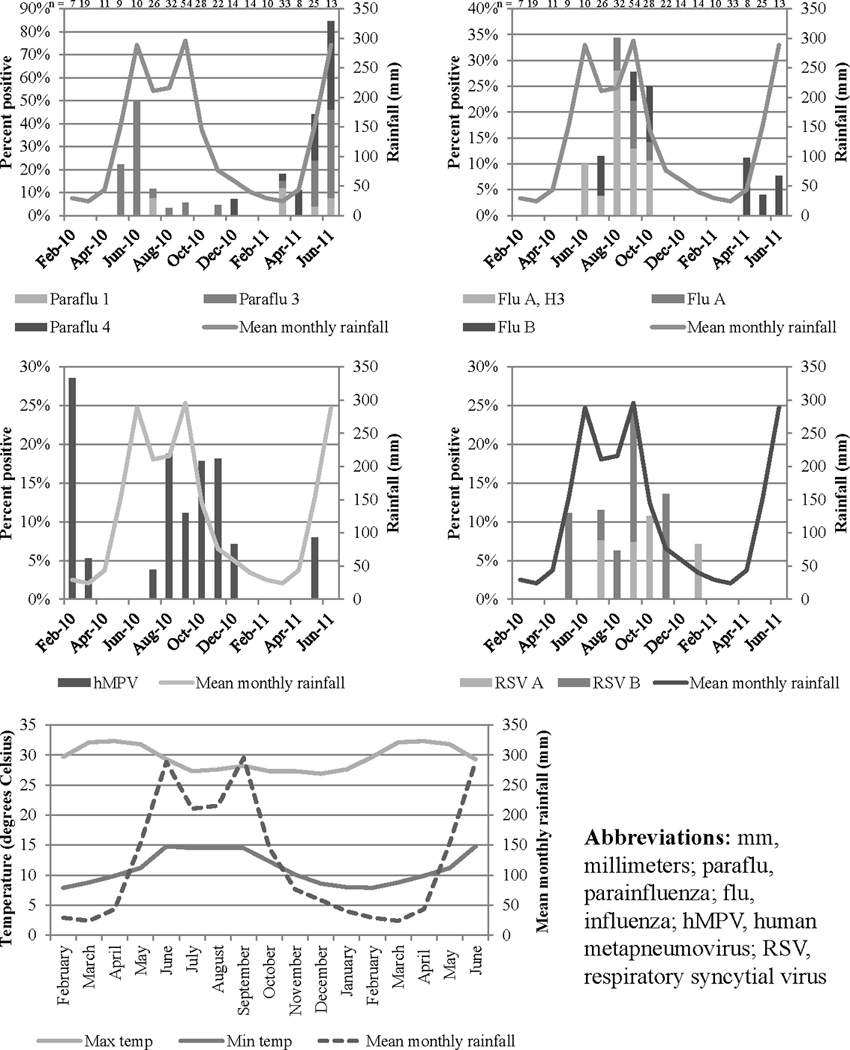
The peaks in influenza and other respiratory viruses coincided with the rainfall peak from June through September.
Parainfluenza peaked in June 2010 and 2011 and was present nine out of 12 months. Influenza peaked in the months of August and September 2010. Influenza rose from 1.8% of isolates in February through June to 25.7% of isolates in July through October. No cases of influenza were identified from February 2010 through May 2010 and November 2010 through March 2011. Influenza B was present in April 2011 through June 2011. Influenza was present seven out of 12 months (June 2010 through October 2010, April 2011 through June 2011). Human metapneumovirus peaked in February 2010 and was present nine out of 12 months. RSV peaked in September 2010 and was present seven out of 12 months.
Rainfall data obtained from Santa Rosa de Copán revealed an increase in rainfall from May to September 2010, as well as an increase beginning in May 2011. The number of influenza cases detected monthly demonstrated a positive correlation with monthly rainfall (R2 = 0.334, p=0.0151). Using Poisson regression, measured rainfall was significantly correlated with circulating influenza(p≤0.0001), parainfluenza (p≤0.0001), hMPV (p= 0.0182), and RSV (p≤0.0001).
DISCUSSION
To our knowledge, these are the first data describing the etiology of acute respiratory infections in rural Honduras.
Clinical and Demographic Characteristics
The most common symptoms reported by the parents were cough, fever, and runny nose. The most common clinical findings reported by the physician were rhinorrhea, wheezing, and pharyngitis. These findings were much more common than difficulty breathing and retractions, suggesting that subjects had more upper respiratory symptoms and were not severely ill. Though all parents reported a recent fever at the time of recruitment, the average temperature of all participants with viruses recorded at the clinic was less than 37.8 degrees Celsius. Only 19.5% of participants had a fever greater than 37.8 degrees Celsius at the time of enrollment. None of the participants required hospitalization, affirming the likelihood of mild respiratory illness. Several factors may explain this finding. Hondurans in this region have had access to primary care since the introduction of the Shoulder to Shoulder health care system in 1990. Because of this availability of care, we speculate that some severe illness is prevented by early medical care. Further studies are needed to analyze the receipt of antibiotics in this population and its relationship to improved outcomes in viral infections. Additionally, we may have failed to capture severely ill children taken directly to the hospital in La Esperanza without initial triage in the outpatient clinics.
Etiology of Acute Respiratory Infections
This study identified a fairly large proportion of respiratory disease caused by parainfluenza (13.3%). Previous studies in Honduras29 and Brazil22 have identified RSV as the most frequent viral agent identified in hospitalized children with respiratory symptoms. This elevated proportion of parainfluenza may be attributable to the outpatient surveillance setting or limited testing capabilities prior to the availability of respiratory multiplex PCR. Reyes et al. tested for parainfluenza using immunofluorescence techniques and failed to identify any cases among hospitalized Honduran and El Salvadoran children29. To fully describe the patterns, further studies and extended surveillance are necessary to determine the relative frequency of influenza, parainfluenza, human metapneumovirus, and RSV over several years.
Influenza was the next most prevalent virus throughout the year (11.9%). This high rate of disease cannot be attributed to the 2009–2010 pandemic H1N1 influenza epidemic, because only a small proportion (2.3%) of the influenza A isolates identified were not H3. When the pandemic H1N1 influenza epidemic spread to Honduras in the spring of 2009, the WHO and the Honduran government provided monovalent H1N1 vaccine in populous areas. Though the vaccine did not reach rural Intibucá, the availability of the vaccine in La Esperanza and other nearby urban regions may have limited spread of the virus prior to study initiation in February 2010.
Reyes et al. investigated children hospitalized in Honduras and El Salvador in 1991–1992, and they detected influenza by immunofluorescence in 29 of 135 (21%) nasopharyngeal samples29. Twenty (69%) of the 29 influenza virus cases occurred during the first three months of life, and influenza and RSV cases peaked at the end of the rainy season. A recent study by Laguna-Torres et al. analyzed etiologic agents associated with influenza-like illness in five participating hospitals or health centers in large urban areas in El Salvador, Honduras, and Nicaragua30. Out of 427 samples tested in Honduras, 66 (15%) were positive for influenza A or B, 37 (9%) for adenovirus, 29 (7%) for parainfluenza, 15 (4%) for RSV, and 1 for hMPV. Our study similarly selected for influenza-like illness in children, possibly increasing the number of children identified with influenza in both studies.
The low prevalence of hMPV in the urban study by Laguna-Torres et al. contrasts with our findings30. We identified hMPV in over 8% of our nasopharyngeal samples, surpassing RSV and adenovirus. Previous studies have failed to identify hMPV due to limited diagnostic techniques, so future studies may further define the prevalence of this virus.
RSV was the most common pathogen detected by Laguna-Torres et al. in children less than five years of age in all three countries30. It is associated with high prevalence, hospitalization, and mortality rates22. However, we detected RSV in 7.5% of nasopharyngeal samples in our study. This finding may reflect the relatively low severity of illness in children identified in our study, as well as the utilization of outpatient clinic data only.
Adenovirus was present in much smaller numbers. It tended to occur in small clusters that can be attributed to the closed nature of this rural community. The individuals in this community have limited access to large cities in Honduras and other surroundings areas. The presence of different viruses may vary year to year based on exposure to outside communities.
Seasonality
Measured rainfall was significantly correlated with circulating influenza, parainfluenza, hMPV, and RSV. These results demonstrated a clear peak of influenza, hMPV, and RSV in the months of July through October, coinciding with the rainy season in this region of Honduras. Parainfluenza peaked in May and June at the onset of the rainy season. Previous research in Brazil has shown that influenza circulation coincides with the rainy season37, but further research is needed to understand if these environmental conditions affect circulation of other respiratory viruses.
This influenza seasonality in rural Intibucá differs from that of urban Honduras, where influenza circulates throughout the year with two clear peaks38. This urban pattern is consistent with data from other tropical countries but may vary year to year20. This pattern may also be explained by travelers between urban settings around the globe39, and the remote setting of Intibucá may influence the local prevalence and seasonality of influenza virus. Further data are needed to assess the current use of the Northern Hemisphere vaccine in Honduras34 as the influenza peak in July and August occurred before Northern Hemisphere vaccines were available.
The statistical correlation between respiratory viruses and rainfall may be due to several factors, including increased crowding with increased transmission of respiratory viruses40. Some have speculated that the seasonality of influenza is mediated by vitamin D levels41, but limited data are available about vitamin D levels by season at high latitudes in Latin American countries to establish a relationship between influenza seasonality and vitamin D. A recent study in older Mayan adults showed lower vitamin D levels in elderly adults42, but we are not aware of published studies of seasonal vitamin D level variation in Honduran children. Further research is needed to confirm these findings and to assess the relationship between vitamin D levels and seasonality of respiratory infection.
There were several limitations to this study. Even though the Clinica Hombro a Hombro and Centro de Salud serve a large catchment area for ill child care, we did not confirm that we sampled all children with respiratory disease. Some ill children may have been unable to travel to the clinic due to severity of illness, and some families may not have sought care for their children if the illness was mild. Our denominator data from the computerized clinic database were obtained retrospectively using diagnostic codes, so the data were limited by physician coding and lack of diagnostic specificity. It is likely that patients with other respiratory symptoms were included in the denominator data, limiting the utility of these data.
This study was also limited by the lack of a healthy comparison group. We collected data for 17 months, making it difficult to fully characterize details of local seasonality of individual virus species. The nearest available weather data were not local, so correlations between viruses and rainfall may not be exact. Our sample size limited our ability to do more detailed analyses examining differences in clinical and demographic features of children with different respiratory viruses.
While epidemiological data are available from industrialized nations, minimal etiologic information is available for respiratory disease in other parts of the world. These unique rural results suggest that the spectrum of viruses in rural Honduran children is similar to that found in North America, though the circulation of viruses coinciding with the rainy season is more typical of previous findings in several tropical regions. Data from this study will guide further research into prevention and treatment of acute viral respiratory diseases, including improved therapeutic options and prevention with immunization.
Supplementary Material
Acknowledgments
Financial support: This work was supported by the Fogarty International Clinical Research Scholars and Fellows program (5 R24 TW007988-03), National Institutes of Health; and the Division of Infectious Diseases at Cincinnati Children’s Hospital Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts of interest or funding to disclose.
REFERENCES
- 1.Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010 Jun 5;375(9730):1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bulletin of the World Health Organization. 2008 May;86(5):408–416. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juven T, Mertsola J, Waris M, et al. Etiology of community-acquired pneumonia in 254 hospitalized children. The Pediatric infectious disease journal. 2000 Apr;19(4):293–298. doi: 10.1097/00006454-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Tajima T, Nakayama E, Kondo Y, et al. Etiology and clinical study of community-acquired pneumonia in 157 hospitalized children. J Infect Chemother. 2006 Dec;12(6):372–379. doi: 10.1007/s10156-006-0476-5. [DOI] [PubMed] [Google Scholar]
- 5.Bonzel L, Tenenbaum T, Schroten H, Schildgen O, Schweitzer-Krantz S, Adams O. Frequent detection of viral coinfection in children hospitalized with acute respiratory tract infection using a real-time polymerase chain reaction. The Pediatric infectious disease journal. 2008 Jul;27(7):589–594. doi: 10.1097/INF.0b013e3181694fb9. [DOI] [PubMed] [Google Scholar]
- 6.Arnold JC, Singh KK, Spector SA, Sawyer MH. Undiagnosed respiratory viruses in children. Pediatrics. 2008 Mar;121(3):e631–e637. doi: 10.1542/peds.2006-3073. [DOI] [PubMed] [Google Scholar]
- 7.Tsolia MN, Psarras S, Bossios A, et al. Etiology of community-acquired pneumonia in hospitalized school-age children: evidence for high prevalence of viral infections. Clin Infect Dis. 2004 Sep 1;39(5):681–686. doi: 10.1086/422996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott JA, Brooks WA, Peiris JS, Holtzman D, Mulholland EK. Pneumonia research to reduce childhood mortality in the developing world. J Clin Invest. 2008 Apr;118(4):1291–1300. doi: 10.1172/JCI33947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poehling KA, Edwards KM, Weinberg GA, et al. The underrecognized burden of influenza in young children. The New England journal of medicine. 2006 Jul 6;355(1):31–40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- 10.Viboud C, Alonso WJ, Simonsen L. Influenza in tropical regions. PLoS medicine. 2006 Apr;3(4):e89. doi: 10.1371/journal.pmed.0030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks WA, Steinhoff M. Epidemiology of Influenza in Tropical and Subtropical Low-Income Regions. In: Rappuoli R, Giudice GD, editors. Influenza Vaccines for the Future. Second ed. Basel, Switzerland: Springer; 2011. [Google Scholar]
- 12.Chiu SS, Lau YL, Chan KH, Wong WH, Peiris JS. Influenza-related hospitalizations among children in Hong Kong. The New England journal of medicine. 2002 Dec 26;347(26):2097–2103. doi: 10.1056/NEJMoa020546. [DOI] [PubMed] [Google Scholar]
- 13.Nascimento-Carvalho CM, Ribeiro CT, Cardoso MR, et al. The role of respiratory viral infections among children hospitalized for community-acquired pneumonia in a developing country. The Pediatric infectious disease journal. 2008 Oct;27(10):939–941. doi: 10.1097/INF.0b013e3181723751. [DOI] [PubMed] [Google Scholar]
- 14.Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. The New England journal of medicine. 2008 Oct 9;359(15):1555–1564. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 15.Henkle E, Steinhoff MC, Omer SB, et al. Incidence of influenza virus infection in early infancy: a prospective study in South Asia. The Pediatric infectious disease journal. 2011 Feb;30(2):170–173. doi: 10.1097/INF.0b013e3181f63c39. [DOI] [PubMed] [Google Scholar]
- 16.Tamerius J, Nelson MI, Zhou SZ, Viboud C, Miller MA, Alonso WJ. Global influenza seasonality: reconciling patterns across temperate and tropical regions. Environ Health Perspect. 2011 Apr;119(4):439–445. doi: 10.1289/ehp.1002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Straliotto SM, Siqueira MM, Muller RL, Fischer GB, Cunha ML, Nestor SM. Viral etiology of acute respiratory infections among children in Porto Alegre, RS, Brazil. Revista da Sociedade Brasileira de Medicina Tropical. 2002 Jul-Aug;35(4):283–291. doi: 10.1590/s0037-86822002000400002. [DOI] [PubMed] [Google Scholar]
- 18.de Arruda E, Hayden FG, McAuliffe JF, et al. Acute respiratory viral infections in ambulatory children of urban northeast Brazil. The Journal of infectious diseases. 1991 Aug;164(2):252–258. doi: 10.1093/infdis/164.2.252. [DOI] [PubMed] [Google Scholar]
- 19.Chew FT, Doraisingham S, Ling AE, Kumarasinghe G, Lee BW. Seasonal trends of viral respiratory tract infections in the tropics. Epidemiology and infection. 1998 Aug;121(1):121–128. doi: 10.1017/s0950268898008905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon A, Ortega O, Kuan G, et al. Prevalence and seasonality of influenza-like illness in children, Nicaragua, 2005–2007. Emerging infectious diseases. 2009 Mar;15(3):408–414. doi: 10.3201/eid1503.080238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alonso WJ, Viboud C, Simonsen L, Hirano EW, Daufenbach LZ, Miller MA. Seasonality of influenza in Brazil: a traveling wave from the Amazon to the subtropics. American journal of epidemiology. 2007 Jun 15;165(12):1434–1442. doi: 10.1093/aje/kwm012. [DOI] [PubMed] [Google Scholar]
- 22.Alonso WJ, Laranjeira BJ, Pereira SA, et al. Comparative dynamics, morbidity and mortality burden of pediatric viral respiratory infections in an equatorial city. The Pediatric infectious disease journal. 2012 Jan;31(1):e9–e14. doi: 10.1097/INF.0b013e31823883be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Mello WA, de Paiva TM, Ishida MA, et al. The dilemma of influenza vaccine recommendations when applied to the tropics: the Brazilian case examined under alternative scenarios. PLoS One. 2009;4(4):e5095. doi: 10.1371/journal.pone.0005095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgs ES, Hayden FG, Chotpitayasunondh T, Whitworth J, Farrar J. The Southeast Asian Influenza Clinical Research Network: development and challenges for a new multilateral research endeavor. Antiviral Res. 2008 Apr;78(1):64–68. doi: 10.1016/j.antiviral.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Nair H, Brooks WA, Katz M, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 2011 Dec 3;378(9807):1917–1930. doi: 10.1016/S0140-6736(11)61051-9. [DOI] [PubMed] [Google Scholar]
- 26.Yazdanbakhsh M, Kremsner PG. Influenza in Africa. PLoS medicine. 2009 Dec;6(12):e1000182. doi: 10.1371/journal.pmed.1000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.United Nations Statistics Division. [Accessed January 11, 2012];World Statistics Pocketbook, Honduras country profile. 2009 http://data.un.org/CountryProfile.aspx?crName=HONDURAS.
- 28.United Nations DoEaSA. World Population Prospects: The 2006 Revision. 2007. [Accessed October 14, 2008]. Linear interpolation using the mid-points of the quinquennium. CD-ROM Edition - Extended Dataset (United Nations publications) [Google Scholar]
- 29.Reyes M, Hedlund KO, Lorenzana I, Ehrnst A. Respiratory infection and iatrogenic diarrhea in Honduras and El Salvador during the 1991–1992 season. The American journal of tropical medicine and hygiene. 1996 Mar;54(3):260–264. doi: 10.4269/ajtmh.1996.54.260. [DOI] [PubMed] [Google Scholar]
- 30.Laguna-Torres VA, Sanchez-Largaespada JF, Lorenzana I, et al. Influenza and other respiratory viruses in three Central American countries. Influenza Other Respi Viruses. 2011 Mar;5(2):123–134. doi: 10.1111/j.1750-2659.2010.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams BG, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. The Lancet infectious diseases. 2002 Jan;2(1):25–32. doi: 10.1016/s1473-3099(01)00170-0. [DOI] [PubMed] [Google Scholar]
- 32.Cashat-Cruz M, Morales-Aguirre JJ, Mendoza-Azpiri M. Respiratory tract infections in children in developing countries. Seminars in pediatric infectious diseases. 2005 Apr;16(2):84–92. doi: 10.1053/j.spid.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 33.GAVI Alliance. [Accessed April 16, 2012];Honduras tackles leading killer of children: Pneumococcal vaccines introduced in seventh GAVI-eligible country. 2011 http://fr.gavialliance.org/media_centre/statements/honduras_pneumococcal.php.
- 34.Ropero-Alvarez AM, Kurtis HJ, Danovaro-Holliday MC, Ruiz-Matus C, Andrus JK. Expansion of seasonal influenza vaccination in the Americas. BMC Public Health. 2009;9:361. doi: 10.1186/1471-2458-9-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahony J, Chong S, Merante F, et al. Development of a respiratory virus panel test for detection of twenty human respiratory viruses by use of multiplex PCR and a fluid microbead-based assay. Journal of clinical microbiology. 2007 Sep;45(9):2965–2970. doi: 10.1128/JCM.02436-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. The New England journal of medicine. 2008 Oct 9;359(15):1555–1564. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 37.Moura FE, Perdigao AC, Siqueira MM. Seasonality of influenza in the tropics: a distinct pattern in northeastern Brazil. The American journal of tropical medicine and hygiene. 2009 Jul;81(1):180–183. [PubMed] [Google Scholar]
- 38.World Health Organization. FluNet, Global Influenza Surveillance and Response System (GISRS) [Accessed January 27, 2012];Influenza Laboratory Surveillance Information, Honduras. 2012 http://www.who.int/influenza/gisrs_laboratory/flunet/charts/en/index.html.
- 39.Viboud C, Bjornstad ON, Smith DL, Simonsen L, Miller MA, Grenfell BT. Synchrony, waves, and spatial hierarchies in the spread of influenza. Science. 2006 Apr 21;312(5772):447–451. doi: 10.1126/science.1125237. [DOI] [PubMed] [Google Scholar]
- 40.Mikolajczyk RT, Akmatov MK, Rastin S, Kretzschmar M. Social contacts of school children and the transmission of respiratory-spread pathogens. Epidemiology and infection. 2008 Jun;136(6):813–822. doi: 10.1017/S0950268807009181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juzeniene A, Ma LW, Kwitniewski M, et al. The seasonality of pandemic and non-pandemic influenzas: the roles of solar radiation and vitamin D. Int J Infect Dis. 2010 Dec;14(12):e1099–e1105. doi: 10.1016/j.ijid.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Sud SR, Montenegro-Bethancourt G, Bermudez OI, Heaney RP, Armas L, Solomons NW. Older Mayan residents of the western highlands of Guatemala lack sufficient levels of vitamin D. Nutr Res. 2010 Nov;30(11):739–746. doi: 10.1016/j.nutres.2010.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


