Abstract
Prostaglandins (PGs) are ubiquitous membrane-derived, lipid-signaling molecules with wide ranging effects throughout the body. In the brain, PGE2 is the key regulator of fever after inflammation but is also implicated in neural development and synaptic plasticity. The steroid hormone estradiol is also a key regulator of neural development and synaptic plasticity. Recently, we showed that administering cyclooxygenase (COX) inhibitors to block PGE2 production increased the total length of Purkinje cell dendrites, the number of dendritic spines, and the level of spinophilin protein, which is enriched in dendritic spines. Correspondingly, PGE2 administration into the cerebellum decreased spinophilin protein content. We now report that PGE2 stimulates estradiol synthesis in the immature rat cerebellum via enhanced activity of the aromatase enzyme. Treatment with cyclooxygenase inhibitors reduced cerebellar aromatase activity and estradiol content whereas PGE2 administration increased both. Treatment with either PGE2 or estradiol stunted Purkinje neuron dendritic length and complexity and produced a corresponding reduction in spinophilin content. Treatment with formestane to inhibit aromatase activity led to excessive sprouting of the dendritic tree, whereas elevated estradiol had the opposite effect. Electrophysiological measurements from Purkinje neurons revealed novel sex differences in input resistance and membrane capacitance that were abolished by estradiol exposure, whereas a sex difference in the amplitude of the afterhyperpolarization after an action potential was not. Correlated changes in action potential threshold suggest that prolonged alterations in neuronal firing activity could be a consequence of increased estradiol content during the second week of life. These findings reveal a previously unappreciated role for PG-stimulated steroidogenesis in the developing brain and a new potential route for inflammation-mediated disruption of neuronal maturation.
Prostaglandins (PGs) are membrane-derived lipid-signaling molecules formed from arachidonic acid via the sequential coupling of the cyclooxygenase (COX) isoenzymes, COX-1 and COX-2 to the terminal PG synthases (45). PGE2 is the principal mediator of fever and inflammation but also contributes to fundamental neurological processes, including long-term potentiation in the hippocampus (1) and synaptogenesis in the developing preoptic area (2, 3). Receptors for PGE2 (EP1–4) are expressed in the developing sheep cerebellum (4) and in adult rat Purkinje cells (5), but a role for PGE2 signaling in cerebellar synaptic connectivity had not been demonstrated until recently. Treatment with nimesulide, a COX-2 inhibitor, increases both spine numbers and the total length of all the dendrites by about 30–50% in the posterior vermis and cerebellar hemispheres (6). Reciprocal alterations were observed in PGE2-treated animals when assessing spinophilin protein, a validated proxy for dendritic spine levels in the cerebellum. We now determine how PGE2 signals for these profound changes.
Cerebellar development is delayed relative to the rest of the central nervous system and undergoes significant cytoarchitectural changes after birth. The second postnatal week is a particularly dynamic period as granule cells are born and make contact with Purkinje cells, and the multiple climbing fibers synapsing on a single Purkinje cell are pruned to one (7–9). The cerebellum is also notable for the presence of estrogen receptors (ERs) (10) despite its having no role in reproduction. The mRNA levels of the ER receptors, ERα and ERβ, peak during the second postnatal week, and ER protein is detected within Purkinje cells at this time (9). In register with the peak in ER expression during the second postnatal week is a late emergence of expression of the estradiol-synthesizing enzyme, aromatase, which peaks around postnatal day (PN)10 (11), compared with a perinatal peak in other brain regions. Estradiol is an essential determinant of many developmentally established sex differences in the brain, particularly in the rodent, with the estradiol being derived from testicularly derived androgens (12, 13). However, the cerebellum has recently been identified as one of a few brain regions capable of de novo synthesis of estradiol, meaning it is synthesized locally from cholesterol with no requirement for gonadally derived upstream precursors such as testosterone or androstenedione (11, 14). Aromatase is the rate-limiting enzyme in estradiol production and hence subject to extensive regulation, by substrate and product, as well as by neuronal activity (15, 16). In the ovary and breast, PGs stimulate estradiol synthesis by increasing the expression of aromatase (17, 18). To our knowledge there have been no reports of PG stimulation of either aromatase synthesis or activity in the brain. We report here that PGE2 stimulates aromatase activity within the developing cerebellum and the synthesized estradiol plays a role in shaping the dendritic architecture of developing cerebellar Purkinje cells with associated changes in neurophysiological parameters. We also report novel sex differences in Purkinje neuron membrane input resistance and capacitance as well as the amplitude of the action potential afterhyperpolarization. Some of these sex differences were ablated by exposure to estradiol, and estradiol treatment increased the action potential threshold in both sexes. These results suggest complex interactions between neurosteriodogenesis and sex in the development and intrinsic excitability of the cerebellum.
Materials and Methods
Animals and treatment
All procedures used in these studies were approved by the Institutional Animal Care and Use Committee of the University of Maryland, Baltimore (Baltimore, MD). Animals were kept under a reverse 12-h light, 12-h dark cycle with ad libitum access to food and water. Sprague Dawley rats mated in our facility were allowed to deliver normally under standard laboratory conditions. Sesame oil was the vehicle for all drugs injected sc unless otherwise noted. Pups were injected sc daily for 3–7 consecutive days starting on PN7. Treatment groups for the sc injections were either 1) 0.1 ml of sesame oil vehicle; 2) the COX-2-specific inhibitor nimesulide (5 mg/kg); 3) the COX-1/-2 inhibitor indomethacin (2 mg/kg); 4) 17β-estradiol-3 benzoate (estradiol: 10 μg); 5) the aromatase inhibitor formestane (20 μg/kg); or 6) a combination of estradiol (10 μg) and nimesulide (5 mg/kg). The exact injection schedules, treatment groups, and doses are described at the beginning of Results for each experiment.
Intracerebellar injections
Pups were injected on PN10 and PN12 with low doses of ketamine (0.05 mg/kg) plus acepromazine (0.0125 mg/kg) ip to prevent distress or movement that could cause injury. A sharp, beveled needle attached to a Hamilton syringe was inserted 2 mm at the base of the skull into the foramen magnum. Pups then received a volume no greater than 1 μl of either 1) 1% dimethylsulfoxide vehicle; 2) PGE2 (2.5 μg); or, depending upon the experiment, 3) PGE2 (2.5 μg) combined with the ER antagonist ICI 182780 (50 ng). The doses of PGE2 and ICI 182780 were based on previous studies involving other brain regions (3, 19). Animals were monitored under a heat lamp until recovery from anesthesia (pups are mobile, vocalize, and urinate at least once) and then returned to their dam.
Cerebellar culture conditions
Whole cerebellums from PN0 mixed sex neonates were processed for primary dispersed mixed neuronal/glial cultures as described (20). Plates were maintained at 37 C and 5% CO2 for 14 d in vitro (DIV; time of plating, DIV0). Cells were treated once a day from DIV7-10 with 10.5 nm PGE2 or 0.1 m PBS vehicle. Cells were collected in lysis buffer at DIV11 for aromatase assay.
RIA
Animals were treated sc with either 1) sesame oil vehicle; 2) 5 mg/kg nimesulide; or 3) 2 mg/kg indomethacin daily from PN7-12 as described above. In a separate experiment they received PN10 and PN12 intracerebellar injections of PGE2 (2.5 μg) or vehicle as described above. Animals were anesthetized 24 h after the last treatment via overdose with Nembutal and then exsanguinated by transcardiac perfusion with ice-cold PBS. Cerebellums were collected and estradiol was extracted via a previously published method (21) with some modifications. Briefly, cerebellums were homogenized in radioimmune precipitation assay buffer (50 mm Tris-HCl, 1% Na-deoxycholate, 0.25% Nonidet P-40, 150 mm NaCl, 1 mm EDTA), protein concentration was determined via Bradford assay, and 100 μl of homogenate were collected. Diethyl ether (4 ml) was added to each homogenate in Teflon-capped glass extraction tubes and rotated horizontally for 30 min. Tubes were placed vertically and allowed to stand for 15 min to ensure complete separation. The aqueous phase was frozen within a dry ice and isopentane slurry, and the organic phase was decanted. Ether was allowed to evaporate overnight, after which 2 ml of 5% methanol in water were added to each tube and tubes were agitated for 1 h; steroids previously extracted into the ether were visible as white flaky precipitate. Precipitate in 5% methanol was added to previously conditioned 500 mg bed weight octadecyl C18 minicolumns according to the manufacturer's instructions (Amersham Biosciences, Piscataway, NJ). Columns were washed sequentially with 10 ml of 40% and 85% methanol to wash out sulfate esters and nonconjugated esters. A final 10 ml of 100% methanol were passed through the columns and collected. Samples were maintained at 37 C and methanol was allowed to evaporate. Samples were reconstituted in 0.1 m PBS. Samples were assayed in triplicate for estradiol by the Center for Research in Reproduction (University of Virginia, Charlottesville, VA), using commercially available doublelabel immunoassay kit (DPC-TKE21, Diagnostic Products Corp., Los Angeles, CA).
Aromatase assay
Pups received daily sc injections of either 5 mg/kg nimesulide or vehicle from PN7-9. On PN10, pups were killed by rapid decapitation, and fresh cerebellums were collected in 300 μl ice-cold buffer TEK buffer (150 mm KCl, 10 mm Tris-HEPES, pH 7.2). Aromatase activity was quantified by measuring tritiated water production using a previously validated method with modifications (22). Samples were incubated in duplicate for 18 h at 37 C with 125 nm [1β3′-3H]androstenedione and 2.4 mm reduced nicotinamide adenine dinucleotide phosphate. An aliquot of the same samples was incubated as above but with the addition of the aromatase inhibitor 1.6 mm letrozole (Sigma Chemical Co., St. Louis, MO). The long incubation period was empirically determined to provide the most reliable and robust measure but was not linear over time. The reaction was stopped by cooling the samples in an ice bath and adding 400 μl of ice-cold 10% trichloroacetic acid containing 2% activated charcoal. The tritiated water produced was isolated by centrifugation and column chromatography. Tubes were centrifuged at 1200 × g for 15 min. Supernatant was applied to small columns made of Pasteur pipettes plugged with glass beads and filled (3 cm high) with a Dowex cation exchange resin AG 50 W-X4, 100–200 mesh (Bio-Rad Laboratories, Richmond, CA). The columns were eluted with 3 × 0.6 ml distilled water and effluent collected in scintillation vials. Ecoscint A (10 ml, National Diagnostics, Atlanta, GA) was added, and vials were counted for 4 min on a Packard Tri-Carb 1600 TR Liquid Scintillation analyzer (Packard Instruments, Meriden, CT). The moles of heavy water and estrone produced by aromatase were calculated by the following: 1) the disintegrations per minutes from the letrozole-incubated samples were subtracted from the average values from the duplicates; 2) these values were divided by the product of the following variables: a) 2.2 × 1012 dpm/Ci of tritium; b) the specific activity of the androstenedione in Ci/mmol; c) the recovery yield of fluid from a column; d) the fraction of tritium with β-decay; and e) the volume of the homogenate; 3) finally these values were normalized to the amount of protein loaded into each reaction quantified by a Bradford assay. In a separate experiment, cultures were maintained until DIV10 as above, and six coverslips of like treatment were collected in TEK buffer and combined to obtain a single sample for aromatase assay. Assay was performed as above except samples were incubated for 48 h.
Golgi-Cox impregnation
Neonates were injected daily from PN7-13 with sc treatments of either 1) vehicle, 2) formestane, 3) estradiol alone, 4) nimesulide alone, or 5) coadministered estradiol and nimesulide. For the fourth group, nimesulide alone, we present data previously reported in Ref. 6 except that we combined the previous vehicle data with those presented here. Some of these animals were from the same litters as those in the current report. The inclusion of the previous vehicle animals and the nonsubjective nature of Golgi impregnated neuronal reconstruction assures no systematic bias. Alternatively, neonates were injected on PN10 and PN 12 intracerebrally with either 1) sterile saline or 2) PGE2 (2.5 μg). Brains were collected on PN14 and Golgi-Cox impregnated for 30 d using an established method (23) with described modifications (24). Brains were cut in 100 μm thick sagittal sections. Posterior vermal Purkinje cells were identified by 1) their lobule; 2) having cell bodies between the granule and molecular layer; and 3) their large size and complex dendritic trees with many spines. Five posterior vermal Purkinje neurons per animal were selected randomly by a researcher blinded to sex and treatment, excluding neurons that were entangled with others, and traced using Neurolucida (MBF Bioscience, Williston, VT) under a Nikon 100× oil objective (Nikon, Melville, NY), incorporating the complete dendrite from soma to distal ends. Spines were operationally defined as protrusions off the dendritic tree of less than 5 μm. Neuroexplorer software (MBF Bioscience,) was used to analyze the file of the trace of each individual Purkinje cell to quantify 1) the total length of all the dendritic segments from individual Purkinje cells; 2) the total number of dendritic spines per Purkinje cell; and 3) Sholl analysis of the number of times the dendritic tree intersects a series of concentric circles spaced 10 μm apart and centered around the perikaryon. Each morphological measurement was averaged from the five traced neurons per animal so that individual animals were treated as the subjects for statistical analyses. For Sholl analysis, concentric circles of increasing radius centered on the cell body were superimposed over traced neurons, and the number of intersections with each circle was counted. Images of Purkinje cell neurons representative of the group means shown in figures were captured with a Coolsnap CF camera (Photometrics, Tucson, AZ) under a Nikon 40× objective to allow for visualization of the entire dendritic tree. Images were then imported into Photoshop and adjusted for contrast, brightness, and color balance. All adjustments were applied to whole images and never to parts and were applied to allow greater ease of distinguishing characteristics of printed images. To save space, images were cropped to include only the neuron in focus.
Western blot
Western analysis was used to quantify spinophilin in neonatal and juvenile tissue as described elsewhere (2). Phototype Chemiluminescence System (New England Biolabs, Danvers, MA) was used to detect protein recognized with antisera at a concentration of 1:1000 (polyclonal rabbit, Upstate Biotechnology, Inc., Lake Placid, NY). Blots were exposed on Hyperfilm-ECL (Amersham) for varying durations (1–15 min.) The protein appeared as a band with a relative molecular mass of 120 kDa and the grayscale integrative area density (iad) with a charge-coupled device camera and quantified with National Institutes of Health IMAGE software. Blots were dyed with 0.1% Ponceau S in 5% acetic acid to visualize other proteins. iad of the dominant Ponceau band representing more than 200 proteins was measured, and spinophilin iad was normalized to these results to control for differences in total protein loading.
Slice preparation and electrophysiological recording
Sagittal slices of the cerebellar vermis 300 μm thick were made by a Leica VT1200S (Buffalo Grove, IL) in ice-cold cutting solution containing (in mm concentration): 75 sucrose, 85 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 4 MgCl2, 0.5 CaCl2, 25 d-glucose equilibrated under 95% O2/5% CO2 after cerebellar hemispheres were removed. Slices were kept at room temperature under the oxygenated artificial cerebrospinal fluid containing (in mm concentration) 120 NaCl, 3 KCl, 1 NaH2PO4, 2 MgSO4, 2.5 CaCl2, 25 NaHCO3, 20 d-glucose for 1 h before recording. Whole-cell patch clamp recordings were initially made under voltage clamp using a Axopatch-1 C amplifier (Axon Instruments, Union City, CA) and patch pipettes (pipette resistance in the bath was in the range of 4–8 MOhm) filled with (in mm concentration) 130 K-gluconate, 2.9 KCl, 10 HEPES-Na, 3 MgATP, 0.3 Tris-GTP, 0.2 CaCl2, 2 BAPTA-K, 2 MgSO4-7 H2O, and 0.1% wt/vol biocytin. Purkinje cells were observed under differential interference contrast with a Nikon Eclipse E600PN with a 40× water immersion objective before patching and were selected for by having round, teardrop-shaped cell bodies with little to no shadows in the contrast around the cell membrane. Data were filtered at 2 kHz and digitized at 10 kHz. Hyperpolarization steps (5-mV) were used to monitor input resistance (Rin), capacitance (Cin), access resistance (Ra), and the membrane time constant (τm). After switching to current clamp recording mode and sequentially giving inward current steps (30–300 pA at 20-pA intervals 50–110 msec duration), action potential threshold and amplitude were recorded. The afterhyperpolarization (AHP) was recorded from the first evoked action potential as described by Ovsepian and Friel (25).
Statistical analysis
All experiments with more than two groups were analyzed with a two-way ANOVA or analysis of covariance (ANCOVA) followed by either a Bonferroni-Sidak adjusted t test or Tukey's honest-significant difference multiple-comparison test to determine significance between groups. When an ANCOVA was used, the post hoc was a Bonferroni-Sidak t test for the marginal means that were adjusted by regression to the covariate to remove false-positive results caused by biases in the covariate alone. Aromatase assay results were analyzed using a Mann-Whitney U test due to nonhomogeneous variances between groups. Sholl analysis results were subject to Friedman's tests for repeated measures and Wilcoxon-Ranked Sign tests for post hoc analysis with Bonferroni-Sidak correction to avoid family-wise error because of nonhomogeneous variances. All statistical tests used a value of P < 0.05 as the criterion for significance, except for the cumulative frequency results of Sholl measures, which was P < 0.001.
Results
PGE2 stimulates aromatase activity and estradiol synthesis in the developing cerebellum
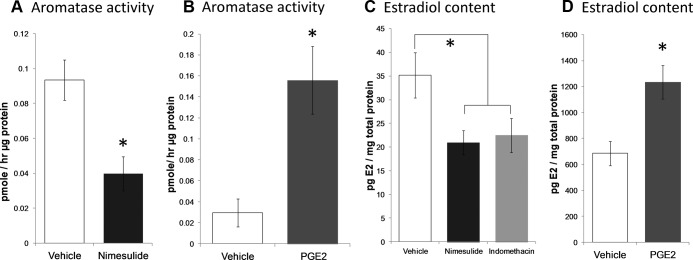
To assess the ability of PGE2 to stimulate aromatase activity and thereby increase estradiol production in the cerebellum, we used two approaches: 1) treatment of neonates with COX inhibitors or direct infusion of PGE2 into the cerebellum; and 2) in vitro cerebellar cultures obtained from day-of-birth rat pups and treated with PGE2 from day in vitro (DIV)7–10. For in vivo study, pups were treated from PN7–9 daily with sc injections of nimesulide (5 mg/kg) or vehicle and euthanized 24 h later on PN10. Whole cerebellums were flash frozen and stored at −80 C until aromatase activity assay (n = 4 male and 4 females for each treatment). Nimesulide treatment significantly reduced aromatase activity 57% from baseline (two-way ANOVA, Ftreatment=9.74; P = 0.009; Fig. 1A). No effects of sex or interactions between sex and nimesulide treatment were observed (Fint = 0.06; P = 0.811; Fsex = 0.28; P = 0.606).
Fig. 1.
PGE2 stimulates and COX inhibitors reduce aromatase activity and lower estradiol content in the developing cerebellum. Aromatase activity was measured by conversion of tritiated androstenedione to heavy water. A, Daily treatment of pups with 5 mg/ kg body weight (b.w.) nimesulide from PN7–9 reduced aromatase activity in the PN10 cerebellum compared with vehicle-treated controls (n = 3–4; two-way ANOVA; *, P = 0.02). B, Three days of treatment of cerebellar cultures with PGE2 increased aromatase activity compared with vehicle-treated controls. (n = 4 for each group; Mann-Whitney U; *, P = 0.05). C, Daily treatment for 3 d with the COX-2 inhibitor nimesulide (*, P = 0.04 compared with vehicle) or the COX-1/2 inhibitor indomethacin reduced estradiol content in the cerebellums of PN10 rats (two-way ANOVA; *, P = 0.03 compared with vehicle). D, Intracerebellar injection of PGE2 on PN10 and PN12 increased estradiol levels on PN13 (two-way ANOVA; *, P = 0.0007). Data are presented as means ± sems after collapsing across sex following a two-way ANOVA for treatment and sex with no significant effects of sex or interactions.
To determine whether the effects of COX inhibition and PGE2 availability were localized within the cerebellum, cerebellar cultures were generated as described above, and on DIV7-10 were treated with 10.5 nm PGE2 or 0.1 m PBS. Cells were collected in lysis buffer at DIV11 for aromatase assay. Cerebellar cultures treated with PGE2 had significantly increased aromatase activity compared with vehicle-treated controls (n = 4/group; Mann Whitney U = 0; P < 0.05; Fig. 1B).
To test the prediction that reduced aromatase activity resulted in decreased estradiol production, pups were treated daily from PN7-12 with either; 1) 0.1 ml sesame oil vehicle; 2) the COX-2 selective inhibitor, nimesulide (5 mg/kg); or 3) the COX-1 and -2 inhibitor indomethacin (2 mg/ kg) injected sc (n = 4 male and 4 female per treatment group). On PN13, 24 h after the last injection, cerebellums were collected and assessed for estradiol content by RIA. Inhibition of PG production with indomethacin (two-way ANOVA; Ftreatment = 3.42; P < 0.05; Bonferroni P = 0.0231; n values = 5–14) or nimesulide (P = 0.0132) significantly reduced cerebellar estradiol content compared with vehicle (Fig. 1C). Reciprocally, pups were injected intracerebellarly (n = 3–6 per treatment per sex) with either; 1) 0.1 m PBS; or 2) PGE2 (2.5 μg) on PN10 and PN12, and animals were killed 24 h later on PN13. Compared with vehicle injections, PGE2 injected locally into the cerebellum significantly increased cerebellar estradiol content (two-way ANOVA, Ftreatment = 9.94; P = 0.0071; Fig. 1D). There were no significant differences due to sex or interactions between treatment and sex. We therefore conclude that PGs stimulate cerebellar aromatase activity and locally increase estradiol production in both sexes.
Changes in PG synthesis and/or estradiol during the second postnatal week modulate the size of the dendritic tree of Purkinje neurons
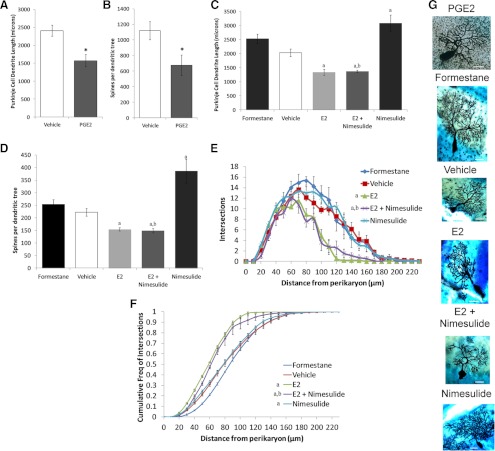
Previously, we showed that nimesulide exposure increased the size of the Purkinje cell dendritic tree without affecting morphological characteristics of other cell types. Our hypothesis predicts that exposure to either PGE2 or estradiol will encumber the development of Purkinje neuron dendrites. We again used two complimentary approaches to test this prediction; 1) morphological reconstruction of Purkinje cells impregnated using the Golgi-Cox reaction; and 2) Western blot quantification of spinophilin, a protein not only enriched in dendritic spines but also verified to correlate with the total number of dendritic spines in this and other brain areas (2, 3, 26, 27). Pups were injected on PN10 and PN12 with PGE2 or vehicle directly into the fluid surrounding the cerebellum, and brains were collected on PN14. PGE2 treatment significantly reduced the sum length of all dendritic segments (Ftreatment = 11.48; P = 0.0061) and total spine numbers (Ftreatment = 7.04; P = 0.024) in Golgi-impregnated Purkinje cells. There was no significant influence of sex (length: Fsex = 0.5; P = 0.49; spines: Fsex = 1.09; P = 0.32) nor an interaction between sex and PGE2-treatment (length: Finteraction = 0.42; P = 0.53; spines: Finteraction = 0.55; P = 0.48) for either measure. The decrease in dendritic spine number is secondary to the decrease in length because the density of dendritic spines per unit length of dendrite was not influenced by PGE2 treatment (vehicle: 4.62 ± 0.22 spines/μm vs. PGE2: 4.26 ± 0.58 spines/μm; Ftreatment = 0.21; P = 0.66) or sex (Fsex = 0.45; P = 0.52) or an interaction between the sex and treatment (Finteraction = 0.82; P = 0.38). The number of branch points per unit length of dendrite was unaffected by PGE2 treatment (vehicle: 0.0513 ± 0.0011 branch points/μm dendrite vs. PGE2: 0.494 ± 0.0013 branch points/μm dendrite; Ftreatmetn = 0.844; P = 0.37); sex (Fsex = 0.035, P = 0.85) or an interaction between the two (Finteraction = 1.109; P = 0.31), suggesting that the decrease in dendritic length was not due to a reduction in the number of branches from the primary dendrite, but instead a result of decreasing the size of the tree evenly.
We next sought to determine whether formestane treatment would increase the complexity of the dendritic arbor compared with vehicle and whether estradiol treatment would mimic PGE2 treatment as would be predicted from our model. Because these were different questions, separate omnibus and post hoc analyses were performed. From PN7 to PN13, pups were injected sc daily with either; 1) 0.1 ml sesame oil vehicle (see notes in Materials and Methods regarding this group); 2) the aromatase inhibitor formestane (20 μg/kg); 3) estradiol (10 μg) alone; 4) nimesulide (5 mg/kg) alone (see notes in Materials and Methods regarding this group); or 5) estradiol (10 μg) and the COX-2 inhibitor nimesulide (5 mg/kg) combined. Brains were also collected on PN14, 24 h after the last injection. Formestane treatment trended toward increasing Purkinje cell dendritic length (Ftreatment = 3.34; P = 0.086) compared with length from vehicle treatment (Fig 2C). There was no effect of sex or an interaction between the two on dendritic length (Fint = 0.07; P = 0.79; Fsex = 0.08; P = 0.79). The total number of dendritic spines per tree was unaffected by sex alone and there was no interaction between sex and treatment (Ftreatment = 1.21; P = 0.29; Fsex = 0.07; P = 0.80; Fint = 0.01; P = 0.99, Fig 2D). Purkinje cell dendrite length (Ftreatment = 11.74; P < 0.0001) and total spine numbers (Ftreatment = 11.92; P < 0.001) were significantly affected by treatment with either estradiol alone, nimesulide alone, or estradiol combined with nimesulide, but there were no interactions between sex and treatment (length: Fint = 0.09; P = 0.96; spines: Fint = 0.08; P = 0.97) or effects of sex alone (length: Fsex = 0.02; P = 0.89; spines: Fsex = 0.0001; P = 0.99). A priori, we limited our post-hoc Sidak-Bonferroni comparisons to the following: each treatment condition vs. vehicle; estradiol alone vs. estradiol combined with nimesulide, and nimesulide alone vs. estradiol combined with nimesulide. Dendritic length and spine numbers were significantly decreased after treatment with estradiol, (length: P = 0.004; spines: P < 0.001, respectively) or estradiol combined with nimesulide (P < 0.001 for both) whereas dendritic lengths and spine counts significantly increased with nimesulide treatment (length: P < 0.001; spines: P = 0.01) relative to vehicle. Dendritic length and total spine numbers both decreased significantly with treatment of a combination of estradiol and nimesulide compared with nimesulide alone (length: P < 0.001; spines: P = 0.0015) but not compared with estradiol alone (length: P = 0.85; spines: P = 0.60). Similar to PGE2 treatment above, the density of dendritic spine numbers per unit length of dendrite was also unaffected by treatment (F = 1.59; P = 0.20), sex (F = 0.29, P = 0.87), or an interaction between sex and treatment (F =1.89; P = 0.14).
Fig. 2.
Exogenous estradiol inhibits Purkinje cell dendritic development and endogenous estradiol is necessary for normal development. A, PGE2 treatment significantly reduces the length of Purkinje dendrites regardless of sex (*, P < 0.05, two-way ANOVA). B, PGE2 treatment also significantly reduced the total number of dendritic spines per Purkinje neuron when counted at ×100 magnification (*, P < 0.05, two-way ANOVA). C, Estradiol treatment significantly reduced the length of Purkinje dendrites, regardless of whether it was combined with the COX inhibitor, nimesulide (two-way ANOVA, a priori comparisons with Bonferroni-Sidak correction: a, P < 0.01 compared to vehicle; b, P < 0.01 compared to nimesulide). D, In the same animals, estradiol also decreased the number of spines per Purkinje neuron regardless of nimesulide coadministration (two-way ANOVA, a priori comparisons with Bonferroni-Sidak corrections; a, P < 0.01 compared to vehicle; b, P < 0.01 compared to nimesulide) when counted at ×60 magnification. The lack of effect of a COX inhibitor on estradiol action indicates that prostaglandin synthesis is not necessary for estradiol-induced reductions in Purkinje cell dendritic arbor size and suggests PGE2 synthesis is upstream of estradiol signaling. E, Sholl analysis characterizing the number of intersection of dendrites with 10 μm spaced concentric circles relative to their distance from the soma. Estradiol administration or coadministration of estradiol and nimesulide reduced the number of intersections as a function of their distance, such that the dendritic tree is restricted to a smaller area and branches less frequently (Friedman Test, post hoc: a priori Wilcoxon-Signed Rank Tests: a, P < 0.01 compared to vehicle; b, P < 0.01 compared to nimesulide). F, The cumulative frequency of intersections of dendrite segments with concentric shifts to the left in estradiol treated animals regardless of nimesulide treatment, better depicting that the neuron's dendritic arbor is restricted to a smaller area (Friedman Test; post hoc: a priori Wilcoxon-Signed Rank Tests: a, P < 0.001 compared to vehicle; b, P < 0.001 compared to nimesulide). G, Images of typical Golgi-Cox impregnated Purkinje cells from the same treatment groups representing the group means. Scale bar, 25 microns. Data are presented as means ± sems after collapsing across sex following a two-way ANOVA for treatment and sex. No significant effects of sex or interactions between sex and treatment were found. E2, Estradiol.
In-depth Sholl analysis also buttresses the finding that treatment does not affect the complexity of the dendritic tree by pruning secondary branches, but rather reduces the size of the tree overall. In formestane vs. vehicle comparisons, formestane treatment had no effect on the intersections between the dendritic tree and concentric circles spaced 10 μm around the perikaryon as a function of distance (Wilcoxon's Signed Rank test: W = −44; z = −0.76; P = 0.44, Fig 2E), and the cumulative frequency of curve of these measures (W = 118; z = 2.19; P = 0.028 vs. critical P < 0.001; Fig 2F). Similarly, treatment with estradiol, nimesulide, or the combination of the two significantly affected the number of intersections between the dendrite and concentric circles spaced 10 μm around the perikaryon as a function of distance from the perikaryon (Freidman's χ2 = 33.39; P < 0.0001), such that measures from treatment with estradiol alone (W = 181; z =3.14; P = 0.002) and the combination of estradiol and nimesulide (W = 227; z = 3.91; P = 0.0001) but not nimesulide alone significantly differed from measures from vehicle treatment. Coadministration of estradiol with nimesulide (W = −24; z = 0.51; P = 0.61) did not affect the measures of intersections when compared against measures from estradiol alone but when compared against measures from nimesulide alone, effect size differed (W = −226; z = −3.92; P < 0.0001). Intersections for nimesulide treatment did not differ from those of vehicle (W = −85; z = −1.47; P = 0.14). Treatment also affected the curve of the cumulative frequency of intersections as a function of distance (Friedman's χ2 = 51.13; P < 0.0001). The curves for estradiol compared with the curve of vehicle (Wilcoxon-Signed Rank: W = −210; z = 3.91; P = 0.0001) indicating that the dendritic arbor was both smaller and terminated closer to the cells' perikaryon. This effect of estradiol was not altered by simultaneous administration of the COX inhibitor, nimesulide, such that the curve of the combination of estradiol and nimesulide was to the left of both vehicle (W = −208; z = −3.87; P = 0.0001) and nimesulide (W = 194; z = 3.61; P = 0.0003). The curve for nimesulide was to the significantly right of that of vehicle (W = 210; z = 3.91; P = 0.0001). In sum, estradiol and estradiol + nimesulide treatment restricts the dendritic arbor to a smaller area such that the density of branching per unit area does not appreciably increase. Photomicrographs of representative Purkinje neurons are shown in Fig. 2G and Supplemental Fig. 1.
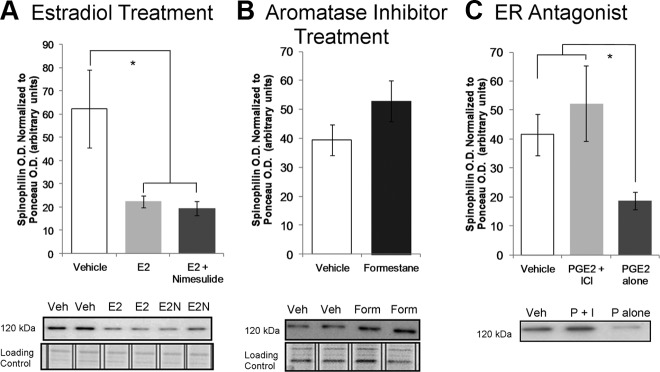
Similar effects were seen when using Western blot analysis for spinophilin as a proxy marker for dendritic morphology. Pups were injected daily from PN7 to PN13 with either 1) 0.1 ml sesame oil; 2) estradiol (10 μg) alone; or 3) a combination of estradiol (10 μg) and nimesulide (5 mg/kg), and on PN14 the pups were killed and spinophilin content of the posterior vermis assessed by Western blot. Treatment with estradiol significantly decreased spinophilin levels, compared with the levels after saline treatment, and this decrease was neither enhanced nor blocked by simultaneous treatment with nimesulide; no effects of sex or interactions between sex and treatment were observed (two-way ANOVA; Ftreatment = 4.64; P = 0.0156; Fint = 0.70; P = 0.50; Fsex = 0.01; P = 0.92; Tukey's honest significant difference, P < 0.05; n = 14–16; Fig. 3A). The lack of effect of the COX inhibitor nimesulide on the effects of estradiol is consistent with the interpretation that estradiol acts downstream of PGs. On the other hand, treatment with formestane from PN7–13 produced a trend toward increased cerebellar spinophilin content compared with vehicle treated (Ftreatment = 3.821; P = 0.0624), but again there was no effect of sex or an interaction between treatment and sex (Fint = 0.657; P = 0.4255; Fsex = 1.623; P = 0.215; n = 6–9 per sex per treatment; Fig. 3B).
Fig. 3.
PGE2-induced estradiol synthesis reduces spinophilin. A, Treatment with either E2 or E2 + nimesulide during the second postnatal week reduced spinophilin protein content (two-way ANOVA; *, P < 0.05 compared with vehicle by Tukey's honest significant difference), which is consistent with dendritic spine numbers observed by Golgi-Cox impregnation. Representative Western blots reveal lower spinophilin iad in estradiol-treated groups with no change in loading control (Ponceau staining). B, Effect of treatment with an inhibitor of estrogen synthesis, formestane, on spinophilin content (two-way ANOVA; P = 0.0624 compared with vehicle). C, To assess whether E2 synthesis is downstream of PGE2 signaling, animals were treated with either PGE2 or PGE2 + ICI 182,780, an E2 receptor antagonist. Exposure to PGE2 mimicked estradiol by reducing spinophilin level in the cerebellum, and coadministration of ICI with PGE2 prevented the decrease in spinophilin levels by PGE2 alone (two-way ANOVA; *, P < 0.05 compared with PGE2 treatment alone by Sidak Bonferroni). Above, Data are presented as group means ± sems after collapsing across sex following a two-way ANOVA for treatment and sex with no significant effects of sex or interactions. Below, Immunoblots of spinophilin protein and loading control Ponceau staining protein representing the group means. E2, Estradiol; E2N, E2 + nimesulide; Veh, vehicle.
If PGE2 affects cerebellar development via estradiol, its effects should be mediated via ER. Therefore, we next sought to determine whether PGE2 affects cerebellar development independently of the effects of estradiol on ER. Rat pups were treated on PN10 and PN12 via intracerebellar injection with either 1) 1 μl of 1% dimethylsulfoxide/99% 0.1 m PBS vehicle; 2) PGE2 (2.5 μg) alone; or 3) a combination of PGE2 (2.5 μg) and the ER antagonist ICI 182,870 (50 ng). Again no effects of sex or interactions between sex and treatment were observed (two-way ANOVA: Fint = 0.6; P = 0.558; Fsex = 1.65; P = 0.214), but infusion of PGE2 directly into the cerebellum significantly reduced spinophilin in the posterior vermis (Ftreatment = 3.57; P = 0.047; Sidak-Bonferroni: P = 0.0159; n = 8–10); this effect was blocked by simultaneous administration of ICI 182,780 (P = 0.014 compared with PGE2 alone; Fig 3C) such that levels were equivalent to those of vehicle treatment. We therefore conclude that the effect of PGE2 on Purkinje cell dendrites is mediated by induction of aromatase activity directly within the cerebellum, which in turn increases estradiol production to affect spinophilin levels.
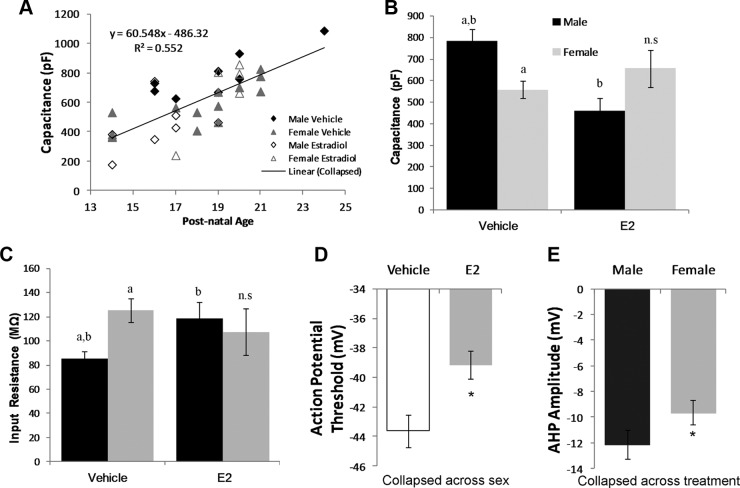
Treatment with estradiol during the second postnatal week modulates the passive and active electrophysiological properties of Purkinje cells between PN14 and PN24
Because estradiol treatment significantly reduced the size of the dendritic arbor of Purkinje cells, we predicted that the same treatment would change the passive and active electrophysiological properties of these cells, including action potential threshold, action potential amplitude, afterhyperpolarization amplitude, resting membrane potential, input capacitance (Cin), input resistance (Rin), and the membrane time constant (τm) (See Table 1). Comparisons were made between Purkinje cells in slices prepared from male and female animals treated with vehicle or estradiol. Because we could not measure all cells on the same developmental day, electrophysiological measures were tested for a correlative relationship with postnatal age to remove any bias of age on treatment effects (See Table 2 for average postnatal ages for each treatment). Pups were injected sc daily from PN7-13 with either 1) 0.1 ml sesame oil vehicle; or 2) estradiol (10 μg) and euthanized between PN14 and PN24 for in vitro slice preparation.
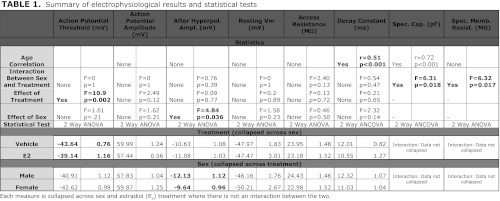
Table 1.
Summary of electrophysiological results and statistical tests
Each measure is collapsed across sex and estradiol (E2) treatment where there is not an interaction between the two.
Table 2.
Summary of the postnatal ages of the animals used in electrophysiological recordings by group
| Male vehicle | Female vehicle | Male E2 treated | Female E2 treated | |
|---|---|---|---|---|
| Average postnatal day | 18.88 | 17.79 | 16.56 | 19.43 |
| sem | 0.93 | 0.74 | 0.58 | 0.48 |
E2, Estradiol. Where there was a correlation between age and an electrophysiological measure, such as input capacitance, we compared the estimated marginal means after regression by Bonferroni tests to remove any bias that postnatal age might have on the raw means.
There were no significant effects of treatment, sex, or an interaction between the two on the amplitude of the action potential (Ftreatment = 2.49; P = 0.12; Fsex = 1.62; P = 0.214; Fint = 0.01; P = 0.97), the resting membrane potential (Ftreatment = 0.2; P = 0.889; Fsex = 1.58; P = 0.225; Fint = 0.01; P = 0.97), or the access resistance (Ftreatment = 0.13; P = 0.721; Fsex = 0.46; P = 0.501; Fint = 2.40; P = 0.131; Table 1). There also was no correlation between these parameters and age of animals at the time of recording. In contrast, for both the Cin and Rin, there was a significant interaction between sex and treatment (Fint = 6.32; P = 0.017; two-way ANOVA for Rin and Fint = 5.007; P = 0.032; two-wayANCOVA for Cin); additionally Cin but not Rin significantly and positively correlated with postnatal age (r = 0.72; P < 0.001; Fig 4A). The Cin of vehicle-treated males was significantly higher than that of vehicle-treated females (P = 0.025; Bonferroni-Sidak of marginal means adjusted for age; Fig 4B and Table 2 for ages). Estradiol treatment reduced the Cin of males such that they were significantly below those of control males (P = 0.032) and equivalent to both vehicle- and estradiol-treated females. Estradiol treatment did not reduce the Cin of female Purkinje cells, but measures did not differ from vehicle-treated males either. Correspondingly, the Rin of males was significantly lower than that of females (Bonferroni Sidak; P < 0.01), but treatment with estradiol significantly increased it in males to the level seen in either estradiol- or vehicle-treated females (Bonferroni Sidak, P < 0.01). There was no effect of estradiol treatment in females (Fig 4C). The decay time constant, τm, also correlated significantly with postnatal age (r = 0.51; P < 0.001) such that the time constant increased with age, but there were no effects of either treatment or sex alone or an interaction between the two (Ftreatment = 0.21; P = 0.65; Fsex = 2.32; P = 0.137; Fint = 0.54; P = 0.47 by ANCOVA; Table 2). Estradiol treatment significantly increased evoked action potential threshold of Purkinje neurons regardless of sex or age (Ftreatment = 10.9; P = 0.0024; Fsex = 1.61; P = 0.214; Fint = 0.01; P = 0.97; Fig 4D). Lastly, the AHP amplitude was significantly deeper for males than females, regardless of estradiol treatment (Fsex = 4.84; P = 0.036; Ftreatment = 0.203; P = 0.66; Fint = 0.01; P = 0.99; Fig 4E).
Fig. 4.
Passive and active electrophysiological properties of Purkinje cells following estradiol treatment. A, Animals ranged in age from PN14 to PN24 and the input capacitance was found to be positively correlated with postnatal age (two-way ANCOVA n = 8–14 per sex per treatment; r = 0.72; P < 0.001). Vehicle-treated males exhibit a higher input capacitance (Cin, inset B) and a lower input resistance (Rin, inset C) than vehicle-treated females (a, P < 0.05 difference from other groups designated “a”). Estradiol treatment significantly modified male (b, P < 0.05 difference from other groups designated “b”) but not female measures and as a result the basal sex differences were eliminated [two-way ANOVA; Bonferroni test for either means (Rin) or estimated marginal means (Cin) P < 0.05]. D, Estradiol treatment raised the threshold for action potential initiation regardless of sex (*, P < 0.05, two-way ANOVA). E, The amplitudes of Purkinje cell afterhyperpolarizations were greater in males compared to females. (*, P < 0.05, two-way ANOVA). E2, Estradiol.
Discussion
The second postnatal week is an important time in cerebellar development. The dendritic tree is maturing, and the multiple climbing fibers from the inferior olive that innervated the same Purkinje cell are pruned to a single contact (7). In the mature cerebellum the climbing fiber provides a powerful source of excitation for Purkinje cells that induces a characteristic burst of activity known as the complex spike. Also during the second postnatal week the bulk of cerebellar granule cells are born, make contact with their Purkinje cell partners in spatially restricted rows of parallel fibers, and migrate to the internal granule cell layer (28). This complex series of events requires a coordinated developmental program and, as such, represents a period of heightened vulnerability to disturbance. Both intrinsic and extrinsic factors impact on the course of Purkinje cell development, further increasing the vulnerability of this critical brain area. Here we identify a common pathway between the intrinsic factor, estradiol, and upstream PGE2, an important intrinsic signaling molecule that is also subject to extrinsic regulation by infectious agents, injury, or trauma. Although it is known that PGE2 regulates estradiol synthesis in the ovary and breast cancer cells, this is the first report of such a role in the CNS.
Estradiol is both a gonadally and neuronally synthesized signaling molecule derived by aromatization of its precursor, testosterone (13). We observed that PGE2 stimulated cerebellar aromatase activity during the second postnatal week and that convergent evidence suggests subsequent estradiol production reduced not only the size of the dendritic trees of Purkinje cells but also the total number of dendritic spines. Because there were no changes in the density of spines, the PGE2-estradiol-induced reduction in the total number of dendritic spines appears to be a by product of the reduced size of the dendritic tree. We used the dendritic spine proxy marker, spinophilin, which is concentrated in dendritic spines and positively correlates with the overall number of dendritic spines in several brain regions (2, 3, 27), including the cerebellum (6). In animals treated with intracerebellar PGE2, we observed reductions in the sum length of the dendritic tree, numbers of spine, and protein content for spinophilin. Moreover, the reduction in spinophilin was prevented by cotreatment with PGE2 and the ER antagonist, ICI 182,870. In contrast, combined treatment with the COX inhibitor, nimesulide, and estradiol failed to nullify the decreases in spinophilin or total dendrite length observed after estradiol treatment alone. We interpret these results to indicate that estradiol action on Purkinje dendritic development is downstream of PGE2, and consistent with the ability of PGE2 signaling to increase the activity of aromatase (Fig 5). Contrary to our expectation, the effects of PGE2 on estradiol production and associated neuroanatomical measures were independent of sex.
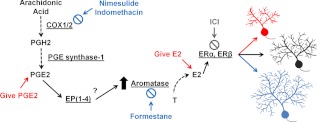
Fig. 5.
Working model of PGE2-E2 pathway in the cerebellum. Arachidonic acid is converted to PGH2 by the enzymes COX1/2, and further metabolized to PGE2 via PGE synthase-1. PGE2 interacts with its receptors EP(1–4), increasing aromatase activity to convert testosterone to estradiol, which binds to its receptors ERα and ERβ and regulates Purkinje cell dendritic growth. Estradiol acts as a brake on growth; changes to the pathway that increase E2 stunt dendritic growth (red), and changes to the pathway that decrease E2 allow for dendritic overgrowth (blue). E2, Estradiol; ICI, ICI 182,780.
An important question is the functional impact of altered Purkinje neuron development. We previously observed that stunting of the Purkinje dendritic tree in response to elevated PGE2 correlates with impairments in social play behavior and somatosensory threshold in males (6). In the current study, we assessed passive and active electrophysiological properties of Purkinje cells and observed changes consistent with a net decrease in Purkinje cell excitability in estradiol-treated animals. Estradiol exposure raised the threshold for an action potential further away from the membrane resting potential. The increased action potential threshold likely reflects a lower density of voltage-gated sodium channels at the soma. The input resistance (Rin) was lower and input capacitance (Cin) was higher in males than females, and there was an interaction between sex and estradiol treatment such that only males responded to estradiol treatment by significantly increasing Rin and decreasing Cin to levels equivalent to those of females. The capacitance and decay constant positively correlated with developmental age whereas the input resistance did not, likely because normal dendritic outgrowth during this time increases the total membrane surface area and capacitance. To keep the total input resistance stable with age, Purkinje cells adjust by also increasing specific resistance per unit area of membrane with age, as demonstrated by the correlation between age and the decay constant. The lack of interaction between sex and estradiol treatment for the decay constant undercuts the possibility that the change in Rin across sex and estradiol treatment results from a greater density or unitary conductance of leak channels because the decay constant is the product of the specific resistance and an invariable constant, the specific capacitance. The electrophysiological measurements were made between PN14 and PN24, after our morphological measurement of Golgi-Cox impregnated neurons on PN14, so we cannot preclude the possibility that increased input resistance and decreased capacitance in females or estradiol-treated males could reflect a late-emerging change in the size of the cell, especially considering that we previously observed a sex difference in the overall size of the cerebellum, which was larger in males at PN40 (6). However, this also assumes that sex and estradiol do not affect the properties of complex electrophysiological models, such as the electrotonic compactness of Purkinje neurons (29). Moreover, unlike in females, the increase in resistance observed in males after estradiol treatment might somewhat offset the effect of the increased in action potential threshold. Males also had a significantly greater amplitude of the AHP than females, regardless of estradiol treatment. The calcium-chelating protein calbindin, found particularly in Purkinje cells, is lower in male cerebellum than female (30) and may contribute to the sex difference in AHP by allowing for greater increases in free cytosolic calcium in males than females (31). The activation of Ca2+-activated K+ channels would be greater in males resulting in larger AHPs and could shut down burst-firing activity sooner, thus decreasing the intrinsic excitability and the bursting firing rate of their Purkinje neurons (31).
How these estradiol-induced changes in basic cellular parameters would affect social play behavior and other brain regions important for the behavior remains a difficult prediction. Electrical stimulation of Purkinje cells increases extracellular levels of dopamine in the medial prefrontal cortex (MPFC), a brain area implicated in social play behavior (32, 33). The targets of dopaminergic activity in the MPFC, including synapsin II, regulate social play behavior (33, 34). Moreover, decreases in Purkinje cells, and therefore activity, mimic the effects of excitotoxic lesions to the MPFC and result in increased repetitive behavior, a hallmark of autism (35).
The ability of the brain to locally synthesize steroids is of increasing interest, but there are relatively few examples of either physiological regulation of local steroidogenesis or a functional impact of such. The cerebellum is poised to provide unique insights on both levels, because it is not only capable of the in situ aromatization of estradiol from testosterone, but also the complete repertoire of enzymatic steps to synthesize cholesterol into gonadal steroids. Purkinje cells expresses multiple neurosteroidogenic enzymes including steroidogenic acute regulatory protein, cytochrome P450 side-chain cleavage family 11 subfamily A polypeptide 1 enzyme (P450scc or Cyp11a1) (36), 3-β hydroxysteriod dehydrogenase (3β-HSD) (37), 17β-HSD (38), and aromatase (39, 40). Estradiol levels are higher in the neonatal cerebellum than in the plasma (41), and expression of some of these enzymatic isoforms are higher in the cerebellum than in endocrine tissues (38). The regulation of these enzymes is also important for a number of cellular functions and may mediate the phenotype of certain genetic mutations. Estradiol administration to males, but not females, restores deficiencies in reelin mRNA levels caused by heterozygote expression of the null mutation and thereby increases the survival of Purkinje cells (42). Moreover, other laboratories administered estradiol to rats at earlier developmental time points than here and observed dendritic outgrowth (39). The source of variation could also be related to route of administration, dose, timing, or methods for measuring dendrites. In zebra finches, cerebellar lesions up-regulate steroidogenic acute regulatory protein, P450scc, 3β-HSD, and aromatase, and estrogen signaling is necessary for improvements in cognitive and motor function after these injuries (43). Here we show the inflammatory mediator PGE2 initiates the neurosteroidogenesis of estradiol, which affects neuronal morphology and activity in the developing cerebellum. PGE2 is also one of the principal signaling molecules released after neuronal hyperexcitability, injury, or oxygen/glucose deprivation because it increases, in part, cerebral blood flow (44). During development, the neuronal responses from the PGE2-regulated biosynthesis of estradiol could represent a double-edged sword because the increase in action potential threshold and decreases in excitatory axo-dendritic synapses could temporarily stave off neuronal hyperexcitability and loss after inflammatory insults, but, in the long-run, also negatively impact juvenile and adult cognitive processes such as those that encode for social play and somatosensory thresholds. Thus the significance of PGE2-mediated steroidogenesis within the cerebellum may have wide-ranging consequence for both normal and pathological functioning of this brain region.
Supplementary Material
Acknowledgments
We thank M. Vogel for help and advice on cerebellar dispersed cultures. We also thank Aleisha Shoenfelder and the Center for Research in Reproduction Ligand Assay and Analysis Core for their measurement of cerebellar estradiol content (University of Virginia, Charlottesville, VA).
This work was supported by grants from the US National Institutes of Health (R01 MH52716). S.L.D. was supported by the Training Grant for Integrative Membrane Biology to the University of Maryland (T32 GM081810).
Author Contributions: S.L.D., M.M.M. and B.E.A. designed the experiments. S.L.D., J.F.H. and M.W. performed the experiments. C.L.W. and S.L.D. analyzed the data. S.L.D., C.L.W. and M.M.M. wrote the paper. M.M.M. and B.E.A. supervised the project. All authors offered editorial advice.
Disclosure Summary: The authors do not report any conflicts of interest.
Footnotes
- AHP
- Afterhyperpolarization
- ANCOVA
- analysis of covariance
- COX
- cyclooxygenase
- DIV
- days in vitro
- ER
- estrogen receptor
- HSD
- hydroxysteroid dehydrogenase
- iad
- integrative area density
- MPFC
- medial prefrontal cortex
- PN
- postnatal day.
References
- 1. Chen C, Magee JC, Bazan NG. 2002. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J Neurophysiol 87:2851–2857 [DOI] [PubMed] [Google Scholar]
- 2. Amateau SK, McCarthy MM. 2002. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J Neurosci 22:8586–8596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amateau SK, McCarthy MM. 2004. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci 7:643–650 [DOI] [PubMed] [Google Scholar]
- 4. Tai TC, Lye SJ, Adamson SL. 1998. Expression of prostaglandin E2 receptor subtypes in the developing sheep brainstem. Brain Res Mol Brain Res 57:161–166 [DOI] [PubMed] [Google Scholar]
- 5. Candelario-Jalil E, Slawik H, Ridelis I, Waschbisch A, Akundi RS, Hull M, Fiebich BL. 2005. Regional distribution of the prostaglandin E2 receptor EP1 in the rat brain: accumulation in Purkinje cells of the cerebellum. J Mol Neurosci 27:303–310 [DOI] [PubMed] [Google Scholar]
- 6. Dean SL, Knutson JF, Krebs-Kraft DL, McCarthy MM. 2012. Prostaglandin E2 is an endogenous modulator of cerebellar development and complex behavior during a sensitive postnatal period. Eur J Neurosci 35:1218–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crepel F, Delhaye-Bouchaud N, Dupont JL. 1981. Fate of the multiple innervation of cerebellar Purkinje cells by climbing fibers in immature control, x-irradiated and hypothyroid rats. Brain Res 227:59–71 [DOI] [PubMed] [Google Scholar]
- 8. Altman J. 1972. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol 145:399–463 [DOI] [PubMed] [Google Scholar]
- 9. Ikeda Y, Nagai A. 2006. Differential expression of the estrogen receptors α and β during postnatal development of the rat cerebellum. Brain Res 1083:39–49 [DOI] [PubMed] [Google Scholar]
- 10. Jakab RL, Wong JK, Belcher SM. 2001. Estrogen receptor beta immunoreactivity in differentiating cells of the developing rat cerebellum. J Comp Neurol 430:396–409 [DOI] [PubMed] [Google Scholar]
- 11. Lavaque E, Mayen A, Azcoitia I, Tena-Sempere M, Garcia-Segura LM. 2006. Sex differences, developmental changes, response to injury and cAMP regulation of the mRNA levels of steroidogenic acute regulatory protein, cytochrome p450scc, and aromatase in the olivocerebellar system. J Neurobiol 66:308–318 [DOI] [PubMed] [Google Scholar]
- 12. Phoenix CH, Goy RW, Gerall AA, Young WC. 1959. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65:369–382 [DOI] [PubMed] [Google Scholar]
- 13. McEwen BS, Lieberburg I, Chaptal C, Krey LC. 1977. Aromatization: important for sexual differentiation of the neonatal rat brain. Horm Behav 9:249–263 [DOI] [PubMed] [Google Scholar]
- 14. Tsutsui K, Sakamoto H, Ukena K. 2003. Biosynthesis and action of neurosteroids in the cerebellar Purkinje neuron. J Steroid Biochem Mol Biol 85:311–321 [DOI] [PubMed] [Google Scholar]
- 15. Charlier TD, Harada N, Balthazart J, Cornil CA. 2011. Human and quail aromatase activity is rapidly and reversibly inhibited by phosphorylating conditions. Endocrinology 152:4199–4210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dickens MJ, Cornil CA, Balthazart J. 2011. Acute stress differentially affects aromatase activity in specific brain nuclei of adult male and female quail. Endocrinology 152:4242–4251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cai Z, Kwintkiewicz J, Young ME, Stocco C. 2007. Prostaglandin E2 increases cyp19 expression in rat granulosa cells: implication of GATA-4. Mol Cell Endocrinol 263:181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brueggemeier RW, Richards JA, Joomprabutra S, Bhat AS, Whetstone JL. 2001. Molecular pharmacology of aromatase and its regulation by endogenous and exogenous agents. J Steroid Biochem Mol Biol 79:75–84 [DOI] [PubMed] [Google Scholar]
- 19. Speert DB, Konkle AT, Zup SL, Schwarz JM, Shiroor C, Taylor ME, McCarthy MM. 2007. Focal adhesion kinase and paxillin: novel regulators of brain sexual differentiation? Endocrinology 148:3391–3401 [DOI] [PubMed] [Google Scholar]
- 20. Heitz S, Gautheron V, Lutz Y, Rodeau JL, Zanjani HS, Sugihara I, Bombarde G, Richard F, Fuchs JP, Vogel MW, Mariani J, Bailly Y. 2008. BCL-2 counteracts Doppel-induced apoptosis of prion-protein-deficient Purkinje cells in the Ngsk Prnp(0/0) mouse. Dev Neurobiol 68:332–348 [DOI] [PubMed] [Google Scholar]
- 21. Amateau SK, Alt JJ, Stamps CL, McCarthy MM. 2004. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology 145:2906–2917 [DOI] [PubMed] [Google Scholar]
- 22. Baillien M, Balthazart J. 1997. A direct dopaminergic control of aromatase activity in the quail preoptic area. J Steroid Biochem Mol Biol 63:99–113 [DOI] [PubMed] [Google Scholar]
- 23. Glaser EM, Van der Loos H. 1981. Analysis of thick brain sections by obverse-reverse computer microscopy: application of a new, high clarity Golgi-Nissl stain. J Neurosci Methods 4:117–125 [DOI] [PubMed] [Google Scholar]
- 24. Mong JA, Glaser E, McCarthy MM. 1999. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci 19:1464–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ovsepian SV, Friel DD. 2008. The leaner P/Q-type calcium channel mutation renders cerebellar Purkinje neurons hyper-excitable and eliminates Ca2+-Na+ spike bursts. Eur J Neurosci 27:93–103 [DOI] [PubMed] [Google Scholar]
- 26. Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P. 2000. Spinophilin regulates the formation and function of dendritic spines. Proc Natl Acad Sci USA 97:9287–9292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schwarz JM, Liang SL, Thompson SM, McCarthy MM. 2008. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron 58:584–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Altman J. 1972. Postnatal development of the cerebellar cortex in the rat. 3. Maturation of the components of the granular layer. J Comp Neurol 145:465–513 [DOI] [PubMed] [Google Scholar]
- 29. Roth A, Häusser M. 2001. Compartmental models of rat cerebellar Purkinje cells based on simultaneous somatic and dendritic patch-clamp recordings. J Physiol 535:445–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abel JM, Witt DM, Rissman EF. 2011. Sex differences in the cerebellum and frontal cortex: roles of estrogen receptor α and sex chromosome genes. Neuroendocrinology 93:230–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hosy E, Piochon C, Teuling E, Rinaldo L, Hansel C. 2011. SK2 channel expression and function in cerebellar Purkinje cells. J Physiol 589:3433–3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rogers TD, Dickson PE, Heck DH, Goldowitz D, Mittleman G, Blaha CD. 2011. Connecting the dots of the cerebro-cerebellar role in cognitive function: neuronal pathways for cerebellar modulation of dopamine release in the prefrontal cortex. Synapse 65:1204–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bell HC, Pellis SM, Kolb B. 2010. Juvenile peer play experience and the development of the orbitofrontal and medial prefrontal cortices. Behav Brain Res 207:7–13 [DOI] [PubMed] [Google Scholar]
- 34. Dyck BA, Tan ML, Daya RP, Basu D, Sookram CD, Thomas N, Mishra RK. 2012. Behavioral effects of non-viral mediated RNA interference of synapsin II in the medial prefrontal cortex of the rat. Schizophr Res 137:32–38 [DOI] [PubMed] [Google Scholar]
- 35. Martin LA, Goldowitz D, Mittleman G. 2010. Repetitive behavior and increased activity in mice with Purkinje cell loss: a model for understanding the role of cerebellar pathology in autism. Eur J Neurosci 31:544–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ukena K, Usui M, Kohchi C, Tsutsui K. 1998. Cytochrome P450 side-chain cleavage enzyme in the cerebellar Purkinje neuron and its neonatal change in rats. Endocrinology 139:137–147 [DOI] [PubMed] [Google Scholar]
- 37. Ukena K, Kohchi C, Tsutsui K. 1999. Expression and activity of 3β-hydroxysteroid dehydrogenase/δ5-δ4-isomerase in the rat Purkinje neuron during neonatal life. Endocrinology 140:805–813 [DOI] [PubMed] [Google Scholar]
- 38. Dinkel K, Rickert M, Möller G, Adamski J, Meinck HM, Richter W. 2002. Stiff-man syndrome: identification of 17β-hydroxysteroid dehydrogenase type 4 as a novel 80-kDa antineuronal antigen. J Neuroimmunol 130:184–193 [DOI] [PubMed] [Google Scholar]
- 39. Sakamoto H, Mezaki Y, Shikimi H, Ukena K, Tsutsui K. 2003. Dendritic growth and spine formation in response to estrogen in the developing Purkinje cell. Endocrinology 144:4466–4477 [DOI] [PubMed] [Google Scholar]
- 40. Azcoitia I, Yague JG, Garcia-Segura LM. 2011. Estradiol synthesis within the human brain. Neuroscience 191:139–147 [DOI] [PubMed] [Google Scholar]
- 41. Tsutsui K, Sakamoto H, Shikimi H, Ukena K. 2004. Organizing actions of neurosteroids in the Purkinje neuron. Neurosci Res 49:273–279 [DOI] [PubMed] [Google Scholar]
- 42. Biamonte F, Assenza G, Marino R, D'Amelio M, Panteri R, Caruso D, Scurati S, Yague JG, Garcia-Segura LM, Cesa R, Strata P, Melcangi RC, Keller F. 2009. Interactions between neuroactive steroids and reelin haploinsufficiency in Purkinje cell survival. Neurobiol Dis 36:103–115 [DOI] [PubMed] [Google Scholar]
- 43. Mirzatoni A, Spence RD, Naranjo KC, Saldanha CJ, Schlinger BA. 2010. Injury-induced regulation of steroidogenic gene expression in the cerebellum. J Neurotrauma 27:1875–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lecrux C, Toussay X, Kocharyan A, Fernandes P, Neupane S, Lévesque M, Plaisier F, Shmuel A, Cauli B, Hamel E. 2011. Pyramidal neurons are “neurogenic hubs” in the neurovascular coupling response to whisker stimulation. J Neurosci 31:9836–9847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pike JE. 1970. Total synthesis of prostaglandins. Fortschr Chem Org Naturst 28:313–342 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.