Abstract
Adverse childhood experiences and substance use have been identified as potential causal risk factors for early-onset sexual intercourse. While it is possible that exposure to these risk factors directly increases the likelihood of engaging in early intercourse, an alternative explanation is that observed associations between these variables are due to shared familial confounds. These unmeasured confounds may increase the likelihood of being exposed to these risk factors and of engaging in early intercourse. Participants drawn from a population-based study of Swedish adult twins (ages 19–47 years; N = 12,126) reported on their history of exposure to early physical and sexual trauma, cigarette use, and cannabis use. We investigated the nature of the association between these risk factors and young age at first intercourse, using a comparison of twins differentially exposed to each risk factor. When compared to non-exposed, unrelated individuals, participants who reported adverse childhood experiences or who engaged in early cigarette use or cannabis use were more likely to engage in early intercourse. However, co-twin comparisons indicated that observed associations between these risk factors and early intercourse may be due to familial factors shared within twin pairs, and risk factor exposure may not lead directly to early intercourse. Our results suggest that preventing trauma exposure or preventing or delaying adolescents’ cigarette smoking or cannabis use may not effectively delay intercourse onset; instead, other aspects of the adolescent’s environment should be addressed.
Keywords: adolescence, sexual behavior, substance use, child abuse, co-twin comparison, behavioral genetics
INTRODUCTION
While the majority of individuals in Sweden and the United States have sexual intercourse by age 18 (Centers for Disease Control and Prevention, 2007; Danielsson, Rogala, & Sundström, 2001), younger age at first intercourse predicts greater likelihood of using birth control inconsistently and having multiple partners (Centers for Disease Control and Prevention, 2007; Kaiser Family Foundation, 2008). These behaviors increase the likelihood of adverse outcomes, such as unplanned pregnancy or sexually transmitted infection (Buston, Williamson, & Hart, 2007; Danielsson et al., 2001; Hollander, 2009; Kaiser Family Foundation, 2008). Because of the large public-health burden associated with these outcomes, leaders in the field have targeted reduction of sexual risk behaviors, including increasing the proportion of adolescents who abstain from intercourse, as a national priority in health research (U.S. Department of Health and Human Services, 2000). A more nuanced understanding of modifiable risk factors causally associated with different sexual risk behaviors is needed so that the most appropriate and effective targets may be identified in the development of sexual health education and prevention programs for young people (Bailey, 2009; Biglan, Mrazek, Carnine, & Flay, 2003; British Academy of Science Working Group, 2010; Kirby, Laris, & Rolleri, 2007; National Campaign to Prevent Teen Pregnancy, 2002; Rutter & The Academy of Medical Sciences Working Group, 2007; Santelli, Carter, Orr, & Dittus, 2009).
Adverse childhood experiences, including sexual or physical abuse and trauma (Anda, Butchart, Felitti, & Brown, 2010; Senn, Carey, & Vanable, 2008) and substance use (e.g., tobacco and cannabis use) (Capaldi, Crosby, & Stoolmiller, 1996; Hansen et al., 2010; Siebenbruner, Zimmer-Gembeck, & Egeland, 2007; Zimmer-Gembeck & Helfand, 2008), are risk factors for beginning to engage in intercourse in early adolescence vs. later in adolescence or as a young adult. Among adolescents who are already sexually active, these risk factors are also associated with inconsistent contraceptive use, substance use during intercourse, an increased number of sexual partners, and a greater likelihood of adolescent pregnancy (Anda et al., 2010; Anderson & Stein, 2011; Hansen et al., 2010; Senn et al., 2008; Siebenbruner et al., 2007).
Previous studies investigating risk factors for early intercourse (Zimmer-Gembeck & Helfand, 2008) have compared unrelated individuals to one another. While these traditional designs have a number of advantages, researchers cannot use them to infer a causal relationship between a risk factor and early intercourse because these designs cannot account for between-family differences that could be driving observed associations (Rutter, 2007; Rutter et al., 2010; Shadish, Cook, & Campbell, 2002). If a risk factor causes younger age at first intercourse, intervening to modify the risk factor would delay intercourse onset. If the risk factor and younger age at first intercourse are associated only because they are both correlated with the true cause, modifying the risk factor would not delay intercourse onset. Because of the possibility of alternative, non-causal mechanisms related to sexual risk behavior, additional research is needed before we can make strong causal inferences (McGue, Iacono, & Krueger, 2006; Mendle, Turkheimer, & Emery, 2007; Santelli et al., 2009).
While it is possible that trauma exposure or early substance use leads directly to engaging in early intercourse, children and adolescents are not exposed to trauma, drugs, or sexual risk behaviors at random. Previous research has found that familial factors (i.e., genetic and/or shared environmental influences) account for a significant amount of the variance in age at first intercourse (Bricker et al., 2006; Dunne et al., 1997; Guo & Tong, 2006; Mustanski, Viken, Kaprio, Winter, & Rose, 2007; Rodgers, Rowe, & Buster, 1999), exposure to early sexual and/or physical assaultive trauma and maltreatment (Jaffee et al., 2004; Stein, Lang, Taylor, Vernon, &Livesley, 2002), and adolescent marijuana and tobacco use (Maes et al., 1999; McGue, Elkins, & Iacono, 2000; Rhee et al., 2003).
It is possible that common genetic and environmental factors could lead to a shared vulnerability for both exposure to adverse childhood experiences, tobacco use, or marijuana use and for engaging in early intercourse. The co-occurrence of sexual risk behavior and adolescent delinquency, for example, may be due to common genetic influences related to personality traits, such as sensation seeking and impulsivity (Verweij, Zietsch, Bailey, & Martin, 2009). Environmental factors shared by family members (Dick, Johnson, Viken, & Rose, 2000), such as growing up in a low-income family, living in a disadvantaged or unstable neighborhood, having parents with low education, or being exposed to certain parental attitudes about parenting and risk behaviors, could also explain the co-occurrence of these behaviors (Kirby, 2003; Roche et al., 2005). Research on potential causes of early intercourse, therefore, must rule out these possible confounds using novel approaches, such as quasi-experimental methods, including the comparison of twins discordant for risk-factor exposure (Lahey, D'Onofrio, & Waldman, 2009; Rutter, 2007; Shadish et al., 2002).
Recent studies highlight the usefulness of quasi-experimental designs in sexual behavior research. In one study, early intercourse was not associated with later delinquency after controlling for genetic and environmental confounds shared by twins (Harden, Mendle, Hill, Turkheimer, & Emery, 2008), in contrast to results found when comparing unrelated individuals (Armour & Haynie, 2007). A co-twin comparison investigating the relationship between non-heterosexual behavior and poor mental health suggested that this association may be heavily confounded by shared genetic and environmental influences (Frisell, Lichtenstein, Rahman, & Långström, 2010).
In the current study, we examined the mechanisms through which putative risk factors—specifically, adverse childhood experiences and early substance use—are associated with early age at first intercourse, using a genetically informative sample of Swedish adult twins. To determine whether the association between these risk factors and early intercourse may be accounted for by familial confounds, we tested whether twins exposed to these risk factors were more likely to engage in early intercourse than their non-exposed co-twins. Comparing differentially exposed twins allows us to test causal inferences by automatically controlling for unmeasured genetic and shared environmental characteristics that could explain the associations between these risk factors and early intercourse that have been observed in previous studies (Lahey et al., 2009; Rutter, 2007).
METHOD
Participants
Participants were drawn from the Study of Twin Adults: Genes and Environment (STAGE), a study of twins born in Sweden between 1959 and 1985 (Lichtenstein et al., 2006). All eligible twins (i.e., all twins born in Sweden between 1959 and 1985, N = 42,582) were invited to complete a Web-based survey between November 2005 and March 2006. The survey consisted of approximately 1,300 possible questions across 34 modules related to sociodemographic characteristics, physical and mental health, substance use, trauma, and sexual behavior. Many of the items were follow-up questions not relevant to all respondents. Participants were given the option of completing a phone interview instead of the Web-based survey, and participants completing the phone interview were also mailed a separate paper questionnaire assessing potentially sensitive information (e.g., sexual risk behaviors and stressful and traumatic life events). For participants completing the Web-based survey, these items were included in the full survey and not assessed separately. For more details regarding survey content and administration, cf. Lichtenstein et al. (2006). Data collection was approved by the Swedish Data Inspection Board and the Regional Ethics Committee of the Karolinska Institutet, and participants provided consent while completing the Web survey or the telephone interview. The total response rate was 59.6% (Web survey, 43.1%; phone interview, 16.5%), with data available from 25,381 individuals, comprised of 11,235 complete twin pairs and 2,911 individuals whose co-twin did not participate. Previous analyses have shown that STAGE responders did not differ from eligible non-responders with regard to age, birth weight, or whether they had ever been diagnosed with a neurological condition (Furberg et al., 2008). However, non-responders were more likely to be male, have one or both parents born outside of Sweden, been convicted of a crime, or been diagnosed with a psychiatric disorder. Non-responders were also less educated and non-responding males had lower intellectual performance scores at the time of conscription.
Given the relatively low response rate for completed STAGE assessments, we also compared individuals completing STAGE assessments (N = 25,381) to all individuals born in Sweden between 1959 and 1985 (N = 2,859,123) through an anonymized merge with data from the Swedish National Crime Register (Fazel & Grann, 2006) and Education Register (Statistics Sweden, 2011). Based on data obtained in the national registers, STAGE participants were roughly equivalent to all individuals in the selected birth cohort with regard to highest level of education completed (4.5/4.2 on a scale of 1 to 7, with a value of 4 corresponding to three years of upper secondary education). Based on data obtained in the national registers, STAGE participants were slightly less likely to have been convicted of a crime (violent and nonviolent, including driving-related offenses) than individuals in the selected birth cohort (16.2%/21.8%), which may be an artifact of the slightly higher percentage of females in the STAGE sample (55.8%/48.6%)—among only females, rates of criminal conviction were comparable in both groups (8.1%/10.0%) and also lower than rates among males (26.5%/32.9%). Compared to all individuals in the birth cohort, STAGE participants were also comparable with regard to family background characteristics, including maternal education (3.3/3.2), paternal education (3.2/3.1), maternal criminal history (8.4%/9.4%), and paternal criminal history (27.1%/29.7%). No identified father was available for 0.7% of the STAGE sample and 1.6% of the birth cohort.
The current analyses included only MZ and same-sex DZ twin-pairs from the STAGE sample in which both twins participated in assessments (N = 12,126 individuals from 3,548 MZ pairs and 2,515 DZ pairs). Of these individuals, 60.4% were female, and the average age at assessment was 32.6 years.
Measures
Early sexual intercourse
Participants were asked whether they had ever engaged in voluntary sexual intercourse and, if so, at what age they first had intercourse. Participants reporting intercourse onset before age 16 were considered to have engaged in early intercourse, consistent with measurement in other studies of sexual risk behavior (Zimmer-Gembeck & Helfand, 2008). Consistent with previous data from Swedish samples (Danielsson et al., 2001), participants reported an average age at first intercourse of 17.5 years. Early intercourse was reported by 23.9% of individuals.
Adverse childhood experiences
History of physical trauma or violence was assessed by participant report of having witnessed physical violence between family members (e.g., hitting, kicking, or punching), having been physically neglected (e.g., not fed, not properly clothed, or left to take care of themselves when they felt they were too young or too ill), or having been physically abused (e.g., hit, choked, burned, beaten, severely punished—for example, locked up, shut in a closet, tied up, or chained–by someone who knew them well such as a parent, sibling, boyfriend or girlfriend). History of sexual trauma was assessed by participant report of having been touched or made to touch someone else in a sexual way or if they had ever had oral, anal, or genital sex because they felt forced in some way or threatened by harm to themselves or someone else.
To increase statistical power in the analyses of differentially exposed twin pairs, physical and sexual trauma were combined, in keeping with a recent call to conceptualize child maltreatment and related events as a coherent set of experiences with important implications for social development and public health (Anda et al., 2010). Participants who reported any trauma were asked during which age period they first experienced the event: before age 7 (before starting school), ages 7 to 12 (in elementary or grammar school), ages 13 to 15 (in junior-high or middle school), or ages 16 to 18 (in high school). To reduce potential temporal overlap between trauma onset and intercourse onset, participants exposed to trauma before age 13 were considered to have experienced adverse childhood experiences (18.3%; physical trauma or violence = 17.9%; sexual trauma = 2.5%).
Early cigarette use
Participants were asked whether they had ever smoked cigarettes and to what degree (tried smoking only, smoke[d] now and then or at parties, smoke[d] regularly). Participants reporting any history of cigarette use were asked to provide the age at which they smoked their first cigarette. Participants who reported smoking their first cigarette prior to age 13 (9.0%) were considered to have engaged in early cigarette use.
Early cannabis use
Participants were asked whether they had ever tried marijuana or hash, and participants who reported using one or both substances were considered to have engaged in cannabis use. Participants reporting having tried cannabis were asked to provide the age at which they first tried using cannabis. Due to low prevalence rates at earlier ages, participants reporting cannabis use before age 16 (2.8%) were considered to have engaged in early cannabis use.
Statistical Analyses
We used a co-twin comparison approach to estimate whether the association between early intercourse and each risk factor was reduced after controlling for unmeasured confounds shared within twin pairs. For each risk factor, we ran four models using Mplus version 6.1 software (Muthén & Muthén, 2010). We first estimated the magnitude of the association between early sex and the risk factor across the overall sample, while controlling for participant gender and age. Next, to account for the clustered nature of the data (i.e., individuals nested within twin pairs), we used multilevel modeling (Snijders & Bosker, 1999) to compare DZ twins and MZ twins discordant for risk factor exposure, with models run separately by zygosity. For these models, we calculated a mean risk factor exposure score within each twin pair (0.0 = concordant for nonexposure, 0.5 = discordant, 1.0 = concordant for exposure). For each participant, we then calculated a deviation score from the pair-mean exposure score. A deviation score greater than 0 indicated a participant who engaged in early cigarette use whose co-twin did not.
Each two-level logistic regression model used in the co-twin comparisons included a between-pair and a within-pair component. The between-pair component corresponded to the regression of the random intercept on the participant’s pair-mean score. Here we controlled for participant gender, as only same-sex twin pairs were included in the analyses and gender could only vary between twin pairs. The within-pair component corresponded to the regression of the outcome on the participant’s deviation score, with the intercept treated as a random effect varying across twin pairs and the slope treated as a fixed effect not varying across twin pairs (i.e., the effect of exposure was assumed to be equivalent across all twin pairs). This within-pair parameter is equivalent to fitting econometric fixed-effects models (Neuhaus & McCulloch, 2006). Here we also controlled for participant age, as assessments occurred within a four-month window of time, and exact age at assessment could vary within twin pairs. The within-pair estimate obtained in these models would correspond to the change in odds of engaging in early sex associated with risk factor exposure relative to no exposure within a twin pair.
We then calculated an interaction term representing a zygosity × deviation score in order to test whether the within-twin effect of risk-factor exposure differed by zygosity type. Both MZ and DZ pairs were included in this model and the interaction term was added as a third parameter in the within-pair component of the two-level model. A significant interaction parameter would indicate a significant difference in the within-pair estimates for MZ vs. DZ twins, and a significantly larger association in DZ twins than in MZ twins would indicate genetic confounding. A nonsignificant interaction term would indicate no difference in the within-pair estimates by zygosity type and would suggest confounding due to shared environmental influences.
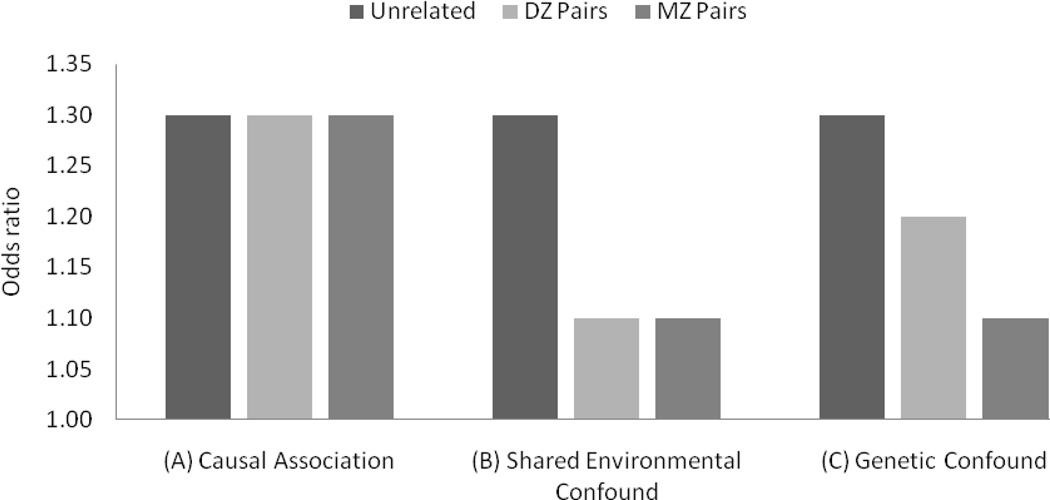
Potential outcomes for the co-twin comparisons are shown in Fig. 1 (Young-Wolff, Kendler, Ericson, & Prescott, 2011). An odds ratio significantly greater than 1 would indicate that the risk factor was associated with a significantly increased likelihood of early intercourse. In the case of a causal association (Fig. 1A), the risk factor would be associated with increased likelihood of early intercourse among unrelated individuals as well as among both MZ and DZ pairs discordant for risk factor exposure (Lahey & D'Onofrio, 2010). In the case of familial confounding, the association between risk factor exposure and likelihood of early intercourse would be lower among MZ and DZ pairs than among unrelated individuals (Fig. 1B & C). More specifically, if the confound was environmental in nature (Fig. 1B), likelihood of early intercourse would not be higher among discordant DZ twins compared to MZ twins, since both twins from the discordant pair would be exposed to these shared environmental factors. Alternatively, in the case of a genetic confound (Fig. 1C), likelihood of early intercourse would be greater among discordant DZ twins than among discordant MZ twins because comparing discordant MZ twins controls for 100% of shared genetic influences, while comparing discordant DZ twins controls for an average of 50%.
Figure 1.
Hypothetical odds ratios representing likelihood of engaging in early intercourse among risk-factor–exposed individuals relative to non-exposed individuals, by degree of relatedness. An odds ratio equal to 1 represents no difference in likelihood of early intercourse based on risk-factor exposure, while an odds ratio greater than 1 represents increased likelihood of early intercourse among risk-factor exposed individuals.
RESULTS
Descriptive statistics for all variables are shown in Table 1. Within-pair correlations for each variable are shown in Table 2. Twin-pair correlations were stronger in MZ pairs than in DZ pairs, suggesting that genetic factors influence variability in these behaviors. Additionally, the magnitudes of all DZ correlations were greater than half the size of the MZ correlations, suggesting that variability in these behaviors is also influenced by shared environmental influences. These findings are consistent with previous research (Guo & Tong, 2006; Jaffee et al., 2004; Maes et al., 1999; McGue et al., 2000; Nelson et al., 2006; Rhee et al., 2003; Rodgers et al., 1999; Stein et al., 2002).
Table 1.
Descriptive Statistics for Measured Variables
| Variable | Prevalence: % |
M (SD), Range | Correlation with early intercourse: ra(SE) |
|---|---|---|---|
| Sexual behaviorb | |||
| Early intercourse | 24.0% | -- | |
| Age at first intercourse | 17.5 (3.1), 10–46 | -- | |
| Adverse childhood experiencesc | |||
| Any early trauma | 18.3% | 0.13 (0.02) | |
| Early physical trauma | 17.9% | 0.13 (0.02) | |
| Early sexual trauma | 2.5% | 0.17 (0.04) | |
| Substance use | |||
| Early cigarette smoking | 9.0% | 0.14 (0.03) | |
| Age first tried cigarettesd | 15.3 (3.2), 10–46 | −0.15 (0.02) | |
| Early cannabis use | 2.1% | 0.46 (0.03) | |
| Age first tried cannabise | 21.1 (6.0), 10–46 | −0.15 (0.02) | |
| Covariates | |||
| Female | 60.4% | 0.13 (0.01) | |
| Age at assessment | 32.6 (7.6), 19–47 | 0.07 (0.01) | |
Pearson correlations are provided for continuous variables, and tetrachoric correlations are provided for dichotomous variables.
Individuals missing sexual intercourse data (21.8%) were more likely to be male (OR = 1.95, p < .001).
Individuals missing abuse timing data (21.5%) were more likely to be female (OR = 1.79, p < .001).
Individuals missing cigarette history (18.8%) were on average 1.7 years older (t = −12.00, p < .001) and were more likely to be female (OR = 1.31, p < .001).
Individuals missing cannabis use data (3.5%) were on average 0.6 years older (t = −2.10, p = .04) and were more likely to be male (OR = 1.36, p < .001) than individuals with available data.
Table 2.
Twin-Pair Similarity for Measured Variables
|
ra(SE) |
||||
|---|---|---|---|---|
| Within-pair similarity |
||||
| Variable | N | % of pairs discordant |
MZ pairs | DZ pairs |
| Early intercourse | 3,822 | 20.0% | 0.78 (0.02) | 0.53 (0.04) |
| Adverse childhood experiences | 3,989 | 17.0% | 0.71 (0.03) | 0.58 (0.04) |
| Early cigarette use | 3,297 | 11.2% | 0.60 (0.05) | 0.34 (0.07) |
| Early cannabis use | 5,611 | 3.1% | 0.65 (0.06) | 0.58 (0.07) |
Note: N = number of twin pairs in which both twins provided data.
Tetrachoric correlation (r) representing twin liability for exposure based on co-twin exposure.
Co-twin Comparisons
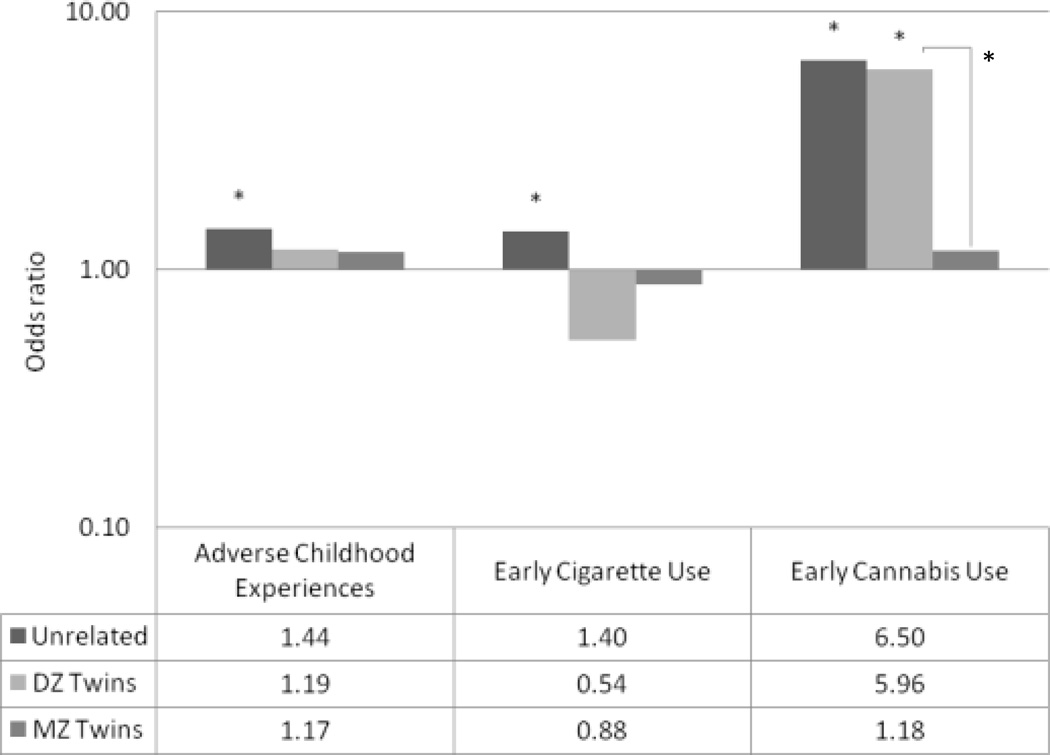
Odds ratios for all risk factors are displayed in Fig. 2 and corresponding logit estimates are presented in the text and in Table 3.
Figure 2.
Odds of reporting early intercourse based on differential risk-factor exposure, by degree of relatedness. A logarithmic scale was used on the y-axis to illustrate the relative magnitude of effect of odd ratios less/greater than 1.0.
* p < .05
Table 3.
Estimated Effects of Risk-Factor Exposure on Early Intercourse
| Unrelated individuals |
DZ twins |
MZ twins |
Interaction of exposure and zygosity |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE | p | B | SE | p | B | SE | p | B | SE | p | |
| Adverse childhood experiences |
ExposureU | 0.36 | 0.07 | < 0.001 | |||||||||
| ExposureW | 0.18 | 0.26 | 0.49 | 0.16 | 0.26 | 0.56 | 0.14 | 0.23 | 0.56 | ||||
| ExposureB | 0.60 | 0.23 | 0.01 | 1.19 | 0.29 | < 0.001 | 0.90 | 0.18 | < 0.001 | ||||
| ExposureW × zygosity | 0.08 | 0.38 | 0.84 | ||||||||||
| N: | 6,868 | 2,646 | 4,222 | 6,868 | |||||||||
| Early cigarette use |
ExposureU | 0.34 | 0.15 | 0.02 | |||||||||
| ExposureW | −0.63 | 0.40 | 0.12 | −0.13 | 0.49 | 0.79 | −0.11 | 0.40 | 0.80 | ||||
| ExposureB | 1.63 | 0.48 | 0.17 | 0.80 | 0.66 | 0.22 | 1.26 | 0.41 | < 0.001 | ||||
| ExposureW × zygosity | −0.70 | 0.65 | 0.28 | ||||||||||
| N: | 4,058 | 1,528 | 2,530 | 4,058 | |||||||||
| Early cannabis use |
ExposureU | 1.87 | 0.15 | < 0.001 | |||||||||
| ExposureW | 1.79 | 0.50 | < 0.001 | 0.16 | 0.53 | 0.76 | 0.14 | 0.46 | 0.76 | ||||
| ExposureB | 4.23 | 0.57 | < 0.001 | 5.86 | 0.88 | < 0.001 | 5.07 | 0.51 | < 0.001 | ||||
| ExposureW × zygosity | 2.02 | 0.76 | 0.01 | ||||||||||
| N: | 7,356 | 2,896 | 4,460 | 7,356 | |||||||||
Note DZ, dizygotic; MZ, monozygotic; B, unstandardized logit estimate; SE, standard error; N, number of individuals included in model; ExposureU = risk factor exposure (0/1); ExposureW = within-pair deviation; ExposureB = twin-pair mean. All models included covariates of age (within) and gender (between). Logit estimates in bold indicate significance at p < .05.
Association with adverse childhood experiences
Individuals reporting adverse childhood experiences were nearly 1.5 times more likely to engage in early intercourse than unrelated, non-exposed individuals (B = 0.36, SE = 0.07). However, twins exposed to adverse childhood experiences were not significantly more likely to engage in early intercourse than their non-exposed co-twins. This was true for both DZ pairs (B = 0.18, SE = 0.26) and MZ pairs (B = 0.16, SE = 0.26). The within-twin effect of exposure to adverse childhood experiences did not differ by zygosity (B = 0.08, SE = 0.38). These results suggest that adverse childhood experiences may not have a causal influence on engaging in early intercourse because shared environmental influences may contribute to the association between adverse childhood experiences and early intercourse.
Additional analyses were conducted to test for the possibility of differential effects of early exposure to physical trauma vs. early exposure to sexual trauma. Exposure to early physical trauma (B = 0.38, SE = 0.07) and early sexual trauma (B = 0.51, SE = 0.17) were each associated with significantly higher risk of early intercourse when comparing unrelated individuals and controlling for age and gender. However, exposure to each trauma type no longer predicted early intercourse risk after controlling for genetic and environmental influences shared by twins.
Association with early cigarette use
Individuals who smoked cigarettes before age 13 were nearly 1.5 times more like to engage in early intercourse than unrelated, non-exposed individuals (B = 0.34, SE = 0.15). In contrast, twins who engaged in early cigarette use were not significantly more likely to engage in early intercourse than their non-exposed co-twin, in both DZ pairs (B = −0.63, SE = 0.40) and MZ pairs (B = −0.13, SE = 0.49). The within-twin effect of exposure to adverse childhood experiences did not differ by zygosity (B = −0.70, SE = 0.65), indicating that shared environmental influences may explain the co-occurrence of early cigarette smoking and early intercourse.
Association with early cannabis use
Individuals who used cannabis before age 16 were over 6.5 times more likely to engage in early intercourse than unrelated, non-exposed individuals (B = 1.87, SE = 0.15). DZ twins who engaged in early cannabis use were more likely to engage in early intercourse than their non-exposed co-twins (B = 1.79, SE = 0.50), while MZ twins who engaged in early cannabis use did not show a significantly greater risk (B = 0.16, SE = 0.76). The effect of exposure was significantly higher among DZ pairs (B = 2.02, SE = 0.76), suggesting that genetic influences may explain the association between early cannabis use and early intercourse.
DISCUSSION
We examined the nature of the association between three putative risk factors (adverse childhood experiences, cigarette use, and cannabis use) and early age at first intercourse, using a population-based sample of 19- to 47-year-old Swedish twins. Specifically, we tested whether the association between these risk factors and early intercourse may be causal in nature or if potential genetic and shared environmental confounds may explain the association. This approach has not been used in previous research examining predictors of sexual risk behavior in adolescence, despite recent calls for studies testing alternative explanations (Bailey, 2009; Biglan et al., 2003; Kirby et al., 2007; National Campaign to Prevent Teen Pregnancy, 2002; Santelli et al., 2009).
STAGE respondents who reported adverse childhood experiences (i.e., physical or sexual trauma by the age of 13), began smoking cigarettes before the age of 13, or used cannabis before age 16 were more likely to have sexual intercourse by 16 when compared to unrelated individuals not exposed to these risk factors. However, when comparing differentially exposed twins, the associations between each early risk factor and early intercourse were attenuated. The lack of association after accounting for familial confounding suggests that the link between these risk factors and early intercourse is not causal. Instead, additional factors shared by individuals within families must explain exposure to the risk factors and early intercourse. These results suggest that these unmeasured confounds are likely environmental with regard to adverse childhood experiences and early cigarette use and genetic with regard to early cannabis use.
This study contributes to an emerging line of research investigating the contribution of genetic and environmental confounds to the association between sexual risk behavior and putative risk factors. Our results suggest that preventing trauma exposure or delaying adolescents’ cigarette smoking or cannabis use may not effectively delay intercourse onset if other aspects of the adolescent’s environment are also not addressed. These environmental factors may include general levels of adversity faced by the adolescent’s family (Kirby, 2003; Roche et al., 2005), including neighborhood safety or disadvantage and parents’ attitudes about parenting and risk behaviors. Children reared in high-stress environments characterized by lack of resources, marital discord, and inconsistent and harsh parenting may be more likely to engage in early intercourse (e.g., Belsky, Steinberg, & Draper, 1991), and the lack of a within-pair effect of adverse childhood experiences, for example, may reflect that exposure to contextual factors associated with adverse childhood experiences is more predictive of early intercourse than exposure to adverse childhood experiences specifically. In other words, all of the offspring raised in an environment in which adverse childhood experiences are more likely occur are at heightened risk for engaging in intercourse at an earlier age.
Results from the co-twin comparison model examining the effect of early cannabis use on early intercourse suggest that genetic confounding may explain the association between these two variables. Polymorphisms in the dopamine receptor D4 gene (DRD4) have been associated with heightened risk of early intercourse (Guo & Tong, 2006) and polymorphisms in the promoter region of the serotonin transporter gene (5-HTTLPR) associated with risk-taking behavior have been found to moderate the effect of early substance use on sexual risk behaviors in early adolescence (Kogan et al., 2010). The DRD4 and 5-HTTLPR polymorphisms are genetic vulnerability factors that may interact with contextual factors, such as the environmental influences described above, to place certain individuals at greater risk for engaging in both cannabis use and sexual intercourse during early adolescence. Effective interventions aimed at delaying age at first intercourse, then, must target these other correlated, causal risk factors.
Although these results suggest that adverse childhood experiences and early substance use may not lead directly to early intercourse, previous research points toward a possible causal connection between these risk factors and other outcomes. Co-twin comparisons have found that childhood sexual abuse predicts increased risk for smoking, illicit drug use, drug abuse and dependence, and a variety of psychiatric disorders (Kendler et al., 2000; Nelson et al., 2006), although common environmental factors may explain the association between childhood maltreatment and alcohol-related disorders in adulthood (Young-Wolff et al., 2011).
Several limitations of the study design should be addressed. The STAGE sample relied upon retrospective reports of lifetime health-related behaviors assessed during adulthood and, given that many individuals were reporting on behaviors that occurred an average of 20 years earlier, it is possible that recall bias contributed to error in reported age at initiation of all measured variables. Potential bias may also have contributed to an underestimation of early risk factor exposure and early intercourse, and we were also not able to assess whether current functioning biased reports of exposure to any of the measured variables. However, the prevalence of early sexual activity reported retrospectively in this sample was similar to rates of early intercourse reported prospectively in adolescent samples, suggesting that any bias produced by the retrospective assessment of sexual behavior may be minimal. Prospective reporting of substance use, sexual behavior, and other variables gathered at multiple time points would provide a more time-sensitive assessment of the link between these risk factors and sexual risk behavior. However, few population-based datasets meet these criteria and also use quasi-experimental designs.
Questions regarding the importance of frequency or intensity of risk factor exposure on sexual risk behaviors could also not be addressed using this sample. Future research using similar analyses should also assess the effect of other substances, particularly alcohol, which may have more direct—and potentially causal—effects on adolescents’ sexual decision-making (George et al., 2009). Additionally, while early intercourse has often been used as an index of sexual risk, related outcomes, such as contraceptive use during intercourse, number of sexual partners, or the frequency of intercourse occurring within or outside of the context of monogamous relationships, should also be considered, as they may represent more direct measures of risk behavior.
The twin comparisons in the present study could be completed only when both twins provided information for all relevant variables. In additional bivariate twin analyses (results not shown), we estimated the degree to which the co-variation between each early risk factor and early intercourse was due to genetic, shared environmental, and unique environmental influences. Results from a bivariate common and specific factors model suggested that there was significant co-variation between each risk factor and early intercourse, although low rates of risk-factor exposure (in the case of cannabis use), the categorical nature of the measured variables, and twin pairs lacking complete data may have made precise partitioning of the source of covariation into genetic vs. environmental influences difficult.
Although the overall response rate among those contacted to participate in STAGE was roughly 60%, basic demographic characteristics of STAGE responders were comparable to those of all individuals born in Sweden belonging to the same birth cohort. This suggests that results obtained using the STAGE sample are likely missing at random and may be generalizeable to the broader Swedish population.
Conclusion
The current study represents a novel approach to investigating risk factors of early sexual behavior. These results suggest that familial confounds should be considered and previous studies not accounting for these factors may have overstated the effect of risk factor exposure on sexual behavior. Genetically informative designs, such as the comparison of twins differentially exposed to a risk factor, can shed light on the nature of the mechanisms responsible for the connection of these risk factors to subsequent sexual health outcomes.
Acknowledgments
This research was supported by award numbers F31 DA 029376-01 from the National Institute on Drug Abuse and T32 HD 007475 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Kelly Donahue received the 2012 Sandra R. Leiblum Student Research Award from the Society for Sex Therapy and Research (SSTAR) in recognition of her work on this article. Portions of the article were presented at the March 2012 SSTAR meeting, Chicago, IL.
REFERENCES
- Anda RF, Butchart A, Felitti VJ, Brown DW. Building a framework for global surveillance of the public health implications of adverse childhood experiences. American Journal of Preventive Medicine. 2010;39:93–98. doi: 10.1016/j.amepre.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Stein MD. A behavioral decision model testing the association of marijuana use and sexual risk in young women. AIDS and Behavior. 2011;15:875–884. doi: 10.1007/s10461-010-9694-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armour S, Haynie DL. Adolescent sexual debut and later delinquency. Journal of Youth and Adolescence. 2007;36:141–152. [Google Scholar]
- Bailey JA. Editorial: Addressing common risk and protective factors can prevent a wide range of adolescent risk behaviors. Journal of Adolescent Health. 2009;45:107–108. doi: 10.1016/j.jadohealth.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy: An evolutionary theory of socialization. Child Development. 1991;62:647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Biglan A, Mrazek PJ, Carnine D, Flay B. The integration of research and practice in the prevention of youth problem behaviors. American Psychologist. 2003;58:433, 440. doi: 10.1037/0003-066x.58.6-7.433. [DOI] [PubMed] [Google Scholar]
- Bricker JB, Stallings MC, Corley RP, Wadsworth SJ, Bryan A, Timberlake DS, DeFries JC. Genetic and environmental influences on age at sexual initiation in the Colorado Adoption Project. Behavior Genetics. 2006;36:820–832. doi: 10.1007/s10519-006-9079-2. [DOI] [PubMed] [Google Scholar]
- British Academy of Science Working Group. Social science and family policy. London: British Academy Policy Center; 2010. [Google Scholar]
- Buston K, Williamson L, Hart G. Young women under 16 years with experience of sexual intercourse: Who becomes pregnant? Journal of Epidemiology & Community Health. 2007;61:221–225. doi: 10.1136/jech.2005.044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi DM, Crosby L, Stoolmiller M. Predicting the timing of first intercourse for at-risk adolescent males. Child Development. 1996;67:344–359. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Youth risk behavior surveillance: United States. Atlanta, GA: Author; 2007. [Google Scholar]
- Danielsson M, Rogala C, Sundström K. Teenage sexual and reproductive behavior in developed countries: Country report for Sweden [Occasional Report] New York: Guttmacher Institute; 2001. [Google Scholar]
- Dick DD, Johnson JK, Viken RJ, Rose RJ. Testing between-family associations in within-family comparisons. Psychological Science. 2000;11:409–413. doi: 10.1111/1467-9280.00279. [DOI] [PubMed] [Google Scholar]
- Dunne MP, Martin NG, Statham DJ, Slutske WS, Dinwiddie SH, Bucholz KK, Heath AC. Genetic and environmental contributions to variance in age at first sexual intercourse. Psychological Science. 1997;8:211–216. [Google Scholar]
- Fazel S, Grann M. The population impact of severe mental illness on violent crime. American Journal of Psychiatry. 2006;163:1397–1403. doi: 10.1176/ajp.2006.163.8.1397. [DOI] [PubMed] [Google Scholar]
- Frisell T, Lichtenstein P, Rahman Q, Långström N. Psychiatric morbidity associated with same-sex sexual behavior: Influence of minority stress and familial factors. Psychological Medicine. 2010;40:315–324. doi: 10.1017/S0033291709005996. [DOI] [PubMed] [Google Scholar]
- Furberg H, Lichtenstein P, Pedersen NL, Thornton L, Bulik CM, Lerman C, Sullivan PF. The STAGE cohort: A prospective study of tobacco use among Swedish twins. Nicotine and Tobacco Research. 2008;10:1727–1735. doi: 10.1080/14622200802443551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George WH, Davis KC, Norris J, Heiman JR, Stoner SA, Schacht RL, Kajumulo KF. Indirect effects of acute alcohol intoxication on sexual risk-taking: The roles of subjective and physiological sexual arousal. Archives of Sexual Behavior. 2009;38:498–513. doi: 10.1007/s10508-008-9346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G, Tong Y. Age at first sexual intercourse, genes, and social context: Evidence from twins and the dopamine D4 receptor gene. Demography. 2006;43:747–769. doi: 10.1353/dem.2006.0029. [DOI] [PubMed] [Google Scholar]
- Hansen BT, Kjær SK, Munk C, Tryggvadottir L, Sparén P, Hagerup-Jenssen M, Nygård M. Early smoking initiation, sexual behavior and reproductive health: A large population-based study of Nordic women. Preventive Medicine. 2010;51:68–72. doi: 10.1016/j.ypmed.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Harden KP, Mendle J, Hill JE, Turkheimer E, Emery RE. Rethinking timing of first sex and delinquency. Journal of Youth and Adolescence. 2008;37:373–385. doi: 10.1007/s10964-007-9228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander D. In the period before age 21, women, but not men, may have elevated STD risks. Perspectives on Sexual and Reproductive Health. 2009;41:129–130. [Google Scholar]
- Jaffee SR, Caspi A, Moffitt TE, Polo-Tomas M, Price TS, Taylor A. The limits of child effects: Evidence for genetically mediated child effects on corporal punishment but not on physical maltreatment. Developmental Psychology. 2004;40:1047–1058. doi: 10.1037/0012-1649.40.6.1047. [DOI] [PubMed] [Google Scholar]
- Kaiser Family Foundation. Sexual health of adolescents and young adults in the United States [Occasional Report] Washington, DC: Author; 2008. [Google Scholar]
- Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, Prescott CA. Childhood sexual abuse and adult psychiatric and substance use disorders in women. Archives of General Psychiatry. 2000;57:953–959. doi: 10.1001/archpsyc.57.10.953. [DOI] [PubMed] [Google Scholar]
- Kirby D. Risk and protective factors affecting teen pregnancy and the effectiveness of programs designed to address them. In: Romer D, editor. Reducing adolescent risk: Toward an integrated approach. Thousand Oaks, CA: Sage Publications; 2003. pp. 265–283. [Google Scholar]
- Kirby D, Laris BA, Rolleri LA. Sex and HIV education programs: Their impact on sexual behaviors of young people throughout the world. Journal of Adolescent Health. 2007;40:206–217. doi: 10.1016/j.jadohealth.2006.11.143. [DOI] [PubMed] [Google Scholar]
- Kogan SM, Beach SRH, Philibert RA, Brody GH, Chen Y, Lei H. 5-HTTPLR status moderates the effect of early adolescent substance use on risky sexual behavior. Health Psychology. 2010;29:471–476. doi: 10.1037/a0020594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, D'Onofrio BM. All in the family: Comparing siblings to test causal hypotheses regarding environmental influences on behavior. Current Directions in Psychological Science. 2010;19:319–323. doi: 10.1177/0963721410383977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, D'Onofrio BM, Waldman ID. Using epidemiologic methods to test hypotheses regarding causal influences on child and adolescent mental disorders. Journal of Child Psychology and Psychiatry. 2009;50:53–62. doi: 10.1111/j.1469-7610.2008.01980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, Sullivan PF, Cnattingius S, Gatz M, Johansson S, Carlström E, Pedersen NL. The Swedish Twin Registry in the third millenium: An update. Twin Research and Human Genetics. 2006;9:875–882. doi: 10.1375/183242706779462444. [DOI] [PubMed] [Google Scholar]
- Maes HH, Woodard CE, Murrelle L, Meyer JM, Silberg JL, Hewitt JK, Eaves LJ. Tobacco, alcohol, and drug use in 8- to 16-year-old twins: The Virginia Twin Study of Adolescent Behavioral Development. Journal of Studies on Alcohol. 1999;60:293–305. doi: 10.15288/jsa.1999.60.293. [DOI] [PubMed] [Google Scholar]
- McGue M, Elkins I, Iacono WG. Genetic and environmental influences on adolescent substance use and abuse. American Journal of Medical Genetics. 2000;96:671–677. doi: 10.1002/1096-8628(20001009)96:5<671::aid-ajmg14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Krueger RF. The association of early adolescent problem behavior and adult psychopathology: A multivariate behavioral genetic perspective. Behavior Genetics. 2006;36:591–602. doi: 10.1007/s10519-006-9061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle JM, Turkheimer E, Emery RE. Detrimental psychological outcomes associated with early pubertal timing in adolescent girls. Developmental Review. 2007;27:151–171. doi: 10.1016/j.dr.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustanski B, Viken RJ, Kaprio J, Winter T, Rose RJ. Sexual behavior in young adulthood: A population-based twin study. Health Psychology. 2007;26:610–617. doi: 10.1037/0278-6133.26.5.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L, Muthén B. Mplus users' guide. 6th ed. Los Angeles, CA: Author; 2010. [Google Scholar]
- National Campaign to Prevent Teen Pregnancy. Halfway there: A prescription for progress in preventing teen pregnancy. Washington, DC: Author; 2002. [Google Scholar]
- Nelson EC, Heath AC, Lynskey MT, Bucholz KK, Madden PAF, Statham DJ, Martin NG. Childhood sexual abuse and risks for licit and illicit drug-related outcomes: a twin study. Psychological Medicine. 2006;36:1473–1483. doi: 10.1017/S0033291706008397. [DOI] [PubMed] [Google Scholar]
- Neuhaus JM, McCulloch CE. Separating between- and within-cluster covariate effects by using conditional and partitioning methods. Journal of the Royal Statistical Society. 2006;68:859–872. [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Archives of General Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Roche KM, Mekos D, Alexander CS, Astone NM, Bandeen-Roche K, Ensminger ME. Parenting influences on early sex initiation among adolescents: How neighborhood matters. Journal of Family Issues. 2005;26:32–54. [Google Scholar]
- Rodgers JL, Rowe DC, Buster M. Nature, nurture and first sexual intercourse in the USA: Fitting behavioral genetic models to NLSY kinship data. Journal of Biosocial Science. 1999;31:29–41. doi: 10.1017/s0021932099000292. [DOI] [PubMed] [Google Scholar]
- Rutter M. Proceeding from observed correlation to causal inference: The use of natural experiments. Perspectives on Psychological Science. 2007;2:377–395. doi: 10.1111/j.1745-6916.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Belsky J, Brown G, Dunn J, D'Onofrio BM, Eekelaar J, Witherspoon S. Social science and family policies. London: British Academy Policy Centre; 2010. [Google Scholar]
- Rutter M The Academy of Medical Sciences Working Group. Identifying the environmental causes of disease: How should we decide what to believe and when to take action? London: The Academy of Medical Sciences; 2007. [Google Scholar]
- Santelli J, Carter M, Orr M, Dittus P. Trends in sexual risk behaviors, by nonsexual risk behavior involvement, U.S. high school students, 1991–2007. Journal of Adolescent Health. 2009;44:372–379. doi: 10.1016/j.jadohealth.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Senn TE, Carey MP, Vanable PA. Childhood and adolescent sexual abuse and subsequent sexual risk behavior: Evidence from controlled studies, methodological critique, and suggestions for research. Clinical Psychology Review. 2008;28:711–735. doi: 10.1016/j.cpr.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for generalized causal inference. Boston, MA: Houghton Mifflin; 2002. [Google Scholar]
- Siebenbruner J, Zimmer-Gembeck MJ, Egeland B. Sexual partners and contraceptive use: A 16-year prospective study predicting abstinence and risk behavior. Journal of Research on Adolescence. 2007;17:179–206. [Google Scholar]
- Snijders T, Bosker R. Multilevel analysis: An introduction to basic and advanced multilevel modeling. Thousand Oaks, CA: Sage Publishers; 1999. [Google Scholar]
- Statistics Sweden. Educational attainment of the population, from http://www.scb.se/Pages/Product____9577.aspx. 2011
- Stein MB, Lang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: A twin study. American Journal of Psychiatry. 2002;159:1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Healthy people 2010: Understanding and improving health. 2nd ed. Washington, DC: U.S. Government Printing Office; 2000. [Google Scholar]
- Verweij KJH, Zietsch BP, Bailey JM, Martin NG. Shared aetiology of risky sexual behaviour and adolescent misconduct: Genetic and environmental influences. Genes, Brain and Behavior. 2009;8:107–113. doi: 10.1111/j.1601-183X.2008.00456.x. [DOI] [PubMed] [Google Scholar]
- Young-Wolff KC, Kendler KS, Ericson ML, Prescott CA. Accounting for the association between childhood maltreatment and alcohol-use disorders in males: A twin study. Psychological Medicine. 2011;41:59–70. doi: 10.1017/S0033291710000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer-Gembeck MJ, Helfand M. Ten years of longitudinal research on U.S. adolescent sexual behavior: Developmental correlates of sexual intercourse, and the importance of age, gender, and ethnic background. Developmental Review. 2008;28:153–224. [Google Scholar]