Abstract
Macrophage-induced bystander effects have been implicated as an important mediator of chromosomal instability and colon cancer triggered by Enterococcus faecalis a human intestinal commensal bacteria. There is little understanding about how inflammatory cytokines mediate bystander effects, but questions in this area are important because of the pivotal contributions made by inflammatory processes to cancer initiation and progression. Here we report that the central pro-inflammatory cytokine TNF-α acts as a diffusible mediator of the bystander effects induced by macrophages, an effect caused by a proliferation of macrophages that trigger epithelial cell production of Netrin-1, a neuronal guidance molecule. TNF-α-mediated bystander assays employed a murine co-culture system of primary colonic epithelial cells and E. faecalis-infected macrophages (in vitro), with an IL-10-deficient mouse model of colon cancer that involves long-term colonization with E. faecalis (in vivo). In cell co-cultures, we observed increased expression of the TNF-α receptor Tnfrsf1b and Netrin-1. These effects were blocked by anti-TNF-α antibody or by pretreatment with an inhibitor of NF-κB signaling. RNAi-mediated attenuation of Tnfrsf1b decreased TNF-α-induced netrin-1 production and augmented epithelial cell apoptosis in culture. Extending these observations, colon biopsies from E. faecalis-colonized IL-10−/− mice exhibited crypt hyperplasia and increased staining for macrophages, TNF-α, netrin-1, NF-κB, Tnfrsf1b and the proliferation marker PCNA, also displaying a reduction in epithelial cell apoptosis. Together, our results define a pathway for macrophage-induced bystander effects in which TNF-α triggers TNFRSF1b receptor signaling leading to increased production of Netrin-1, crypt hyperplasia and decreased epithelial cell apoptosis. In elucidating an important commensal-associatedpro-inflammatory mechanism in the intestinal microenvironment, our work highlights the role of Netrin-1 and a specific TNF-α receptor as candidate targets to prevent or treat colorectal cancer.
Keywords: CRC Model, Bystander effect, TNF-α, Netrin-1, Tnfrsf1b, macrophage, tumorigenesis, colorectal cancer
Introduction
Accumulating evidence implicates intestinal commensals and pathogens as important triggers forepithelial cell transformationleading to colorectal cancer (1–5),adiagnosiswith an overall lifetime risk of 5.3% for persons in the United States (6). Enterococcus faecalis is acommon human intestinal commensal that producesDNA damage in epithelial cells and generateschromosomal instability through a macrophage-induced bystander effect (5, 7–9).The generation of extracellular superoxide by this microorganism—an unusual phenotype resultingfrom constrained respiration—substantially contributes to bystander effects and makes this commensal a usefulmodel forstudying bacterial mechanisms that initiate and/or promote colorectal cancer (5, 8, 10).
Bystander effects arising from bacterial activation of macrophages lead to aneuploidy, tetraploidy, and chromosomal instability in neighboring epithelial cells (5, 8, 9).This is analogous to observations by radiation biologists that irradiating myeloid cells or fibroblasts causes clastogens ( i.e. chromosome-breaking factors) to be produced that diffuse into neighboring unirradiated cells and cause CIN (11). This phenomenon is termed the radiation-induced bystander effect. In mice theprooxidant metabolism of E. faecalis can acutely activate nuclear factor (NF)-κB in colonic macrophages and induce a mucosal gene network associated with inflammation, apoptosis, and cell-cycle regulation (12). Changes in gene regulation are chronically sustained ininterleukin (IL)-10 knockout mice colonized with E. faecalis where activated gene networks include tumor necrosis factor (TNF)-α, IL-1, interferon-γ, and IL-6 (13). The long-term result is colonic inflammation, dysplasia, and colorectal cancer (5, 13–15).Several enterococcal determinants have been implicated in the development of inflammation and cancer including an extracellular serine proteinase and extracellular superoxide production (5, 16).One mediator for macrophage-induced bystander effects has been identified, 4-hydroxy-2-nonenal, a mutagenic aldehyde produced by E. faecalis-infected macrophages and observed in the mucosa of colonized Il10−/− mice (5).However, other potential mediators that may modulate proliferative or anti-apoptotic pathways and promote carcinogenesis remain uncharacterized.
A plausible mediator for macrophage-induced bystander effects is TNF-α. In murine models of colitis, TNF-α has been linked to tumorigenesis through NF-κB activation and induction of inflammation-associated cytokines and anti-apoptotic proteins (17–19). TNF-α is also known to promote carcinogenesis by damaging DNA, stimulating cancer growth, and promoting invasiveness (20–22).TNF-α signaling occurs through Tnfrsf1a (Tnfr1)and/or Tnfrsf1b (Tnfr2) receptors that, when bound, recruit intracellular adaptor proteins to activate multiple signal transduction pathways including NF-κB (23).Tnfrsf1b receptor signaling in particular has been linked to NF-κB activation in the a zoxymethane-dextran sodium sulfate model of colitis-associated carcinogenesis (24).The potential relevance of such signaling in human colorectal cancer was recently noted in the Nurses’ Health Study cohort where surrogate markers for cytokine activation were assessed. In that population, baseline plasma TNFRSF1B (TNFR2), a marker of inflammation and soluble TNF antagonist, was significantly associated with an increased risk of human colorectal cancer (25), whereas no association was found for C-reactive protein or interleukin-6.
One mechanism by which TNF-α signaling and NF-κB activation could contribute to colorectal carcinogenesis is through the inhibition of apoptosis (20).One key regulator of apoptosis in the intestine is netrin-1. This diffusible protein was initially discovered as an attractant for commissural axons (26). More recent data implicates netrin-1 and its dependence receptors in cancer initiation and progression (27).Many human cancers, including inflammation-associated colorectal cancer, are associated with upregulation of netrin-1 or loss of one or more of its cognate receptors (28–30).Increased binding of netrin-1 to dependence receptors—including deleted in colorectal cancer (DCC)and the UNC5H family members—inhibits p53-dependent apoptosis and promotes epithelial cell proliferation (27). The oncogenic properties of netrin-1 were noted in mice containing a germline mutation in Apc (Apc +/1638N) that overexpressed netrin-1, and in an azoxymethane-dextran sodium sulfate model of colitis-associated carcinogenesis wherein netrin-1 binding was blocked using a protein fragment of DCC (29, 30). In both models, netrin-1 inhibited colonic epithelial cell apoptosis and promoted high grade adenomas with focal adenocarcinoma. In our work, we observed increased expression of Ntn1 in colonic epithelial cells both in vitro and in vivo using superoxide-producing E. faecalis (12).
It remains to be determined whether TNF-α can mediate intestinal commensal-triggered bystander effects to modulate netrin-1 production and promote colorectal carcinogenesis. To further investigate this potential mechanism we measured TNF-α production by E. faecalis-infected macrophages and noted marked increases in vitro and in colonic macrophages from Il10−/− mice colonized with E. faecalis . This finding was associated with colonic crypt hyperplasia and increased netrin-1 production. During the in vitro infection of macrophages by E. faecalis inhibition of NF-κB or blocking Tnfrsf1b, but not Tnfrsf1a, abrogated this increase in netrin-1. Finally, Tnfrsf1b receptor signaling resulted in the activation of anti-apoptotic survival proteins. The pro-proliferative consequences of netrin-1 confirm TNF-α as an important mediator of macrophage-induced bystander effects. These findings substantially add to our understanding of the mechanisms by which intestinal commensals contribute to autochthonous carcinogenesis in the colon.
Materials and Methods
Cell lines and bacteria
YAMC primary mouse colon epithelial cells (obtained from the Ludwig Institute for Cancer Research, New York in 2007). YAMC cells were last authenticated in 2011 by continuous growth at 33°C, senescence at 39.5°C, and PCR amplification of the transforming SV40 large T antigen. RAW264.7 cells were obtained from the American Type Culture Collection in 2003 and not further authenticated. Both cell lines were grown as previously described (8, 31). E. faecalis strain OG1RFSS was spontaneously derived from OG1RF—a protease-positive, Gram-positive human commensal—was grown in vitro as previously described (7, 8). A dual-chamber co-culture system for exposing YAMC cells to macrophages was used as previously described (8).
Assay for TNF-α
RAW264.7 murine macrophages were infected with sham or E. faecalis OG1RF at a multiplicity of infection (MOI) of 1000. Supernatants were collected over 72 hrs and TNF-α concentration determined using an ELISA kit according to the manufacturer’s instructions (eBioscience, San Diego, CA) with protein content normalized using recombinant mouse TNF-α (Cell Signaling Technology, Danvers, MA). NF-κB was irreversibly blocked using BAY11-7085 as a selective inhibitor of IκB-α phosphorylation (Santa Cruz Biotechnology, Santa Cruz, CA) (32).
Gene silencing
Gene expression was silenced using Tnfrsf1b siRNA with mouse specific reagents (Santa Cruz Biotechnology) or scrambled pooled non-targeting siRNA (Ambion, Austin, TX). Transient transfections were performed using lipofectamin 2000 reagent according to the manufacturer (Invitrogen, Camarillo, CA) and gene silencing confirmed by Western blot.
Western blotting
Protein extraction and Western blotting were performed as previously described using antibody to netrin-1 (Neuromics, Edina, MN); Tnfrsf1a, Tnfrsf1b, p-NF-κB p65, and cleaved caspase-3 (Cell Signaling Technology) (9). Murine β-actin antibody was purchased from BioVision (Milpitas, CA).
RT-PCR
Total RNA was extracted from YAMC cells using the NucleoSpin RNA II Kit (BD Biosciences, Palo Alto, CA) and cDNA synthesized using TaqMan Reverse Transcription Reagents according to manufacturer’s instructions (Applied Biosystems, Bedford, MA). Primers and PCR product sizes are listed in the Supplementary Table. PCR was performed by preheating to 94°C for 1 min followed by 30 cycles at 94°C for 20 sec, 56°C for 20 sec, and 72°C for 30 sec. PCR products were separated in a 2.5% agarose gel.
Apoptosis assay
Apoptosis was assessed by flow cytometry using the Annexin V FITC apoptosis detection kit according to the manufacturer’s instructions (EMD Biosciences) and quantified by flow cytometry (CellQuest Pro, San Jose, CA). The effect of TNF-α-induced netrin-1 expression on colonic epithelial cells was determined using YAMC cells after silencing Tnfrsf1b, or using cells treated with scrambled pooled non-targeting siRNA, 24 hrs following exposure to 100 ng/ml TNF-α. For each sample, >10,000 events were collected and groups compared using the Student’s t-test.
Colonization of Il10−/− mice
Conventionally housed Il10−/− mice develop aggressive enterocolitis (33). These same mice, when housed in specific-pathogen-free or germ-free environments show no pathology (34), with many commensals or mixtures of commensals failing to cause inflammation or cancer (34). Colonization with E. faecalis induces progressive colitis that results in colorectal cancer (14, 15). E. faecalis specifically triggers macrophages while many other intestinal commensals do not (5, 14, 15).
Specific pathogen-free Il10−/− knockout mice (C57BL, Jackson Laboratory, Bar Harbor, ME) were colonized with E. faecalis OG1RFSS as previously described (7). In brief, OG1RFSS expresses high-level resistance to spectinomycin and streptomycin (i.e, >500 mg L−1). When these antibiotics are administered together in drinking water, sensitive intestinal gram-positive and gram-negative microaerophilic intestinal flora are suppressed permitting OG1RFSS to colonize. These antibiotics are inactive against anaerobes (that comprise >99% of the microbiota). The use of two antibiotics reduces the probability of spontaneous resistance or superinfection by the minority population of microaerophilic intestinal microbiota. As these bacteria are sensitive to these antibiotics, the probability of spontaneous double-resistance events is extremely low (estimated at <10−12 per bacterium or <1 resistance event per 103 mice). This strategy, when coupled with contact barrier precautions and autoclaved food/water, permits long-term colonization. These antibiotics are not absorbed and so mice show no ill effect. The protocol was initiated for 10 week old male mice by the orogastric administration of OG1RFSS after 2 days on antibiotics. High density fecal colonization developed within 1 week at an average of 109 colony forming units per gram for mice receiving OG1RFSS. E. faecalis was never recovered from mice receiving sham. Colonization was checked every 8 weeks and at necropsy and remained unchanged. Colon biopsies were obtained at necropsy after 3 and 9 months of colonization. Crypt lengths in photomicrographs of colon sections were measured using a Photoshop CS3 ruler tool (Adobe, version 10.0.1) and converted to microns by magnification (100X). At least 50 crypts from three mice per group were analyzed. Protocols were approved by the University of Oklahoma Health Sciences Center and Oklahoma City Department of Veterans Affairs animal studies committees.
Immunochemistry
Immunohistochemical staining was performed as previously described (9). Primary antibodies included F4/80 and anti-mouse TNF-α (eBioscience); netrin-1, TNF-R1 (Tnfrsf1a), TNF-R2 (Tnfrsf1b), and PCNA (Santa Cruz Biotechnology); nuclear localization sequence (NLS)-specific anti-NF-κB/p65 (Rockland Immunochemicals, Gilbertsville, PA); and cleaved caspase-3 (Cell Signaling). Secondary antibodies were conjugated to horseradish peroxidase and visualized using DAB Enhanced liquid substrate with nuclei counterstained using Mayer’s hematoxylin solution (Sigma, St. Louis, MO).
For Immunofluorescence tissue sections were incubated in 10% normal serum with 0.3 M glycine and 0.1% Tween in PBS for 30 min. A 1:50 dilution of anti-mouse TNF-α antibody and 1:100 dilution of anti-mouse F4/80 antigen FITC antibody were used as primary antibodies (eBioscience) and anti-rat IgG Texas Red (Santa Cruz Biotechnology) as secondary antibody. Nuclei were counterstained with 4,6-diamidino-2-phenylindole prior to laser-scanning confocal microscopy (Carl Zeiss Microscopy, Thornwood, NY).
Statistical analysis
Data were expressed as means with standard deviations. Student’s t test was used for comparison between experimental and control groups. P values less than 0.05 were considered statistically significant.
Results
Macrophages produce TNF-α in E. faecalis-colonized Il10−/− mice
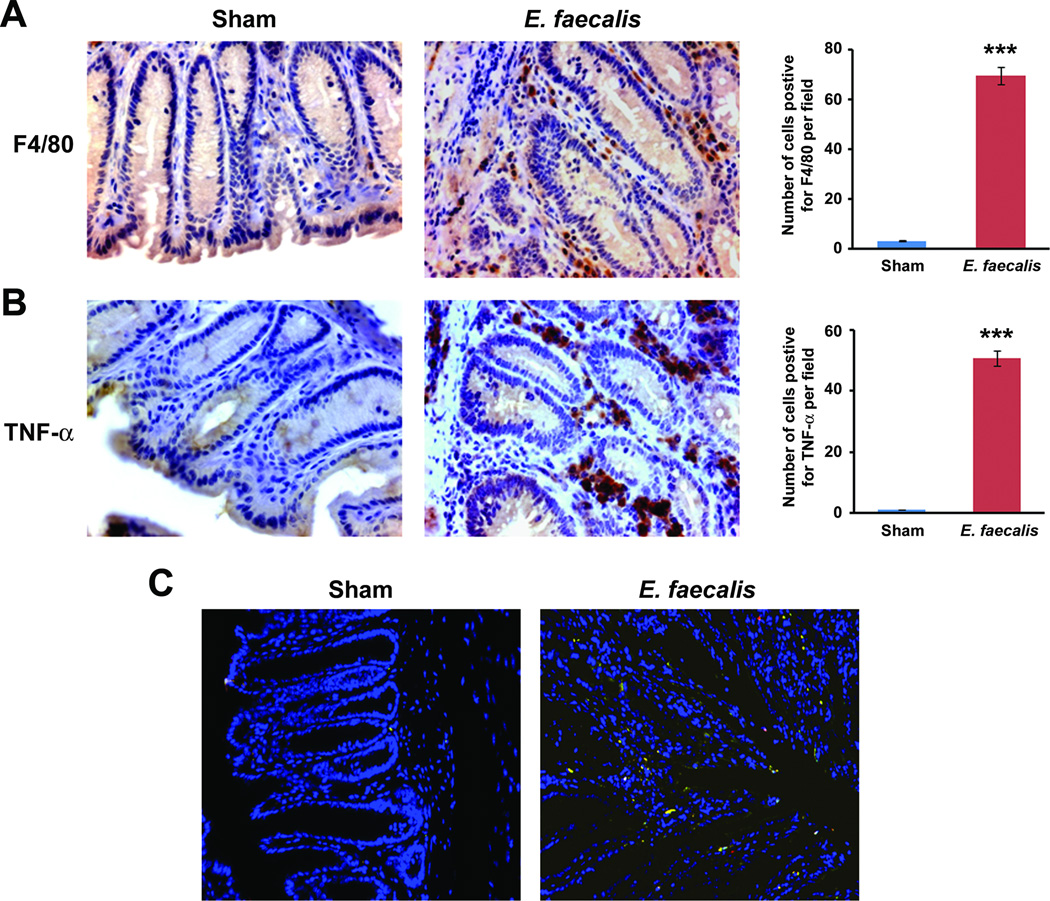
TNF-α is a key mediator of inflammation that contributes to carcinogenesis through the activation of NF-κB (20, 22). To investigate whether TNF-α was involved in E. faecalis-triggered and macrophage-induced bystander effects, we noted, as expected, increased production of TNF-α by E. faecalis-infected macrophages (Supplementary Fig. S1). Untreated macrophages produced scant TNF-α. We colonized Il10−/− mice with E. faecalis, or administered sham, and biopsied colons after 3 and 9 months. We found inflammation and dysplasia in the distal colons of colonized mice at 3 months and cancer at 9 months; no abnormal pathology was noted among shams. Using F4/80 staining, we found significantly increased numbers of macrophages in the lamina propria of colons compared to shams. For colonized mice, these same cells showed intense staining for TNF-α compared to sham (P < 0.001, Fig. 1A 1B, and Supplementary Fig. S2). Immunofluorescence confirmed TNF-α production in lamina propria macrophages (Fig. 1C), suggesting activation of these cells by colonizing E. faecalis.
Figure 1. E. faecalis-infected macrophages produce TNF-α.
A Immunohistochemical staining of colons from Il10−/− mice colonized with E. faecalis for 9 months; increased numbers of macrophages (F4/80 positive, brown) and B, staining for TNF-α (brown) are noted in the lamina propria (40X), but not seen in shams. Numbers of F4/80 and TNF-α-positive cells per 20X field are increased in colons from Il10−/− mice colonized with E. faecalis for 9 months compared to sham (***P < 0.001). C Immunofluorescence staining of colon biopsies from E. faecalis colonized Il10−/− mice using F4/80 (red) and TNF-α (green) confirm co-localization of TNF-α to macrophages (yellow).
E. faecalis-infected macrophages induce netrin-1
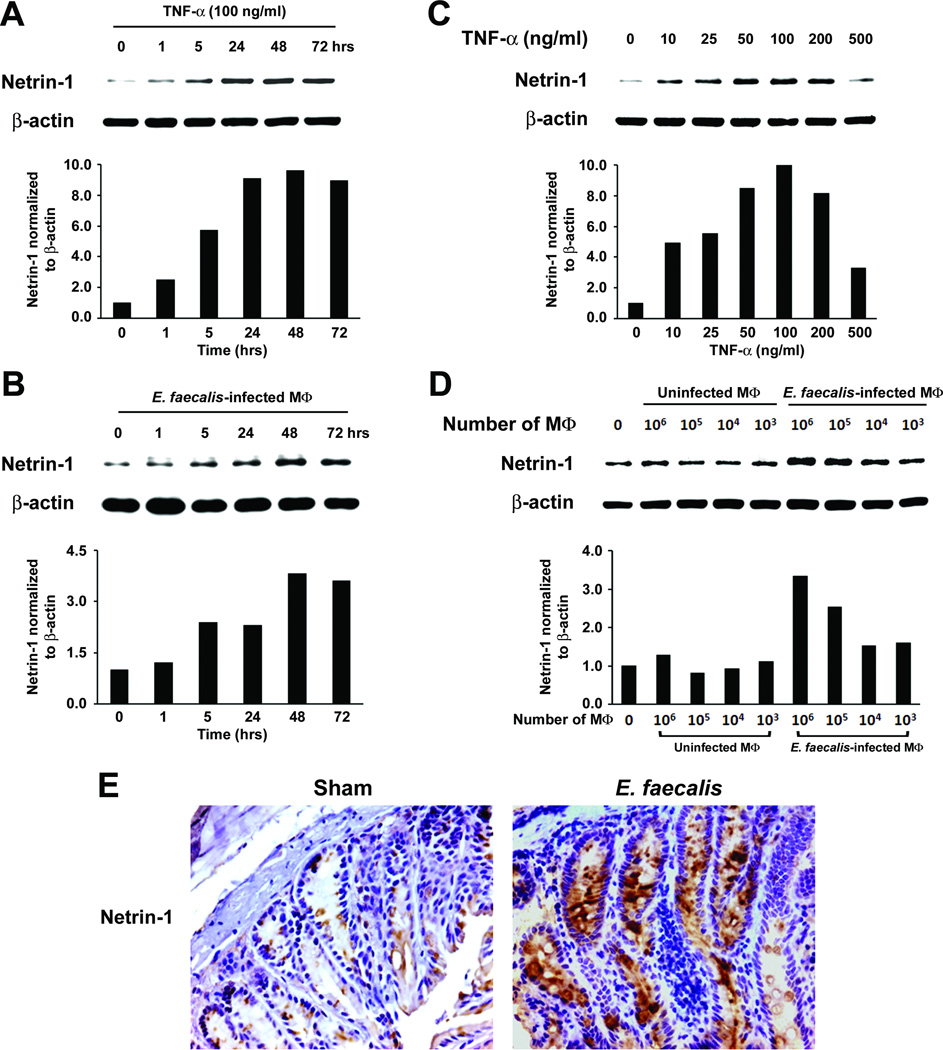
To explore mechanisms by which TNF-α might contribute to bystander effects, we measured netrin-1 production in YAMC epithelial cells treated with TNF-α. We noted substantial increases after only 5 hrs that persisted through 72 hrs (Fig. 2A). Similar results were found following exposure of these cells to E. faecalis-infected macrophages in the dual-chamber co-culture system (Fig. 2B). Increases in netrin-1 occurred in a dose-dependent fashion except for the highest TNF-α concentration tested (Fig. 2C). Finally, production of netrin-1 by YAMC cells increased following exposure to greater numbers of infected macrophages (Fig. 2D). RT-PCR demonstrated increased Ntn1 expression by TNF-α and E. faecalis-infected macrophages (Supplementary Fig. S3A–D). Colon biopsies from E. faecalis colonized Il10−/− mice showed intense staining for netrin-1 in epithelial cells compared to sham (Fig. 2E and Supplementary Fig. S4), and were consistent with in vitro results obtained using YAMC cells exposed to E. faecalis-infected macrophages.
Figure 2. TNF-α increases netrin-1.
A, YAMC cells increase netrin-1 production when treated with 100 ng/ml TNF-α. B In the dual-chamber co-culture system, E. faecalis-infected macrophages show peak production of netrin-1 at 48–72 hrs. C Netrin-1 production increases with higher concentrations of TNF-α except at 500 ng/ml when inhibition is noted. D, E. faecalis-infected macrophages similarly increase netrin-1 in a dose-dependent fashion. E, Immunohistochemical staining shows increased netrin-1 in colonic epithelial cells for Il10−/− mice colonized with E. faecalis for 9 months compared to sham (40X).
TNF-α induces netrin-1 production
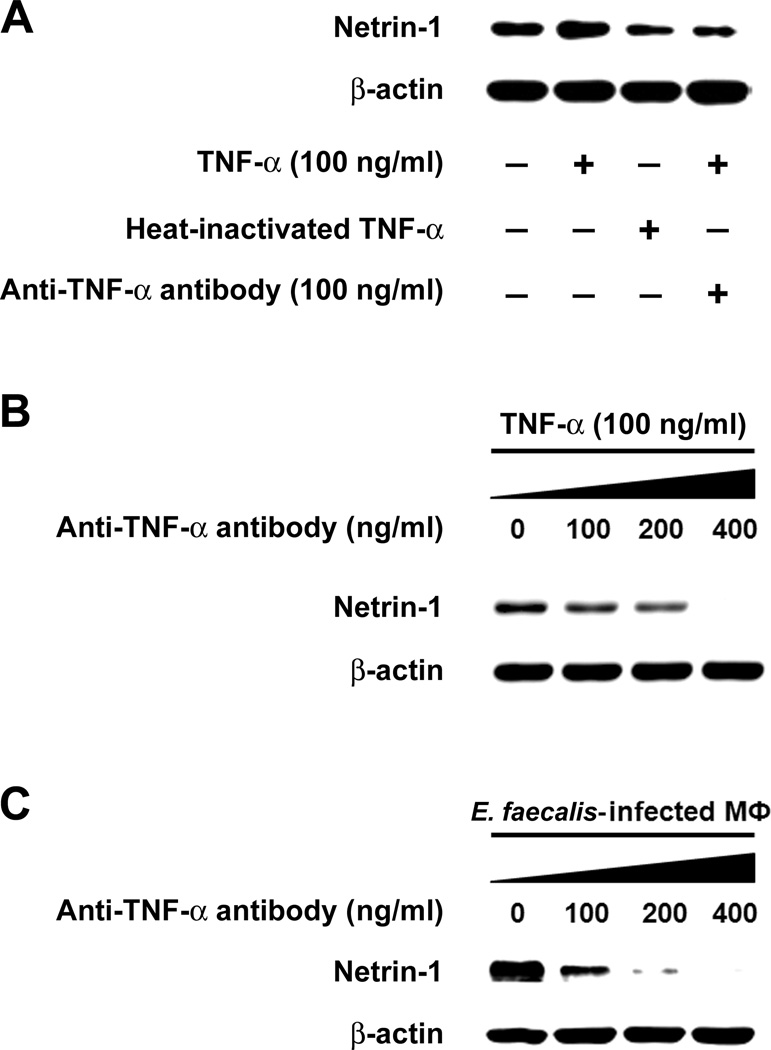
To confirm these results, we measured netrin-1 production by YAMC epithelial cells that had been treated with TNF-α and anti-TNF-α antibody or heat-denatured TNF-α. Both controls led to significantly decreased netrin-1 production (Fig. 3A). Anti-TNF-α antibody blocked netrin-1 production in a dose-dependent manner (Fig. 3B). Of note, the scant netrin-1 produced by untreated YAMC cells that was blocked by anti-TNF-α antibody ( Supplementary Fig. S5), suggesting in vitro autocrine production of TNF-α by YAMC cells. Finally, in the dual-chamber co-culture system, this antibody was able to completely block netrin-1 production in YAMC cells exposed to E. faecalis-infected macrophages (Fig. 3C), confirming specificity for this cytokine on netrin-1 production via a bystander effect.
Figure 3. Anti-TNF-α blocks TNF-α-induced netrin-1 production.
A Treatment of YAMC cells with murine TNF-α increases netrin-1 production; heat-inactivation of TNF-α and anti-TNF-α antibody block netrin-1 compared to control. B Anti-TNF-α antibody blocks netrin-1 by YAMC cells in a dose-dependent manner. C In the dual-chamber co-culture system, increasing anti-TNF-α antibody similarly blocks netrin-1 production by YAMC cells exposed to E. faecalis-infected macrophages cells.
TNF-α promotes netrin-1 via NF-κB
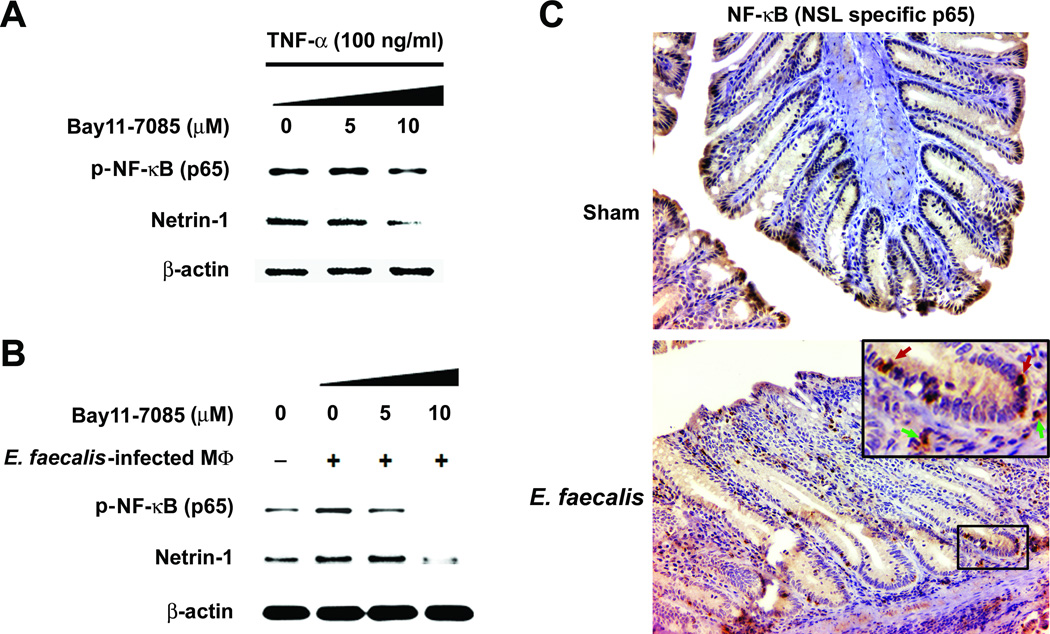
Activation of NF-κB contributes to tumor formation, at least in part, by inducing anti-apoptotic factors in epithelial cells. To establish whether netrin-1 was induced through NF-κB activation, we exposed YAMC cells with an irreversible inhibitor of NF-κB and followed by TNF-α treatment. After 24 hrs, netrin-1 significantly decreased (Fig. 4A), a finding in agreement with previous observations showing Ntn1 as an NF-κB target (28). Similar results were seen using the dual-chamber co-culture system (Fig. 4B). We next performed immunohistochemical staining on colon sections from Il10−/− mice colonized with E. faecalis for 3 or 9 months using antibody to the nuclear localization signal of p65 (RelA). Lamina propria macrophages and epithelial cells stained intensely for this marker compared to sham (Fig. 4C and Supplementary Fig. S6), a finding consistent with the concept that NF-κB activation results in TNF-α production by colonic macrophages and, via bystander effects, increased netrin-1 production by epithelial cells.
Figure 4. TNF-α induces netrin-1 production via NF-κB activation.
A TNF-α-induced netrin-1 decreases in YAMC cells at 24 hrs when pre-treated with Bay11-7085; NF-κB activation decreases using antibody to nuclear localization signal of NF-κB/p65. B Similarly, Bay11-7085 blocks netrin-1 in YAMC cells exposed to E. faecalis-infected macrophages using the dual-chamber co-culture system. C Colon macrophages from Il10−/− mice colonized with E. faecalis for 9 months (bottom, inset, green arrows, 40X) stain intensely for nuclear localization sequence of NF-κB/p65 (20X); epithelial cells also stain strongly (bottom, inset, red arrows); in contrast, weak or no staining is seen for sham-treated mice (top).
TNF-α induces Tnfrsf1b
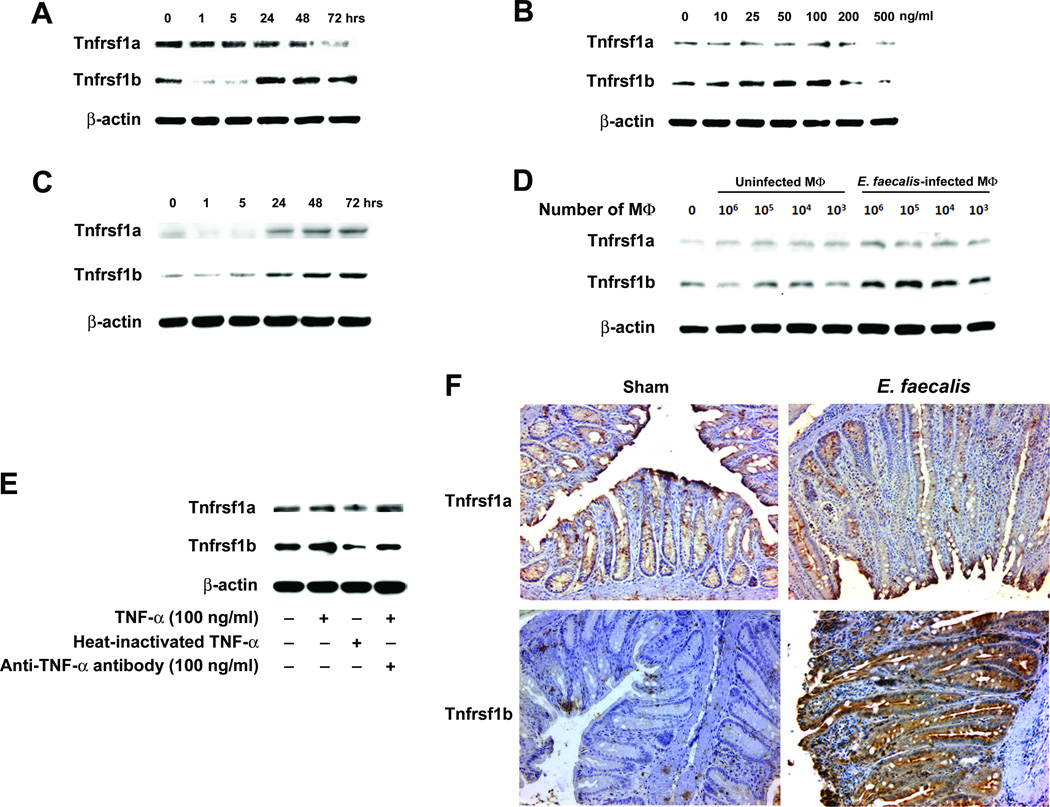
The activation of NF-κB in intestinal epithelial cells by TNF-α through Tnfrsf1b receptor signaling has been previously linked to carcinogenesis (24). To determine whether TNF-α binding to epithelial cells was involved in increased netrin-1 production, we initially assayed YAMC cells for Tnfrsf1a and Tnfrsf1b and noted strong induction of Tnfrsf1b by TNF-α (Fig. 5A and B and Supplementary Fig. S3A and C). In contrast, expression of Tnfrsf1a was slightly reduced by treatment with TNF-α. Similarly, Tnfrsf1b production increased in YAMC cells co-cultured with E. faecalis-infected macrophages while Tnfrsf1a only slightly increased (Fig. 5C and D). A similar change, however, was not seen in mRNA (Supplementary Fig. 3B and D), suggesting post-transcriptional modifications of this receptor due to TNF-α and/or other cytokines from E. faecalis-infected macrophages. Induction of Tnfrsf1b was specific for TNF-α as anti-TNF-α antibody, or heat-inactivated TNF-α, blocked the increase Tnfrsf1b expression (Fig. 5E). Colon biopsies from Il10−/− mice colonized with E. faecalis confirmed these in vitro findings. Increased staining for Tnfrsf1b was noted in colonic epithelial cells compared to sham while there were no changes in Tnfrsf1a staining (Fig. 5F). These results are consistent with colonization by E. faecalis causing enhanced Tnfrsf1b receptor expression on colonic epithelial cells.
Figure 5. TNF-α increases Tnfrsf1b receptors.
A Expression of Tnfrsf1b receptors increases in YAMC cells at 100 ng/ml TNF-α at 24, 48, and 72 hrs whereas Tnfrsf1a receptors are slightly reduced.B At 48 hrs, TNF-α concentrations ≤100 ng/ml increase Tnfrsf1b in YAMC cells in a dose–dependent fashion. No significant increases are seen for Tnfrsf1a.C, Tnfrsf1b production increases for YAMC cells co-cultured with E. faecalis-infected macrophages at 24, 48, and 72 hrs. D, At 48 hrs, Tnfrsf1b production increases in YAMC cells co-cultured with E. faecalis-infected macrophages in a dose-dependent manner.E, Heat-inactivated TNF-α and anti-TNF-α antibody block TNF-α induction of Tnfrsf1b compared to control. F Immmunohistochemical staining shows increased Tnfrsf1b in colonic epithelial cells from Il10−/− mice colonized with E. faecalis for 9 months compared to sham; no change is seen for Tnfrsf1a (20X).
Tnfrsf1b promotes TNF-α-induced apoptosis
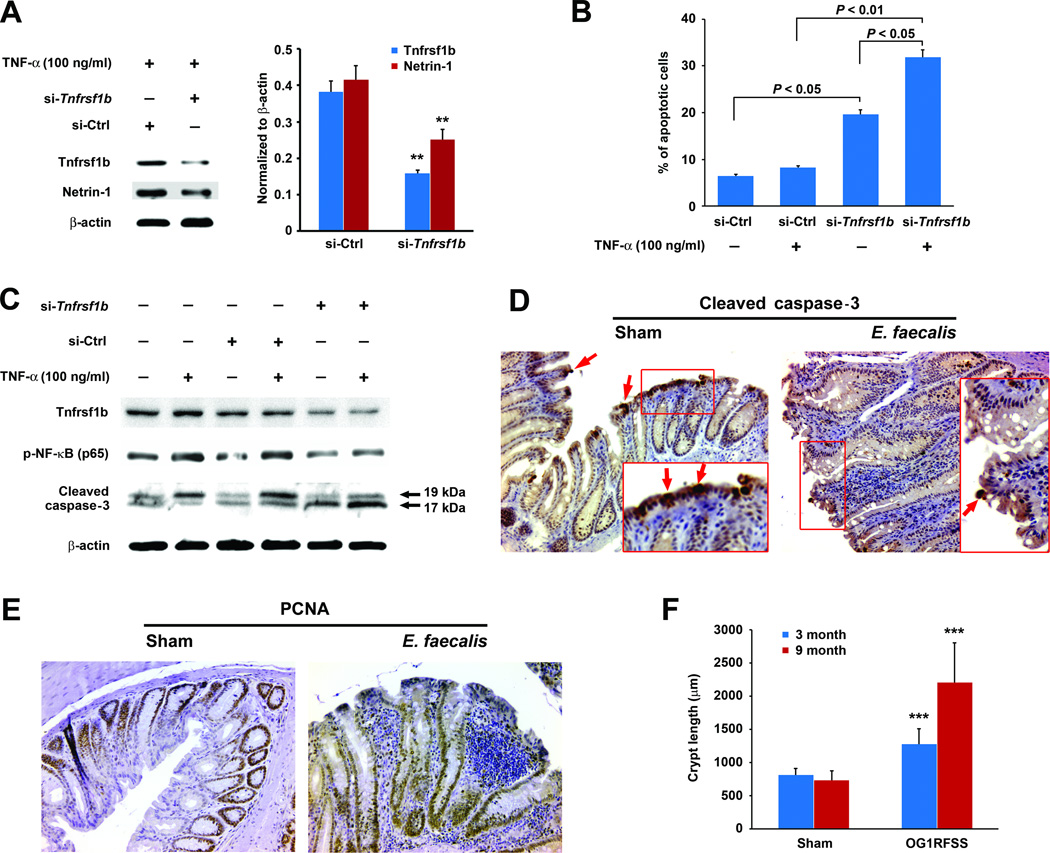
The primary mechanism by which netrin-1 promotes colorectal carcinogenesis is through the pro-proliferative modulation of apoptosis (29, 30, 35). To establish whether TNF-α signaling through Tnfrsf1b receptors was responsible for the enhanced production of netrin-1 by epithelial cells, we silenced Tnfrsf1b in YAMC cells using Tnfrsf1b-specific siRNA. TNF-α treatment of these cells showed a 60% decrease in Tnfrsf1b expression and concomitant 40% decrease in netrin-1 compared to cells transfected with non-targeting siRNA (Fig. 6A). This observation confirmed the predominant role of Tnfrsf1b receptor in TNF-α-induced netrin-1 production for these primary colonic epithelial cells.
Figure 6. TNF-α signaling through Tnfrsf1b blocks apoptosis and promotes proliferation.
A Tnfrsf1b receptors and netrin-1 are reduced 60% and 40% (**P < 0.01), respectively, in YAMC cells following transfection with Tnfrsf1b-specific siRNA (si-Tnfrsf1b) compared to non-targeting siRNA (si-Ctrl) in the presence of 100 ng/ml TNF-α. B Flow cytometry shows increased numbers of apoptotic cells for Tnfrsf1b-silenced YAMC cells compared to cells transfected with non-targeting siRNA; the proportion of apoptotic YAMC cells increases at 24 hrs following treatment with 100 ng/ml TNF-α for Tnfrsf1b-silenced cells compared to cells transfected with non-targeting siRNA. C Phosphorylated NF-κB (p65) decreases in Tnfrsf1b-silenced cells compared to cells transfected with non-targeting siRNA. No increase in p-NF-κB (p65) is seen in these cells following treatment with TNF-α. Cleaved caspase-3 increases in Tnfrsf1b-silenced cells compared to cells transfected with non-targeting siRNA and with additional increases following treatment with 100 ng/ml TNF-α. D, Immmunohistochemical staining shows decreased cleaved caspase-3 at the tops of colonic crypts (arrows) from colon sections for Il10−/− mice colonized with E. faecalis for 9 months compared to sham (20X). E, PCNA staining increases in epithelial cells for Il10−/− mice colonized with E. faecalis for 9 months compared to sham (20X). F Colon crypt length increases for mice colonized with E. faecalis at 3 (blue) and 9 months (red) compared to sham (***P < 0.001).
We next determined the effect of Tnfrsf1b receptor signaling on apoptosis in YAMC cells. Tnfrsf1b-silenced YAMC cells were stained with annexin V-FITC. As shown in Fig. 6B following treatment with TNF-α, the percentage of apoptotic cells significantly increased for Tnfrsf1b-silenced cells compared to cells transfected with non-targeting siRNA (31.9 ± 2.0 vs. 8.2 ± 0.7, P < 0.01), supporting induction of netrin-1 by TNF-α through the action of Tnfrsf1b. Notably, the percentage of apoptotic cells for Tnfrsf1b-silenced cells also increased in the absence of TNF-α compared to cells transfected with non-targeting siRNA (19.7 ± 1.2 vs. 6.4 ± 3.2, P < 0.05). This might be due to scant production of TNF-α by YAMC cells ( Supplementary Fig. S5).
Finally, to investigate whether netrin-1 contributed to cellular proliferation through anti-apoptotic signaling, we measured p-NF-κB (p65) and cleaved caspase-3 in Tnfrsf1b-silenced YAMC cells. We found decreased p-NF-κB (p65) in Tnfrsf1b-silenced cells compared to cells transfected with non-targeting siRNA and no additional induction by TNF-α, implying impaired NF-κB activation when Tnfrs1b was silenced. Moreover, we noted increased cleaved caspase-3 compared to cells transfected with non-targeting siRNA and additional increases when Tnfrsf1b-silenced cells were treated with TNF-α (Fig. 6C). We next stained colon crypts from E. faecalis colonized Il10−/− mice for cleaved caspase-3 and noted marked decreases in staining at the tops of crypts (Fig. 6D). In addition, PCNA staining was increased in colonic epithelial cells (Fig. 6E). Finally, these pro-proliferative effects were associated with colon crypt hyperplasia as measured by increased crypt length in mice colonized with E. faecalis for 3 and 9 months compared to sham (Fig. 6F).
Discussion
Substantial credible evidence identifies inflammation as pivotal to the initiation and promotion of many common tumors including colorectal cancer (17, 18). However, interactions among diverse factors involved in colorectal carcinogenesis, including host determinants (e.g., age and genetic predisposition), dietary composition, intestinal microbiota, and pharmacological intervention, have challenged mechanistic studies in this field. Recently, we used Il10−/− mice colonized with E. faecalis to investigate bystander effects as a model for endogenous (or autochthonous) carcinogenesis triggered by a human commensal (5, 8, 9). In this model, local inflammation generates chromosomal instability and promotes colorectal cancer through bystander effects.
E. faecalis is particularly relevant to the study of colon carcinogenesis because the microorganism belongs to a small number of human commensals, including E. coli and Bacteroides fragilis that promote colorectal adenomas and cancer in murine models (3, 4, 15). IL-10−/− mice are attractive for modeling bacterial triggers because loss of this cytokine allows colonic macrophages to be activated by the intestinal microbiota (33, 36). These triggers, however, appear to be highly specific with pathology only developing with E. faecalis or E. coli are used and not with other members of the pathogen-free flora (34, 37). Finally, the IL-10 knockout model avoids potent exogenous carcinogens (e.g. azoxymethane) and allows dietary control. One potential limitation for conventionally housed Il10−/− mice colonized with E. faecalis is the need to use antibiotics to maintain high-density colonization. Without such pressure, exogenous E. faecalis only transiently colonizes mice. Potential side-effects, however, are minimized by using oral non-absorbable antibiotics and are further controlled by treating shams similarly.
Our study further expands the conceptual basis for macrophage-induced bystander effects. This phenomenon was initially observed by radiation biologists who found chromosomal instability in non-irradiated cells exposed to diffusible mediators from irradiated cells (11). Microbeam irradiation experiments, where only the cellular cytoplasm is targeted, clearly demonstrate that DNA damage is not necessary to generate these diffusible mediators. The first reports were from studies of individuals exposed to low-linear-energy transfer whole body irradiation (e.g. x-rays) (38, 39). High linear-energy-transfer radiation (e.g. α-particles), however, appears more efficient at producing mediators. Bystander effects occur in vivo as has been confirmed in experiments using syngenic mice (40, 41). Finally, both radiation-induced and E. faecalis-triggered bystander effects depend on cyclooxygenase-2 (8, 42), and may well provide insights into the established role for this enzyme in carcinogenesis (43).
TNF-α and NF-κB are key contributors to inflammation-associated tumorigenesis (20, 44). In the Mdr2-knockout model of hepatocellular carcinoma, TNF-α activates NF-κB and is essential for tumor formation (20). Similarly, blocking TNF-α in a carcinogen-colitis model of colorectal cancer reduces tumor formation (19). TNF-α may contribute to carcinogenesis by inducing apoptosis. In our system, TNF-α suppressed apoptosis in YAMC cells through Tnfrsf1b receptor signaling. Although we saw markedly increased Tnfrsf1b receptor expression in the colonic epithelial cells of E. faecalis colonized Il10−/− mice, a direct linkage of TNF-α signaling through these receptors awaits further investigation. NF-κB in epithelial cells was activated in vitro and in vivo by E. faecalis-triggered (and TNF-α-mediated) bystander effects. We found that NF-κB led to induction of netrin-1, a known transcriptional target for this pleiotrophic transcription factor (30). These findings are also consistent with previous results that show netrin-1 is acutely upregulated by superoxide-producing E. faecalis (12).
Netrin-1 is an oncogene that is overexpressed in 70% of colorectal adenocarcinomas from patients with inflammatory bowel disease (30). Approximately one-quarter of sporadic colorectal cancers overexpress netrin-1 (28, 30). The loss of netrin-1 receptors that act as tumor suppressors, however, is more common and found in the majority of these cancers. We noted that E. faecalis-infected macrophages specifically induced netrin-1 in a time- and dose–dependent fashion and, compared to shams, was visible by immunostaining in the colonic epithelial cells of Il10−/− mice colonized with E. faecalis . These findings indicate that, in addition to 4-hydroxy-2-nonenal, a known mutagen and spindle poison (5), TNF-α also contributes to macrophage-induced bystander effects via the anti-apoptotic action of netrin-1.
The contributions of TNF-α toward carcinogenesis are multifactoral and include initiation and promotion of malignant cells, development of angiogenesis, and modulation of adaptive immunity including responses to hormones and chemotherapeutics (18, 20–22, 44). Many of these cellular events are orchestrated by NF-κB. We investigated signaling pathways for TNF-α as a potential mediator of bystander effects and noted that anti-TNF-α antibody blocked the nuclear localization of NF-κB/p65. In addition, the NF-κB inhibitor Bay11-7085 decreased netrin-1, presumably by blocking TNF-α-induced phosphorylation of IκBα (32). These findings agree with previous reports that show NF-κB drives netrin-1 expression (28).
TNF-α can bind at least two receptors on colonic epithelial cells. Tnfrsf1a receptors possess a cytoplasmic death domain that rapidly engages apoptotic signaling, whereas Tnfrsf1b receptors recruit adaptor proteins that activate NF-κB and promote gene transcription to protect against apoptosis. The importance of TNFRSF1B in carcinogenesis was recently noted in a large cohort study where cleaved soluble receptor was associated with an increased risk for colorectal cancer (25). We investigated changes in Tnfrsf1a and Tnfrsf1b on YAMC cells after treatment with TNF-α and found that only Tnfrsf1b was induced. Similarly, E. faecalis colonization of Il10−/− mice only appeared to increase Tnfrsf1b expression on colonic epithelial cells. For the macrophage-induced bystander effect, Tnfrsf1b receptor signaling was specific for netrin-1 since silencing Tnfrsf1b decreased production and increased apoptosis. However, we cannot entirely exclude the possibility that increased apoptosis may have resulted from pro-apoptotic signaling through Tnfrsf1a receptors.
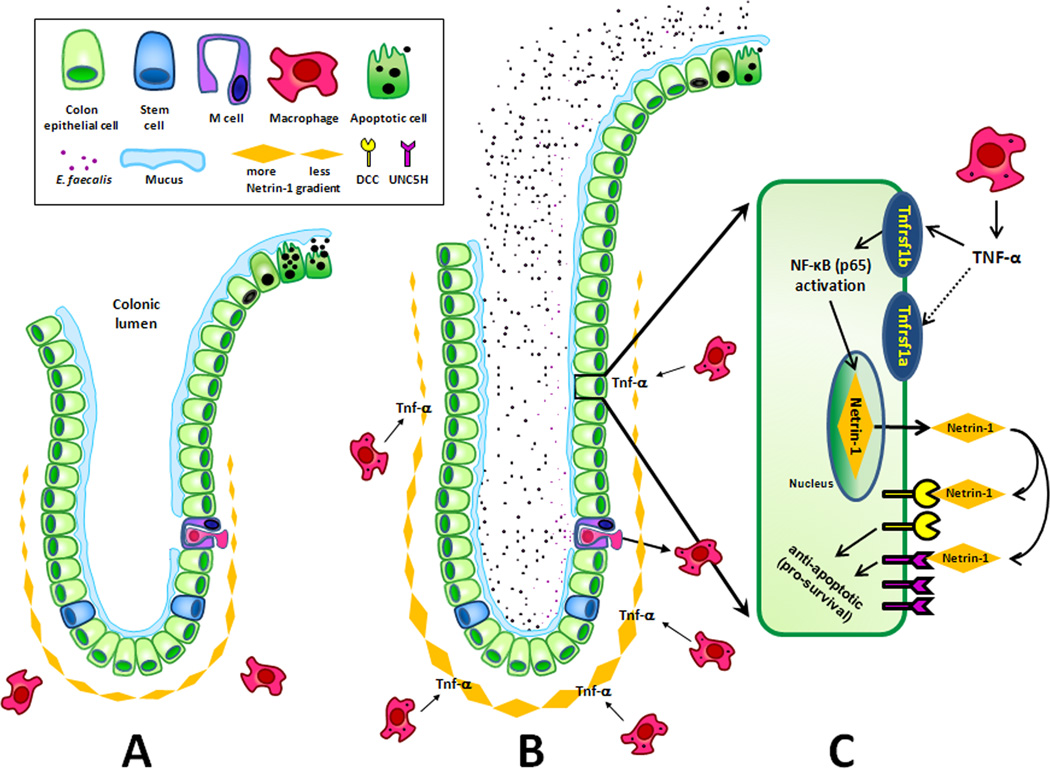
In this study we found that TNF-α acted as a diffusible mediator for macrophage-induced bystander effects (Fig. 7). This inflammatory cytokine was triggered by E. faecalis and led to colonic epithelial cell proliferation through the anti-apoptotic action of netrin-1 (35). Netrin-1 was up-regulated through Tnfrsf1b receptor signaling and activation of NF-κB. Netrin-1 is an anti-apoptotic ligand that binds DCC and the UNC5H family of dependence receptors and in murine models promotes progression of colon adenomas to high grade cancers (29, 30). In conclusion, our findings show that TNF-α is an important mediator for macrophage-induced bystander effects. Through Tnfrsf1b receptor signaling initiated by E. faecalis-infected macrophages, TNF-α leads to increased epithelial cells production of netrin-1 causing colonic crypt hyperplasia and a reduction in epithelial cell apoptosis. TNF-α is a diffusible mediator for macrophage-induced bystander effects in the colon and provides additional potential targets for the prevention of colorectal cancer.
Figure 7. TNF-α promotes netrin-1 through bystander effects.
A Normal colon crypt: stem cells produce the terminally differentiated epithelial cells within a crypt; netrin-1 is constitutively produced by epithelial cells at crypt base and blocks apoptosis until cells migrate to the top of the crypt; macrophages are quiescent in the absence of a specific bacterial trigger. B In Il10−/− mice, colonizing E. faecalis translocate intact epithelium (presumably through M cells as overlying mucus poses a barrier to commensal bacterial interaction with epithelial cells) and activates tissue macrophages to produce TNF-α. C Inset of epithelial cell: TNF-α mediates bystander effects by binding Tnfrsf1b receptors on epithelial cells to activate NF-κB and increase netrin-1; netrin-1 acts in an autocrine and/or paracrine fashion to bind dependence receptors (e.g., DCC or UNC5H) and provides anti-apoptotic signals for epithelial cells resulting in elongated crypts.
Supplementary Material
Acknowledgments
We thank Jim Henthorn in the Flow Cytometry Laboratory at the University of Oklahoma Health Sciences Center and Robert Whitehead at Vanderbilt University and Ludwig Institute for their gift of YAMC cells.
Funding:Supported by National Institutes of Health grant CA127893 (to M.M.H.), the Oklahoma Center for the Advancement of Science and Technology grant HR10-032 (to X.W.), and funds from the Frances Duffy Endowment.
Footnotes
Author Contributions: Mark M. Huycke and Yonghong Yang: study concept and design, analysis and interpretation of data, writing of the manuscript; Yonghong Yang, Xingmin Wang, Danny R. Moore: acquisition of data; Stanley A. Lightfoot: analysis and interpretation of data.
Conflicts of Interest:The authors disclose no conflicts.
References
- 1.Burnett-Hartman AN, Newcomb PA, Potter JD. Infectious agents and colorectal cancer: a review of Helicobacter pylori, Streptococcus bovis, JC virus, and human papillomavirus. Cancer Epidemiol Biomarkers Prev. 2008;17:2970–2979. doi: 10.1158/1055-9965.EPI-08-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinicrope FA. Sporadic colorectal cancer: an infectious disease? Gastroenterology. 2007;132:797–801. doi: 10.1053/j.gastro.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuevas-Ramos G, Petit CR, Marcq I, Boury M, Oswald E, Nougayrëde JP. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc Natl Acad Sci USA. 2010;107:11537–11542. doi: 10.1073/pnas.1001261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Yang Y, Moore DR, Nimmo SL, Lightfoot SA, Huycke MM. 4-Hydroxy-2-nonenal mediates genotoxicity and bystander effects caused by Enterococcus faecalis-infected macrophages. Gastroenterology. 2012;142:543–551. doi: 10.1053/j.gastro.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SEER Cancer Statistics Review, 1975–2008 [cited based on November 2010 SEER data submission, posted to the SEER web site. 2011 Available from: http://seer.cancer.gov/csr/1975_2008/
- 7.Huycke MM, Abrams V, Moore DR. Enterococcus faecalis produces extracellular superoxide and hydrogen peroxide that damages colonic epithelial cell DNA. Carcinogenesis. 2002;23:529–536. doi: 10.1093/carcin/23.3.529. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Huycke MM. Extracellular superoxide production by Enterococcus faecalis promotes chromosomal instability in mammalian cells. Gastroenterology. 2007;132:551–561. doi: 10.1053/j.gastro.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Allen TD, May RJ, Lightfoot S, Houchen CW, Huycke MM. Enterococcus faecalis induces aneuploidy and tetraploidy in colonic epithelial cells through a bystander effect. Cancer Res. 2008;68:9909–9917. doi: 10.1158/0008-5472.CAN-08-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huycke MM, Moore D, Joyce W, Wise P, Shepard L, Kotake Y, et al. Extracellular superoxide production by Enterococcus faecalis requires demethylmenaquinone and is attenuated by functional terminal quinol oxidases. Mol Microbiol. 2001;42:729–740. doi: 10.1046/j.1365-2958.2001.02638.x. [DOI] [PubMed] [Google Scholar]
- 11.Lorimore SA, Wright EG. Radiation-induced genomic instability and bystander effects: related inflammatory-type responses to radiation-induced stress and injury? A review. Int J Radiat Biol. 2003;79:15–25. [PubMed] [Google Scholar]
- 12.Allen TD, Moore DR, Wang X, Casu V, May R, Lerner MR, et al. Dichotomous metabolism of Enterococcus faecalis induced by haematin starvation modulates colonic gene expression. J Med Microbiol. 2008;57:1193–1204. doi: 10.1099/jmm.0.47798-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnett MP, McNabb WC, Cookson AL, Zhu S, Davy M, Knoch B, et al. Changes in colon gene expression associated with increased colon inflammation in interleukin-10 gene-deficient mice inoculated with Enterococcus species. BMC Immunol. 2011;11:39. doi: 10.1186/1471-2172-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balish E, Warner T. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am J Pathol. 2002;160:2253–2257. doi: 10.1016/S0002-9440(10)61172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SC, Tonkonogy SL, Albright CA, Tsang J, Balish EJ, Braun J, et al. Variable phenotypes of enterocolitis in IL-10 deficient mice monoassociated with two different commensal bacteria. Gastroenterology. 2005;128:891–906. doi: 10.1053/j.gastro.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Steck N, Hoffmann M, Sava IG, Kim SC, Hahne H, Tonkonogy SL, et al. Enterococcus faecalis metalloprotease compromises epithelial barrier and contributes to intestinal inflammation. Gastroenterology. 2011;141:959–971. doi: 10.1053/j.gastro.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 17.Terzič J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 19.Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, et al. Blocking TNF-α in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-κ functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 21.Yan B, Wang H, Rabbani ZN, Zhao Y, Li W, Yuan Y, et al. Tumor necrosis factor-α is a potent endogenous mutagen that promotes cellular transformation. Cancer Res. 2006;66:11565–11570. doi: 10.1158/0008-5472.CAN-06-2540. [DOI] [PubMed] [Google Scholar]
- 22.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 23.Thommesen L, Laegreid A. Distinct differences between TNF receptor 1- and TNF receptor 2-mediated activation of NF-κB. J Biochem Mol Biol. 2005;38 doi: 10.5483/bmbrep.2005.38.3.281. 281-189. [DOI] [PubMed] [Google Scholar]
- 24.Onizawa M, Nagaishi T, Kanai T, Nagano K, Oshima S, Nemoto Y, et al. Signaling pathway via TNF-α/NF-κB in intestinal epithelial cells may be directly involved in colitis-associated carcinogenesis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G850–G859. doi: 10.1152/ajpgi.00071.2008. [DOI] [PubMed] [Google Scholar]
- 25.Chan AT, Ogino S, Giovannucci EL, Fuchs CS. Inflammatory markers are associated with risk of colorectal cancer and chemopreventive response to anti-inflammatory drugs. Gastroenterology. 2011;140:799–808. doi: 10.1053/j.gastro.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990;4:61–85. doi: 10.1016/0896-6273(90)90444-k. [DOI] [PubMed] [Google Scholar]
- 27.Mehlen P, Delloye-Bourgeois C, Chédotal A. Novel roles for slits and netrins: axon guidance cues as anticancer targets? Nat Rev Cancer. 2011:188–197. doi: 10.1038/nrc3005. [DOI] [PubMed] [Google Scholar]
- 28.Paradisi A, Maisse C, Bernet A, Coissieux MM, Maccarrone M, Scoazec JY, et al. NF-κB regulates netrin-1 expression and affects the conditional tumor suppressive activity of the netrin-1 receptors. Gastroenterology. 2008;135:1248–1257. doi: 10.1053/j.gastro.2008.06.080. [DOI] [PubMed] [Google Scholar]
- 29.Mazelin L, Bernet A, Bonod-Bidaud C, Pays L, Arnaud S, Gespach C, et al. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis. Nature. 2004;431:80–84. doi: 10.1038/nature02788. [DOI] [PubMed] [Google Scholar]
- 30.Paradisi A, Maisse C, Coissieux MM, Gadot N, Lepinasse F, Delloye-Bourgeois C, et al. Netrin-1 up-regulation in inflammatory bowel diseases is required for colorectal cancer progression. Proc Natl Acad Sci USA. 2009;106:17146–17151. doi: 10.1073/pnas.0901767106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitehead RH, VanEeden PE, Noble MD, Ataliotis P, Jat PS. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc Natl Acad Sci USA. 1993;90:587–591. doi: 10.1073/pnas.90.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, et al. Novel inhibitors of cytokine-induced IκBα phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272:21096–21103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- 33.Kühn R, Jürgen L, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 34.Sellon RK, Tonkonogy SL, Schultz M, Dieleman LA, Grenther WB, Balish E, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infection and immunity. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehlen P, Llambi F. Role of netrin-1 and netrin-1 dependence receptors in colorectal cancers. Br J Cancer. 2005;93:1–6. doi: 10.1038/sj.bjc.6602656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanikawa T, Wilke CM, Kryczek I, Chen GY, Kao J, Nunez G, et al. Interleukin-10 ablation promotes tumor development, growth, and metastasis. Cancer Res. 2012;72:420–429. doi: 10.1158/0008-5472.CAN-10-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, Danon SJ, Grehan M, Chan V, Lee A, Mitchell H. Natural colonization with Helicobacter species and the development of inflammatory bowel disease in interleukin-10-deficient mice. Helicobacter. 2005;10:223–230. doi: 10.1111/j.1523-5378.2005.00314.x. [DOI] [PubMed] [Google Scholar]
- 38.Goh K, Sumner H. Breaks in normal human chromosomes: are they induced by a transferable substance in the plasma of persons exposed to total-body irradiation? Radiat Res. 1968;35:171–181. [PubMed] [Google Scholar]
- 39.Pant GS, Kamada N. Chromosome aberrations in normal leukocytes induced by the plasma of exposed individuals. Hiroshima J Med. 1977;26:149–154. [PubMed] [Google Scholar]
- 40.Watson GE, Lorimore SA, Macdonald DA, Wright EG. Chromosomal instability in unirradiated cells induced in vivo by a bystander effect of ionizing radiation. Cancer Res. 2000;60:5608–5611. [PubMed] [Google Scholar]
- 41.Lorimore SA, Chrystal JA, Robinson JI, Coates PJ, Wright EG. Chromosomal instability in unirradiated hemaopoietic cells induced by macrophages exposed in vivo to ionizing radiation. Cancer Res. 2008;68:8122–8126. doi: 10.1158/0008-5472.CAN-08-0698. [DOI] [PubMed] [Google Scholar]
- 42.Zhou H, Ivanov VN, Gillespie J, Geard CR, Amundson SA, Brenner DJ, et al. Mechanism of radiation-induced bystander effect: role of the cyclooxygenase-2 signaling pathway. Proc Natl Acad Sci USA. 2005;102:14641–14646. doi: 10.1073/pnas.0505473102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fischer SM, Hawk ET, Lubet RA. Coxibs and other nonsteroidal anti-inflammatory drugs in animal models of cancer chemoprevention. Cancer Prev Res. 2011;4:1728–1735. doi: 10.1158/1940-6207.CAPR-11-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.