Abstract
Estrogens rapidly affect dopamine (DA) neurotransmission in the dorsal striatum (dSTR) and DA-related diseases, such as Parkinson's disease and schizophrenia. How estrogens influence DA function remains unclear, in part, because the ultrastructural localization of estrogen receptors (ER) in the dSTR is not known. Light microscopic studies of the dSTR have suggested the presence of ER. This experiment used electron microscopy to determine whether these ER are at extranuclear sites in the dSTR, providing evidence for a mechanism through which estrogen could rapidly affect DA transmission. The dSTR was labeled with antibodies for ERα, ERβ, and G protein-coupled ER 1 (GPER-1) to confirm whether these ER were present in this brain area. After this, the dSTR was dual labeled with antibodies for ERα or GPER-1 and tyrosine hydroxylase or vesicular acetylcholine transporter to determine whether ER are localized to dopaminergic and/or cholinergic processes, respectively. Ultrastructural analysis revealed immunoreactivity (IR) for ERα, ERβ, and GPER-1 exclusively at extranuclear sites throughout the dSTR. ERα-, ERβ-, and GPER-1-IR are mostly frequently observed in axons and glial profiles but are also localized to other neuronal profiles. Dual labeling revealed that ERα- and GPER-1-IR is not associated with DA axons and terminals but is sometimes associated with cholinergic neurons. Because these receptors are exclusively extranuclear in the dSTR, binding at these receptors likely affects neurotransmission via nongenomic mechanisms.
Estrogens affect dopamine (DA)-dependent behaviors, such as response memory (1, 2) and selective attention (3, 4). They are also implicated in DA-related diseases, such as Parkinson's disease (for review see Ref. 5) and schizophrenia (6). Estrogens increase DA neurotransmission in the dorsal striatum (dSTR) (also known as caudate/putamen) (7), which may contribute to these effects. However, how estrogens influence DA neurotransmission remains unclear. Because it is believed that they act through estrogen receptors (ER) to influence DA function, it is important to examine the ultrastructural localization of these receptors in the dSTR.
The localization of ER in the dSTR is of particular interest, because estrogens modulate dopaminergic activity at various steps in neurotransmission in this brain area. Both natural increases in estrogens across the estrous cycle and estradiol (E2) replacement in ovariectomized rats attenuate DA reuptake in the dSTR (7, 8), possibly by reducing the availability of the DA transporter (9). Furthermore, chronic E2 treatment results in significant increases in DA D2 receptor binding in the dSTR (10, 11), with no corresponding increase in D2 mRNA in the dSTR. These authors suggested that this indicates that E2-induced increases in D2 receptors occur through nongenomic mechanisms (12). Finally, systemic injections of E2 are associated with higher levels of amphetamine-induced DA release in the dSTR (7, 13). These E2-induced increases in DA release occur rapidly, which further supports the idea that estrogens act through nongenomic mechanisms in this region (7).
Estrogens may affect DA transmission in the dSTR via binding at the classical ER, ERα and ERβ, or the more recently discovered G protein-coupled ER 1 (GPER-1), formerly known by its orphan receptor name, G protein-coupled receptor 30. Using in situ hybridization, Shughrue et al. (14) examined the distribution of ERα and ERβ throughout the central nervous system of the female rat and found no evidence of mRNA for these receptors in the dSTR. However, mRNA for ERα and ERβ was found in the dSTR of female mice using real-time PCR (15), and limited nuclear immunolabeling for ERα and ERβ was observed in the in the dSTR of adult female mice using light microscopy (16). Immunohistochemical studies using light microscopy also have assessed GPER-1 distribution in the brain and have shown this receptor to be abundant in the dSTR (17). Thus, light microscopy data indicate that GPER-1 receptors are present in the dSTR, and ERα and ERβ also may be found there at very low levels. Establishing whether and where these ER are located on striatal neurons would contribute to our understanding of how estrogens affect dopaminergic activity in this region.
Three experiments were conducted using immunolabeling techniques and electron microscopy (EM) to examine the distribution of ER in the dSTR. Experiment 1 was conducted to determine whether ER are found in this brain area and, if so, whether they are located on neurons or glia. Because these experiments revealed that ERα and GPER-1 are in numerous axon terminals, experiment 2 used dual immunolabeling EM to determine whether these ER are colocalized with tyrosine hydroxylase (TH), a marker of dopaminergic terminals in the dSTR (18). Moreover, acetylcholine (ACh) has modulatory effects on dopaminergic transmission in the dSTR (19), and ERα has been localized to cholinergic terminals in the hippocampus (20), whereas GPER-1 has been localized to cholinergic neurons in medial septum, nucleus basalis magnocellularis, and STR (21). Therefore, experiment 3 used dual-labeling EM to determine whether ERα or GPER-1 is localized to profiles containing vesicular ACh transporter (VAChT), a marker of cholinergic neurons.
Materials and Methods
Animals
Adult female (225–250 g; ∼60 d old; n = 6) Sprague Dawley rats from Charles River Laboratories (Wilmington, MA) were pair-housed with ad libitum access to food and water and with 12-h light, 12-h dark cycles (lights on 0600–1800 h). All procedures were in accordance with the National Institutes of Health guidelines and approved by the Weill Cornell Medical College Institutional Animal Care and Use Committee. The rats used in these experiments are the same as those used by Williams et al. (22). After arrival, rats acclimated 1 wk to the vivarium, after which estrous cycle phase was determined using vaginal smear cytology (23, 24). Tissue from rats in the diestrus 2 phase of the estrous cycle was analyzed for these experiments. Estrous phase was verified by measuring uterine weights and plasma E2 levels from blood samples (see Ref. 22).
Antisera
Estrogen receptor α
A rabbit polyclonal antiserum (AS409) produced against almost the full peptide for the native rat ERα (amino acid 61 through the C terminus) was supplied by S. Hayashi. This antibody was previously tested for specificity and shown to recognize both ligand bound and unbound receptors (25, 26). On immunoblots of uterine lysates, this antiserum recognizes one major band migrating at approximately 67 kDa (the molecular mass of ERα) (27). Preadsorption of the antibody with purified ERα resulted in no detectable bands in any of these locations (27).
Estrogen receptor β
A rabbit polyclonal antiserum produced against a peptide sequence in the C terminus (amino acid 468–485) of the mouse ERβ protein was used (Z8P; Zymed Laboratories, San Francisco, CA) (28). This antibody has been shown to be specific for ERβ (∼60 kDa) using Western blot analyses, double label with ERβ-mRNA using in situ hybridization, preadsorption control, and absence of labeling in fixed brain sections prepared from ERβ knockout mice (28, 29).
GPER-1. Two antisera were used
Experiment 1
An affinity purified rabbit polyclonal antiserum produced against the N-terminal extracellular domain of the human GPER-1 receptor (LifeSpan BioSciences, Inc., Seattle, WA) was used in experiment 1. This antibody recognized GPER-1-green fluorescent protein transfected COS7 cells and showed identical patterns of labeling to an antibody generated against the C terminus of the GPER-1 protein (30).
Experiments 2 and 3
These experiments used a rabbit polyclonal antiserum generated against a synthetic peptide, CAVIPDSTEQSDVRFSSAV (Multiple Peptide Systems, San Diego, CA), derived from the C terminus of the human GPER-1 receptor (31). In Western blot analyses, this affinity purified antibody specifically recognizes a 38-kDa band that corresponds to the mature 351-amino acid GPER-1 polypeptide and does not recognize either ERα or ERβ (31). In brains fixed with 4% paraformaldehyde, immunoreactivity (IR) was greatly reduced when the antibody was preadsorbed with 10 mg/ml of purified C-terminal peptide (32).
Vesicular ACh transporter
A goat polyclonal antiserum against the C-terminal synthetic peptide sequence corresponding to amino acids 511–530 of the rat VAChT (33, 34) was used. This antibody was obtained commercially from Incstar (Stillwater, MN; now Millipore) and has been used in our previous studies using identical labeling conditions (19).
Tyrosine hydroxylase
A mouse monoclonal antiserum against the full length of the peptide TH in the rat (Immunostar, Inc., Hudson, WI) was used. This antibody has been characterized extensively in fixed rat brain (35).
Tissue preparation
Rats were deeply anesthetized with sodium pentobarbital (150 mg/kg, ip) and were perfused through the ascending aorta sequentially with: 10 ml of heparin (1000 U/ml) in saline, 50 ml of 3.75% acrolein (Polysciences, WA, PA) in 2% paraformaldehyde and 0.1 m phosphate buffer (PB) (pH 7.4), and 200 ml of 2% paraformaldehyde in PB. Brains were removed, cut into four 5-mm blocks, and postfixed in 2% paraformaldehyde in PB for 30 min. The brains were sectioned coronally at 40-μm thickness on a vibrating microtome (Vibratome; Leica, Heerbrugg, Switzerland) and stored in 30% sucrose and 30% ethylene glycol in PB (36) at −80 C.
Tissue sections containing the dSTR (Fig. 1F) were rinsed in PB and coded with hole punches so that they could be pooled in single containers. Additionally, in single-labeling experiments for ERα, a section containing the ventromedial and arcuate nuclei of the hypothalamus was included in analyses; at the light microscopic level, there is abundant ERα labeling in this region (37), so the success of immunolabeling could be confirmed at the light microscopic level before processing the tissue for EM. Similarly, in the experiment examining ERβ, a section containing the supraoptic nucleus was included, because previous light microscopy experiments have observed abundant imunolabeling for ERβ in this region (14). Sections were incubated in 1% sodium borohydride in PB for 30 min to remove any active aldehydes. Tissue then was rinsed in PB, followed by 0.1 m Tris-buffered saline (TBS) (pH 7.6), and was incubated for 30 min in 1% BSA in TBS to reduce nonspecific labeling.
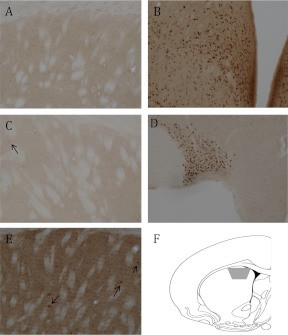
Fig. 1.
Light microscopic localization of ERs. A, Neither nuclear nor extranuclear ERα-IR is detected in the dSTR. B, Dense nuclear ERα-IR in the ventromedial and arcuate nuclei of the hypothalamus. C, No extranuclear ERβ-IR is detected in the dSTR. However, rarely a nucleus with ERβ-IR (arrow) is detected. D, Dense nuclear ERβ-IR in the supraoptic nucleus. E, Dense extranuclear GPER-1-IR is detected in the neuropil of the dSTR. Moreover, several cells with GPER-1-IR (arrows) are seen. F, A coronal schematic of the striatum (atlas level 14; AP 1.00 mm from bregma) (59) indicated the region (gray trapezoid) analyzed.
Immunohistochemical labeling
Experiment 1
Free-floating tissue sections containing the dSTR from three rats were processed for immunohistochemical localization of ERα, ERβ, or GPER-1. Tissue sections from each rat were incubated in antirabbit ERα (1:10,000 dilution), ERβ (1:2000 dilution), or GPER-1 (1:1000 dilution; LifeSpan BioSciences, Inc., Seattle, WA) for 24 h at room temperature and 4 d at 4 C in 0.1% BSA in TBS. ER were visualized using the avidin-biotin complex method (36). Briefly, the tissue was incubated in a 1:400 dilution of biotinylated donkey antirabbit IgG (Jackson ImmunoResearch, West Grove, PA) in 0.5% BSA in TBS for 30 min. Tissue was then incubated in peroxidase-avidin complex (Vector, Burlingame, CA) for a further 30 min, and 3,3-diaminobenzidine (Aldrich, Milwaukee, WI) and H2O2 in TBS for 6 min.
Experiments 2 and 3
Tissue from three rats was processed for immunohistochemical localization of ERα or GPER-1 and TH or VAChT. Immunohistochemical procedures for ER were identical to experiment 1 above. One day before processing, either TH antisera (1:2000 dilution) or VAChT antisera (1:3000 dilution) was added to the diluent.
For immunohistochemical localization, this experiment used preembedding dual-labeling methods (36). The same avidin-biotin complex method described above for experiment 1 was used to visualize the ER. TH and VAChT were detected using silver-enhanced immunogold. Briefly, tissue sections were incubated for 2 h in a 1:50 dilution donkey antimouse or donkey antigoat IgG conjugated to 1-nm colloidal gold particles [Electron Microscopy Sciences (EMS), Fort Washington, PA] in 0.001% gelatin and 0.08% BSA in 0.01 m PBS. Tissue sections then were rinsed in PBS, incubated in 1.25% glutaraldehyde in PBS for 10 min, rinsed again in PBS, followed by a brief wash in 0.2 m sodium citrate (pH 7.4). A 7-min incubation in a silver solution (IntenSE; GE Healthcare, Waukesha, WI) was used to enhance the conjugated gold particles.
Tissue fixation and embedding for ultrastructural analysis
After immunolabeling, tissue sections from all three experiments were fixed for 60 min in 2% osmium tetroxide in PB, dehydrated through a graded series of ethanols and propylene oxide, and embedded in EMbed 812 (EMS) between two sheets of Aclar (36). Ultrathin sections (∼70 nm thick) were taken through the dorsal region of the STR (Fig. 1) using a Leica UCT ultratome. The tissue was collected on copper grids (EMS) and then was counterstained using Reynolds' lead citrate and uranyl acetate. These grids were examined under a Philips CM10 EM with an AMT digital camera. Final photomicrographs were generated from digital images, where brightness and contrast were adjusted using Windows Live Photo Gallery 2011. Adjusted images were assembled in Microsoft PowerPoint 2010.
Data analysis
The subcellular distribution of ERα, ERβ, and GPER-1 alone, and ERα and GPER-1 colocalized with either TH or VAChT, were examined in the dSTR. A profile was considered positive for immunogold labeling if it contained two or more gold particles. Two dSTR sections of 54 μm2, from either the right or left hemisphere, were analyzed for each rat. For quantification analyses, ER-labeled profiles in each section were counted and categorized as: dendrites, dendritic spines, axons, axon terminals, or glia. The total number of labeled profiles was averaged for all six tissue sections (two sections × three rats). The number of each type of single- or dual-labeled profile was divided by the total number of ER-IR profiles to determine the relative proportion of each type of labeled profile. Tissue selected for counting was taken from a depth of 0.2–1.5 μm from the plastic-tissue interface, and only samples that were thin-sectioned evenly across the plastic tissue interface were included in these analyses.
The type of neuronal profile was determined using the description of ultrastructural morphology from Peters et al. (38). Dendrites were large profiles (usually between 1.0 and 2.0 μm) that contained regular microtubule arrays and were sometimes contacted by terminals. Dendritic spines were small (usually between 0.3 and 0.4 μm), sometimes contained a spine apparatus or budded from dendritic shafts and formed synaptic contacts with axon terminals. Axon profiles were less than 0.2 μm in diameter, contained a few small vesicles, and did not form synapses within the plane of section. Axon terminals had a cross-sectional diameter greater than 0.3 μm and contained numerous synaptic vesicles and sometimes formed synapses with other neuronal profiles. Glial profiles were recognized by their conformation to the boundaries of other profiles and their lack of microtubules. Finally, soma were identified by their extremely large size, a lack of microtubules and high numbers of cellular organelles. All sections were assessed for nuclear labeling. However, soma were not included in the single label or TH quantification analyses, because they frequently occupy more than half of the area counted for analysis, reducing the overall number of ER-IR profiles. Soma were included in the analyses with VAChT, because high levels of colocalization were observed in these profiles, and we did not want to underestimate the colocalization between the ER and VAChT. Contact between neuronal profiles refers to synapses identified by synaptic density, and appositions were defined as adjacent profiles that did not form a synapse in the plain of section.
Results
Experiment 1. Single labeling for ER
By light microscopy, dense GPER-1, but almost no ERα or ERβ, is seen in the dSTR
By light microscopy, no nuclear or extranuclear labeling for ERα was observed in the dSTR (Fig. 1A). However, in the sections containing the ventromedial and arcuate regions of the hypothalamus, abundant nuclei containing ERα-IR were seen, indicating that immunolabeling for this receptor was successful (Fig. 1B). Similarly, no extranuclear ERβ-IR was observed, although a rare ERβ-labeled nucleus was seen (Fig. 1C). However, many nuclei containing ERβ-IR were seen in the supraoptic nucleus, confirming that labeling for this antibody was successful (Fig. 1D). In contrast to ERα or ERβ, IR for GPER-1 was observed throughout the neuropil in the dSTR (Fig. 1E). GPER-1-IR was in the cytoplasm, but not the nuclei, of perikarya.
By EM, extranuclear ERα is observed in the dSTR
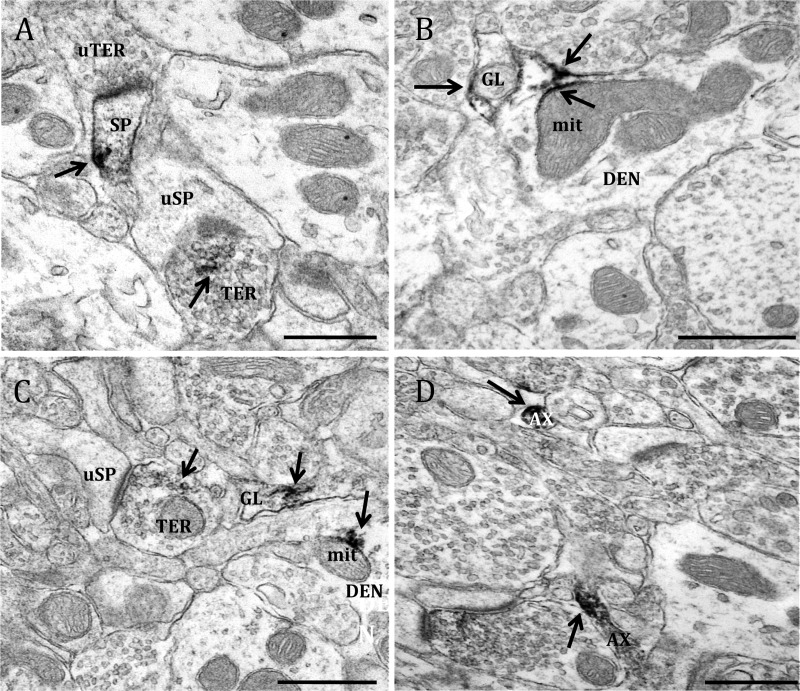
ERα-IR was present in all types of neuronal processes and glia in the dSTR (Table 1). Semiquantitative analysis demonstrated that 35% of ERα-IR profiles were axons, and 20% of ERα-IR was localized axon terminals. In axons (<0.15 μm in diameter), IR was typically discrete and was affiliated with the plasma membrane or clusters of small vesicles (Fig. 2D). Axon terminals in the dSTR had cross-sectional diameters that ranged from approximately 0.4 to 1.5 μm and contained numerous small synaptic vesicles (SSV) and occasionally mitochondria but no dense-core vesicles (Fig. 2, A and C). ERα-IR was commonly found in clusters of reaction product around SSV (Fig. 2, A and C) and was occasionally associated with the plasma membrane.
Table 1.
ERα, ERβ, and GPER-1 distribution in neuronal profiles and glia
| Receptor | ERβ | ERα | GPER-1 |
|---|---|---|---|
| Dendrites | 13.2 | 9.4 | 18.7 |
| 2.3 ± 0.6 | 10.6 ± 1.7 | 17.3 ± 3.8 | |
| Spines | 1.9 | 8.3 | 9.3 |
| 0.3 ± 0.6 | 8.3 ± 0.9 | 10.1 ± 1.7 | |
| Axons | 49 | 35 | 36.4 |
| 8.7 ± 1.1 | 32.3 ± 3.4 | 33.6 ± 4.7 | |
| Terminals | 13.2 | 20.1 | 11.2 |
| 2.3 ± 0.9 | 20.7 ± 1.9 | 10.3 ± 1.9 | |
| Glia | 22.6 | 27.8 | 23.1 |
| 4 ± 1.2 | 27.7 ± 0.9 | 21.3 ± 2.0 | |
| Total | 100 | 100 | 100 |
| 17.7 ± 2.7 | 99.7 ± 4.9 | 92.3 ± 12.3 |
The percentage of total IR profiles (first row) and number of IR profiles and the corresponding se (second row) observed in approximately 3000-μm area of the dorsal STR, averaged across rats.
Fig. 2.
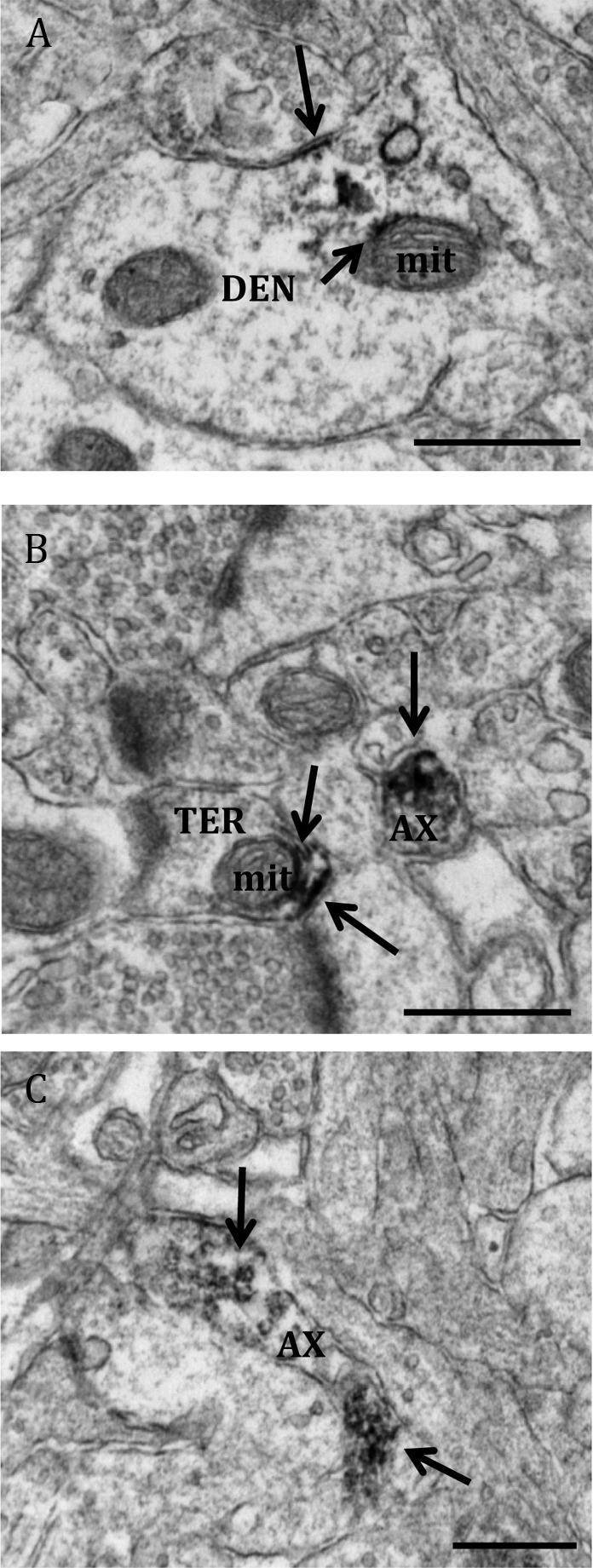
Electron micrographs show examples of ERα-containing profiles. ERα-IR is observed in (A) a dendritic spine (SP) that is contacted by an unlabeled axon terminal (uTER), and an axon terminal (TER) that forms an asymmetric synapses with an unlabeled dendritic spine (uSP); (B) a dendritic shaft (DEN), where it is affiliated with the plasma membrane and a mitochondria (mit), and in a glial process (GL); (C) an axon terminal (TER) forming an asymmetric synapse with an unlabeled dendritic spine, a glial profile, and on a mitochondria in a dendritic shaft; and (D) two unmyelinated axons (AX). In this and subsequent figures, labels are placed approximately in the center of the profile, whereas arrows point directly to immunoperoxidase/immunogold labeling. Black arrow, Immunoperoxidase for ERα. Scale bar, 500 μm.
Peroxidase labeling for ERα was observed in neuronal perikarya, exclusively in the cytoplasm. This imunoreactivity was discrete and was usually associated with the plasma membrane or with mitochondria. Dendritic shafts accounted for approximately 9.4% of ERα-labeled profiles and dendritic spines accounted for 8.3% of ERα-labeled profiles. In the dendritic shafts, peroxidase reaction product was often affiliated with the mitochondrial and plasma membranes (Fig. 2, B and C) and microtubules. In dendritic spines, immunolabeling for ERα accumulated in the spine head and was observed on the plasma membrane particularly near the postsynaptic density (Fig. 2A). ERα-IR was found at asymmetric synapses, where it was seen both pre- and postsynaptically. Occasionally, ERα-IR axon terminals synapsed onto ERα-IR spines. Finally, one quarter (27.8%) of ERα-IR was observed in glial cells of the dSTR. Labeling was primarily at the plasma membranes of glia (Fig. 2B).
By EM, extranuclear ERβ is observed in the dSTR
At the ultrastructural level, ERβ-IR was observed at extranuclear sites in some neuronal profiles and in glial cells in the dSTR. Although ERβ-IR is observed in the dSTR, the number of profiles labeled for ERβ was 5-fold less than profiles for ERα or GPER-1 (Table 1). ERβ-IR was most commonly observed in axons, where it constituted 49% of the total ERβ-IR profiles. In axons (<0.15 μm in diameter), immunolabeling was discrete and was localized primarily to the plasma membrane but was also affiliated with clusters of small vesicles (Fig. 3, B and C). IR for ERβ also was found in axon terminals, which accounted for approximately 13% of the total immunolabeling. Axon terminals containing ERβ-IR ranged from 0.3 to 0.6 μm and contained numerous SSV and occasional mitochondria but did not contain dense-core vesicles. ERβ-IR was found in clusters of reaction product associated with SSV and was sometimes affiliated with mitochondria (Fig. 3B).
Fig. 3.
Electron micrographs show examples of profiles containing ERβ. Rarely, ERβ-IR was detected in (A) a dendritic shaft (DEN) and (B) an axon terminal (TER). Within both profiles, ERβ-IR associated with mitochondria (mit). C, ERβ-IR was observed in unmyelinated axons (AX). Black arrow, Immunoperoxidase for ERβ. Scale bar, 500 μm.
ERβ-IR was not observed in the perikarya of the dSTR. However, ERβ-IR was observed in dendrites, where it accounted for 13% of immunolabeling, and was rarely observed in dendritic spines, where it accounted for 2% of immunolabeling. In dendrites, IR was typically associated with the plasma membrane or with mitochondria (Fig. 3A). Finally, IR for ERβ in glial cells also was frequently observed (23% of the total). In glial cells, labeling was discrete and was localized primarily at the plasma membrane.
By EM, extrasynaptic GPER-1 is observed in the dSTR
Immunoperoxidase labeling for GPER-1 also was observed throughout the dSTR (Table 1). This labeling was associated with both neurons and glia and was found exclusively at extranuclear sites. Like ERα and ERβ, most GPER-1-IR was presynaptic; axons and axon terminals accounted for 36.4 and 11.2% of the GPER-1-labeled profiles, respectively. Axons containing GPER-1-IR were small (<0.15 μm) and almost always unmyelinated. The labeling in axonal profiles was usually discrete and often associated with small clusters of vesicles (Fig. 4C). Axon terminals containing GPER-1-IR ranged from 0.3 to 0.6 μm and contained numerous SSV, occasional mitochondria, but did not contain dense-core vesicles. GPER-1 labeling in terminals was most commonly clustered on groups of SSV or the plasma membrane (Fig. 4C).
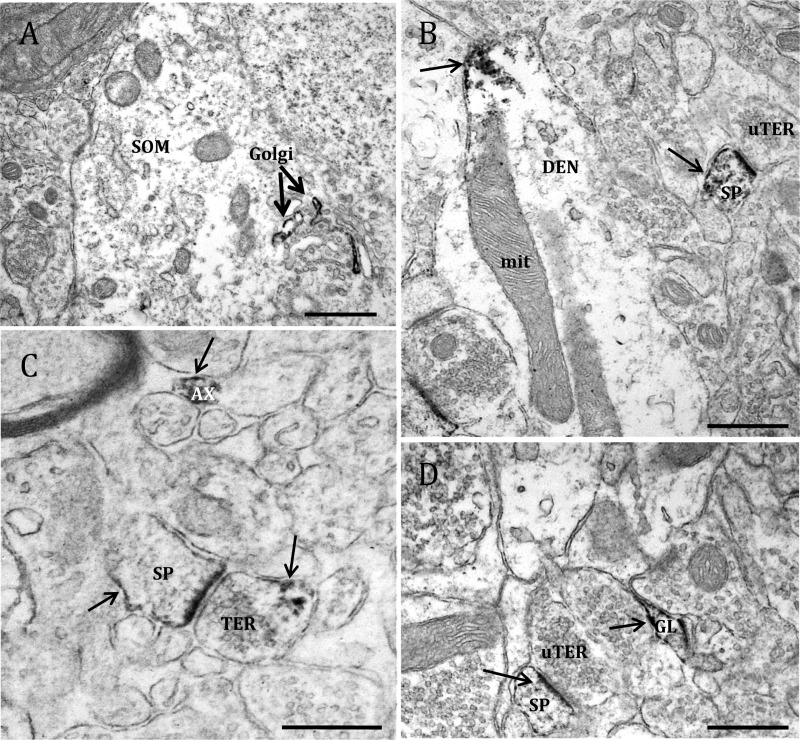
Fig. 4.
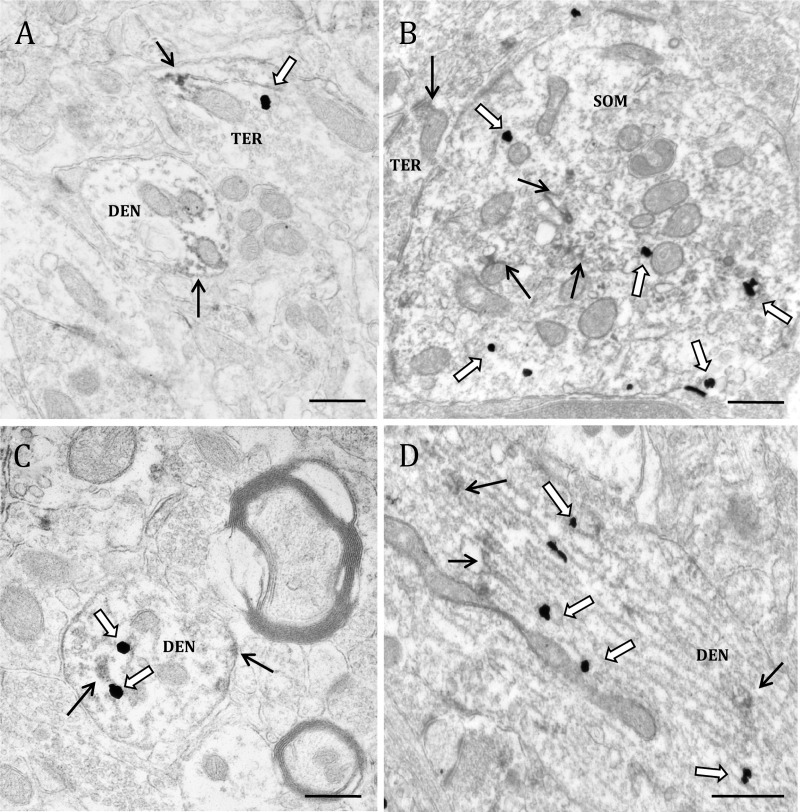
Electron micrographs showing examples of GPER-1-containing profiles. GPER-1-IR is localized to (A) Golgi bodies (Golgi) in a soma (SOM); (B) a dendritic shaft (DEN) at the plasma membrane, and in a dendritic spine (SP) forming an asymmetric synapse with an unlabeled axon terminal (uTER); (C) an unmyelinated axon (AX) and an axon terminal (TER) forming an asymmetric synapse with a dendritic spine; and (D) a glial process (GL) and dendritic spine contacted by an unlabeled terminal. Black arrow, Immunoperoxidase for GPER-1. Scale bar, 500 μm.
GPER-1-IR was observed in neuronal perikarya exclusively in the cytoplasm; it was discrete, and was affiliated with Golgi apparati, mitochondria, and the plasma membrane (Fig. 4A). GPER-1-IR was also observed in dendritic shafts, where it constituted 18.7% of total GPER-1-labeled profiles, and in dendritic spines, where it constituted 9.3% of the total profiles. In the dendritic shafts, GPER-1 was typically associated with the plasma membrane but also was affiliated with microtubules, mitochondrial membranes, and Golgi apparati (Fig. 4B). In dendritic spines, GPER-1-IR peroxidase reaction product accumulated in the spine head and was associated with the plasma membrane, particularly near the postsynaptic density (Fig. 4, B– D). Although GPER-1-IR was observed both pre- and postsynaptically, it was rare for GPER-1-IR terminals to synapse onto GPER-1-IR spines. Finally, 23.1% of GPER-1-IR was observed in glia in the dSTR; the labeling in glial cells was discrete and was observed at the plasma membrane (Fig. 4D).
The total proportion of ERα-IR and GPER-1-IR profiles were very similar in the dSTR. However, there was a higher proportion of GPER-1-IR than ERα-IR in dendritic profiles and a greater proportion of ERα-IR than GPER-1-IR in axon terminal profiles (see Table 1).
Experiment 2. Dual labeling for ER and TH
In dual-labeled sections, IR for both ERα and GPER-1 had a similar distribution to that seen in experiment 1. In agreement with previous studies (39), TH-IR also was also observed throughout the dSTR, exclusively in axons and axon terminals. These TH-labeled terminals were 0.4–1.5 μm in diameter and contained numerous closely packed round SSV. TH terminals typically formed symmetric synapses with dendrites and perikarya. TH labeling was also infrequently observed in unmyelinated axons (0.1–0.15 μm in diameter). Although IR for TH, ERα, and GPER-1 were observed individually throughout the dSTR, immunolabeling for TH and ERα or TH and GPER-1 in the same profile was rarely, if ever, observed (see Table 2). TH-labeled axons and terminals were often found in close proximity to processes containing ERα-IR or GPER-1-IR, although contacts between these profiles were very rarely seen in the plane of section.
Table 2.
ERα and GPER-1 distribution in profiles containing TH or VAChT
| Receptor | ERα or GPER-1 + TH |
ERα or GPER-1 + VAChT |
||||||
|---|---|---|---|---|---|---|---|---|
| ERα | GPER-1 | ERα + TH | GPER-1 + TH | ERα | GPER-1 | ERα + VAChT | GPER-1 + VAChT | |
| Dendrites | 18.8 | 18.1 | 24.7 | 27.3 | 4.8 | 13.2 | ||
| 13.3 ± 2.3 | 10.2 ± 1.4 | 19.7 ± 4.9 | 20.2 ± 1.2 | 1.0 ± 0.4 | 2.6 ± 0.2 | |||
| Spines | 8.4 | 9.4 | 7.8 | 5.2 | 2.6 | 4.3 | ||
| 6.0 ± 0.4 | 5.3 ± 1.6 | 6.2 ± 0.6 | 3.8 ± 1.3 | 0.2 ± 0.2 | 0.2 ± 0.2 | |||
| Axons | 40.1 | 44.4 | 0.7 | 2 | 30.4 | 33.1 | 2 | |
| 28.3 ± 7.0 | 25.0 ± 2.3 | 0.2 ± 0.2 | 0.7 ± 0.3 | 24.2 ± 6.5 | 24.5 ± 2.9 | 0.5 ± 0.4 | ||
| Terminals | 14.6 | 14.2 | 1.6 | 2.8 | 15.5 | 20.5 | 5.1 | 3.3 |
| 10.3 ± 1.4 | 8.0 ± 1.3 | 0.2 ± 0.2 | 0.3 ± 0.3 | 12.3 ± 2.9 | 15.2 ± 2.6 | 0.7 ± 0.4 | 0.5 ± 0.2 | |
| Glia | 17.4 | 17.2 | 17.8 | 12.6 | ||||
| 12.3 ± 1.6 | 9.7 ± 5.8 | 14.2 ± 3.3 | 9.3 ± 2.3 | |||||
| Soma | N/A | N/A | N/A | N/A | 1.3 | 1.4 | 14.3 | 17 |
| N/A | N/A | N/A | N/A | 1.0 ± 0.3 | 1.0 ± 0.3 | 0.2 ± 0.2 | 0.2 ± 0.2 | |
| Total | 99.4 | 97.1 | 0.6 | 1.7 | 97.5 | 94.1 | 2.5 | 5.9 |
| 70.7 ± 11.6 | 56.3 ± 6.1 | 0.4 ± 0.4 | 1.0 ± 0.6 | 77.5 ± 17.7 | 74.0 ± 6.4 | 2.0 ± 1.2 | 4.7 ± 1.0 | |
The percentage of total IR profiles (first row) and number of IR profiles and the corresponding se (second row) observed in approximately 3000-μm area of the dorsal STR, averaged across rats.
Experiment 3. Dual labeling for ER and VAChT
VAChT labeling was observed in multiple types of profiles, including axon terminals, dendrites, and perikarya, which is in agreement with previous studies examining cholinergic neurons in the dSTR (19, 39). In axon terminals, VAChT-IR was associated with the membranes of SSV (Figs. 5D and 6A). VAChT-IR was scattered throughout dendrites and soma, sometimes affiliated with microtubules and the endoplasmic reticulum (Fig. 5, A–C, and 6, A–C). A low proportion of ERα-IR was observed in VAChT-containing profiles, with the greatest proportion of colocalization in dendrites and axon terminals (see Table 2 and Fig. 5, A and D). Low levels of colocalization were observed between GPER-1 and VAChT-IR, primarily in dendrites and perikarya (see Table 2 and Fig 6, B–D). GPER-1-VAChT-IR profiles were observed twice as frequently as ERα-VAChT-IR profiles. In rare instances, VAChT-IR profiles were observed in apposition to either ERα-IR or GPER-1-IR profiles (Fig. 6B).
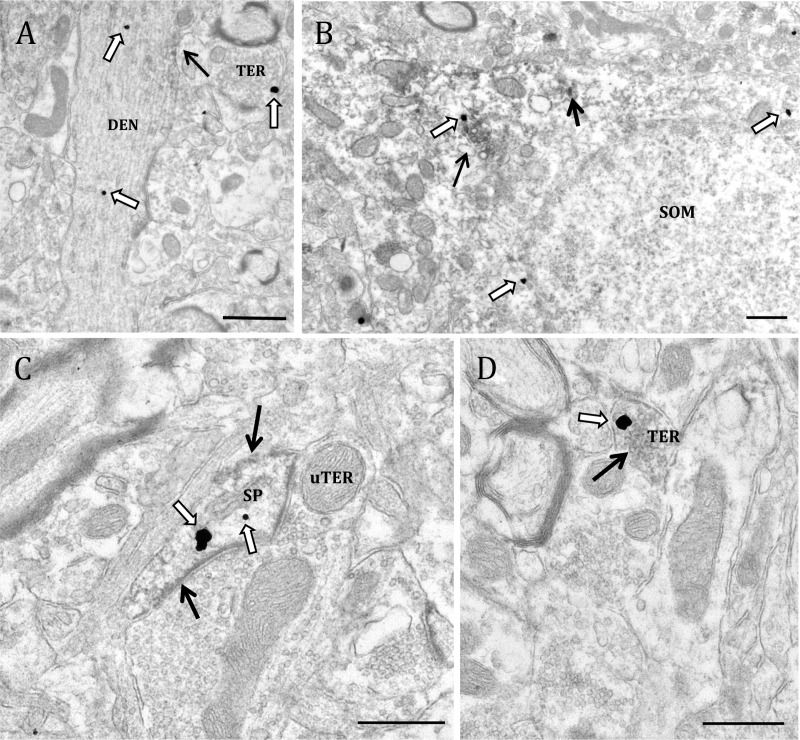
Fig. 5.
Electron micrographs show examples of profiles containing ERα and VAChT-IR. A, ERα localized to a VAChT-IR dendrite (DEN) and a VAChT positive terminal (TER). B, A soma (SOM) containing immunogold labeling for VAChT and immunoperoxidase labeling for ERα. C, A dendritic spine (SP) containing ERα-IR and VAChT-IR synapsing onto an unlabeled axon terminal (uTER). D, An axon terminal containing IR for both ERα and VAChT. Black arrow, Immunoperoxidase for ERα; white arrow, immunogold for VAChT. Scale bar, 500 μm.
Fig. 6.
Electron micrographs show examples of GPER-1- and VAChT-containing profiles. A, GPER-1 localized to a VAChT-IR terminal (TER) and a dendrite containing GPER-1-IR (DEN). B, A soma (SOM) containing immunogold labeling for VAChT and immunoperoxidase labeling for GPER-1. A terminal containing GPER-1-IR is in apposition to the soma. C, A dendrite that contains GPER-1-IR and VAChT-IR. D, A large dendrite containing IR for both GPER-1 and VAChT. Black arrow, Immunoperoxidase for GPER-1; white arrow, immunogold for VAChT. Scale bar, 500 μm.
Discussion
These experiments demonstrated that at the ultrastructural level, ERα, ERβ, and GPER-1-IR is localized exclusively to extranuclear sites in both neuronal and glial profiles in the dSTR of female rats. Labeling for ERα and GPER-1 is not detected in dopaminergic terminals but is found in a small proportion of cholinergic interneurons.
Methodological considerations
To determine whether ERα, ERβ, and GPER-1 are found in the dSTR, and to localize ERα or GPER-1 to TH- or VAChT-containing neurons, the present study used both immunoperoxidase and immunogold labels and preembedding methods. The ERα antibody and the two GPER-1 antibodies had similar cellular and subcellular localizations when viewed by EM, increasing confidence in the accuracy of these findings. Additionally, in accordance with previous research (39), TH labeling is restricted to axons and terminals of the dSTR, whereas VAChT is seen in neuronal profiles.
The preembedding EM immunohistochemical methods used in these experiments have been demonstrated to result in excellent cellular morphology and allow for discrete subcellular localization of antigens (40). To ensure that any differences in number of labeled profiles were not due to differences in antibody penetration or sample size, all tissue samples analyzed for quantification were identical in size and taken from near to the plastic/tissue interface. This methodology tends to underestimate the absolute number of peroxidase-labeled profiles and underestimates immunogold labeling to a greater extent, because immunogold is more limited in penetration and sensitivity (40). IR for ERα, ERβ, and GPER-1 are discrete, so the absence of ER labeling within cellular profiles does not demonstrate that these profiles lack ER. This does not negatively impact the findings of these experiments, because the goal was to investigate whether these receptors were found in this region and the type of neurons where they were localized. However, the quantification analyses presented here are likely conservative values, underestimating the actual numbers of profiles containing these ER and the frequency with which ERα and GPER-1 are localized to cholinergic profiles.
ERα is detected at extranuclear sites
At the ultrastructural level, the location and types of profiles containing ERα-IR in the dSTR were consistent in both single- and dual-labeled tissue. Extranuclear ERα is observed in all types of neuronal profiles and glial cells. This finding contrasts previous light microscopic and in situ hybridization studies, which observed almost no ERα in the dSTR (14, 16). The discrepant findings in the present study and previous studies are likely because of the greater sensitivity and resolution of EM. In fact, in this experiment, this discrepancy was also found, because light microscopy was not sufficient to observe ERα-IR, but EM allowed for the detection of discrete ERα-IR in the dSTR.
The majority of ERα-IR profiles are axons, axon terminals, and glia. The presence of ERα in axons may simply reflect the transportation of these receptors from the perikarya to the terminal, but binding at these receptors may also alter protein transport or the transduction of electrochemical signals (41, 42). Additionally, these presynaptic receptors may be important in the local control of transmitter release, because estrogens have been shown to decrease γ-aminobutyric acid (GABA) transmission in the dSTR (43). ERα-IR is observed exclusively at extranuclear sites in the dSTR, which is in congruence with previous findings that have localized this receptor to extranuclear sites in other brain regions, such as the hippocampus of rodents (27) and the prefrontal cortex of rhesus monkeys (44). Binding at these receptors on the plasma membrane could rapidly alter dopaminergic transmission in the dSTR, which provides a possible mechanism for estrogens' rapid effects on transmission in this brain area (7).
ERβ is detected at extranuclear sites
ERβ-IR was observed exclusively at extranuclear sites neuronal and glia in the dSTR when examined via EM. Similar to ERα, IR for ERβ was rarely observed at the light level, which explains why previous light microscopy experiments did not observe this receptor in the dSTR (16). Additionally, the number of profiles labeled for ERβ was 5-fold less than that seen for ERα or GPER-1. This likely contributes to the lack of detection of ERβ-IR by light microscopy in the dSTR (14, 16).
The highest proportion of ERβ-IR was observed in axons and glial cells. Like ERα, the presence of ERβ-IR in axons could reflect receptors in transport or could suggest that ERβ has a role in conduction of electrochemical signals (41, 42). However, the scarcity of ERβ-IR in axon terminals suggests ERβ has a limited role in directly modulating synaptic transmission. The localization of ERβ exclusively to extranuclear sites in the dSTR agrees with previous studies in the hippocampus and rostral ventrolateral medulla (35, 45). Binding at these membrane-associated ERβ receptors could contribute to estrogens rapid effects on dopaminergic transmission in the dSTR.
GPER-1 is detected at extranuclear sites
GPER-1-IR is seen throughout the dSTR, which agrees with previous light microscopic findings (17). At the ultrastructural level, GPER-1 is observed at the plasma membrane and in the cytoplasm of various neuronal profiles, corresponding to previous research examining the distribution of GPER-1 (46–48). GPER-1 is also observed at the plasma membrane of glial cells.
The highest proportion of GPER-1-IR is observed in dendrites and on glial cells. This suggests that binding at GPER-1 in the dSTR is more likely to affect neurotransmission through postsynaptic mechanisms. Additionally, GPER-1 is associated with Golgi apparati in the dSTR, similar to findings in hippocampus (48). However, in contrast to findings in COS7, HEC50, and CHO cell cultures (49), and the hippocampal formation (48, 50), GPER-1-IR is not observed to be associated with the endoplasmic reticulum in the dSTR. It was hypothesized that regulatory steps in the biosynthesis of this protein occur at the endoplasmic reticulum (47), which would imply that GPER-1 should be present at this site in the dSTR. It is unclear why GPER-1 was not observed in this organelle in here.
Extranuclear ER are associated with mitochondria
ERα, ERβ, and GPER-1 are all localized to mitochondrial membranes and to the plasma membrane of glial cells in the dSTR. Estrogens have been implicated in mitochondrial functioning and cellular metabolism (51, 52), and to our knowledge, this is the first time that GPER-1 have been observed on mitochondria. This provides a mechanism through which estrogens could affect mitochondrial functioning. Additionally, E2 is known to mediate glial-induced neuroprotection (53, 54), in part through binding at GPER-1 (54). Thus, the localization of ER to the plasma membrane of glia could contribute to the explanation of how estrogens are involved in glial-mediated neuroprotection.
Both ERα-IR and GPER-1-IR are found in cholinergic, but not dopaminergic, profiles
DA terminals in the dSTR predominantly have cell bodies originating in the substantia nigra (SN) pars compacta, although some axon collaterals originate from the ventral tegmental area. These DA terminals form synapses primarily with GABAergic medium spiny projection and interneurons (18) but also interact with cholinergic interneurons (19). Increases in systemic E2 have been consistently shown to increase DA availability in the dSTR (7, 13, 55), and it was hypothesized that estrogens might have these effects through binding at receptors found on dopaminergic terminals in the dSTR. However, neither ERα nor GPER-1-IR is observed in dopaminergic terminals, insofar as they are not colocalized with TH. Consequently, this suggests that estrogens are acting at receptors on other neurons in the dSTR (e.g. cholinergic neurons), or at receptors in other brain regions, to elicit these effects. One potential alternate region where estrogens could be acting to affect dSTR DA transmission is the SN, because ERα and GPER-1 are shown to be localized in the SN (17, 56). Moreover, estrogens can directly target dopaminergic neurons in the SN, which could alter DA release and reuptake in the dSTR (56, 57). Previous studies that have found estrogen-induced effects on DA release and (DA transporter) functioning in the dSTR used systemic injections of E2 (7, 9, 13), and consequently, estrogens could have been acting on receptors in the SN to have these effects on DA transmission in the dSTR. Further research is needed to determine whether estrogens effects on DA transmission in the dSTR result from estrogens binding in the SN.
Both ERα-IR and GPER-1-IR are localized to cholinergic profiles in the dSTR. The localization of ERα to cholinergic neurons agrees with findings in the hippocampus (20), and the localization of GPER-1-IR to cholinergic neurons agrees with findings in the medial septum, nucleus basalis magnocellularis, and STR (21). This finding suggests that estrogens could have rapid effects on cholinergic transmission by binding at extranuclear ER on these neurons. Almost all profiles containing either ERα or GPER-1 with VAChT are dendrites, indicating that estrogens binding at these receptors would affect postsynaptic cholinergic transmission. ACh has modulatory effects on dopaminergic activity in the dSTR (19), so estrogen-induced changes in striatal cholinergic transmission could, theoretically, alter dopaminergic transmission in this brain region, providing an alternate mechanism for the rapid effects of estrogens on DA in the dSTR.
Less than 10% of ER-labeled profiles are cholinergic. This could partially be due to our immunolabeling yielding conservative estimates of both the ER and VAChT but does imply that a large proportion of ER-IR in the dSTR is localized to an unknown neuron type. Over 95% of neurons in the dSTR are GABAergic interneurons and projection neurons (18). Systemic injections of E2 rapidly reduce GABA concentrations in the dSTR (43), and antagonizing GABAergic neurons in the dSTR increases DA levels in this brain area (58). These results indicate that estrogens alter GABAergic transmission in the dSTR, which could indirectly alter DA transmission. Only GABA neurons and cholinergic interneurons have their soma and dendrites in the dSTR; IR for both ER is observed in dendritic profiles and soma that do not contain VAChT-IR, so it is reasonable to hypothesize that these remaining ER-labeled profiles are associated with GABA neurons or interneurons. Future research from our group will address whether ERα, ERβ, and GPER-1 are localized to GABA neurons in the dSTR.
Conclusions
These experiments demonstrate the presence of ER in the striatum with ERα and GPER-1 predominating. All three receptors are localized exclusively to extranuclear sites, in various neuronal profiles and on glial cells, providing a mechanism through which estrogens could rapidly alter transmission in the dSTR. ERα and GPER-1 are not localized to DA processes in this brain area but are found in a small proportion of ACh neurons. ACh has strong modulatory effects on DA transmission in the dSTR, so estrogens could indirectly affect DA transmission through altering cholinergic transmission.
Acknowledgments
We thank Dr. Tanya Williams for providing the rat brain tissue and Ms. Louisa Thompson for technical assistance.
This work was supported by a Natural Sciences and Engineering Research Council of Canada discovery grant (W.G.B.) and National Institutes of Health Grants CA119165 (to E.J.F.) and DA08259 and HL096571 (to T.A.M.). The Centre for Studies in Behavioral Neurobiology is a Groupe de Recherche funded by the Fonds de Recherche du Québec-Santé.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACh
- Acetylcholine
- DA
- dopamine
- dSTR
- dorsal striatum
- E2
- estradiol
- EM
- electron microscopy
- EMS
- Electron Microscopy Sciences
- ER
- estrogen receptor
- GABA
- γ-aminobutyric acid
- GPER-1
- G protein-coupled ER 1
- IR
- immunoreactivity
- SN
- substantia nigra
- SSV
- small synaptic vesicle
- TBS
- Tris-buffered saline
- TH
- tyrosine hydroxylase
- VAChT
- vesicular ACh transporter.
References
- 1. Quinlan MG, Hussain D, Brake WG. 2008. Use of cognitive strategies in rats: the role of estradiol and its interaction with dopamine. Horm Behav 53:185–191 [DOI] [PubMed] [Google Scholar]
- 2. Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. 2007. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience 144:26–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nofrey BS, Ben-Shahar OM, Brake WG. 2008. Estrogen abolishes latent inhibition in ovariectomized female rats. Brain Cogn 66:156–160 [DOI] [PubMed] [Google Scholar]
- 4. Quinlan MG, Duncan A, Loiselle C, Graffe N, Brake WG. 2010. Latent inhibition is affected by phase of estrous cycle in female rats. Brain Cogn 74:244–248 [DOI] [PubMed] [Google Scholar]
- 5. Bourque M, Dluzen DE, Di Paolo T. 2009. Neuroprotective actions of sex steroids in Parkinson's disease. Front Neuroendocrinol 30:142–157 [DOI] [PubMed] [Google Scholar]
- 6. Seeman MV. 2004. Gender Differences in the Prescribing of Antipsychotic drugs. Am J Psychiat 161:1324–1333 [DOI] [PubMed] [Google Scholar]
- 7. Becker JB, Rudick CN. 1999. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav 64:53–57 [DOI] [PubMed] [Google Scholar]
- 8. Thompson TL. 1999. Attenuation of dopamine uptake in vivo following priming with estradiol benzoate. Brain Res 834:164–167 [DOI] [PubMed] [Google Scholar]
- 9. Watson CS, Alyea RA, Hawkins BE, Thomas ML, Cunningham KA, Jakubas AA. 2006. Estradiol effects on the dopamine transporter—protein levels, subcellular location, and function. J Mol Signal 1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Landry M, Lévesque D, Di Paolo T. 2002. Estrogenic properties of raloxifene, but not tamoxifen, on D2 and D3 dopamine receptors in the rat forebrain. Neuroendocrinology 76:214–222 [DOI] [PubMed] [Google Scholar]
- 11. Le Saux M, Morissette M, Di Paolo T. 2006. ERb mediates the estradiol induced increase of D2 receptors in rat striatum and nucleus accumbens. Neuropharmacology 50:451–457 [DOI] [PubMed] [Google Scholar]
- 12. Lammers CH, D'Souza U, Qin ZH, Lee SH, Yajima S, Mouradian MM. 1999. Regulation of striatal dopamine receptors by estrogen. Synapse 34:222–227 [DOI] [PubMed] [Google Scholar]
- 13. Becker JB. 1990. Direct effect of 17β-estradiol on striatum: sex differences in dopamine release. Synapse 5:157–164 [DOI] [PubMed] [Google Scholar]
- 14. Shughrue PJ, Lane MV, Merchenthaler I. 1997. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol 388:507–525 [DOI] [PubMed] [Google Scholar]
- 15. Küppers E, Beyer C. 1999. Expression of estrogen receptor-α and β mRNA in the developing and adult mouse striatum. Neurosci Lett 276:95–98 [DOI] [PubMed] [Google Scholar]
- 16. Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. 2003. Immunolocalization of estrogen receptor β in the mouse brain: comparison with estrogen receptor. Endocrinology 144:2055–2067 [DOI] [PubMed] [Google Scholar]
- 17. Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. 2007. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol 193:311–321 [DOI] [PubMed] [Google Scholar]
- 18. Gerfen CR, Wilson CJ. 1996. The rat nervous system: the basal ganglia. St. Louis: Academic Press [Google Scholar]
- 19. Threlfell S, Cragg SJ. 2011. Dopamine signaling in dorsal versus ventral striatum: the dynamic role of cholinergic interneurons. Front Syst Neurosci 5:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Towart LA, Alves SE, Znamensky V, Hayashi S, McEwen BS, Milner TA. 2003. Subcellular relationships between cholinergic terminals and estrogen receptor-α in the dorsal hippocampus. J Comp Neurol 463:390–401 [DOI] [PubMed] [Google Scholar]
- 21. Hammond R, Nelson D, Gibbs RB. 2011. GPER-1 co-localizes with cholinergic neurons in the basal forebrain and enhances potassium-stimulated acetylcholine release in the hippocampus. Psychoneuroendocrinology 36:182–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Williams TJ, Torres-Reveron A, Chapleau JD, Milner TA. 2011. Hormonal regulation of δ opioid receptor immunoreactivity in interneurons and pyramidal cells in the rat hippocampus. Neurobiol Learn Mem 95:206–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Turner CD, Bagnara JT. 1971. General endocrinology. Philadelphia: W. B. Saunders [Google Scholar]
- 24. Marcondes FK, Bianchi FJ, Tanno AP. 2002. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol 62:609–614 [DOI] [PubMed] [Google Scholar]
- 25. Okamura H, Yamamoto K, Hayashi S, Kuroiwa A, Muramatsu M. 1992. A polyclonal antibody to the rat oestrogen receptor expressed in Escherichia coli: characterization and application to immunohistochemistry. J Endocrinol 135:333–341 [DOI] [PubMed] [Google Scholar]
- 26. Alves SE, Weiland NG, Hayashi S, McEwen BS. 1998. Immunocytochemical localization of nuclear estrogen receptors and progestin receptors in the rat dorsal raphe nucleus. J Comp Neurol 391:322–334 [PubMed] [Google Scholar]
- 27. Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. 2001. Ultrastructural evidence that hippocampal α estrogen receptors are located at extranuclear sites. J Comp Neurol 429:355–371 [PubMed] [Google Scholar]
- 28. Shughrue PJ, Merchenthaler I. 2001. Distribution of estrogen receptor β immunoreactivity in the rat central nervous system. J Comp Neurol 436:64–81 [PubMed] [Google Scholar]
- 29. Creutz LM, Kritzer MF. 2002. Estrogen receptor-β immunoreactivity in the midbrain of adult rats: regional, subregional, and cellular localization in the A10, A9, and A8 dopamine cell groups. J Comp Neurol 446:288–300 [DOI] [PubMed] [Google Scholar]
- 30. Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. 2005. A transmembrane intracellular estrogen receptor mediates rapid cell signalling. Science 307:1625–1630 [DOI] [PubMed] [Google Scholar]
- 31. Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr 2000. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPER-1, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14:1649–1660 [DOI] [PubMed] [Google Scholar]
- 32. Hammond R, Gibbs RB. 2011. GPER-1 is positioned to mediate estrogen effects on basal forebrain cholinergic neurons and cognitive performance. Brain Res 1379:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gilmor ML, Nash NR, Roghani A, Edwards RH, Yi H, Hersch SM, Levey AI. 1996. Expression of the puative vesicular acetylcholine transporter in rat brain and localization in cholinergic synaptic vessicles. J Neurosci 16:2179–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arvidsson U, Riedl M, Elde R, Meister B. 1997. Vesicular acetylcholine transporter (VAChT) protein: a novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J Comp Neurol 378:454–467 [PubMed] [Google Scholar]
- 35. Wang G, Drake CT, Rozenblit M, Zhou P, Alves SE, Herrick SP, Hayashi S, Warrier S, Iadecola C, Milner TA. 2006. Evidence that estrogen directly and indirectly modulates C1 adrenergic bulbospinal neurons in the rostral ventrolateral medulla. Brain Res 1094:163–178 [DOI] [PubMed] [Google Scholar]
- 36. Milner TA, Waters EM, Robinson DC, Pierce JP. 2011. Degenerating processes identified by electron microscopic immunocytochemical methods. Method Mol Biol 793:23–59 [DOI] [PubMed] [Google Scholar]
- 37. Yaghmaie F, Saeed O, Garan SA, Voelker MA, Sternberg H, Timiras PS. 2010. Estrogen receptor-α immunoreactivity in the arcuate hypothalamus of young and middle-aged female mice. Neuroendocrinol Lett 31:56–62 [PubMed] [Google Scholar]
- 38. Peters A, Palay SL, Webster HD. 1991. The fine structure of the nervous system. 3rd ed New York: Oxford University Press [Google Scholar]
- 39. Pickel VM, Chan J. 1990. Spiny neurons lacking choline acetyltransferase immunoreactivity are major targets of cholinergic and catecholaminergic terminals in rat striatum. J Neurosci Res 25:263–280 [DOI] [PubMed] [Google Scholar]
- 40. Leranth C, Pickel VM. 1989. in Heimer L, Zaborsky L, eds. Neuroanatomical tract tracing-tracing methods II: recent progress. New York: Plenum; 129–172 [Google Scholar]
- 41. Verdier D, Lund JP, Kolta A. 2003. GABAergic control of action potential propagation along axonal branches of mammalian sensory neurons. J Neurosci 23:2002–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheung DW. 1990. Synaptic transmission in the guinea-pig vas deferens: the role of nerve action potentials. Neuroscience 37:127–134 [DOI] [PubMed] [Google Scholar]
- 43. Hu M, Watson CJ, Kennedy RT, Becker JB. 2006. Estradiol attenuates the K+-induced increase in extracellular GABA in rat striatum. Synapse 59:122–124 [DOI] [PubMed] [Google Scholar]
- 44. Wang AC, Hara Y, Janssen WG, Rapp PR, Morrison JH. 2010. Synaptic estrogen receptor-α levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. J Neurosci 30:12770–12776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. 2005. Ultrastructural localization of estrogen receptor β immunoreactivity in the rat hippocampal formation. J Comp Neurol 491:81–95 [DOI] [PubMed] [Google Scholar]
- 46. Filardo EJ, Graeber CT, Quinn JA, Resnick MB, Giri D, DeLellis RA, Steinhoff MM, Sabo E. 2006. Distribution of GPER-1, a seven membrane-spanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clin Cancer Res 12:6359–6366 [DOI] [PubMed] [Google Scholar]
- 47. Filardo EJ, Thomas P. 2012. G-protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology 153:2953–2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matsuda K, Sakamoto H, Mori H, Hosokawa K, Kawamura A, Itose M, Nishi M, Prossnitz ER, Kawata M. 2008. Expression and intracellular distribution of the G protein-coupled receptor 30 in rat hippocampal formation. Neurosci Lett 441:94–99 [DOI] [PubMed] [Google Scholar]
- 49. Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH. 2008. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology 149:4846–4856 [DOI] [PubMed] [Google Scholar]
- 50. Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. 2006. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun 346:904–910 [DOI] [PubMed] [Google Scholar]
- 51. Araújo GW, Beyer C, Arnold S. 2008. Oestrogen influences on mitochondrial gene expression and respiratory chain activity in cortical and mesencephalic astrocytes. J Neuroendocrinol 20:930–941 [DOI] [PubMed] [Google Scholar]
- 52. Razmara A, Sunday L, Stirone C, Wang XB, Krause DN, Duckles SP, Procaccio V. 2008. Mitochondrial effects of estrogen are mediated by estrogen receptor α in brain endothelial cells. J Pharmacol Exp Ther 325:782–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Arevalo MA, Santos-Galindo M, Bellini MJ, Azcoitia I, Garcia-Segura LM. 2010. Actions of estrogens on glial cells: implications for neuroprotection. Biochim Biophys Acta 1800:1106–1112 [DOI] [PubMed] [Google Scholar]
- 54. Liu SB, Han J, Zhang N, Tian Z, Li XB, Zhao MG. 2011. Neuroprotective effects of oestrogen against oxidative toxicity through activation of G-protein-coupled receptor 30 receptor. Clin Exp Pharmacol Physiol 38:577–585 [DOI] [PubMed] [Google Scholar]
- 55. Becker JB. 1999. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav 64:803–812 [DOI] [PubMed] [Google Scholar]
- 56. Küppers E, Ivanova T, Karolczak M, Beyer C. 2000. Estrogen: a multifunctional messenger to nigrostriatal dopaminergic neurons. J Neurocytol 29:375–385 [DOI] [PubMed] [Google Scholar]
- 57. Becker JB, Beer ME. 1986. The influence of estrogen on nigrostriatal dopamine activity: behavioural and neurochemical evidence for both pre- and postsynaptic components. Behav Brain Res 19:27–33 [DOI] [PubMed] [Google Scholar]
- 58. Adermark L, Clarke RB, Ericson M, Söderpalm B. 2011. Subregion-specific modulation of excitatory input and dopamine output in the striatum by tonically activated glycine and GABA(A) receptors. Front Syst Neurosci 5:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Paxinos G, Watson C. 1998. The rat brain in stereotaxic coordinates, fourth ed New York: Academic Press; 16 [Google Scholar]