Abstract
The pigment content of dark-grown primary needles of Pinus jeffreyi L. and Pinus sylvestris L. was determined by high-performance liquid chromatography. The state of protochlorophyllide a and of chlorophylls during dark growth were analyzed by in situ 77 K fluorescence spectroscopy. Both measurements unambiguously demonstrated that pine primary needles are able to synthesize chlorophyll in the dark. Norflurazon strongly inhibited both carotenoid and chlorophyll synthesis. Needles of plants treated with this inhibitor had low chlorophyll content, contained only traces of xanthophylls, and accumulated carotenoid precursors. The first form of chlorophyll detected in young pine needles grown in darkness had an emission maximum at 678 nm. Chlorophyll-protein complexes with in situ spectroscopic properties similar to those of fully green needles (685, 695, and 735 nm) later accumulated in untreated plants, whereas in norflurazon-treated plants the photosystem I emission at 735 nm was completely lacking. To better characterize the light-dependent chlorophyll biosynthetic pathway in pine needles, the 77 K fluorescence properties of in situ protochlorophyllide a spectral forms were studied. Photoactive and nonphotoactive protochlorophyllide a forms with emission properties similar to those reported for dark-grown angiosperms were found, but excitation spectra were substantially red shifted. Because of their lower chlorophyll content, norflurazon-treated plants were used to study the protochlorophyllide a photoreduction process triggered by one light flash. The first stable chlorophyllide photoproduct was a chlorophyllide a form emitting at 688 nm as in angiosperms. Further chlorophyllide a shifts usually observed in angiosperms were not detected. The rapid regeneration of photoactive protochlorophyllide a from nonphotoactive protochlorophyllide after one flash was demonstrated.
In contrast to angiosperm tissues, gymnosperms are able to synthesize Chl in darkness and in light (for review, see Schoefs and Bertrand, 1997). The ability to synthesize Chl in darkness is linked to the presence of three genes (chlL, chlN, and chlB) in the chloroplast genome coding for subunits of the light-independent Pchlide reductase (for review, see Armstrong, 1998). At least two of these genes are absent from the angiosperm chloroplast genome, which consequently are unable to synthesize Chl in darkness (Shimada and Sugiura, 1991; Suzuki and Bauer, 1992). Both angiosperms and gymnosperms are, however, able to synthesize carotenoids in darkness, although the synthesis in angiosperms is enhanced by light (for review, see Young, 1993). In most cases, Chl synthesis in gymnosperms was studied in cotyledons (Bogorad, 1950; Nikolic and Bogdanovic, 1972; Michel-Wolwertz, 1977; Selstam et al., 1987; Spano et al., 1992; Schoefs et al., 1995a; Raskin and Marder, 1997), and it remains unclear whether gymnosperm primary needles are able to synthesize Chl in the absence of light (Mohr and Schopfer, 1995; Ou and Adamson, 1995; Hudak, 1997). To our knowledge, there is neither a study about Pchlide a or Chl(ide) a spectral forms in dark-grown primary needles nor an analysis of the pigment composition of such needles.
In this study we first established and then compared the pigment composition of dark-grown primary needles of two pine species, Pinus jeffreyi and Pinus sylvestris. The spectral forms of Pchlide a in dark-grown pine primary needles were studied by 77 K fluorescence spectroscopy and analyzed by Gaussian deconvolution. Light-induced transformation of photoactive Pchlide a to Chlide a and the subsequent photoactive Pchlide a regeneration in darkness have also been studied. The results are discussed by comparison with the situation found in angiosperm and other gymnosperm tissues.
MATERIALS AND METHODS
Culture Conditions
Pinus jeffreyi L. and Pinus sylvestris L. seeds were purchased from Versepuy (Le Puy en Vellay, France), sown on tap-watered vermiculite, and placed in a dark room with the thermostat set at 298 K (±2 K). After 3 (P. jeffreyi) or 5 weeks (P. sylvestris) of growth, primary needles appeared (Fig. 1).
Figure 1.
Five-week-old dark-grown seedlings of P. sylvestris. Some cotyledons were removed to expose the primary needles (arrow). Bar = 5 mm.
The seeds of norflurazon-treated plants were sprayed with norflurazon every 2 d during the entire dark-growth period. This compound was first dissolved in ethanol (Pro Analysis, Merck, Darmstadt, Germany) and then in distilled water. The final concentrations in norflurazon and ethanol were 200 μm and 1%. Control plants were sprayed with the same ethanolic solution without norflurazon. Before experiments, the seedlings were dissected under a dim green light.
Pigment Extraction and Analysis by HPLC
Sample Preparation
To prevent Pchlide a photoreduction, dark-grown pine seedlings were dissected under a dim green light and the primary needles were hand-ground at 277 K in methanol (HPLC grade, UCB, Leuven, Belgium). Small amounts of MgCO3 were added to avoid pigment degradation. The extract was clarified by centrifugation at 50,000g for 15 min at 273 K. After the sample was centrifuged we verified that the pellet was devoid of pigments by 77 K fluorescence spectroscopy. If pigments were still present the pellet was extracted once more.
The supernatant was filtered through a 0.45-μm polytetrafluoroethylene filter membrane (Millipore), vacuum dried under a nitrogen stream, and solubilized again in 0.5 mL of methanol. When the sample was not immediately used for HPLC analysis, it was stored under nitrogen in an amber-colored bottle at 243 K in the dark as recommended by Schoefs and Bertrand (1996) and Bertrand and Schoefs (1997). Under these conditions the pigments remained stable for at least 1 month.
HPLC Setup and Pigment Analysis
All of the pigment separations were done according to the method of Schoefs et al. (1995b, 1996). Separations were carried out with a reversed-phase column (particle size of the packing: 4.65 μm; 250 × 4.6 mm i.d.; Zorbax, Hewlett-Packard). The detector was a UV-Vis diode array detector (190–800 nm, model 991–25, Waters). Solvent A (acetonitrile:methanol, 70:30, v/v) was mixed with an increasing proportion of solvent B (methylene chloride) during all runs. Solvent A was delivered isocratically from 0 to 7 min and then by a 6-min linear gradient of 0% to 10% solvent B, immediately followed by a 2-min linear gradient to 20% of solvent B. This solvent mixture was maintained isocratically until 30 min. The column was reequilibrated between analyses for a minimum of 20 min with solvent A. All runs were performed at 293 K. The flow rate was 1 mL min−1. Methanol (HPLC grade), methylene chloride (HiPerSolv), and acetonitrile (HPLC grade) were purchased from Merck, BDH (Poole, UK), and Baker (Deventer, The Netherlands), respectively.
Standard pigment preparations for calibrations were prepared according to the method of Schoefs et al. (1995b, 1996).
Quantification
Quantifications were performed using external standards. The resulting calibration curves were linear over the concentration range tested with a linear coefficient between 0.998 and 0.999. The different pigments were quantified on the basis of their elution peak recorded at 410 nm (pheophytin a), 430 nm (Pchl[ide] a, Chl[ide] a, and cis-violaxanthin), 437 nm (neoxanthin, trans-violaxanthin, lutein-5,6-epoxide, cis-antheraxanthin, and cis-lutein), 450 nm (trans-antheraxanthin, trans-lutein, zeaxanthin, αcarotene, and β-carotene-5,6-epoxide), and 458 nm (Chl b, β-carotene). Before peak integrations, all of the chromatograms were corrected for the baseline recorded at 520 nm. A typical chromatogram of sample containing Pchlide a, Chl(ide) a, and carotenoids has been published elsewhere (Schoefs et al., 1996).
77 K Fluorescence Spectrophotometry
Except for the experiments reported in Figure 7, 77 K fluorescence spectra were recorded using a Perkin-Elmer spectrofluorimeter (model LS 50 B). For the emission spectra, the excitation and emission slits were set at 10 and 3 nm, respectively. For the excitation spectra, the excitation and emission slits were set at 3 and 10 nm, respectively. All spectra were recorded on intact primary needles and were corrected for the sample baseline and for the photomultiplier response.
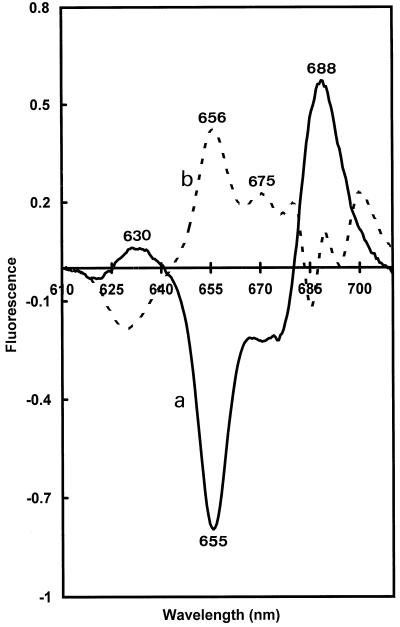
Figure 7.
Difference fluorescence spectra recorded after a flash (flash-minus-dark; curve a) and after 30 s of darkness (30-s dark-minus-flash; curve b).
In the experiments designed to study the transformation and the regeneration of photoactive Pchlide a and the Chlide a spectral shifts, the dark-grown samples were illuminated with a white flash (Portable Multibliz Electronic Flash, 125 J, 1.5-ms duration) at room temperature, immediately frozen at 77 K or placed in darkness (298 K) after the flash for definite times (up to 30 min), and then frozen at 77 K. In the experiments designed to study Chlide a spectral shifts (Fig. 7), the spectra were recorded with an optical multichannel analyzer (OMA 2, EG&G Princeton Applied Research, Princeton, NJ) under excitation at 440 nm with a spectral resolution of 0.5 nm.
The smoothed and fully corrected fluorescence spectra were resolved into Gaussian components using the Dataplot freeware (B. Van Dijk, State University of Leiden, The Netherlands). The spectrum resolutions were made as a function of wave numbers. In the first step of the deconvolution procedure, a Gaussian fitting approximating the main emission band was subtracted from the experimental spectrum. The operation was repeated with the remaining bands until the difference between the Gaussian sum and the original spectrum was minimal. Then the Gaussian half-bandwidths were fixed and a first series of iterations was performed. During this calculation, the Gaussian positions and amplitudes were optimized. Iterations were repeated until convergence with all of the free parameters was achieved. Only the final result is presented.
RESULTS AND DISCUSSION
Analysis of the Pigment Content of Dark-Grown Pine Primary Needles
Figure 1 illustrates that dark-grown primary needles accumulate green pigments. To determine their chemical nature, total pigment extracts were prepared from dark-grown P. sylvestris primary needles and analyzed by HPLC. The pigments and their respective concentrations are summarized in Table I. Both carotenoids (xanthophylls and carotenes) and tetrapyrroles (Pchl[ide] a, Chls, pheophytin a, and pheophytin b) were observed. Qualitatively, the pigment composition of dark-grown primary needles was similar to that found earlier in dark-grown pine cotyledons (Schoefs et al., 1997a) and in greening leaves (Schoefs et al., 1996, 1998), except that antheraxanthin and zeaxanthin were not observed.
Table I.
Pigment composition of 5-week-old dark-grown P. sylvestris primary needles
| Pigment | log k′a | Concentration |
|---|---|---|
| pmol/plant | ||
| Chlide a | −0.53 | 86.86 |
| Pchlide a | −0.32 | 5.01 |
| trans-Neoxanthin | −0.03 | 58.13 |
| trans-Violaxanthin | 0.07 | 168.81 |
| lutein-5,6-epoxide | 0.22 | 87.92 |
| Pheophytin b | 0.33 | NDb |
| trans-Lutein | 0.41 | 340.85 |
| Chl b | 0.72 | 20.66 |
| Chlide a tetrahydrogeranylgeraniol | 0.83 | 183.65 |
| Chlide a phytol | 0.86 | 100.20 |
| α-Carotene | 1.04 | 4.42 |
| β-Carotene | 1.05 | 30.45 |
| Pheophytin a | 1.08 | 16.31 |
| Chl a/bc | 10.35 | |
| Xanthophyll/carotenoid | 18.8 | |
| Carotenoid/Chl | 0.34 |
k′ = (tR − t0)/t0, where t0 and tR represent the retention time of an unretained and a retained peak, respectively.
ND, Not determined.
Chlide a esters/Chl b ratio.
The HPLC method used is unable to separate MV- from DV-Pchlide a (Schoefs et al., 1995b). The absorbance maximum of Pchlide a was at 440 nm (in the eluent), whereas it was at 431 nm for both Chlide a esters (in the eluent). From these data we can assume that the main part of the Pchlide a pool was under the DV form of Pchlide a. whereas Chlide a esters were mainly in MV form. From these maxima we can conclude that Pchlide a was mainly in the DV form, whereas Chlide a esters were in the MV form. This conclusion is based on the fact that, on the one hand, MV-Pchlide a and MV-Chlide a have the same Soret absorbance maximum and, on the other hand, MV pigments are blue shifted compared with DV pigments (Table II; for review, see Jeffrey et al., 1997). Furthermore, esterification of tetrapyrroles by an alcohol moiety does not modify the spectroscopic properties. No attempt was made to determine the MV/DV ratio, but the above data suggest that both Pinus species belong to the plant group that accumulates DV-Pchlide a in darkness. Other gymnosperms also fall into this category (Fasoula et al., 1997), and according to these authors, gymnosperms reduce their DV-Pchlide a to DV-Chlide a, which is in turn transformed to MV-Chlide a. Because 8-vinyl-Chlide reductase, the enzyme catalyzing this reaction in angiosperms, is specific for Chlide a (Parham and Rebeiz, 1992; for review, see Rebeiz et al., 1994), we suggest that the 8-vinyl reduction occurs first and is followed by esterification.
Table II.
Spectroscopic characteristics of MV- and DV-Pchlide a and Chlide a
| Solvent | Absorbance Maximum
in the Soret Region (400–500 nm)
|
References | |||
|---|---|---|---|---|---|
| Pchlide a
|
Chlide a
|
||||
| MV | DV | MV | DV | ||
| Acetone | — | 437.7 | 431.0 | 436.0 | Jeffrey et al. (1997) |
| HPLC eluent | 431.0 | — | 432.0 | — | Schoefs et al. (1995b) |
| — | 440.0 | 431.0 | — | This study | |
HPLC analysis showed that the Chl a pool was heterogeneous and composed of Chlide a tetrahydrogeranylgeraniol and Chlide a phytol. Chlide a tetrahydrogeranylgeraniol is probably not a degradation product, since it is generally accepted that the first step of Chl breakdown consists of removal of the alcohol moiety (for review, see Gossauer and Engel, 1996; Bertrand and Schoefs, 1998). The peculiar accumulation of Chlide a tetrahydrogeranylgeraniol can reflect a pool of intermediates before the last hydrogenation step, suggesting that several hydrogenases are implied in the geranylgeraniol-to-phytol transformation.
A similar situation was found in a rice mutant that mainly accumulated Chlide a geranylgeraniol, Chlide a dihydrogeranylgeraniol, and Chlide a tetrahydrogeranylgeraniol instead of Chlide a phytol (Shibata et al., 1995). Since in the fluorescence spectra presented in Figure 2 no distinct emission band (approximately 675 nm) reflecting free pigments was observed, we must conclude that most of both Chlide a esters are integrated into pigment-protein complexes. The stability of pigment-protein complexes should not be affected by the type of alcohol moiety esterifying Chl, since it has been shown in vitro that light-harvesting complexes could be reconstituted using different Chlide a esters without significant loss of stability (Paulsen et al., 1992).
Figure 2.
The 77 K fluorescence spectra during the development of dark-grown primary needles. Excitation wavelength: 440 nm. The spectra were normalized to their fluorescence emission maximum. A, P. jeffreyi. Curve a, 3 weeks old; curve b, 4 weeks old; curve c, 5 weeks old. B, P. sylvestris. Curve a, 5 weeks old; curve b, 6 weeks old; curve c, 7 weeks old.
Our analysis confirms and extends a recent observation of Chl in dark-grown Pinus pinea needles (Ou and Adamson, 1995). It is interesting that only the phytol ester of Chlide b was detected in our analysis. Pchlide a and Chlide a were also found in the primary needles. In this study we found a Chlide a phytol/Chl b ratio of approximately 10. A similar ratio was reported for P. pinea of a similar age (Ou and Adamson, 1995). Unusual amounts of pheophytin a, pheophytin b, and lutein-5,6-epoxide were measured, suggesting that a small part of the pigments was not stabilized but degraded. It is very unlikely that degradation arose during sample preparations because they were made in the presence of MgCO3 and the same composition was obtained when injection was made directly after grinding.
The HPLC method used for pigment separations was also able to resolve the cis-/trans-carotenoid isomers. The main xanthophylls found were trans-violaxanthin and trans-lutein. The α- to β-carotene ratio was similar to that found in dark-grown P. sylvestris cotyledons (Schoefs et al., 1997a). Qualitative analysis of P. jeffreyi primary needles gave the same results (data not shown).
Changes in the in Situ 77 K Fluorescence Spectrum of Pchlide a and Chl(ide) a during Primary Pine Needle Growth in the Dark
To determine which spectral forms of Pchlide a and Chlide a were present in dark-grown pine primary needles, we recorded 77 K fluorescence spectra of P. jeffreyi and P. sylvestris primary needles during dark growth (Fig. 2). In both species, very similar spectra were observed, with a delay of about 2 weeks in the case of P. sylvestris.
The 77 K fluorescence spectra of young needles presented three main bands at approximately 630, 655, and 678, reflecting the presence of nonphotoactive Pchlide a, photoactive Pchlide a, and Chl(ide) a, respectively (Fig. 2, curve a). The main band was that of nonphotoactive Pchlide a (emission approximately 630 nm). The Chlide a emission band appeared heterogeneous since a shoulder at approximately 675 nm was observed. As dark growth proceeded, the emission at 678 nm was replaced by three bands at approximately 685, 695, and 735 nm. At this developmental stage Pchlide a emission bands (630 and 655 nm) were still detected but became minor compared with the Chl bands (Fig. 2, curves b and c). At the later stages of needle development the relative amplitude of the 685-/695-nm bands decreased with respect to that at 735 nm. The evolution of the fluorescence spectrum was very similar to that found with P. jeffreyi cotyledons cultured in similar conditions (Michel-Wolwertz, 1977; Schoefs et al., 1995a) and in greening angiosperm leaves (Thorne and Boardman, 1971; Schoefs and Franck, 1991; Schoefs et al., 1992). By analogy with previous studies done on etiolated, greening, and fully green leaves (Govindjee and Wasielewski, 1989; Schoefs et al., 1992, 1994; Dreyfuss and Thornber, 1994), we attributed the different bands to nonphotoactive Pchlide a (630 nm), to photoactive Pchlide a (654 nm), to the internal PSII light-harvesting complexes (CP43 and CP47, 685 and 695 nm, respectively), and to the PSI light-harvesting complex (735 nm). The detection of characteristic fluorescence emission of pigment-protein complexes belonging to PSI and PSII demonstrates that Chlide a esters and Chlide b phytol, synthesized in darkness, bind light-harvesting proteins that are in turn assembled into photosynthetic antenna in a way similar to that in dark-grown pine cotyledons and during light-induced angiosperm leaf greening (Michel-Wolwertz, 1977; Schoefs and Franck, 1991; Canovas et al., 1993; Schoefs et al., 1995a). The mechanisms by which Chl and Chl-binding polypeptide synthesis are coregulated remain to be studied in gymnosperms, but we can assume that Chl(ide) a is the factor triggering Chl-binding polypeptide accumulation and assembly, as shown in vitro with higher plant etioplasts fed with exogenous Chlide a (Klein et al., 1988; Eichacker et al., 1992).
Analysis of the Pchlide a Spectral Forms of Dark-Grown Primary Needles
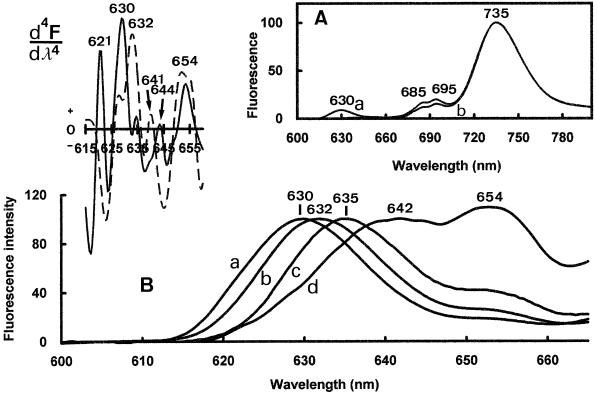
We recently reported that the nonphotoactive Pchlide a pool was spectrally heterogeneous in dark-grown pine cotyledons, as it is in higher plants (Schoefs et al., 1995a). To determine whether a similar heterogeneity also occurred in dark-grown pine primary needles, we recorded 77 K emission spectra in the Pchlide a region (600–665 nm) under four excitation wavelengths (440, 450, 460, and 470 nm). When the excitation wavelength was changed, the position of the emission maximum of the short-wavelength emission band Pchlide a shifted from 630 to 642 nm, with intermediate maxima at 632 and 635 nm (Fig. 3B). The photoactive Pchlide a emission band position (654 nm), as well as that of PSI (735 nm) and PSII (685 and 695 nm) remained unchanged (Fig. 3A). Fourth-order-derivative calculations confirmed the heterogeneity of the fluorescence emission in the Pchlide region and provided approximate positions of the emission maxima of different components (Fig. 3, top left).
Figure 3.
The 77 K fluorescence spectra of dark-grown primary needles of 6-week-old of P. sylvestris recorded with excitation wavelengths at 440 nm (curve a), 450 nm (curve b), 460 nm (curve c), and 470 nm (curve d). The spectra were normalized at 735 nm (A) and on the nonphotoactive Pchlide a fluorescence emission maximum (B). Top left, The 4th-derivative spectra of emission spectra recorded under 440 nm (solid line) and 470 nm (dashed line).
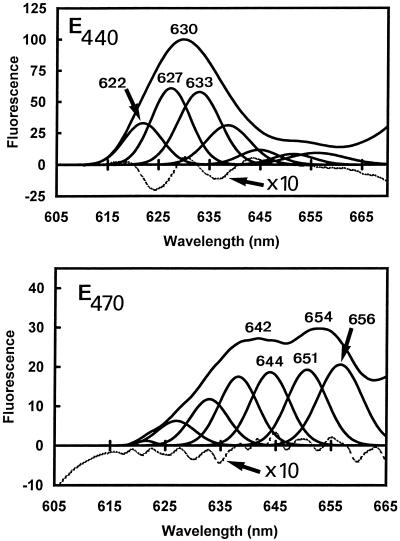
To avoid artifactual attributions of components due to overlapping of primary and secondary peaks in the derivative spectra (Böddi and Franck, 1997), Gaussian deconvolutions of the Pchlide a region (600–665 nm) of the spectra recorded under the four different excitations were performed to confirm the spectral heterogeneity (Fig. 4; Table III). Under each excitation, seven components were found, having maxima at 622, 627, 633, 637, 645, 651, and 656 nm (Fig. 4; Table III). A good correspondence of results obtained by both methods was found (compare with Fig. 3, top left). Only Pchl(ide) a and Chl b have their emission maximum in this region. Chl b emits fluorescence at approximately 653 nm (Fradkin et al., 1969; Larkum and Anderson, 1982). The case of Pchlide a emission was more complex because several spectral forms of this pigment have been reported and usually occur simultaneously. On the basis of the literature (El Hamouri and Sironval, 1979; Cohen and Rebeiz, 1981; Böddi et al., 1993; Böddi and Franck, 1997) we assign six of the seven bands found by Gaussian deconvolutions to nonphotoactive or photoactive Pchlides (Table III).
Figure 4.
Gaussian deconvolution of the spectrum recorded with excitation at 440 nm (top) and 470 nm (bottom) excitation lights. The difference between the Gaussian sum and the original data is presented in each case.
Table III.
Characteristics of the different components found by Gaussian deconvolutions of the fluorescence spectra and the percentage of each component in the emision spectra recorded under the 440-nm and 470-nm excitation light
| Component | Gaussian Maximum | Half-Bandwidth | Percentage
of Total Area
|
Pchlide Type | |
|---|---|---|---|---|---|
| Excitation at 440 nm | Excitation at 470 nm | ||||
| nm | % | ||||
| G622 | 621.89 ± 0.80 | 15.52 ± 2.23 | 15 | 1 | ? |
| G627 | 626.96 ± 0.56 | 16.90 ± 0.98 | 28 | 7 | Pchlide photoinactive |
| G632 | 631.95 ± 1.15 | 18.11 ± 1.56 | 28 | 12 | Pchlide photoinactive |
| G638 | 637.25 ± 1.34 | 18.08 ± 1.46 | 15 | 18 | Pchlide photoinactive |
| G643 | 643.78 ± 1.05 | 16.53 ± 3.01 | 5 | 19 | Pchlide photoactive |
| G651 | 650.48 ± 0.88 | 18.29 ± 1.18 | 4 | 20 | Pchlide photoinactive |
| G656 | 656.14 ± 0.80 | 19.50 ± 1.77 | 5 | 23 | Pchlide photoactive |
Further experiments are needed to define the physicochemical nature and the role of the different spectral forms. The fact that similar spectral forms of nonphotoactive Pchlide a have been found in leaves of dark-grown angiosperms that have only the light-dependent NADPH: Pchlide oxidoreductase does not allow us to assign any of the Pchlide a forms found in pine to specific precursor forms involved in the light-independent Chl synthesis pathway.
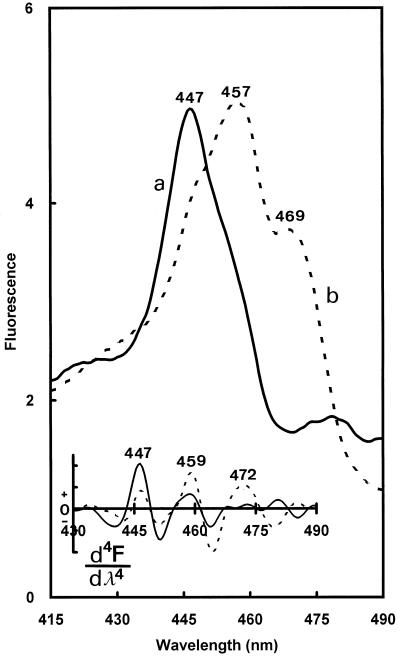
The proportion of the band amplitudes varied with the excitation wavelength, strongly influencing the nonphotoactive/photoactive-Pchlide emission ratio (Table III). Under a 440-nm light, the main emissions were from the 627- and 632-nm forms, whereas under the 470-nm light, the emissions came mainly from the 637-, 644-, 651-, and 656-nm forms. These dramatic changes in the 630-/654-nm fluorescence-intensity ratio were due to the very different excitation spectra of the 630- and 654-nm emission bands in the Soret region (Fig. 5). The excitation spectrum of the fluorescence at 633 nm had its maximum at 447 nm (Fig. 5, curve a). In contrast, the excitation spectrum of the fluorescence at 656 nm was largely red shifted and showed two maxima at 457 and 469 nm (Fig. 5, curve b). These maxima were confirmed by the fourth-order-derivative calculations, which, moreover, revealed the presence of additional shoulders at approximately 459 nm in the excitation spectrum of nonphotoactive Pchlide a (630 nm) and approximately 447 nm in that of photoactive Pchlide a (654 nm; Fig. 3, top left). It was reported previously that in leaves of dark-grown angiosperms photoactive and nonphotoactive Pchlide a have different excitation spectra in the Soret region (Böddi et al., 1993; Durchan and Lebedev, 1995). The above results show that this is also true for pine, but both maxima were red shifted about 9 nm. These shifts were unlikely to be due to contributions of Chl b (which, like photoactive Pchlide a, emits in the 650-nm region and is excited at approximately 470 nm) since, as will be shown below (Fig. 8), most of the fluorescence at 656 nm disappears after one light flash and therefore originates to a large extent from photoactive Pchlide a. It is relevant to add that similar excitation spectra of photoactive Pchlide a as shown here in pine needles have been obtained during photoactive Pchlide a regeneration in spinach cotyledons (Schoefs et al., 1997b) and in cotyledons of dark-grown Arabidopsis transgenic plants in which type B NADPH:Pchlide oxidoreductase has been overexpressed (F. Franck, U. Sperling, B. van Cleve, G. Nelson, G. Frick, K. Apel and G.A. Armstrong, unpublished results). In these two cases, the samples were devoid of Chl b. The reason for the red shift of Pchlide a excitation bands in pine needles remains unclear. It may result from unusual circumstances regarding energy migration processes between different Pchlide forms and/or aggregation states of the pigment.
Figure 5.
The 77 K fluorescence excitation spectra at 630 nm (curve a) and 654 nm (curve b). Bottom, Fourth-order-derivative spectra. The excitation was set at 440 nm.
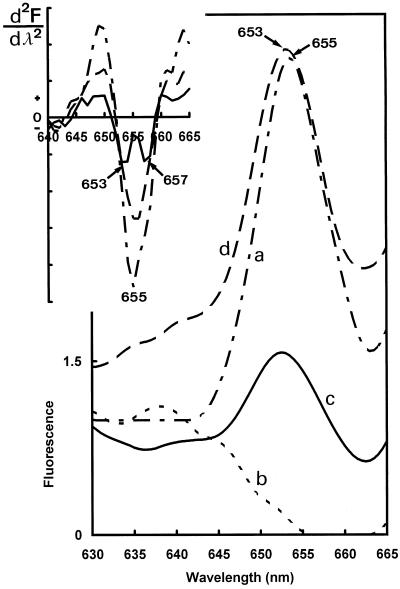
Figure 8.
The 77 K fluorescence spectra recorded with excitation at 470 nm. Curve a, Before the illumination (— – — –); curve b, after a saturating flash (– – – – –); curve c, after 30 s of darkness and the saturating flash (— — —); curve d, after 30 min of darkness and the saturating flash (———). Top left, Corresponding second-derivative calculations.
Photoreduction and Regeneration of Photoactive Pchlide a Triggered by a Short Light Flash
It is well known that in angiosperms a single light flash triggers the phototransformation of photoactive Pchlide a to a Chlide a species that emits fluorescence at 688 nm. This is followed by a series of Chlide a spectral shifts in the second and minute time scale (for review, see Schoefs and Bertrand, 1997).
To determine precisely the emission maximum of the first Chlide a photoproduct in pine needles, we calculated the “flash-minus-dark” difference spectrum in control (not treated with norflurazon) plants. A negative peak was found at approximately 655 nm and a positive peak at approximately 690 nm, reflecting the disappearance of photoactive Pchlide a and the appearance of Chlide a emitting at 688 nm during the flash. No reproducible difference in the Chlide region was observed in the difference spectra between needles frozen 30 s after one flash or immediately after the flash (data not shown). We concluded that the large Chl a fluorescence present in the samples prevented the observation of clear and reproducible differences in this region. To overcome this difficulty, we used norflurazon-treated pine seedlings, which contained less Chl (about 2% of untreated samples) and had a less complex structure of the Chl emission in the 670- to 800-nm region, making the observation of the Chlide a spectral shifts easier. The decrease in carotenoids was due to the inhibitory action of norflurazon on the phytoene desaturase activity (for review, see Bramley, 1994). HPLC analysis revealed that, in addition to the large amounts of carotenoid precursors (i.e. phytoene and phytofluene), only traces of violaxanthin and lutein were observed.
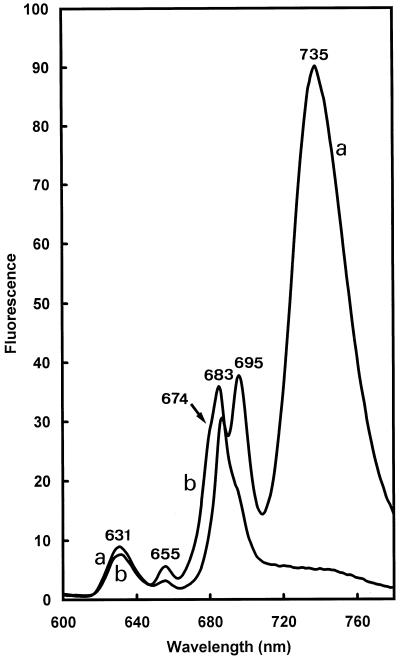
A similar result was also obtained with higher plants treated with norflurazon (Kümmel and Grimme, 1974; Karapetyan et al., 1991). The Chl content was also lowered by the norflurazon treatment. Very small amounts of Chlide a esters (Chlide a phytol and Chlide a tetrahydrogeranylgeraniol) were detected (data not shown). The Pchlide a content was strongly reduced, suggesting a feedback action of unbound Chl(ide) a on the Pchlide biosynthetic pathway. It was observed that the absence of carotenoids impair photosystem assembly (Karapetyan et al., 1991). This was also the case in dark-grown primary needles, as shown by comparison of the 77 K fluorescence of treated and untreated plants (Fig. 6). In norflurazon-treated plants the main Chl emission peak was found at 683 nm, with shoulders at 674 and 694 nm. The large emission band at 735 nm from PSI light harvesting was absent. These results indicate that Chl(ide) a can allow CP47 and CP43 polypeptide stabilization and assembly in the almost complete absence of carotenoids. The shoulder at 674 nm could reflect the presence of free or uncoupled monomeric Chl a (Mysliwa-Kurdziel et al., 1997). The absence of other pigment-protein complexes belonging to PSII could be due to a decrease of stability in the absence of xanthophylls, as reconstitution experiments showed (Pagano et al., 1998). The absence of the PSI light-harvesting complex may suggest that a factor other than Chl(ide) a is required to allow import and stabilization of nuclear-encoded proteins. However, it is also possible that the absence of long-wavelength Chl forms in norflurazon-treated plants merely reflects a general arrest in chloroplast development due to the inhibition of Chl synthesis.
Figure 6.
The 77 K fluorescence spectra of 7-week-old dark-grown pine (P. sylvestris) primary needles cultivated in the absence (a) or presence (b) of norflurazon. The spectra were not normalized.
The flash-minus-dark difference fluorescence spectrum was calculated (Fig. 7, curve a). A positive band of Chlide a was found at 688 nm as the result of photoactive Pchlide a transformation (negative band at 655 nm). The obtained difference spectrum was similar to the difference spectra obtained in angiosperms (Sperling et al., 1998). To visualize rapid Pchlide a and Chlide a shifts in darkness after one flash, we calculated difference spectra between samples frozen 30 s and immediately after one flash (30-s darkness-minus-flash, Fig 7, curve b). The partial regeneration of photoactive from nonphotoactive Pchlide a is demonstrated by the positive band at 656 nm and the negative band at 630 nm in such spectra. The complex structure of the 30-s difference spectrum in the Chlide region was difficult to analyze and indicated that more than one spectral shift overlapped during the 30-s dark period. The presence of a positive band at approximately 675 nm probably reflected the release of Chlide a from the site of its synthesis on the light-dependent NADPH:Pchlide oxidoreductase (Schoefs and Franck, 1993).
As the above data show, photoactive Pchlide a was regenerated after one flash. This process was analyzed in more detail. To avoid overlapping of short- and long-wavelength Pchlide a bands and the fluorescence intensity variations due to energy transfer from the former to the latter, we took advantage of the fact that photoactive Pchlide a emitting at 655 nm can be selectively excited by 470-nm light (Fig. 5). Before the illumination, the photoactive Pchlide a maximum was found at 655 nm (Fig. 8, curve a). The flash triggered the disappearance of the photoactive Pchlide a accumulated during dark growth, and only weak bands at shorter wavelengths remained in the emission spectrum (Fig. 8, curve b). After 30 s of darkness, photoactive Pchlide a was partly regenerated (Fig. 8, curve c). The complete pool of photoactive Pchlide a was resynthesized after 30 min of darkness after the flash (Fig. 8, curve d). The second-derivative analysis of the spectra recorded after 30 s of darkness indicated that the fluorescence band at approximately 655 nm was actually composed of two components, since the derivative band in this region was asymmetric and, for short regeneration times (30 s), clearly showed two peaks at approximately 653 and 657 nm (Fig. 8, top left). These two components should correspond to the 651- and 656-nm components already identified by Gaussian deconvolution in dark-grown needles (Fig. 4). The variations of the amplitude of the two derivative peaks or shoulders at approximately 653 and 657 nm in response to the flash and subsequent dark incubation suggest that both components arise from photoactive Pchlide a forms with slightly different emission maxima.
CONCLUSIONS
The chromatographic analysis of the dark-grown primary needle pigments and the 77 K fluorescence spectra recorded in vivo unambiguously demonstrated that pine primary needles synthesize Chl in the dark. Low-temperature spectroscopy showed that Chl synthesized through the light-independent pathway is integrated into pigment-protein complexes of both photosystems. Small amounts of Pchlide a accumulate in darkness in several forms, and some of their spectroscopic properties differ from those usually reported for dark-grown leaves of angiosperms. The most prominent differences concern the presence of two putative photoactive Pchlide a forms in the 655-nm region and different excitation properties than those usually reported for leaves of dark-grown angiosperms. Nonphotoactive Pchlide a in the 633-nm emission region serves as precursors of photoactive Pchlides a. The fact that nonphotoactive Pchlide accumulates in darkness and are used as substrates for photoactive Pchlide a regeneration, although a light-independent Chl synthesis pathway exists, suggests a specific coupling of nonphotoactive and photoactive Pchlide a forms in the light-dependent Chl synthesis pathway.
ACKNOWLEDGMENT
The authors are grateful to Professor Yves Lemoine (University of Lille 1, France) for the use of HPLC.
Abbreviations:
- Chl
chlorophyll
- Chlide
chlorophyllide
- DV
divinyl
- MV
monovinyl
- Pchlide
protochlorophyllide
Footnotes
This work was supported by a grant from the European Community (grant no. CI1*-CT94-0085) and by the Tournesol program cofinanced by the Commissariat Général au Relations Internationales of the French Community of Belgium and the Foreign Office of the French Republic.
LITERATURE CITED
- Armstrong GA. Greening in the dark: light-independent chlorophyll biosynthesis from anoxygenic photosynthetic bacteria to gymnosperms. J Photochem Photobiol B Biol. 1998;43:87–100. [Google Scholar]
- Bertrand M, Schoefs B (1997) Working with photosynthetic pigments: problems and precautions. In M Pessarakli, ed, Handbook of Photosynthesis. Marcel Dekker, New York, pp 151–173
- Bertrand M, Schoefs B (1998) Photosynthetic pigment metabolism in plants under stress. In M Pessarakli, ed, Handbook of Plant and Crop Stress. Marcel Dekker, New York (in press)
- Böddi B, Franck F. Room temperature fluorescence spectra of protochlorophyllide and chlorophyllide forms in etiolated bean leaves. J Photochem Photobiol B Biol. 1997;41:73–82. [Google Scholar]
- Böddi B, Ryberg M, Sundqvist C. Analysis of the 77 K fluorescence emission and excitation spectra of isolated etioplast inner membranes. J Photochem Photobiol B Biol. 1993;21:125–133. [Google Scholar]
- Bogorad L. Factors associated with the synthesis of chlorophyll in the dark in seedlings of Pinus jeffreyi. Bot Gaz. 1950;3:221–241. [Google Scholar]
- Bramley PM. Carotenoid biosynthesis: a target site for bleaching herbicides. Biochem Soc Trans. 1994;22:625–629. doi: 10.1042/bst0220625. [DOI] [PubMed] [Google Scholar]
- Canovas F, McLarney B, Silverthorne J. Light-independent synthesis of LHC II b polypeptides and assembly of the major pigmented complexes during the initial stages of Pinus palustris seedlings development. Photosynth Res. 1993;38:89–97. doi: 10.1007/BF00015065. [DOI] [PubMed] [Google Scholar]
- Cohen CE, Rebeiz CA. Chloroplast biogenesis 34. Spectral fluorometric characterization in situ of the protochlorophyll species in etiolated tissues of higher plants. Plant Physiol. 1981;67:98–103. doi: 10.1104/pp.67.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss BW, Thornber JP. Organization of the light-harvesting complex of photosystem I and its assembly during plastid development. Plant Physiol. 1994;106:841–848. doi: 10.1104/pp.106.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durchan M, Lebedev NN. Changes in the near-UV fluorescence excitation spectrum during protochlorophyllide photoreduction in etiolated cucumber cotyledons. Photosynthetica. 1995;31:599–611. [Google Scholar]
- Eichacker L, Paulsen H, Rüdiger W. Synthesis of chlorophyll a regulates translation of chlorophyll a apoproteins P700, CP47, CP43 and D2 in barley etioplasts. Eur J Biochem. 1992;205:17–25. doi: 10.1111/j.1432-1033.1992.tb16747.x. [DOI] [PubMed] [Google Scholar]
- El Hamouri B, Sironval C. A new non-photoreducible protochlorophyll(ide)-protein: P649–642 from cucumber cotyledons; NADPH mediation of its transformation to photoreducible P657–650. FEBS Lett. 1979;103:345–347. doi: 10.1016/0014-5793(79)81359-9. [DOI] [PubMed] [Google Scholar]
- Fasoula DA, Smyth C, Rebeiz CA (1997) Relationship of the monovinyl protochlorophyllide a content to plant yield. In M Pessarakli, ed, Handbook of Photosynthesis. Marcel Dekker, New York, pp 671–679
- Fradkin L, Shlyk AA, Kalinina LM, Faludi-Daniel A. Fluorescence studies on reaction centres of chlorophyll biosynthesis at the early stages of greening. Photosynthetica. 1969;3:326–337. [Google Scholar]
- Gossauer A, Engel N. Chlorophyll catabolism-structures, mechanisms, conversions. J Photochem Photobiol B Biol. 1996;32:141–151. [Google Scholar]
- Govindjee, Wasielewski MR (1989) Photosystem II: from a femtosecond to a millisecond. In WR Briggs, ed, Photosynthesis, Vol 8. Alan R Liss, New York, pp 71–103
- Hudak J (1997) Photosynthetic apparatus. In M Pessarakli, ed, Handbook of Photosynthesis. Marcel Dekker, New York, pp 27–48
- Jeffrey SW, Mantoura RFC, Bjorland T (1997) Data for the identifcation of 47 key phytoplankton pigments. In SW Jeffrey, RFC Mantoura, SW Wright, eds, Phytoplankton Pigments in Oceanography. UNESCO, Paris, pp 449–559
- Karapetyan NY, Bolychevtseva YV, Rakhimberdieva MG (1991) The necessity of carotenoids for the assembly of active photosystem 2 reaction centers. In R Douglas, ed, Light in Biology and Medicine, Vol 2. Plenum Press, New York, pp 45–54
- Klein RR, Gamble PE, Mullet JE. Light-dependent accumulation of radiolabelled plastid-encoded chlorophyll a-apoproteins requires chlorophyll a. I. Analysis of chlorophyll-deficient mutant and phytochrome. Plant Physiol. 1988;88:1246–1256. doi: 10.1104/pp.88.4.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kümmel HW, Grimme LH. The inhibition of carotenoid biosynthesis in green algae by SANDOZ H6706: accumulation of phytoene and phytofluene in Chlorella fusca. Z Naturforsch. 1974;30c:333–336. [Google Scholar]
- Larkum AWD, Anderson JM. The reconstitution of a photosystem II protein complex P-700-chlorophyll a-protein complex and light harvesting chlorophyll a-b-protein. Biochim Biophys Acta. 1982;379:410–421. [Google Scholar]
- Michel-Wolwertz MR. Chlorophyll formation in cotyledons of Pinus jeffreyi during germination in the dark. Occasional accumulation of protochlorophyll(ide) forms. Plant Sci Lett. 1977;8:125–134. [Google Scholar]
- Mohr H, Schopfer P. Plant Physiology. Berlin: Springer-Verlag; 1995. [Google Scholar]
- Mysliwa-Kurdziel B, Barthélemy X, Strzalka K, Franck F. The early stages of photosystem II assembly monitored by measurements of fluorescence lifetime, fluorescence induction and isoelectric focusing of chlorophyll-proteins in barley etiochloroplasts. Plant Cell Physiol. 1997;38:1187–1196. [Google Scholar]
- Nikolic D, Bogdanovic M. Plastid differentiation and chlorophyll synthesis in cotyledons of black pine seedlings grown in the dark. Protoplasma. 1972;75:205–213. [Google Scholar]
- Ou K, Adamson H. Chlorophyll accumulation in cotyledons, hypocotyls and primary needles of Pinus pinea seedlings in light and dark. Physiol Plant. 1995;93:719–724. [Google Scholar]
- Pagano A, Cinque G, Bassi R. In vitro reconstitution of the recombinant photosystem II light-harvesting complex CP24 and its spectroscopic characterization. J Biol Chem. 1998;273:17154–17165. doi: 10.1074/jbc.273.27.17154. [DOI] [PubMed] [Google Scholar]
- Parham R, Rebeiz CA. Chloroplast biogenesis: chlorophyllide a reductase is a divinyl chlorophyllide a specific NADPH-dependent enzyme. Biochemistry. 1992;31:8460–8464. doi: 10.1021/bi00151a011. [DOI] [PubMed] [Google Scholar]
- Paulsen H, Hobe S, Eisen C (1992) Reconstitution of LHCP-protein complexes with mutant LHCP and chlorophyll analogs. In JH Argyroudi-Akoyunoglou, ed, Regulation of Chloroplast Biogenesis. Plenum Press, New York, pp 343–349
- Raskin VI, Marder JB. Chlorophyll organization in dark-grown and light-grown pine (Pinus brutia) and barley (Hordeum vulgare) Physiol Plant. 1997;101:620–626. [Google Scholar]
- Rebeiz CA, Parham R, Fasoula DA, Ionnides IM. Chlorophyll a biosynthetic heterogeneity. Ciba Found Symp. 1994;180:177–193. doi: 10.1002/9780470514535.ch10. [DOI] [PubMed] [Google Scholar]
- Schoefs B, Bertrand M. Photosynthetic pigments: isolation, purification and quantification. A short practical overview. Bull Soc R Sci Liège. 1996;65:321–329. [Google Scholar]
- Schoefs B, Bertrand M (1997) Chlorophyll biosynthesis. In M Pessarakli, ed, Handbook of Photosynthesis. Marcel Dekker, New York, pp 49–71
- Schoefs B, Bertrand M, Franck F. Plant greening: biogenesis of the photosynthetic apparatus in bean leaves irradiated shortly after the germination. Photosynthetica. 1992;27:497–504. [Google Scholar]
- Schoefs B, Bertrand M, Franck F. Spectral heterogeneity of the photoinactive protochlorophyllide in dark-grown bean leaves and pine cotyledons. In: Mathis P, editor. Photosynthesis: From Light to Biosphere, Vol 3. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995a. pp. 1009–1012. [Google Scholar]
- Schoefs B, Bertrand M, Lemoine Y. Separation of photosynthetic pigments and their precursors by reversed-phase high-performance liquid chromatography using a photodiode array detector. J Chromatogr. 1995b;692A:239–245. [Google Scholar]
- Schoefs B, Bertrand M, Lemoine Y (1998) Changes in the photosynthetic pigments in bean leaves during the first photoperiod of greening and the subsequent dark-phase. Comparison between old (10-d-old) leaves and young (2-d-old) leaves. Photosynth Res (in press)
- Schoefs B, Bertrand M, Lemoine Y, Franck F. Determinations of the pigment composition of dark-grown pine cotyledons by reversed-phase high-performance liquid chromatography. Arch Physiol Biochim. 1997a;105:15. [Google Scholar]
- Schoefs B, Franck F. Photosystem II assembly in 2-days-old bean leaves during the first 16 hrs. of greening. CR Acad Sci Paris Ser III. 1991;314:441–445. [Google Scholar]
- Schoefs B, Franck F. Photoreduction of protochlorophyllide to chlorophyllide in 2-d-old dark-grown bean (Phaseolus vulgaris cv. Commodore) leaves. Comparison with 10-d-old dark-grown (etiolated) leaves. J Exp Bot. 1993;44:1053–1057. [Google Scholar]
- Schoefs B, Funk C, Andersson B. Spectral changes during photoactive protochlorophyllide regeneration in spinach cotyledons. Arch Physiol Biochim. 1997b;105:15. [Google Scholar]
- Schoefs B, Garnir HP, Bertrand M. Comparison of the photoreduction of protochlorophyllide to chlorophyllide in leaves and cotyledons from dark-grown bean as a function of age. Photosynth Res. 1994;41:405–417. doi: 10.1007/BF02183043. [DOI] [PubMed] [Google Scholar]
- Schoefs B, Lemoine Y, Bertrand M. Reversed-phase HPLC separation of photosynthetic pigments and their precursors. Am Biotechnol Lab. 1996;14:18–22. [Google Scholar]
- Selstam E, Widell A, Johansson LBA. A comparison of prolamellar bodies from wheat, Scots pine and Jeffrey pine. Pigment spectra and properties of protochlorophyllide oxidoreductase. Physiol Plant. 1987;70:209–214. [Google Scholar]
- Shibata M, Tsuyama M, Kitagawa Y, Kobayashi Y. Hydrogenation of chlorophyll alcohol side chain and its role on photosynthesis. In: Mathis P, editor. Photosynthesis: From Light to Biosphere, Vol 3. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 997–1000. [Google Scholar]
- Shimada H, Sugiura H. Fine structural features of the chloroplast genome: comparison of the sequenced chloroplast genomes. Nucleic Acids Res. 1991;19:983–995. doi: 10.1093/nar/19.5.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano AJ, He Z, Timko MP. NADPH:protochlorophyllide oxidoreductases in white pine (Pinus strobus) and loblolly pine (P. taeda). Evidence for light and developmental regulation of expression and conservation in gene organization and protein structure between angiosperms and gymnosperms. Mol Gen Genet. 1992;236:86–95. [PubMed] [Google Scholar]
- Sperling U, Franck F, van Cleve B, Frick G, Apel K, Armstrong GA. Etioplast differentiation in Arabidopsis: both PORA and PORB restore the prolamellar body membrane and photoactive protochlorophyllide-F655 to the cop1 photomorphogenic mutant. Plant Cell. 1998;10:283–296. doi: 10.1105/tpc.10.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki JY, Bauer CE. Light-independent chlorophyll biosynthesis: involvement of the chloroplast gene chlL (frxC) Plant Cell. 1992;4:929–940. doi: 10.1105/tpc.4.8.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne SW, Boardman NK. Formation of chlorophyll b, and the fluorescence properties and photochemical activities of isolated plastids from greening pea seedlings. Plant Physiol. 1971;47:252–261. doi: 10.1104/pp.47.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AJ. Factors that affect the carotenoid composition of higher plants and algae. In: Young A, Britton G, editors. Carotenoids in Photosynthesis. London: Chapman & Hall; 1993. pp. 160–205. [Google Scholar]