Abstract
P2X7 receptors (P2X7R) are extracellular ATP-gated ion channels expressed in the immune effector cells that carry out critical protective responses during the early phases of microbial infection or acute tissue trauma. P2X7R-positive cells include monocytes, macrophages, dendritric cells, and T-cells. Given its presence in all host and pathogen cell types, ATP can be readily released into extracellular compartments at local sites of tissue damage and microbial invasion. Thus, extracellular ATP and its target receptors on host effector cells can be considered as additional elements of the innate immune system. In this regard, stimulation of P2X7R rapidly triggers a key step of the inflammatory response: induction of NLRP3/caspase-1 inflammasome signaling complexes that drive the proteolytic maturation and secretion of the proinflammatory cytokines interleukin-1β(IL-1β) and interleukin-18 (IL-18). IL-1β(and IL-18) lacks a signal sequence for compartmentation within the Golgi and classical secretory vesicles and the proIL-1β precursor accumulates within the cytosol following translation on free ribosomes. Thus, ATP-induced accumulation of the mature IL-1β cytokine within extracellular compartments requires non-classical mechanisms of export from the cytosolic compartment. Five proposed mechanisms include: 1) exocytosis of secretory lysosomes that accumulate cytosolic IL-1β via undefined protein transporters; 2) release of membrane-delimited microvesicles derived from plasma membrane blebs formed by evaginations of the surface membrane that entrap cytosolic IL-β; 3) release of membrane-delimited exosomes secondary to the exocytosis of multivesicular bodies formed by invaginations of recycling endosomes that entrap cytosolic IL-β; 4) exocytosis of autophagosomes or autophagolysosomes that accumulate cytosolic IL-1β via entrapment during formation of the initial autophagic isolation membrane or omegasome; and 5) direct release of cytosolic IL-1β secondary to regulated cell death by pyroptosis or necroptosis. These mechanisms are not mutually exclusive and may represent engagement of parallel or intersecting membrane trafficking responses to P2X7 receptor activation.
P2X7 Receptors, Inflammation, and Innate Immunity
Extracellular ATP and other nucleotides participate in multiple types of intercellular communication (Ralevic and Burnstock, 1998). Acute tissue trauma that results in cell lysis is one obvious source of extracellular ATP. However, various non-lytic stimuli also induce ATP release from many cell types via several mechanisms (Lazarowski, 2012). The released ATP and other nucleotides act as paracrine or autocrine agonists for the P2 nucleotide receptors which include eight G protein-coupled P2Y receptors and seven ionotropic P2X receptor subtypes that have been identified in multiple vertebrate genomes (Khakh and North, 2006; von Kugelgen and Wetter, 2000). Given the overlapping roles of stress, cell death, and adaptation to injury in inflammation and P2 receptor signaling, P2 receptors have been linked to multiple inflammatory and immune responses (Dubyak, 2001; Junger, 2011). Within the context of innate immune response to the pathogen-associated molecule patterns (PAMPs) derived from killed or dying microbes, it is important to consider that these microorganisms will additionally release generic intracellular metabolites, such as ATP.
P2X receptors function as ATP-gated non-selective cation channels and the seven different P2X receptor protein subunits (encoded by distinct genes) share a similar structure comprising intracellular amino- and carboxy-termini, two transmembrane segments, and an extracellular loop containing 10 similarly spaced cysteines and glycosylation sites. Functional channels composed of P2X receptor subunits self-assemble during in vivo translation into stable trimeric complexes that predominantly traffic to the plasma membrane (Khakh and North, 2006). All P2X receptor subtypes display a very high selectivity for ATP over other nucleotides. P2X7 receptors are most highly expressed in hematopoietic tissues and cells including monocytes, macrophages, microglia, and T-lymphocytes (Ferrari et al., 2006). When gated to the open state by ATP binding, P2X7R channels facilitate a rapid, non-inactivating influx of Na+ and Ca2+, and efflux of K+; this results in rapid depolarization, decreased cytosolic [K+], and increased cytosolic [Ca2+] and [Na+] (Figure 1). Prolonged stimulation of the receptor additionally induces the flux of molecules up to 800 Da in mass via an as yet unidentified mechanism. Activation of P2X7R in myeloid or lymphoid leukocytes triggers multiple responses that shape the intensity or duration of innate immune and inflammatory responses (Ferrari et al., 2006). Depending on the magnitude and duration of stimulation, P2X7R can also elicit necrotic cytolysis or apoptotic death of activated leukocytes. However, the most extensively characterized innate immune response to P2X7R activation is the rapid and robust assembly of the NLRP3 inflammasome complex that facilitates caspase-1 mediated processing and secretion of the primary proinflammatory cytokine IL-1β (Di Virgilio, 2007).
Figure 1. Regulation of NLRP3 Inflammasome Assembly and Non-Classical Pathways of IL-1β Secretion by the P2X7 Receptor.
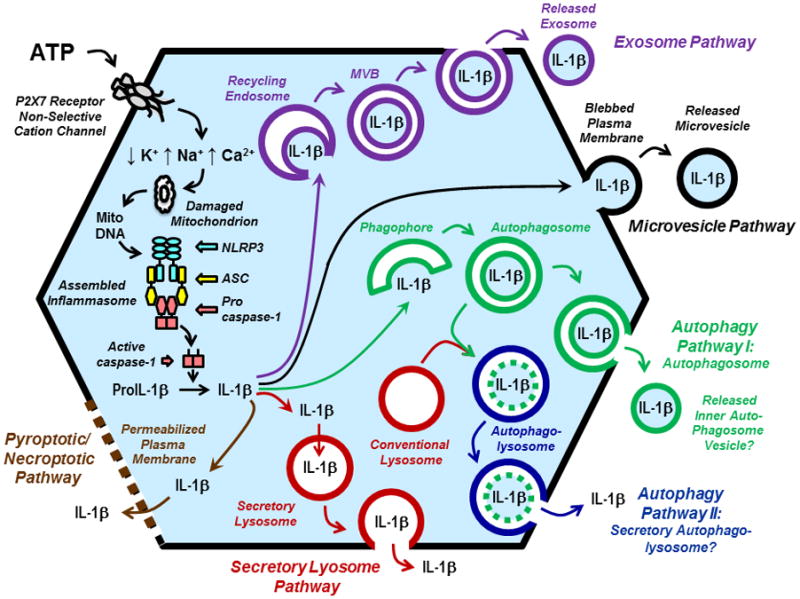
The figure illustrates the signaling cascade (left side of a generic myeloid leukocyte) by which extracellular ATP-induced gating of P2X7 receptor channels serially triggers changes in intracellular cation concentrations, perturbation of mitochondrial function, and assembly of NLRP3/ASC/Procaspase-1 inflammasome complexes in macrophages, monocytes, dendritic cells, or microglial cells. Accumulation of active caspase-1 in the cytosol facilitates efficient proteolytic processing of cytosolic proIL-1β (a 33 kDa protein) to the mature, 17 kDa IL-1β cyotokine. The right sight of the figure illustrates the four major pathways of non-classical secretion (also termed unconventional secretion) by the cytosolic IL-1β can be exported to the extracellular compartment. These include: the 1) exosome pathway (violet); 2) the microvesicle pathway (black); 3) two variants of the autophagy pathway that may involve exocytosis of either autophagosomes (green) or autophagolysosomes (blue); 4) the secretory lysosome pathway (red). The bottom left of the figure illustrates a fifth pathway (brown) for IL-1b export as a secondary sequelae of regulated macrophage death by caspase-1 dependent pyroptosis or, possibly, damaged mitochondria-dependent necroptosis. Abbreviations: MitoDNA (mitochondrial DNA); MVB (multivesicular body).
P2X7 Receptor Regulation of NLRP3 Inflammasome Assembly, IL-1β Processing, and Non-classical IL-β Secretion
IL-1β is a primary proinflammatory cytokine whose local and circulating levels are tightly regulated to prevent aberrant activation of pathways that can lead to chronic inflammatory diseases (Dinarello, 1996). In response to various pathogen-associated molecular pattern (PAMP) molecules that target toll-like receptors (TLR), IL-1β accumulates as a biologically inactive 33 kDa procytokine (proIL-1β) in the cytoplasm of monocytes and macrophages. Conversion to the biologically active 17 kDa form requires proteolytic maturation by caspase-1 which itself is regulated by the assembly of multiprotein complexes termed inflammasomes. Characterization of the multiple adapter proteins that comprise distinct inflammasome complexes, as well as the microbial or sterile stress stimuli that trigger inflammasome assembly, have been extraordinarily active areas of innate immune biology research during the past few years and the subject of many excellent reviews (Franchi et al., 2012; Rathinam et al., 2012; Zitvogel et al., 2012).
Although PAMPs, such as LPS, stimulate rapid toll-like receptor (TLR)-dependent transcription and translation of proIL-1β, they elicit only modest production of mature IL-1β due to inefficient assembly of active caspase-1 inflammasomes. However, when PAMP-primed macrophages are additionally stimulated with extracellular ATP, they rapidly release large amounts of mature IL-1β at rates up to 100 times faster than with PAMP exposure alone (Laliberte et al., 1999; Perregaux and Gabel, 1994). This stimulatory action of ATP is mediated by the P2X7R cation channels which act to rapidly dissipate the normal trans-plasma membrane K+, Na+, and Ca2+ gradients (Solle et al., 2001). Significantly, the ability of ATP/P2X7R to markedly accelerate caspase-1 activation and IL-1β secretion is eliminated in either ASC-null (Mariathasan et al., 2004) or NLRP3-null (Mariathasan et al., 2006) macrophages primed with LPS. Activation of the NLRP3 inflammasome by P2X7R is likely mediated by the secondary effects of altered cytoplasmic [K+]/[Na+] ratios, as well as increased [Ca2+], on the integrity of the mitochondrial outer and inner membranes, which also increases generation of mitochondria-dependent reactive oxygen species (ROS) (Figure 1). Recent studies have demonstrated that P2X7R stimulation triggers rapid release of oxidized mitochondrial DNA (mitoDNA) into the cytosol wherein the mitoDNA facilitates (possibly by direct binding to NLRP3) assembly of active NLRP3/ASC/caspase-1 complexes (Nakahira et al., 2011; Shimada et al., 2012).
A major unresolved issue is how inflammasome-mediated IL-1β processing in response to P2X7R activation and indeed all other inflammasome-inducing stimuli is linked to the efficient export of biologically active IL-1β to the extracellular compartments. IL-1β lacks signal sequences for compartmentation within the Golgi and classical secretory vesicles and the proIL-1β precursor accumulates within the cytosol following translation on free ribosomes. Likewise, the various protein constituents (caspase-1, ASC, and NLRP3) of the NLRP3 inflammasome assemble within the cytosolic compartment. Thus, accumulation of the mature, caspase-1-processed IL-1β cytokine within extracellular compartments requires non-classical mechanisms of export from the cytosolic compartment (Eder, 2009; Rubartelli et al., 1990; Wewers, 2004). Moreover, P2X7R activation additionally stimulates the rapid co-release of active caspase-1, a 60 kDa tetrameric complex, (Kahlenberg et al., 2005; Laliberte et al., 1999; Mariathasan et al., 2004; Mariathasan et al., 2006) as well as the ASC inflammasome scaffolding protein. This indicates a mechanism that involves a coordinated mass transport of the IL-1β together with the caspase-1 inflammasome processing complex. Although P2X7R stimulation can trigger cytolysis (Le Feuvre et al., 2002) via caspase-1 mediated pyroptosis, the early stages of IL-1β release from ATP-activated macrophages can be unequivocally dissociated from ATP-induced cytolysis (Brough and Rothwell, 2007; Pelegrin et al., 2008; Verhoef et al., 2005).
Non-Classical or Unconventional Secretion of Cytosolic Macromolecules and Metabolites
The non-classical secretion of IL-1β raises mechanistic questions that pertain to a broader group of cellular responses that involve non-canonical export of other normally cytosolic macromolecules and metabolites to extracellular compartments in the absence of (or preceding) cytolysis (Nickel and Rabouille, 2009) (Thery et al., 2002b). In addition to IL-1β, other cytokines and growth factors are released via non-classical secretion. These include: 1) IL-18 which is also a substrate for caspase-1 inflammasomes (Mariathasan et al., 2004); 2) IL-1α which binds to the same cell surface receptor as IL-1β, but is not a caspase-1 substrate (Gross et al., 2012; Seki et al., 2001); 3) fibroblast growth factors (Nickel, 2011); and 4) high-mobility group protein B1 (HMGB1), a nuclear protein which acts as a proinflammatory mediator in extracellular compartments (Gardella et al., 2002; Lamkanfi et al., 2010). There is also growing appreciation that cytosolic mRNA and miRNAs can be released from intact cells for extracellular transfer to other cells and tissues (Valadi et al., 2007); this includes transfer of miRNAs between T cells and antigen-presenting cells at immune synapses (Mittelbrunn et al., 2011).
A complete discussion of the diverse cellular pathways described or hypothesized to underlie the extracellular release of these various cytosolic macromolecules is beyond the scope of this short review. However, consideration of the multiple mechanisms linked to the specific response of P2X7 receptor-regulated IL-1β secretion provides a useful overview of five major models of non-classical/unconventional secretion (Figure 1). All of these models include three basic and common reactions: 1) targeting of the IL-1β and/or inflammasome proteins to the interfaces of the cytosol with particular subcellular membrane loci on the plasma membrane or intracellular organelles; 2) entrapment or transfer of the cytosolic IL-1β within membrane-delimited compartments which are distinct from the classical Golgi-derived secretory vesicles; and 3) export of this entrapped or transferred IL-1β to the extracellular space as either directly as a free, soluble protein or, indirectly, as the cargo within a membrane vesicle which is the moiety directly released from the activated macrophage/monocyte.
Experiments over the past 10–15 years have implicated four mechanistically distinct pathways of unconventional IL-1β secretion from macrophages and other monocyte-lineage leukocytes in response to P2X7R activation. The mechanisms, which are considered in more detail in separate sections of this review, include: 1) exocytosis of secretory lysosomes that accumulate cytosolic IL-1β via undefined protein transporters (Figure 1, Secretory Lysosome Pathway in Red); 2) release of membrane-delimited microvesicles derived from plasma membrane blebs formed by evaginations of the surface membrane that entrap cytosolic IL-β(Figure 1, Microvesicle Pathway in Black); 3) release of membrane-delimited exosomes secondary to the exocytosis of multivesicular bodies formed by invaginations of recycling endosomes that entrap cytosolic IL-β(Figure 1, Exosome Pathway Scheme Violet); and 4) exocytosis of autophagosomes (Figure 1, Autophagy Pathway I in Green) or autophagolysomes (Figure 1, Autophagy Pathway II in Blue) that accumulate cytosolic IL-1β via entrapment during formation of the initial autophagic isolation membrane or omegasome. Notably, these mechanisms are not mutually exclusive and may indeed represent engagement of parallel or intersecting membrane trafficking responses to P2X7 receptor activation.
It is also important to note that sustained activation of the P2X7R→NLRP3 inflammasome cascade induces caspase-1 mediated pyroptotic death of monocyte/macrophages (Brough and Rothwell, 2007; Le Feuvre et al., 2002; Verhoef et al., 2005). Thus, direct efflux of cytosolic mature IL-1β across hyper-permeable plasma membranes comprises a fifth pathway of non-classical export (Figure 1, Pyroptotic/Necroptic Pathway in Brown).
Non-classical IL-1β Secretion via P2X7R-Stimulated Exocytosis of Secretory Lysosomes
An early model was based on observations that immunoreactive IL-1β and caspase-1 can be localized within lysosomes of human monocytes with the underlying assumption that proIL-1β and caspase-1 are transported from the cytosol into a subset of secretory lysosomes (Figure 1) (Andrei et al., 1999; Andrei et al., 2004; Carta et al., 2006). Secretory lysosomes comprise specialized subtypes of lysosome-related organelles predominantly found in hematopoietic cells (Blott and Griffiths, 2002). As for other secretory granules released via regulated exocytosis, the fusion of secretory lysosomes with the plasma membrane is regulated by characteristic t- and v-SNARE proteins and Ca2+-dependent synaptotagmins (Andrews, 2000; Blott and Griffiths, 2002). Notably, P2X7R-stimulated exocytosis of secretory lysosomes (as assayed by release of lysosomal proteases) occurs concurrently with the release of IL-1β and caspase-1 in both murine macrophages (Qu et al., 2007) and human monocytes (Carta et al., 2006). In human monocytes, both events are blocked by inhibitors of Ca2+-dependent and Ca2+-independent phospholipase A2 and phosphatidylcholine-specific phospholipase C (Andrei et al., 2004). Suppression of the microtubule-directed movements of secretory lysosomes also attenuates ATP triggered release of IL-1β (Andrei et al., 1999; Carta et al., 2006). The ability of an LPS-dependent and cyclohexamide-sensitive priming process to potentiate the release of both cathepsin B (as a marker of secretory lysosomes) and mature IL-1β further suggested that a common set of rapid-turnover signaling protein(s) may orchestrate the ability P2X7R to coordinately regulate inflammasome assembly, IL-1β export, and lysosome exocytosis (Qu et al., 2007).
In contrast to these multiple correlations between IL-1β release and exocytosis of secretory lysosomes, other findings argue against secretory lysosomes as a central pathway for non-classical IL-1β export in response to P2X7R activation. Notably, removal of extracellular Ca2+ completely abrogated ATP-induced secretion of the lysosomal marker cathepsin B from murine macrophages while minimally affecting IL-1β export (Qu et al., 2007). Moreover, immunofluorescence analyses of intact murine peritoneal macrophages indicated that IL-1β did not significantly co-localize with cathepsin B and LAMP-1 in lysosomal compartments immediately before, or during, P2X7R-triggered activation of caspase-1 (Brough and Rothwell, 2007). Given the defined roles of cytosolic ASC and NLRP3 in P2X7R-regulated caspase-1 activation, it is also unclear how these inflammasome components plus the proIL-1β substrate might be coordinately transported into lysosomes prior to assembly of active inflammasome complexes and IL-1β maturation. Nonetheless, earlier electron microscopy studies based on immunogold labeling as well as conventional western blot analyses provided unequivocal evidence that a significant fraction of intracellular proIL-1β and procaspase-1 colocalizes with lysosomal marker proteins within an endolysosomal fraction of LPS-activated human monocytes (Andrei et al., 1999). However, interpretation of these previous immunolocalization results needs to be revisited in the light of recent findings that pro-IL1β, as well as the protein components of the NLRP3 inflammasome, can be targeted by the basic autophagy machinery of macrophages (Harris et al., 2011; Shi et al., 2012). These new observations indicate that cytosolic pro-IL1β is sequestered within the isolation membranes/phagophores that ultimately enclose to generate the double membrane autophagosomes (Figure 1). Most autophagosomes fuse with conventional lysosomes to facilitate proteolytic degradation of their sequestered cytosolic protein during both basal and stress-stimulated autophagy. Thus, the previously described colocalization of immunoreactive proIL-1β and caspase-1 with lysosomal markers may reflect basal or stress-stimulated autophagic degradation of these proteins, rather than the acutely induced compartmentalization of the IL-1β pool destined for maturation by active inflammasomes and regulated export. On the other hand, there is growing evidence that a subset of autophagosomes may be redirected for fusion with the plasma membrane rather than lysosomes and thereby comprise another route for unconventional protein secretion.
Non-classical IL-1β Secretion via P2X7R Stimulation of Plasma Membrane Blebbing and Microvesicle Release
Studies with human THP1 monocytes (MacKenzie et al., 2001), murine microglial cells (Bianco et al., 2005), or human dendritic cells (DCs) (Pizzirani et al., 2007) revealed that P2X7R stimulation can initiate local accumulation of caspase-1 and IL-1β within the microdomain of the sub-plasma membrane cytosol which is then coordinated with the evagination of plasma membrane blebs that rapidly scission away from the cell surface (Figure 1). The shed blebs comprise a pool of phosphatidylserine-enriched microvesicles that contain entrapped IL-1β and caspase-1. Stimulus-induced shedding of plasma membrane-derived microvesicles has been described in multiple hematopoietic cell types (platelets, DCs, and neutrophils) and non-hematopoietic cell types (Barry et al., 1998; Heijnen et al., 1999; Hess et al., 1999). Microvesicles range in size between 100 nm to 1 μm in diameter. This distinguishes them from both the 1– 4 μm apoptotic bodies derived from fragmented apoptotic cells and the 30–80 nm exosomes (discussed below) derived from the intraluminal vesicles of endosomal multivesicular bodies (MVB).
ATP-induced microvesicle shedding from macrophages and DCs was correlated with decreased plasma membrane capacitance (MacKenzie et al., 2001) and markedly inhibited by the removal of extracellular Ca2+ or the treatment of with P2X7R antagonists (Pizzirani et al., 2007). The released microvesicles have been reported to contain: 1) soluble proteins including unprocessed proIL-1β, caspase-1 processed mature IL-1β, and caspase-1 itself; 2) intrinsic membrane proteins including MHC-II, P2X7R itself, P2Y2 receptors, CD63, CD39, and LAMP-1 (Andrei et al., 2004; Bianco et al., 2005; Carta et al., 2006; Gudipaty et al., 2003; Qu et al., 2007); 3) an increased level of surface-active phospholipids such as phosphatidylserine (MacKenzie et al., 2001). In addition to IL-1β and IL-18, the ATP-mobilized microvesicles comprise a mechanism for extracellular delivery of other danger molecules such as the high mobility group box 1 protein (HMGB1) (Gardella et al., 2002) and intracellular thiol reducing agents (Angelini et al., 2002). P2X7R stimulation of murine dendritic cells or interferon-γ primed murine macrophages also elicits the release of microvesicles enriched in MHCII (major histocompatibility protein II), the intrinsic membrane protein that plays a critical role in presentation of antigens to T cells (Qu et al., 2009; Ramachandra et al., 2010).
The molecular basis for P2X7R-dependent microvesicle shedding remains incompletely characterized. It likely reflects direct effects of altered ion homeostasis, particularly increased cytosolic Ca2+, on proteases that regulate association of the plasma membrane with the sub-membrane cytoskeleton, as well as lipases that induce localized changes in membrane lipid composition and fluidity. A recent analysis of P2X7R-stimulated IL-1β secretion from astrocytes indicated that rapid activation of acid sphingomyelinase was required for efficient formation and shedding of microvesicles containing IL-1β (Bianco et al., 2009). Plasma membrane blebbing, which is a prerequisite for microvesicle shedding, represents a phenomenon wherein sections of plasma membrane reversibly protrude and retract at the cell surface. Studies with different cell types have indicated that P2X7R-stimulated membrane blebbing involves several common cytoskeletal signaling pathways (Morelli et al., 2003; Wilson et al., 2002). Activation of Rho and p38 MAPKs occurred concurrently with P2X7R-induced membrane blebbing (Pfeiffer et al., 2004) while pharmacological inhibitors of p38, Rho, and Rho kinases all reduced P2X7R-stimulated actin reorganization and blebbing (Morelli et al., 2003; Pfeiffer et al., 2004; Verhoef et al., 2003). Deletion of the C terminus of P2X7R completely abrogates its interaction with tetraspanin proteins that are implicated as regulators of rapid blebbing and markers of released microvesicles (Wilson et al., 2002). P2X7R-dependent membrane blebbing in osteoblasts was correlated with serial activation of phospholipase D and A2 to produce and release lysophosphatidic acid (LPA) (Panupinthu et al., 2007). Antagonism of G protein-coupled LPA receptors in these osteoblasts suppressed blebbing in response to either LPA or P2X7R agonists but did not affect the ionotropic actions of P2X7R. Thus, LPA may function as an autocrine mediator downstream of P2X7R to induce signaling cascades that control membrane blebbing and, possibly, microvesicle release and non-classical secretion of inflammatory mediators.
Non-classical IL-1β Secretion via P2X7R Stimulation of the Release of Multivesicular Body-Derived Exosomes
In addition to plasma membrane-derived microvesicles, immune and inflammatory cells release another pool of membranous vesicles termed exosomes (Johnstone, 2006; Thery et al., 2002b). Exosomes are derived from the intraluminal vesicles (ILV) within the multivesicular bodies (MVB) that are generated by invagination of the limit membranes of recycling endosomes and contain a unique proteome (Thery et al., 2001). Although MVBs often fuse with lysosomes to deliver their ILV-associated proteins for degradation, some can be redirected for fusion with the plasma membrane to release the ILVs into extracellular compartment as exosomes. Exosomes also act as yet another non-classical pathway for the P2X7R-stimulated secretion of IL-1β (Qu et al., 2007). These data suggest that invagination of cytosol by recycling endosomes in inflammatory macrophages can entrap caspase-1 inflammasome complexes and the proIL-1β substrate (Figure 1). Significantly, exosomes released from macrophages, dendritic cells, or B lymphocytes also contain intrinsic membrane proteins, such as the type II major histocompatibility complex (MHCII), that play critical roles in immune recognition and antigen presentation (Raposo et al., 1996). P2X7R activation in murine macrophages and dendritic cells triggers the rapid extracellular accumulation of two pools of membranes containing MHCII (Qu et al., 2009).
When released from macrophages or DCs that have internalized and processed microbial or other foreign proteins, exosomes will contain MHCII loaded with antigenic peptide (pMHCII exosomes) (Muntasell et al., 2007). These released pMHCII exosomes can either directly activate T lymphocytes, or indirectly activate T cells following the uptake of the pMHCII exosomes by remote naïve dendritic cells which then process the antigen-loaded exosomes for conventional antigen presentation at DC-T cell synapses (Thery et al., 2002a). In macrophages primed with both interferon-γ and LPS or DC primed with LPS, extracellular ATP stimulated the export of ~15% of the total cellular MHCII pool within 90 min (Qu et al., 2009). The released MHCII was associated with two distinct populations of membrane vesicles: 1) plasma membrane-derived microvesicles (100–500 nm diameter) that contained P2X7R protein, actin, and the LAMP1 lysosomal membrane protein; and 2) multivesicular body-derived exosomes (30–80 nm diameter) that lacked the P2X7R, actin, and LAMP1 markers. Notably, both pools of released MHCII membranes were capable of binding antigenic peptide and activating T cell receptor-dependent IL-2 production in antigen-specific T cell hybridoma cells (Ramachandra et al., 2010). These observations linked the well-characterized ability of the P2X7 receptor to stimulate NLRP3 inflammasome and non-classical IL-1β secretion as innate immune responses with the coordinated release of MHCII-containing exosomes and microvesicles that may engage the adaptive immune response.
The strong repressive effects of ASC or NLRP3 deletion on ATP-induced release of MHCII exosomes suggest the possibility that inflammasome complexes per se may regulate the formation of specialized MVB that accumulate cytosolic IL-1β and caspase-1 within the invaginating exosomes that define MVB (Qu et al., 2009). Such a role for inflammasomes per se in membrane compartmentation and non-classical export may be related to the observations that: 1) caspase-1 targets the Sterol Regulatory Element Binding Proteins (SREBPs) which regulate regulate membrane biogenesis (Gurcel et al., 2006); and 2) NLRP3 inflammasomes also regulate the export of IL-1α which, unlike IL-1β, is not substrate for caspase-1 (Gross et al., 2012).
Non-classical IL-1β Secretion via P2X7R-Stimulated Mobilization of Autophagosomes
The most recently proposed model of non-classical IL-1β secretion utilizes the membrane trafficking machinery of the autophagy pathway (recently reviewed by (Deretic et al., 2012). Basal autophagy comprises a highly conserved pathway for the isolation and degradation of cytosolic proteins (such as aggregated or excess enzymes) and dysfunctional organelles (such as irreversibly damaged mitochondria). Stimulated rates of autophagy in response to metabolic stresses, such as nutrient deprivation, serve to replenish and maintain the levels of amino acids necessary for cell survival and basic function. However, much recent research has identified additional roles for autophagy in the innate immune responses to microbial invasion or sterile inflammatory stress (Levine et al., 2011). Basal and stimulated autophagy involves the assembly of isolation membrane structures or phagophores initiated as so-called omegasome evaginations from the endoplasmic reticulum (Figure 1). As nascent omegasomes grow, they entrap adjacent cytosol and/or cytoplasmic organelles prior to maturation and the end-to-end fusion which generates the double-membrane autophagosomal organelles. In most cases, autophagosomes fuse with lysosomes which deliver the proteases and lipases that degrade the cytosolic proteins or damaged organelles previously sequestered within the autophagosome. The multiple phases of autophagosome initiation and maturation are orchestrated by several key Atg (Autophagy) regulatory factors. Knockout or overexpression of these Atg proteins in immune effector cells provides experimental approaches for assessing roles for autophagy in innate or acquired immunity. Notably, several recent studies have reported that basal autophagy in macrophages and DCs provides an important suppressive mechanism to limit the rate and extent of the NLRP3 inflammasome activation and consequent IL-1β processing in response to P2X7R and other inflasmmasome stimuli. This suppression involves: 1) targeting of ubiquitinated inflammasome proteins for entrapment with developing omegasomes and autophagosomes (Shi et al., 2012); 2) autophagic sequestration of the damaged mitochondria that otherwise release the oxidized mitochondrial DNA which facilitates assembly of NLRP3 inflammasomes in the cytosol (Nakahira et al., 2011); and 3) autophagic sequestration of proIL-1β substrate away from the active cytoplasmic inflammasome processing complexes (Harris et al., 2011).
The above findings obviously support a model wherein autophagy will act to attenuate, rather than promote, secretion of mature IL-1β as consequence of suppressed inflammasome assembly and proteolytic maturation of proIL-1β. In those studies, the knockout or inhibition of various Atg proteins acted to increase the rate and extent of IL-1β secretion in response to P2X7R activation and other stimuli. However, another study has indicated that suppression of Atg factors in macrophages can have the opposite effect, i.e., inhibition of IL-1β export (Dupont et al., 2011). This suggests that a subset of autophagosomes containing entrapped IL-1β and caspase-1 can be redirected from fusing with lysosomes to fusing with the plasma membrane (Figure 1, Autophagy Pathway I). That report analyzed IL-1β secretion in response to multiple NLRP3 inflammasome stimuli other than ATP. However, another study has demonstrated that P2X7 activation of microglial cells triggers a secretion of IL-1β that is temporally correlated with the release of membranes enriched in lipidated LC3B (Atg8), a canonical marker of mature autophagosomes (Takenouchi et al., 2009). This response was linked to a P2X7R-induced suppression of lysosomal acidification that may provide a mechanism attenuating the usual fusion of lysosomes with autophagosomes and thereby redirecting the latter organelles for exocytotic fusion with the plasma membrane. This suggests that exocytosis of mature autophagosomes may lead to the release of the intact inner membrane vesicles containing entrapped IL-1β and other cytosolic proteins. Biochemical comparison of possible autophagosome-derived extracellular vesicles with the microvesicle and exosome pools of vesicles known to be released from ATP-activated macrophages is an important area for future research.
Alternatively, autophagosomes containing IL-1β may canonically fuse with conventional lysosomes leading to dissolution of the inner membrane compartment and consequent generation of autophagosolysosomes. It is possible that a subpool of such organelles may be directed for exocytotic fusion with plasma membrane prior to complete proteolytic degradation of entrapped IL-1β. Such putative “secretory autophagolysosomes” may be mobilized for exocytosis via the same signals, SNARE proteins, and synaptotagmins that regulate the secretory lysosomes described above. The exocytosis of secretory autophagolysosomes containing mature IL-1β would result in the direct extracellular accumulation of the cytokine as a free, soluble protein in contrast to the membrane-entrapped cytokine that would be released via the exocytosis of autophagosomes.
Concluding Remarks and Remaining Questions: Non-classical IL-1β Secretion via P2X7R and Other Inflammasome Activators
The five possible mechanisms described above for non-classical IL-1β secretion are not mutually exclusive and likely represent the engagement of parallel responses of macrophages to P2X7 receptor activation under different physiological or pathophysiological conditions. A critical -- but still poorly understood – set of questions concerns the temporal, spatial, and contextual parameters that define each of these pathways. For example, membrane blebbing and microvesicular release may predominate under conditions where a macrophage, DC, or microglial cell is exposed to very high concentrations of ATP, as may occur during extensive but brief traumatic tissue injury such as spinal cord injury (Wang et al., 2004). High local concentrations of ATP will result in rapid and massive P2X7R-mediated Ca2+ influx and consequent perturbation of the sub-plasma membrane cytoskeleton and lipid remodeling dynamics. Conversely, the exososomal or autophagic pathways may predominate during submaximal, but sustained, gating of P2X7 channels in myeloid cells that sense lower concentrations of local extracellular ATP, as likely occurs during paracrine release of ATP from nearby apoptotic cells (Ghiringhelli et al., 2009) or autocrine release at the immune synapses between T cells and APCs (Piccini et al., 2008). Finally, pyroptotic release of IL-1β(driven by both P2X7R-dependent and P2X7R-independent cascades) may be important under pathological conditions wherein the release of active caspase-1 is attenuated, as occurs in macrophages infected with intracellular bacteria that evade the macrophage’s antimicrobial machinery. Recent studies have indicated that pyroptotic death of such infected macrophages is beneficial to the host by facilitating the release not only of inflammatory cytokines, but also the bacteria per se. The release of bacteria from their protected intracellular niche to the extracellular compartments exposes them to rapid killing by adjacent neutrophils (Miao et al., 2010).
Despite the many recent reports describing the myriad extrinsic stimuli that trigger IL-1β maturation via intracellular assembly of NLRP3 inflammasomes, few have considered how these stimuli elicit extracellular IL-1β accumulation. Although this review has focused on the unconventional secretory mechanisms entrained by P2X7R channel activation, the same pathways are likely induced to varying extents by other stimuli coupled to the NLRP3 inflammasome cascade (Franchi et al., 2012; Rathinam et al., 2012; Zitvogel et al., 2012). For example, pore-forming exotoxins produced by some extracellular bacteria increase permeability of the leukocyte plasma membrane to ions and small molecules. Moreover, a growing body of evidence suggests that activation of caspase-1 per se plays a general role in regulating non-classical export pathways via signaling mechanisms that remain largely undefined (Keller et al., 2008). It is possible that caspase-1 interacts – even perhaps independently of its canonical proteolytic function – with other proteins such as GRASP55 and Rab39 implicated in the non-classical export of various macromolecules (Deretic et al., 2012; Nickel and Rabouille, 2009). Exploring these potential mechanistic links between inflammasome signaling and the more general cell biology of unconventional secretion should provide new insights, and undoubtedly new questions, regarding the extracellular accumulation of IL-1β during physiological and pathological inflammatory responses.
Acknowledgments
This work was supported by NIH grants R01-GM36387
References
- Andrei C, Dazzi C, Lotti L, Torrisi MR, Chimini G, Rubartelli A. The secretory route of the leaderless protein interleukin 1beta involves exocytosis of endolysosome-related vesicles. Mol Biol Cell. 1999;10:1463–1475. doi: 10.1091/mbc.10.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrei C, Margiocco P, Poggi A, Lotti LV, Torrisi MR, Rubartelli A. Phospholipases C and A2 control lysosome-mediated IL-1 beta secretion: Implications for inflammatory processes. Proc Natl Acad Sci U S A. 2004;101:9745–9750. doi: 10.1073/pnas.0308558101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews NW. Regulated secretion of conventional lysosomes. Trends Cell Biol. 2000;10:316–321. doi: 10.1016/s0962-8924(00)01794-3. [DOI] [PubMed] [Google Scholar]
- Angelini G, Gardella S, Ardy M, Ciriolo MR, Filomeni G, Di Trapani G, Clarke F, Sitia R, Rubartelli A. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc Natl Acad Sci U S A. 2002;99:1491–1496. doi: 10.1073/pnas.022630299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry OP, Pratico D, Savani RC, FitzGerald GA. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Invest. 1998;102:136–144. doi: 10.1172/JCI2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, Saglietti L, Schuchman EH, Furlan R, Clementi E, Matteoli M, Verderio C. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009;28:1043–1054. doi: 10.1038/emboj.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, Verderio C. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005;174:7268–7277. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- Blott EJ, Griffiths GM. Secretory lysosomes. Nat Rev Mol Cell Biol. 2002;3:122–131. doi: 10.1038/nrm732. [DOI] [PubMed] [Google Scholar]
- Brough D, Rothwell NJ. Caspase-1-dependent processing of pro-interleukin-1beta is cytosolic and precedes cell death. J Cell Sci. 2007;120:772–781. doi: 10.1242/jcs.03377. [DOI] [PubMed] [Google Scholar]
- Carta S, Tassi S, Semino C, Fossati G, Mascagni P, Dinarello CA, Rubartelli A. Histone deacetylase inhibitors prevent exocytosis of interleukin-1beta-containing secretory lysosomes: role of microtubules. Blood. 2006;108:1618–1626. doi: 10.1182/blood-2006-03-014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V, Jiang S, Dupont N. Autophagy intersections with conventional and unconventional secretion in tissue development, remodeling and inflammation. Trends Cell Biol. 2012 doi: 10.1016/j.tcb.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007;28:465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Dubyak GR. Role of P2 Receptors in the Immune System. In: Abbracchio MP, Williams M, editors. Handbook of Experimental Pharmacology: Purinergic and Pyrimidernergic Signaling II. 151/II. Springer; Berlin: 2001. pp. 323–354. [Google Scholar]
- Dupont N, Jiang S, Pilli M, Ornatowski W, Bhattacharya D, Deretic V. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1beta. EMBO J. 2011;30:4701–4711. doi: 10.1038/emboj.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder C. Mechanisms of interleukin-1beta release. Immunobiology. 2009;214:543–553. doi: 10.1016/j.imbio.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, Perfettini JL, Schlemmer F, Tasdemir E, Uhl M, Genin P, Civas A, Ryffel B, Kanellopoulos J, Tschopp J, Andre F, Lidereau R, McLaughlin NM, Haynes NM, Smyth MJ, Kroemer G, Zitvogel L. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- Gross O, Yazdi AS, Thomas CJ, Masin M, Heinz LX, Guarda G, Quadroni M, Drexler SK, Tschopp J. Inflammasome activators induce interleukin-1alpha secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012;36:388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Gudipaty L, Munetz J, Verhoef PA, Dubyak GR. Essential role for Ca2+ in regulation of IL-1beta secretion by P2X7 nucleotide receptor in monocytes, macrophages, and HEK-293 cells. Am J Physiol Cell Physiol. 2003;285:C286–299. doi: 10.1152/ajpcell.00070.2003. [DOI] [PubMed] [Google Scholar]
- Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126:1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Harris J, Hartman M, Roche C, Zeng SG, O’Shea A, Sharp FA, Lambe EM, Creagh EM, Golenbock DT, Tschopp J, Kornfeld H, Fitzgerald KA, Lavelle EC. Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for degradation. J Biol Chem. 2011;286:9587–9597. doi: 10.1074/jbc.M110.202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- Hess C, Sadallah S, Hefti A, Landmann R, Schifferli JA. Ectosomes released by human neutrophils are specialized functional units. J Immunol. 1999;163:4564–4573. [PubMed] [Google Scholar]
- Johnstone RM. Exosomes biological significance: A concise review. Blood Cells Mol Dis. 2006;36:315–321. doi: 10.1016/j.bcmd.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11:201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-kappaB-driven protein synthesis. J Immunol. 2005;175:7611–7622. doi: 10.4049/jimmunol.175.11.7611. [DOI] [PubMed] [Google Scholar]
- Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- Laliberte RE, Eggler J, Gabel CA. ATP treatment of human monocytes promotes caspase-1 maturation and externalization. J Biol Chem. 1999;274:36944–36951. doi: 10.1074/jbc.274.52.36944. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Sarkar A, Vande Walle L, Vitari AC, Amer AO, Wewers MD, Tracey KJ, Kanneganti TD, Dixit VM. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J Immunol. 2010;185:4385–4392. doi: 10.4049/jimmunol.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012;8:359–373. doi: 10.1007/s11302-012-9304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Feuvre RA, Brough D, Iwakura Y, Takeda K, Rothwell NJ. Priming of macrophages with lipopolysaccharide potentiates P2X7-mediated cell death via a caspase-1-dependent mechanism, independently of cytokine production. J Biol Chem. 2002;277:3210–3218. doi: 10.1074/jbc.M104388200. [DOI] [PubMed] [Google Scholar]
- Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity. 2001;15:825–835. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Miao EA, I, Leaf A, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, Bernad A, Sanchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nature communications. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli A, Chiozzi P, Chiesa A, Ferrari D, Sanz JM, Falzoni S, Pinton P, Rizzuto R, Olson MF, Di Virgilio F. Extracellular ATP causes ROCK I-dependent bleb formation in P2X7-transfected HEK293 cells. Mol Biol Cell. 2003;14:2655–2664. doi: 10.1091/mbc.02-04-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntasell A, Berger AC, Roche PA. T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. Embo J. 2007;26:4263–4272. doi: 10.1038/sj.emboj.7601842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel W. The unconventional secretory machinery of fibroblast growth factor 2. Traffic. 2011;12:799–805. doi: 10.1111/j.1600-0854.2011.01187.x. [DOI] [PubMed] [Google Scholar]
- Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol. 2009;10:148–155. doi: 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- Panupinthu N, Zhao L, Possmayer F, Ke HZ, Sims SM, Dixon SJ. P2X7 Nucleotide Receptors Mediate Blebbing in Osteoblasts through a Pathway Involving Lysophosphatidic Acid. J Biol Chem. 2007;282:3403–3412. doi: 10.1074/jbc.M605620200. [DOI] [PubMed] [Google Scholar]
- Pelegrin P, Barroso-Gutierrez C, Surprenant A. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J Immunol. 2008;180:7147–7157. doi: 10.4049/jimmunol.180.11.7147. [DOI] [PubMed] [Google Scholar]
- Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- Pfeiffer ZA, Aga M, Prabhu U, Watters JJ, Hall DJ, Bertics PJ. The nucleotide receptor P2X7 mediates actin reorganization and membrane blebbing in RAW 264.7 macrophages via p38 MAP kinase and Rho. J Leukoc Biol. 2004;75:1173–1182. doi: 10.1189/jlb.1203648. [DOI] [PubMed] [Google Scholar]
- Piccini A, Carta S, Tassi S, Lasiglie D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci U S A. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzirani C, Ferrari D, Chiozzi P, Adinolfi E, Sandona D, Savaglio E, Di Virgilio F. Stimulation of P2 receptors causes release of IL-1beta-loaded microvesicles from human dendritic cells. Blood. 2007;109:3856–3864. doi: 10.1182/blood-2005-06-031377. [DOI] [PubMed] [Google Scholar]
- Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007;179:1913–1925. doi: 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- Qu Y, Ramachandra L, Mohr S, Franchi L, Harding CV, Nunez G, Dubyak GR. P2X7 receptor-stimulated secretion of MHC class II-containing exosomes requires the ASC/NLRP3 inflammasome but is independent of caspase-1. J Immunol. 2009;182:5052–5062. doi: 10.4049/jimmunol.0802968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Ramachandra L, Qu Y, Wang Y, Lewis CJ, Cobb BA, Takatsu K, Boom WH, Dubyak GR, Harding CV. Mycobacterium tuberculosis synergizes with ATP to induce release of microvesicles and exosomes containing major histocompatibility complex class II molecules capable of antigen presentation. Infect Immun. 2010;78:5116–5125. doi: 10.1128/IAI.01089-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol. 2012;13:333–332. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubartelli A, Cozzolino F, Talio M, Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. Embo J. 1990;9:1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki E, Tsutsui H, Nakano H, Tsuji N, Hoshino K, Adachi O, Adachi K, Futatsugi S, Kuida K, Takeuchi O, Okamura H, Fujimoto J, Akira S, Nakanishi K. Lipopolysaccharide-induced IL-18 secretion from murine Kupffer cells independently of myeloid differentiation factor 88 that is critically involved in induction of production of IL-12 and IL-1beta. J Immunol. 2001;166:2651–2657. doi: 10.4049/jimmunol.166.4.2651. [DOI] [PubMed] [Google Scholar]
- Shi CS, Shenderov K, Huang NN, Kabat J, Abu-Asab M, Fitzgerald KA, Sher A, Kehrl JH. Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol. 2012;13:255–263. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- Takenouchi T, Nakai M, Iwamaru Y, Sugama S, Tsukimoto M, Fujita M, Wei J, Sekigawa A, Sato M, Kojima S, Kitani H, Hashimoto M. The activation of P2X7 receptor impairs lysosomal functions and stimulates the release of autophagolysosomes in microglial cells. J Immunol. 2009;182:2051–2062. doi: 10.4049/jimmunol.0802577. [DOI] [PubMed] [Google Scholar]
- Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002a;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002b;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Verhoef PA, Estacion M, Schilling W, Dubyak GR. P2X7 receptor-dependent blebbing and the activation of Rho-effector kinases, caspases, and IL-1 beta release. J Immunol. 2003;170:5728–5738. doi: 10.4049/jimmunol.170.11.5728. [DOI] [PubMed] [Google Scholar]
- Verhoef PA, Kertesy SB, Lundberg K, Kahlenberg JM, Dubyak GR. Inhibitory effects of chloride on the activation of caspase-1, IL-1beta secretion, and cytolysis by the P2X7 receptor. J Immunol. 2005;175:7623–7634. doi: 10.4049/jimmunol.175.11.7623. [DOI] [PubMed] [Google Scholar]
- von Kugelgen I, Wetter A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, Nedergaard M. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10:821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- Wewers MD. IL-1beta: an endosomal exit. Proc Natl Acad Sci U S A. 2004;101:10241–10242. doi: 10.1073/pnas.0403971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HL, Wilson SA, Surprenant A, North RA. Epithelial membrane proteins induce membrane blebbing and interact with the P2X7 receptor C terminus. J Biol Chem. 2002;277:34017–34023. doi: 10.1074/jbc.M205120200. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Kepp O, Galluzzi L, Kroemer G. Inflammasomes in carcinogenesis and anticancer immune responses. Nat Immunol. 2012;13:343–351. doi: 10.1038/ni.2224. [DOI] [PubMed] [Google Scholar]


