Abstract
Glucocorticoids and their synthetic derivatives are known to alter cardiac function in vivo; however, the nature of these effects and whether glucocorticoids act directly on cardiomyocytes are poorly understood. To explore the role of glucocorticoid signaling in the heart, we used rat embryonic H9C2 cardiomyocytes and primary cardiomyocytes as model systems. Dexamethasone (100 nm) treatment of cardiomyocytes caused a significant increase in cell size and up-regulated the expression of cardiac hypertrophic markers, including atrial natriuretic factor, β-myosin heavy chain, and skeletal muscle α-actin. In contrast, serum deprivation and TNFα exposure triggered cardiomyocyte apoptosis, and these apoptotic effects were inhibited by dexamethasone. Both the hypertrophic and anti-apoptotic actions of glucocorticoids were abolished by the glucocorticoid receptor (GR) antagonist RU486 and by short hairpin RNA-mediated GR depletion. Blocking the activity of the mineralocorticoid receptor had no effect on these glucocorticoid-dependent cardiomyocyte responses. Aldosterone (1 μm) activation of GR also promoted cardiomyocyte hypertrophy and cell survival. To elucidate the mechanism of the dual glucocorticoid actions, a genome-wide microarray was performed on H9C2 cardiomyocytes treated with vehicle or dexamethasone in the absence or presence of serum. Serum dramatically influenced the transcriptome regulated by GR, revealing potential glucocorticoid signaling mediators in both cardiomyocyte hypertrophy and apoptosis. These studies reveal a direct and dynamic role for glucocorticoids and GR signaling in the modulation of cardiomyocyte function.
Glucocorticoids are primary stress hormones that are synthesized and released by the adrenal cortex in response to activation of the hypothalamic-pituitary-adrenal axis. Acting on nearly all tissues and cells, glucocorticoids regulate a diverse array of biological processes including intermediary metabolism, immune function, skeletal growth, reproduction, cognition, behavior, and cell proliferation and survival (1, 2). Due to their potent antiinflammatory and immunosuppressive actions, synthetic glucocorticoids are widely used clinically to treat inflammation, autoimmune diseases, and hematological cancers. The therapeutic benefit of glucocorticoids, however, is limited by adverse effects such as osteoporosis, growth retardation in children, glaucoma, and hypertension (3–5). The physiological and pharmacological effects of glucocorticoids are mediated by the glucocorticoid receptor (GR, NR3C1), which belongs to the nuclear receptor superfamily of ligand-dependent transcription factors (6). Glucocorticoid binding stimulates the translocation of GR from the cytoplasm to the nuclear compartment where it regulates the expression of numerous target genes. GR can activate or repress gene transcription by direct binding to specific hormone response elements on the DNA and/or by interactions with other chromatin-bound transcription factors (7).
The GR is known to be expressed in the heart (8–11), but the specific role of glucocorticoid signaling in cardiomyocytes is poorly understood. Both in vivo and in vitro studies have implicated glucocorticoids in the development of cardiac hypertrophy, a major cause of congestive heart failure (12–18). In fact, the level of circulating glucocorticoids is an independent risk factor for cardiovascular disease (19–21). Conversely, glucocorticoids may have cardioprotective effects by inhibiting apoptosis of cardiomyocytes caused by a variety of conditions including ischemia/reperfusion (22). Whether these cardiovascular outcomes reflect systemic actions of glucocorticoids and/or direct effects of the steroid on cardiomyocytes remains controversial. An additional complexity to elucidating the specific function of GR in the heart is that cardiomyocytes also express the closely related mineralocorticoid receptor (MR) (23, 24). In contrast to GR, which binds glucocorticoids with higher affinity than the mineralocorticoid aldosterone, MR binds these two corticosteroids with comparable affinity (25–28). Glucocorticoids are two to three orders of magnitude more abundant than aldosterone in the circulation, and cardiomyocytes express minimal 11β-hydroxysteroid dehydrogenase type 2, the enzyme that converts glucocorticoids to their inactive metabolite (11, 29, 30). Therefore, cardiac-specific MR is thought to be primarily occupied by glucocorticoids, suggesting that both GR and MR are potential mediators of glucocorticoid signaling in the heart.
The present study was initiated to elucidate the role of glucocorticoids in cardiac physiology and pathology by examining the direct effects of these steroids on cardiomyocyte function. Using a rat embryonic cardiomyocyte cell line (H9C2) and primary rat cardiomyocytes, we investigated the ability of glucocorticoids to regulate hypertrophy and apoptosis. In cells cultured in the presence of serum, glucocorticoids were found to induce cardiomyocyte hypertrophy. Under conditions of serum deprivation, glucocorticoids protected cardiomyocytes from apoptosis. These dual actions of glucocorticoids required GR but not MR. A genome-wide microarray analysis of H9C2 cells revealed not only potential mediators of glucocorticoid-dependent hypertrophy and cell survival but also a serum-dependent reprogramming of the glucocorticoid signaling profile. These findings suggest that glucocorticoids can both positively and negatively influence the function of the heart through direct effects on cardiomyocytes.
Materials and Methods
Reagents
Dexamethasone (Dex) (1,4-pregnadien-9α-fluoro-16α-methyl-11β,17,21-triol-3,20-dione), aldosterone (11β,21-dihydroxy-3,20-dioxopregn-4-en-18-al), and RU486 [11β-[p-(dimethylamino)phenyl]-17β-hydroxy-17-(1-propynyl)estra-4,9-dien-3-one] were purchased from Steraloids (Newport, RI). Eplerenone was purchased from Sigma-Aldrich (St. Louis, MO). Antibodies for skeletal muscle actin and growth arrest-specific protein 2 (Gas2) were purchased from Abcam (Cambridge, MA); caspase-3 and Bcl-xL antibodies were obtained from Cell Signaling (Danvers, MA); and the β-myosin heavy chain (β-MHC) antibody was from Millipore (Billerica, MA). The Alexa Fluor 488 secondary antibody and Alexa Fluor 594 phalloidin antibody were from Invitrogen (Carlsbad, CA). The MR antibody was generously provided by Dr. Celso E. Gomez-Sanchez (31). Generation of the anti-GR antibody has been described previously (32).
Cell culture
The rat embryonic cardiomyocyte H9C2 cells (American Type Culture Collection, Manassas, VA) were cultured in high-glucose (4500 mg/liter) DMEM supplemented with 10% heat-inactivated fetal bovine serum, 1 mm sodium pyruvate, 100 μg/ml streptomycin, and 100 U/ml penicillin (33). Rat primary cardiomyocytes were isolated from 2- to 4-d-old Sprague Dawley rats according to the manufacturer's protocol from Worthington Biochemical (Lakewood, NJ) and cultured in DMEM-H containing Earle's salt with glutamine and 10% horse serum, 5% fetal bovine serum, 100 μg/ml streptomycin, and 100 U/ml penicillin at 37 C and 5% CO2 atmosphere (34).
Immunofluorescence staining
Cardiomyocytes were grown on coverslips and fixed in a 4% paraformaldehyde solution in PBS for 10 min at room temperature. After permeabilizing with 0.2% Triton X-100 in PBS for 10 min at room temperature and blocking with 5% boiled goat serum for 1 h at room temperature, the cells were incubated with primary antibodies (anti-β-MHC and/or Alexa Fluor 488 phalloidin) overnight at 4 C. Cells were washed in PBS and then incubated with a fluorescent secondary antibody (Alexa Fluor 594 goat antimouse antibody) for 1 h at room temperature and subsequently washed with PBS. After incubating cells with the 4′,6-diamidino-2-phenylindole nuclear stain, coverslips were mounted with antifading mounting media (Invitrogen), and images were captured at the same magnification (×40) on a Zeiss LSM 510 UV META confocal microscope and processed by Zeiss LSM software (Carl Zeiss, Oberkochen, Germany).
Cell volume measurement
The volume of H9C2 cells was measured by a Cell Lab Quanta SC flow cytometer (Beckman Coulter, Brea, CA). The electronic volume was calibrated before each experiment by applying 6 μm AlignFlow beads (Invitrogen). For each sample, 5000 cells suspended in culture media were analyzed, and the mean cell volume was calculated using Cell Lab Quanta SC Software.
Real-time PCR
Total RNA was isolated using QIAGEN RNeasy mini Kit (QIAGEN GmbH, Hilden, Germany) according to manufacturer's instructions. All primer sets for PCR were purchased from Applied Biosystems (Foster City, CA). PCR was performed with a TaqMan probe-based detection system and quantified by the ABI Prism 7900HT sequence detection system (Applied Biosystems). The abundance of each gene was normalized to the level of cyclophilin B, which was unchanged under our experimental conditions.
Immunoblotting
Cardiomyocytes were lysed in RIPA buffer [25 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate] supplemented with complete mini protease inhibitor cocktail (Roche, Indianapolis, IN) and sonicated for 10 sec on ice. Equivalent amounts of protein were separated on 4–12% bis-Tris gels (Invitrogen) and transferred to nitrocellulose membranes. Membranes were blocked with 5% milk in 1× Tris-buffered saline with Tween 20 [50 mm Tris-HCl (pH 7.4), 150 mm NaCl, and 0.1% Tween 20] for 1 h at room temperature and incubated with primary antibodies [anti-GR, anti-MR; anti-skeletal muscle α-actin (SKA), anti-Bcl-xL, anti-caspase-3, and anti-Gas 2] overnight at 4 C. Membranes were washed with 1× Tris-buffered saline with Tween 20, incubated with appropriate secondary antibodies for 1 h at room temperature, and developed with an enhanced chemiluminescence kit (Thermo Fisher Scientific, Pittsburgh, PA). Immunoblots depicted are representative of three independent experiments.
Targeted knockdown of GR and MR
A lentiviral-derived short hairpin RNA (shRNA) method was used to knock down GR expression (Sigma-Aldrich). Two shRNA duplexes against GR were used for the knockdown experiments: 5′-AAGGGAACTCCAGTCAGAACT-3′ and 5′-AACGGAGGCAGTGTGAAATTG-3′. H9C2 cells were transduced with lentiviral particles containing shRNA against GR or nontargeting control shRNA and selected in puromycin-containing (1.5 μg/ml) cell culture medium. To deplete the expression of MR, we transiently transfected H9C2 cells with small interfering RNAs (siRNAs) directed against MR (SMARTpool) or nontargeting control siRNA (Thermo Fisher Scientific).
Apoptotic assays
Plasma membrane integrity of H9C2 cells was evaluated by flow cytometric analysis of 10 μg/ml propidium iodide (Sigma-Aldrich) exclusion. To analyze the degraded DNA content, cells were washed in ice-cold PBS and fixed in 70% ethanol at 4 C overnight. Fixed cells were then washed with PBS and incubated with 20 μg/ml propidium iodide solution, 1000 U RNase One (Promega, Madison, WI) in PBS for 20 min in the dark. Cardiomyocyte apoptosis was analyzed using a FACSort flow cytometer with CELLQuest software (Becton Dickinson Immunocytometry Systems, San Jose, CA). The percentage of cells with DNA degradation for each sample was calculated by dividing the number of cells having subdiploid DNA by the total number of cells analyzed.
Microarray analysis
Microarrays were performed on RNA isolated from H9C2 cardiomyocytes treated with or without 100 nm Dex in the presence or absence of serum. Three independent samples were collected from the four experimental groups (vehicle, Dex 6 h, Dex 24 h, or Dex 48 h). Gene expression analysis was done using Agilent Whole Genome Rat 4 × 44 multiplex format oligo arrays (Agilent Technologies, Santa Clara, CA) as described previously (35). Results were acquired by using the Agilent Feature Extraction software (version 9.1) with the default one-color setting for all parameters. Error modeling and noise adjustment were performed by the Agilent Feature Extraction Software. These data were analyzed with the Rosetta Resolver system (version 7.2) (Rosetta Biosoftware, Kirkland, WA). Both an error-weighted ANOVA and Benjamini-Hochberg multiple test correction were applied to decrease the number of false positives. A P value < 0.01 was considered significant. Genes significantly regulated by Dex were analyzed using Ingenuity Pathways Analysis software (Ingenuity Systems, Redwood City, CA).
Statistical analysis
For determining statistical significance (P < 0.05), a Student's t test or one-way ANOVA with Tukey's post hoc analysis was performed using Microsoft Office Excel 2003 or GraphPad Prism software (La Jolla, CA).
Results
Cardiomyocyte hypertrophy is induced by glucocorticoids
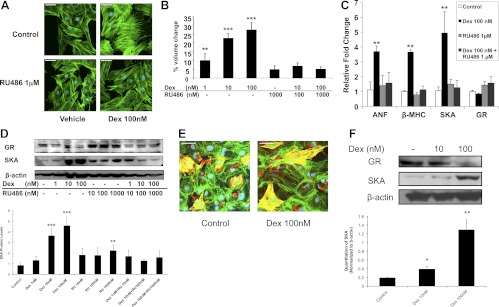
To determine the direct nature and mechanisms of glucocorticoid-induced hypertrophy, we investigated the effect of Dex, a synthetic glucocorticoid used clinically, on H9C2 cardiomyocytes. Figure 1A shows that the surface area of H9C2 cells was increased after 72 h of Dex treatment as determined by immunostaining of the main component of cytoskeleton, filamentous actin. Moreover, Dex increased mean cell volume in a dose-dependent manner, reaching an approximately 25% increase at the 100 nm concentration (Fig. 1B). To determine whether the increased cell size actually resulted from hypertrophy, the mRNA levels of various cardiac hypertrophic marker genes, including atrial natriuretic factor (ANF), β-MHC, and SKA, were measured by real-time PCR. Dex up-regulated the expression of all three mRNAs (Fig. 1C). Moreover, Dex treatment promoted the accumulation of SKA protein, a hallmark of cardiac hypertrophy (Fig. 1D). We next evaluated whether the glucocorticoid-dependent effects on transformed H9C2 cells were observed in primary cardiomyocytes. Neonatal rat cardiomyocytes were isolated and treated with Dex for 72 h. In agreement with the data from H9C2 cells, primary cardiomyocytes exposed to Dex exhibited a marked increase in cell size (Fig. 1E). Consistent with the enlarged size of the cardiomyocytes, SKA protein levels were elevated in the isolated cell population after glucocorticoid treatment (Fig. 1F). These data indicate that glucocorticoids can act directly on cardiomyocytes to induce a hypertrophic response.
Fig. 1.
Glucocorticoids induce hypertrophy in rat cardiomyocytes. Rat H9C2 cells or isolated neonatal cardiomyocytes were treated for 72 h with Dex in the presence or absence of RU486. A, Representative confocal microscopic images of phalloidin-stained H9C2 cells. Scale bar, 45 μm. B, H9C2 cell volumes were measured by flow cytometry and are presented as percent volume change relative to the control cells. C, The mRNA levels in H9C2 cells for GR and the cardiac hypertrophic marker genes ANF, β-MHC, and SKA were determined by real-time PCR and normalized to cyclophilin B. D, GR and SKA protein levels in H9C2 cells were measured by immunoblotting. The lower panel shows quantification of SKA protein normalized to β-actin. E, Representative confocal microscopic images of primary cardiomyocytes double labeled with β-MHC (red) and phalloidin (green). The presence of actin and β-MHC in the same cell (yellow) distinguishes the cardiomyocytes from fibroblasts. Scale bar, 45 μm. F, GR and SKA protein levels in primary cardiomyocytes were measured by immunoblotting. The lower panel shows quantification of SKA protein normalized to β-actin. Data represent the mean ± sd from three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. controls.
GR is required for glucocorticoid-induced cardiomyocyte hypertrophy
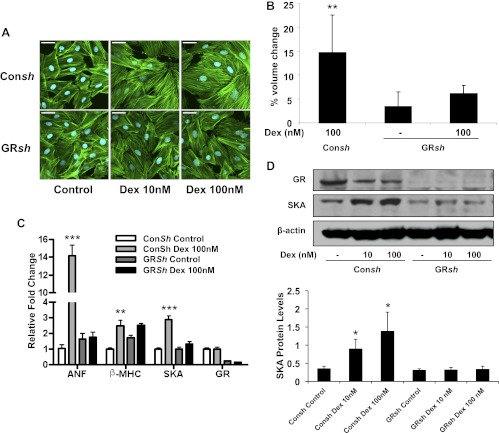
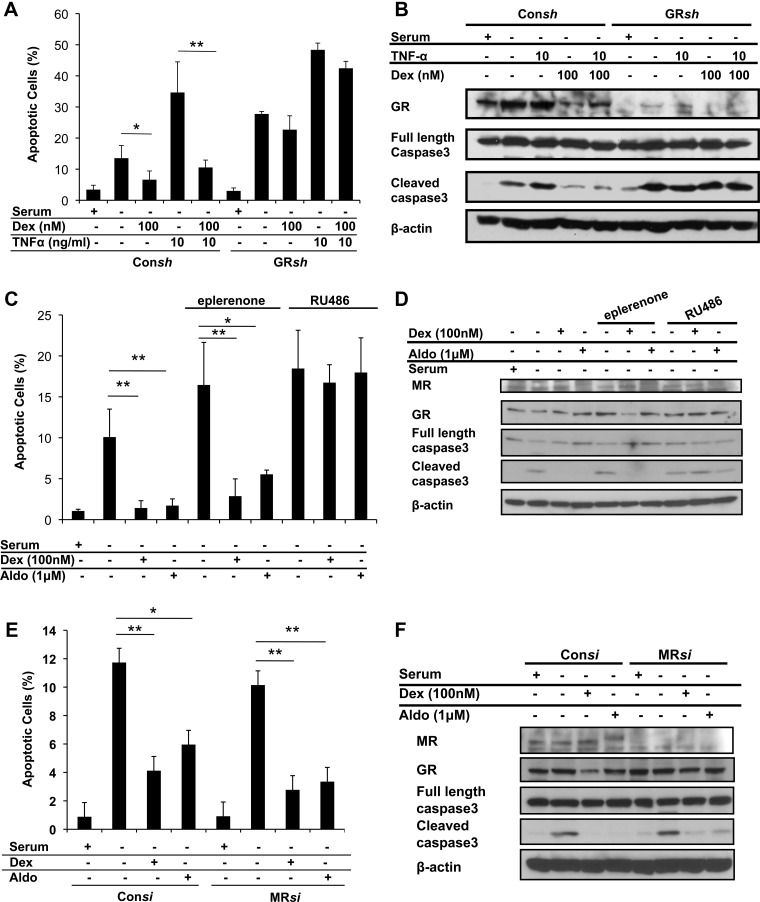
To examine the importance of GR signaling in glucocorticoid-induced hypertrophy, we employed two approaches. First, treatment of H9C2 cells with the GR antagonist RU486 abolished the Dex-induced increase in cell size (Fig. 1, A and B) and the up-regulation of the hypertrophic marker genes (Fig. 1, C and D). Consistent with its ability to function as a partial agonist on certain genes (36, 37), RU486 at a concentration of 1000 nm stimulated a small increase in SKA protein levels (Fig. 1D). Our second approach was to genetically knock down the expression of endogenous GR in H9C2 cells using a lentiviral-derived shRNA system. Cardiomyocytes that received control shRNA had similar glucocorticoid-induced hypertrophic changes when compared with wild-type cells (Fig. 2, A–D). In contrast, cardiomyocytes depleted of GR failed to undergo hypertrophy in response to Dex (Fig. 2, A–D). The substantial decrease in endogenous GR in these cells was confirmed by real-time PCR and immunoblotting (Fig. 2, C and D). These results indicate a necessary role for the GR in the hypertrophic actions of glucocorticoids in cardiomyocytes.
Fig. 2.
GR is required for glucocorticoid-induced cardiomyocyte hypertrophy. H9C2 cardiomyocytes were stably transfected with control shRNA (Consh) or GR shRNA (GRsh) and treated with or without Dex for 72 h. A, Representative confocal microscopic images of phalloidin-stained cells. Scale bar, 45 μm. B, Cell volumes were measured by flow cytometry and are presented as percent volume change relative to the control cells. C, The mRNA levels for GR and the cardiac hypertrophic marker genes ANF, β-MHC, and SKA were determined by real-time PCR and normalized to cyclophilin B. D, GR and SKA protein levels were measured by immunoblotting. The lower panel shows quantification of SKA protein normalized to β-actin. Data represent the mean ± sd from three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. controls.
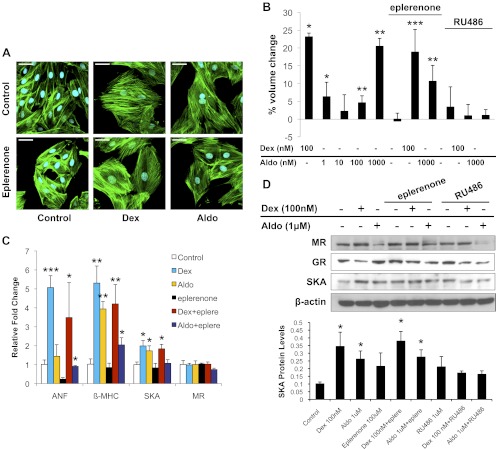
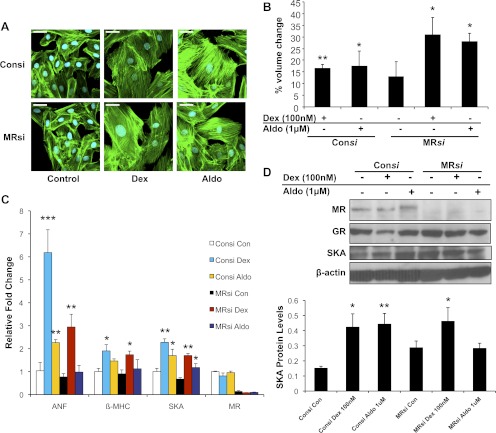
In addition to GR, H9C2 cardiomyocytes also express the closely related MR. The mineralocorticoid aldosterone promoted a small increase in cell size at low doses and a greater response at the higher concentration of 1 μm (Fig. 3, A and B, and Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Aldosterone treatment also enhanced the expression of the hypertrophic marker genes β-MHC and SKA (Fig. 3, C and D). Coadministration of the MR antagonist eplerenone inhibited these hypertrophic changes but did not prevent 1 μm aldosterone from inducing small but significant increases in cell size, β-MHC mRNA, and SKA protein (Fig. 3, A–D, and Supplemental Fig. 1). In addition, 1 μm aldosterone stimulated hypertrophy in cells depleted of MR, although no increases in ANF mRNA and SKA protein were observed (Fig. 4, A–D). The hypertrophic actions of 1 μm aldosterone were also inhibited by RU486 and in cells depleted of GR (Fig. 3, B and D, and Supplemental Fig. 2), suggesting that aldosterone at high concentrations can signal through GR to increase the size of cardiomyocytes. We next evaluated the contribution of MR to the hypertrophic response stimulated by glucocorticoids. Neither eplerenone treatment nor MR knockdown inhibited the Dex-induced cardiomyocyte hypertrophy (Figs. 3, A–D, and 4, A–D), indicating that MR is not involved in the hypertrophic actions of Dex on cardiomyocytes.
Fig. 3.
Glucocorticoid-induced cardiomyocyte hypertrophy is not blocked by eplerenone. H9C2 cardiomyocytes were treated with Dex (100 nm) or aldosterone (1 μm) with or without RU486 (1 μm) or eplerenone (100 μm) for 72 h. A, Representative confocal microscopic images of phalloidin-stained cells. Scale bar, 45 μm. B, Cell volumes were measured by flow cytometry and are presented as percent volume change relative to the control cells. C, The mRNA levels for MR and the cardiac hypertrophic marker genes ANF, β-MHC, and SKA were determined by real-time PCR and normalized to cyclophilin B. D, MR, GR, and SKA protein levels were measured by immunoblotting. The lower panel shows quantification of SKA protein normalized to β-actin. Data represent the mean ± sd from three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. controls.
Fig. 4.
Glucocorticoid-induced cardiomyocyte hypertrophy does not require MR expression. H9C2 cardiomyocytes were transiently transfected with control siRNA (Consi) or MR siRNA (MRsi) and incubated with Dex (100 nm) or aldosterone (1 μm) for 72 h. A, Representative confocal microscopic images of phalloidin-stained cells. Scale bar, 45 μm. B, Cell volumes were measured by flow cytometry and are presented as percent volume change relative to the control cells. C, The mRNA levels for MR and the cardiac hypertrophic marker genes ANF, β-MHC, and SKA were determined by real-time PCR and normalized to cyclophilin B. D, MR, GR, and SKA protein levels were measured by immunoblotting. The lower panel shows quantification of SKA protein normalized to β-actin. Data represent the mean ± sd from three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. controls.
Cardiomyocyte apoptosis is inhibited by glucocorticoids
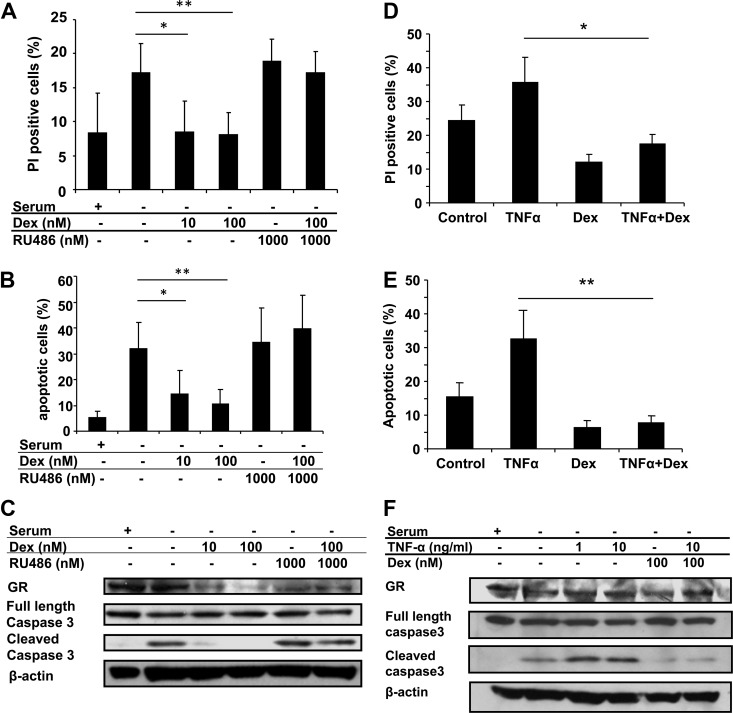
Glucocorticoids have been suggested to protect against cardiac injury by preventing the death of cardiomyocytes (38–41). To investigate the mechanism of this cardioprotective response, we induced H9C2 cells to undergo apoptosis by serum deprivation and examined the role of glucocorticoids in modulating this process. H9C2 cardiomyocytes were cultured in the absence of serum with or without 100 nm Dex for 48 h and then evaluated for apoptosis by multiple approaches including membrane integrity, DNA degradation, and activation of caspase-3. Serum deprivation promoted an increase in cell death and induced the cleavage of caspase-3 (Fig. 5, A–C). Dex treatment inhibited the apoptotic process triggered by serum starvation (Fig. 5, A–C). To determine whether glucocorticoids were able to block apoptosis induced by other stimuli, TNFα was employed in conjunction with serum withdrawal. TNFα was chosen because elevated levels of this cytokine have been linked to increased cardiac apoptosis and heart failure (42–44). The addition of 10 ng/ml TNFα to H9C2 cardiomyocytes cultured in the absence of serum enhanced the percentage of apoptotic cells and the amount of cleaved caspase-3 (Fig. 5, D–F). Despite the enhanced cell death, treatment with 100 nm Dex blocked the apoptotic response (Fig. 5, D–F). These data suggest that glucocorticoids act directly on cardiomyocytes to inhibit cell death triggered by serum starvation and other stimuli such as TNFα.
Fig. 5.
Glucocorticoids inhibit cardiomyocyte apoptosis. A–C, H9C2 cardiomyocytes cultured in the absence of serum were treated with Dex, RU486, or both for 48 h. Cells were analyzed by flow cytometry for propidium iodide staining and DNA content. Levels of GR and both full-length and cleaved caspase-3 were examined by immunoblotting, and β-actin was used as an internal loading control. D–F, H9C2 cardiomyocytes cultured in the absence of serum were treated with TNFα (10 ng/ml), Dex (100 nm), or both for 48 h and analyzed as described above. Data represent the mean ± sd from three independent experiments. *, P < 0.05; **, P < 0.01 vs. control cells in absence of serum or in the absence of serum and treated with TNFα.
GR is required for the anti-apoptotic activity of glucocorticoids
To determine the contribution of GR and MR to the anti-apoptotic action of glucocorticoids on H9C2 cardiomyocytes, we employed receptor antagonists and RNA-mediated gene silencing. The glucocorticoid antagonist RU486 completely blocked the ability of Dex to inhibit apoptosis induced by serum withdrawal (Fig. 5, A–C). Moreover, GR-deficient H9C2 cardiomyocytes were resistant to the anti-apoptotic effects of Dex (Fig. 6, A and B). Treatment of the cells with 1 μm aldosterone also protected cardiomyocytes from cell death (Fig. 6, C and D). This response was blocked by RU486 but not by the mineralocorticoid antagonist eplerenone or by knockdown of MR (Fig. 6, C–F). Lower doses of aldosterone also partially inhibited apoptosis induced by serum withdrawal or TNFα treatment, and both responses were impaired in GR-deficient cells (Supplemental Fig. 3). Finally, neither eplerenone nor MR knockdown altered the ability of Dex to protect cardiomyocytes from the cell-killing effects of serum deprivation (Fig. 6, C–F). Taken together, these results indicate that aldosterone, particularly at high concentrations, can signal through GR to inhibit apoptosis and that GR is required for the anti-apoptotic function of glucocorticoids in cardiomyocytes.
Fig. 6.
GR but not MR is required for the anti-apoptotic effect of glucocorticoids on cardiomyocytes. A and B, H9C2 cardiomyocytes were stably transfected with control shRNA (Consh) or GR shRNA (GRsh) and treated with TNFα, Dex, or both under serum-free conditions for 48 h. Apoptotic cells were determined by DNA content using flow cytometry. Levels of GR and both full-length and cleaved caspase-3 were examined by immunoblotting, and β-actin was used as an internal loading control. C and D, H9C2 cardiomyocytes were treated with Dex or aldosterone, with or without RU486 (1 μm) or eplerenone (100 μm), under serum-free conditions for 48 h and analyzed as described above. MR levels were examined by immunoblotting. E and F, H9C2 cardiomyocytes were transiently transfected with control siRNA (Consi) or MR siRNA (MRsi) and incubated with Dex or aldosterone under serum-free conditions for 48 h and analyzed as described above. Data represent the mean ± sd from three independent experiments. *, P < 0.05; **, P < 0.01 vs. control cells in the absence of serum or in the absence of serum and treated with TNFα or eplerenone.
Genome-wide microarray analysis of glucocorticoid-regulated genes in cardiomyocytes
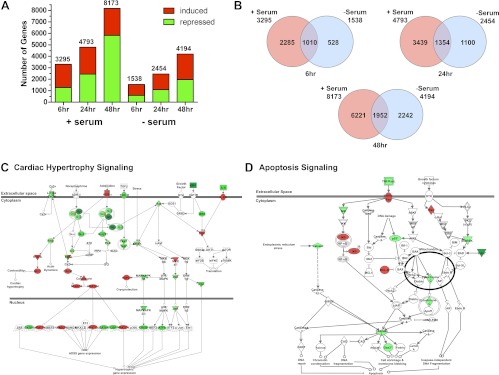
To identify downstream targets involved in the glucocorticoid-dependent effects on cardiomyocyte hypertrophy and apoptosis, we performed a microarray on H9C2 cells treated with vehicle or 100 nm Dex for 6, 24, and 48 h in either the presence or absence of serum. In control cells not exposed to glucocorticoids, removal of serum significantly affected the expression of many genes but did not alter the level of GR (Fig. 5, C and F; Figure 6, B, D, and F; and Supplemental Fig. 4). Dex treatment for 6, 24, and 48 h significantly regulated 3295, 4793, and 8173 genes in serum-supplemented H9C2 cells and 1538, 2454, and 4194 genes in cells cultured without serum, respectively (Fig. 7A). The ratio of induced vs. repressed genes was similar across the different conditions except at the 48-h time point where there was a marked rise in the number of genes repressed by Dex in serum-supplemented cells (Fig. 7A). A comparison of the genes regulated by Dex revealed that serum had a dramatic influence on the transcriptome regulated by glucocorticoids (Fig. 7B). For example, although 1010 genes were commonly regulated by Dex at the 6-h time point, 2285 genes were uniquely regulated by Dex in the presence of serum, and 528 genes were uniquely regulated by Dex in the absence of serum.
Fig. 7.
Microarray analysis of glucocorticoid-regulated genes in H9C2 cardiomyocytes. Global gene expression analysis was performed on RNA isolated from H9C2 cardiomyocytes treated with vehicle or 100 nm Dex for 6, 24, or 48 h in the presence or absence of serum. Genes significantly regulated by Dex were determined by ANOVA (P < 0.01) and analyzed using Ingenuity Pathway Analysis. A, The number of genes induced and repressed by Dex at each time point in cells cultured with or without serum. B, Venn diagram of Dex-regulated genes at 6-, 24-, and 48-h time points in cardiomyocytes cultured in the presence or absence of serum. C, The cardiac hypertrophy signaling canonical pathway was significantly altered in serum-supplemented H9C2 cells treated with glucocorticoids. Shown are the 58 genes in this pathway that were regulated by Dex at the 48-h time point. D, The apoptosis signaling canonical pathway was significantly altered in serum-deprived H9C2 cells treated with glucocorticoids. Shown are the 15 genes in this pathway that were regulated by Dex at the 48-h time point. Red indicates increased expression, and green indicates decreased expression.
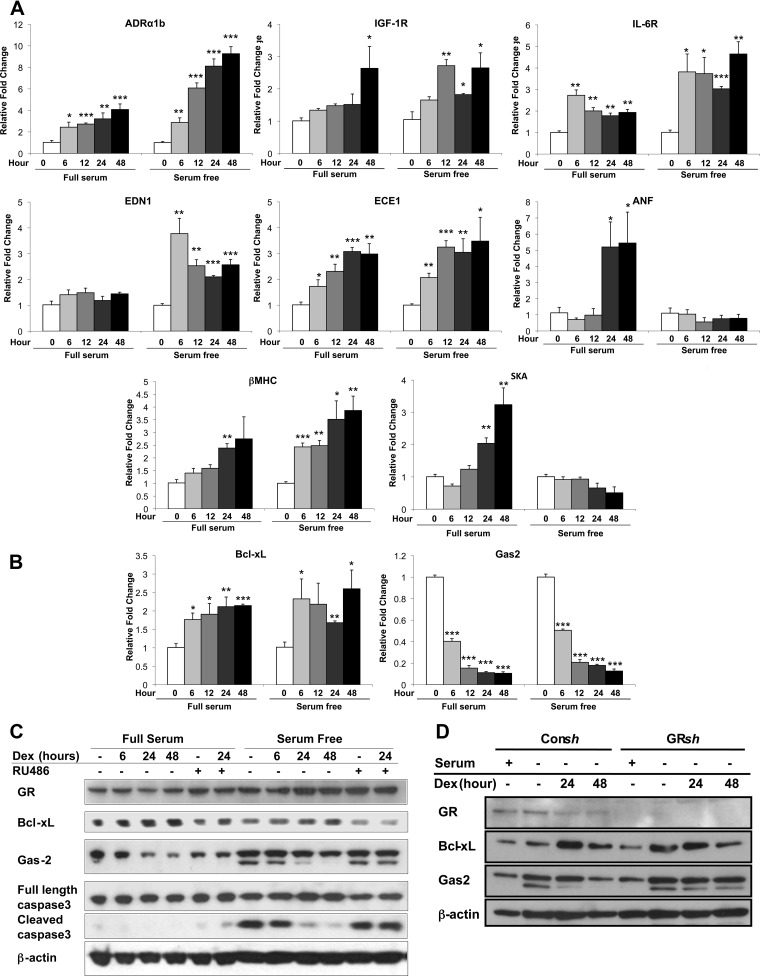
Analysis of the Dex-regulated genes by Ingenuity Pathway Analysis identified cardiac hypertrophy signaling as a canonical pathway significantly altered in serum-supplemented H9C2 cells treated with glucocorticoids. A total of 75 genes in the cardiac hypertrophy signaling pathway were regulated by Dex at the 6-, 24-, and 48-h time points (Fig. 7C and Supplemental Table 1). To validate the glucocorticoid-mediated changes in gene expression detected by the microarray, we treated H9C2 cells with 100 nm Dex and examined by real-time PCR the expression of a subset of genes associated with cardiac hypertrophy. The α1b-adrenergic receptor (ADRα1b), which is known to promote cardiac hypertrophy (45–47), was induced by glucocorticoids at each time point examined (Fig. 8A). The Dex-dependent up-regulation was observed whether serum was present or not but was greater in serum-deprived H9C2 cells. In addition, glucocorticoids up-regulated in a serum-independent manner the expression of the receptors for IGF-I (IGF-IR) and IL-6 (IL-6R), both of which have established roles stimulating cardiac hypertrophy (Fig. 8A) (48–50). We also investigated the expression profile of the pro-hypertrophic gene endothelin-1 (EDN1) (51). In agreement with the microarray data, Dex treatment increased the production of EDN1 mRNA only in cardiomyocytes cultured in the absence of serum (Fig. 8A). However, glucocorticoids stimulated the expression of endothelin-converting enzyme 1 (ECE1), which proteolytically activates EDN1, in a serum-independent manner (Fig. 8A). Finally, we evaluated the expression of the hypertrophic marker genes. As expected from our earlier findings (Fig. 1C), ANF, β-MHC, and SKA were each up-regulated by Dex in H9C2 cells cultured with serum (Fig. 8A). In serum-deprived cells, however, only β-MHC showed a similar pattern of regulation because Dex treatment had no effect on the expression of ANF or SKA at any time point tested. Collectively, these data show that glucocorticoids regulate multiple genes involved in cardiac hypertrophy signaling and that serum has a marked influence on the transcriptional activity of GR on certain target genes.
Fig. 8.
Hypertrophic and apoptotic genes regulated by glucocorticoids in cardiomyocytes. A, H9C2 cardiomyocytes were treated with Dex (100 nm) for varying periods of time in the presence or absence of serum. The mRNA levels for the hypertrophic genes ADRαlb, IGF-IR, IL-6R, EDN1, ECE1, ANF, β-MHC, and SKA were measured by real-time PCR and normalized to cyclophilin B. B, H9C2 cardiomyocytes were cultured as described above, and the mRNA levels for the apoptotic genes Bcl-xL and Gas2 were determined by real-time PCR and normalized to cyclophilin B. C, H9C2 cells were cultured as described above with or without RU486. GR, Bcl-xL, Gas2, and both full-length and cleaved caspase-3 protein levels were examined by immunoblotting, and β-actin was used as an internal loading control. D, H9C2 cells were stably transfected with control shRNA (Consh) or GR shRNA (GRsh) and treated with or without Dex. GR, Bcl-xL, and Gas2 protein levels were examined by immunoblotting, and β-actin was used as an internal loading control. Data represent the mean ± sd from three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. controls.
The canonical pathway apoptosis signaling was significantly altered in serum-deprived H9C2 cells treated with glucocorticoids. A total of 23 genes in the apoptosis signaling pathway were regulated by Dex at the 6-, 24-, and 48-h time points (Fig. 7D and Supplemental Table 2). Validation of the microarray results was performed on two genes with established roles in apoptosis, Bcl-xL and Gas2. Bcl-xL is a key anti-apoptotic factor in cardiac tissues (52–55), whereas Gas2 is a target for caspase-3 and caspase-7 and promotes apoptosis during limb development (56, 57). In accord with its anti-apoptotic actions, Dex treatment of H9C2 cells resulted in an up-regulation in Bcl-xL and a down-regulation in Gas2 mRNA, and these effects occurred independently of serum (Fig. 8B). Moreover, the changes in mRNA expression led to corresponding changes in Bcl-xL and Gas2 protein levels (Fig. 8, C and D). For both genes, the Dex-dependent regulation required GR, because the induction of Bcl-xL and repression of Gas2 was inhibited by the antagonist RU486 and by knockdown of GR (Fig. 8, C and D). These data suggest that Bcl-xL and Gas2 may play an important role in the anti-apoptotic response of cardiomyocytes to glucocorticoids.
Discussion
The present study reveals a direct role for glucocorticoids and GR signaling in the regulation of cardiomyocyte function. In addition, we show that the specific actions of glucocorticoids on cardiomyocytes are strongly affected by serum, suggesting that the transcriptional activity of GR in these cells is dynamic, depending on extracellular cues. In the presence of serum, activated GR induced hypertrophic changes in both H9C2 cells and primary neonatal rat cardiomyocytes. These changes occurred at the mRNA, protein, and morphological levels, because a number of genes known to promote hypertrophy were up-regulated by glucocorticoids. These findings are consistent with the pro-hypertrophic action of glucocorticoids suggested by previous in vitro and in vivo studies (12–18) and indicate that elevated levels of glucocorticoids and enhanced GR signaling in cardiomyocytes could contribute to the development of heart failure. However, in the absence of serum, our data demonstrate that glucocorticoids protect cardiomyocytes from apoptosis through the GR-dependent up-regulation of anti-apoptotic proteins, such as Bcl-xL, and down-regulation of pro-apoptotic proteins, such as Gas2. These anti-apoptotic effects suggest that glucocorticoids may have a direct protective role in the heart by preventing the death of cardiomyocytes triggered by conditions such as ischemia/reperfusion (22).
Elucidating the precise role of glucocorticoids in heart physiology and pathology has been a subject of intense study because of the adverse cardiovascular effects observed in patients on chronic glucocorticoid therapy (58). Glucocorticoids have been implicated in the development of cardiac hypertrophy, which is a major cause of congestive heart failure in man, and increased levels of circulating glucocorticoids are an independent risk factor for cardiovascular disease (19–21). Heart pathologies associated with excess glucocorticoids may be secondary to the adverse systemic actions of these steroids, such as hypertension and metabolic syndrome. Findings from the current study and others suggest that direct activation of GR signaling in cardiomyocytes may also negatively impact heart function by stimulating pathological hypertrophy (15, 18). However, mice genetically engineered to overexpress GR specifically in cardiomyocytes do not display spontaneous cardiac hypertrophy, but they do exhibit conduction defects such as atrioventricular block (59). Several studies have reported that glucocorticoids exert direct beneficial effects on the heart. For example, glucocorticoids positively influence cardiomyocyte contraction through effects on calcium homeostasis (18, 58–62). In addition, results from this and other reports indicate that GR signaling in cardiomyocytes promotes their survival by inhibiting apoptosis triggered by ischemia/reperfusion and TNFα (22, 39, 41). Because apoptosis plays a pivotal role in the development of congestive heart failure (63, 64), local delivery and activation of cardiomyocyte GR signaling may provide an alternative therapy to combat the progression of heart disease. Consistent with the beneficial actions of glucocorticoids on the heart, insufficient GR signaling has been associated with cardiovascular disease in man (65, 66).
Clinically, the importance of MR in cardiomyocyte function is well established and derived from studies reporting an association between elevated MR activity and diverse cardiac abnormalities such as arrhythmias, apoptosis, fibrosis, hypertrophy, and inflammation (29, 67). Additionally, the MR antagonists spironolactone and eplerenone have each been shown to have beneficial outcomes on the progression of cardiac diseases (68, 69). Using specific receptor antagonists and RNA-mediated silencing of GR and MR, we demonstrate that GR can participate in the mineralocorticoid effects on H9C2 cardiomyocytes particularly at high aldosterone concentrations. Consistent with this finding, it was recently reported that GR mediated the hypertrophic response to aldosterone in neonatal rat cardiomyocytes (70). Interestingly, aldosterone may have opposing effects on cardiomyocyte viability depending on whether GR or MR signaling predominates. Although aldosterone activation of MR has been shown to promote apoptosis of cardiomyocytes (71), we show that aldosterone activation of GR protects cardiomyocytes from cell death. GR has much lower affinity for aldosterone than does MR, because it binds with a dissociation constant between 10 and 100 nm (26, 70). Therefore, only under pathological conditions of very high aldosterone levels could GR potentially be activated by mineralocorticoids in vivo and alter cardiomyocyte function. Of note, aldosterone has been reported to reach approximately 300 nm in the plasma from some patients with severe congestive heart failure, and this concentration may be further augmented by the local synthesis of aldosterone in the myocardium (72, 73).
Another significant finding from our study is that serum altered the set of target genes regulated by glucocorticoids in cardiomyocytes. Approximately 75% of the genes regulated by Dex in the presence of serum were not regulated by glucocorticoids in the absence of serum. Conversely, approximately 50% of the genes regulated by Dex in the absence of serum were not regulated by glucocorticoids in the presence of serum. Examples of this reprogramming of the glucocorticoid response include the ANF, SKA, and EDN1 genes (Fig. 8A). The impact of serum on the gene-regulatory profile of GR suggests that growth factors and their activated signaling pathways may cross talk with GR to modulate its response. Indeed, fibroblast growth factor-2 has been shown to synergistically enhance glucocorticoid regulation of the Notch4 gene via activator protein-1-mediated stabilization of GR binding to the Notch4 promoter (74). On the other hand, TGFβ antagonizes the transcriptional activity of GR through mechanisms involving activator protein-1 (75). Although the presence or absence of serum did not significantly affect the expression level of GR, these conditions may also alter posttranslational modifications of the receptor, such as its phosphorylation status, which are known to regulate the function of GR on many target genes (7, 35, 76). Furthermore, given the global impact of serum on gene expression (Supplemental Fig. 4), the availability of various coactivators and corepressors for recruitment to GR may be modified and lead to distinct glucocorticoid signaling profiles in cardiomyocytes cultured with or without serum (77, 78).
In conclusion, we have observed dual actions of glucocorticoids directly on cardiomyocytes. Glucocorticoids induced hypertrophy in the presence of serum and prevented apoptosis triggered by serum depletion. Both the hypertrophic and anti-apoptotic actions of glucocorticoids depended on the activity of GR in the cardiomyocyte. These results suggest that the glucocorticoid signaling pathway in cardiomyocytes may provide an attractive therapeutic target for congestive heart failure arising from hypertrophy and/or ischemic heart disease.
Supplementary Material
Acknowledgments
We thank Dr. Carl Bortner and the Flow Cytometry Center at the National Institute of Environmental Health Sciences for their kind assistance.
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ANF
- Atrial natriuretic factor
- Dex
- dexamethasone
- Gas2
- growth arrest-specific protein 2
- GR
- glucocorticoid receptor
- β-MHC
- β-myosin heavy chain
- MR
- mineralocorticoid receptor
- shRNA
- short hairpin RNA
- siRNA
- small interfering RNA
- SKA
- skeletal muscle α-actin.
References
- 1. Barnes PJ. 1998. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond) 94:557–572 [DOI] [PubMed] [Google Scholar]
- 2. Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89 [DOI] [PubMed] [Google Scholar]
- 3. Miner JN, Hong MH, Negro-Vilar A. 2005. New and improved glucocorticoid receptor ligands. Expert Opin Investig Drugs 14:1527–1545 [DOI] [PubMed] [Google Scholar]
- 4. Rhen T, Cidlowski JA. 2005. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med 353:1711–1723 [DOI] [PubMed] [Google Scholar]
- 5. Schäcke H, Döcke WD, Asadullah K. 2002. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther 96:23–43 [DOI] [PubMed] [Google Scholar]
- 6. Evans RM. 1988. The steroid and thyroid hormone receptor superfamily. Science 240:889–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oakley RH, Cidlowski JA. 2011. Cellular processing of the glucocorticoid receptor gene and protein: new mechanisms for generating tissue-specific actions of glucocorticoids. J Biol Chem 286:3177–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Czerwinski-Helms SM, Hickson RC. 1987. Specificity of activated glucocorticoid receptor expression in heart and skeletal muscle types. Biochem Biophys Res Commun 142:322–328 [DOI] [PubMed] [Google Scholar]
- 9. Katz SE, Penefsky ZJ, McGinnis MY. 1988. Cytosolic glucocorticoid receptors in the developing rat heart. J Mol Cell Cardiol 20:323–328 [DOI] [PubMed] [Google Scholar]
- 10. Kayes-Wandover KM, White PC. 2000. Steroidogenic enzyme gene expression in the human heart. J Clin Endocrinol Metab 85:2519–2525 [DOI] [PubMed] [Google Scholar]
- 11. Sheppard KE, Autelitano DJ. 2002. 11β-Hydroxysteroid dehydrogenase 1 transforms 11-dehydrocorticosterone into transcriptionally active glucocorticoid in neonatal rat heart. Endocrinology 143:198–204 [DOI] [PubMed] [Google Scholar]
- 12. de Vries WB, van der Leij FR, Bakker JM, Kamphuis PJ, van Oosterhout MF, Schipper ME, Smid GB, Bartelds B, van Bel F. 2002. Alterations in adult rat heart after neonatal dexamethasone therapy. Pediatr Res 52:900–906 [DOI] [PubMed] [Google Scholar]
- 13. Gardner DG, Wu JP, LaPointe MC, Hedges BK, Deschepper CF. 1988. Expression of the gene for the atrial natriuretic peptide in cardiac myocytes in vitro. Cardiovasc Drugs Ther 2:479–486 [DOI] [PubMed] [Google Scholar]
- 14. La Mear NS, MacGilvray SS, Myers TF. 1997. Dexamethasone-induced myocardial hypertrophy in neonatal rats. Biol Neonate 72:175–180 [DOI] [PubMed] [Google Scholar]
- 15. Lister K, Autelitano DJ, Jenkins A, Hannan RD, Sheppard KE. 2006. Cross talk between corticosteroids and alpha-adrenergic signalling augments cardiomyocyte hypertrophy: a possible role for SGK1. Cardiovasc Res 70:555–565 [DOI] [PubMed] [Google Scholar]
- 16. Muangmingsuk S, Ingram P, Gupta MP, Arcilla RA, Gupta M. 2000. Dexamethasone induced cardiac hypertrophy in newborn rats is accompanied by changes in myosin heavy chain phenotype and gene transcription. Mol Cell Biochem 209:165–173 [DOI] [PubMed] [Google Scholar]
- 17. Muiesan ML, Lupia M, Salvetti M, Grigoletto C, Sonino N, Boscaro M, Rosei EA, Mantero F, Fallo F. 2003. Left ventricular structural and functional characteristics in Cushing's syndrome. J Am Coll Cardiol 41:2275–2279 [DOI] [PubMed] [Google Scholar]
- 18. Whitehurst RM, Jr, Zhang M, Bhattacharjee A, Li M. 1999. Dexamethasone-induced hypertrophy in rat neonatal cardiac myocytes involves an elevated L-type Ca2+ current. J Mol Cell Cardiol 31:1551–1558 [DOI] [PubMed] [Google Scholar]
- 19. Güder G, Bauersachs J, Frantz S, Weismann D, Allolio B, Ertl G, Angermann CE, Störk S. 2007. Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation 115:1754–1761 [DOI] [PubMed] [Google Scholar]
- 20. Souverein PC, Berard A, Van Staa TP, Cooper C, Egberts AC, Leufkens HG, Walker BR. 2004. Use of oral glucocorticoids and risk of cardiovascular and cerebrovascular disease in a population based case-control study. Heart 90:859–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wei L, MacDonald TM, Walker BR. 2004. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med 141:764–770 [DOI] [PubMed] [Google Scholar]
- 22. Pearl JM, Nelson DP, Schwartz SM, Wagner CJ, Bauer SM, Setser EA, Duffy JY.2002. Glucocorticoids reduce ischemia-reperfusion-induced myocardial apoptosis in immature hearts. Ann Thorac Surg 74:830–836; discussion 836–837 [DOI] [PubMed] [Google Scholar]
- 23. Stockand JD. 2002. New ideas about aldosterone signaling in epithelia. Am J Physiol Renal Physiol 282:F559–F576 [DOI] [PubMed] [Google Scholar]
- 24. Zennaro MC, Farman N, Bonvalet JP, Lombès M. 1997. Tissue-specific expression of alpha and beta messenger ribonucleic acid isoforms of the human mineralocorticoid receptor in normal and pathological states. J Clin Endocrinol Metab 82:1345–1352 [DOI] [PubMed] [Google Scholar]
- 25. Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM. 1987. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science 237:268–275 [DOI] [PubMed] [Google Scholar]
- 26. Gaeggeler HP, Gonzalez-Rodriguez E, Jaeger NF, Loffing-Cueni D, Norregaard R, Loffing J, Horisberger JD, Rossier BC. 2005. Mineralocorticoid versus glucocorticoid receptor occupancy mediating aldosterone-stimulated sodium transport in a novel renal cell line. J Am Soc Nephrol 16:878–891 [DOI] [PubMed] [Google Scholar]
- 27. Krozowski ZS, Funder JW. 1983. Renal mineralocorticoid receptors and hippocampal corticosterone-binding species have identical intrinsic steroid specificity. Proc Natl Acad Sci USA 80:6056–6060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Funder JW. 2006. Aldosterone and mineralocorticoid receptors: lessons from gene deletion studies. Hypertension 48:1018–1019 [DOI] [PubMed] [Google Scholar]
- 29. Funder JW. 2009. Reconsidering the roles of the mineralocorticoid receptor. Hypertension 53:286–290 [DOI] [PubMed] [Google Scholar]
- 30. Stewart PM, Krozowski ZS. 1999. 11 beta-Hydroxysteroid dehydrogenase. Vitam Horm 57:249–324 [PubMed] [Google Scholar]
- 31. Gomez-Sanchez CE, de Rodriguez AF, Romero DG, Estess J, Warden MP, Gomez-Sanchez MT, Gomez-Sanchez EP. 2006. Development of a panel of monoclonal antibodies against the mineralocorticoid receptor. Endocrinology 147:1343–1348 [DOI] [PubMed] [Google Scholar]
- 32. Cidlowski JA, Bellingham DL, Powell-Oliver FE, Lubahn DB, Sar M. 1990. Novel antipeptide antibodies to the human glucocorticoid receptor: recognition of multiple receptor forms in vitro and distinct localization of cytoplasmic and nuclear receptors. Mol Endocrinol 4:1427–1437 [DOI] [PubMed] [Google Scholar]
- 33. Hescheler J, Meyer R, Plant S, Krautwurst D, Rosenthal W, Schultz G. 1991. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res 69:1476–1486 [DOI] [PubMed] [Google Scholar]
- 34. Kedar V, McDonough H, Arya R, Li HH, Rockman HA, Patterson C. 2004. Muscle-specific RING finger 1 is a bona fide ubiquitin ligase that degrades cardiac troponin I. Proc Natl Acad Sci USA 101:18135–18140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Galliher-Beckley AJ, Williams JG, Cidlowski JA. 2011. Ligand-independent phosphorylation of the glucocorticoid receptor integrates cellular stress pathways with nuclear receptor signaling. Mol Cell Biol 31:4663–4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schulz M, Eggert M, Baniahmad A, Dostert A, Heinzel T, Renkawitz R. 2002. RU486-induced glucocorticoid receptor agonism is controlled by the receptor N terminus and by corepressor binding. J Biol Chem 277:26238–26243 [DOI] [PubMed] [Google Scholar]
- 37. Zhang S, Jonklaas J, Danielsen M. 2007. The glucocorticoid agonist activities of mifepristone (RU486) and progesterone are dependent on glucocorticoid receptor levels but not on EC50 values. Steroids 72:600–608 [DOI] [PubMed] [Google Scholar]
- 38. Chen QM, Alexander D, Sun H, Xie L, Lin Y, Terrand J, Morrissy S, Purdom S. 2005. Corticosteroids inhibit cell death induced by doxorubicin in cardiomyocytes: induction of antiapoptosis, antioxidant, and detoxification genes. Mol Pharmacol 67:1861–1873 [DOI] [PubMed] [Google Scholar]
- 39. Kewalramani G, Puthanveetil P, Wang F, Kim MS, Deppe S, Abrahani A, Luciani DS, Johnson JD, Rodrigues B. 2009. AMP-activated protein kinase confers protection against TNF-α-induced cardiac cell death. Cardiovasc Res 84:42–53 [DOI] [PubMed] [Google Scholar]
- 40. Tokudome S, Sano M, Shinmura K, Matsuhashi T, Morizane S, Moriyama H, Tamaki K, Hayashida K, Nakanishi H, Yoshikawa N, Shimizu N, Endo J, Katayama T, Murata M, Yuasa S, Kaneda R, Tomita K, Eguchi N, Urade Y, Asano K, Utsunomiya Y, Suzuki T, Taguchi R, Tanaka H, Fukuda K. 2009. Glucocorticoid protects rodent hearts from ischemia/reperfusion injury by activating lipocalin-type prostaglandin D synthase-derived PGD2 biosynthesis. J Clin Invest 119:1477–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xu B, Strom J, Chen QM. 2011. Dexamethasone induces transcriptional activation of Bcl-xL gene and inhibits cardiac injury by myocardial ischemia. Eur J Pharmacol 668:194–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bryant D, Becker L, Richardson J, Shelton J, Franco F, Peshock R, Thompson M, Giroir B. 1998. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-α. Circulation 97:1375–1381 [DOI] [PubMed] [Google Scholar]
- 43. Krown KA, Page MT, Nguyen C, Zechner D, Gutierrez V, Comstock KL, Glembotski CC, Quintana PJ, Sabbadini RA. 1996. Tumor necrosis factor α-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J Clin Invest 98:2854–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aikawa R, Nitta-Komatsubara Y, Kudoh S, Takano H, Nagai T, Yazaki Y, Nagai R, Komuro I. 2002. Reactive oxygen species induce cardiomyocyte apoptosis partly through TNF-α. Cytokine 18:179–183 [DOI] [PubMed] [Google Scholar]
- 45. Knowlton KU, Rockman HA, Itani M, Vovan A, Seidman CE, Chien KR. 1995. Divergent pathways mediate the induction of ANF transgenes in neonatal and hypertrophic ventricular myocardium. J Clin Invest 96:1311–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Milano CA, Dolber PC, Rockman HA, Bond RA, Venable ME, Allen LF, Lefkowitz RJ. 1994. Myocardial expression of a constitutively active α1B-adrenergic receptor in transgenic mice induces cardiac hypertrophy. Proc Natl Acad Sci USA 91:10109–10113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. O'Connell TD, Ishizaka S, Nakamura A, Swigart PM, Rodrigo MC, Simpson GL, Cotecchia S, Rokosh DG, Grossman W, Foster E, Simpson PC. 2003. The α(1A/C)- and α(1B)-adrenergic receptors are required for physiological cardiac hypertrophy in the double-knockout mouse. J Clin Invest 111:1783–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Colao A, Ferone D, Marzullo P, Lombardi G. 2004. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev 25:102–152 [DOI] [PubMed] [Google Scholar]
- 49. Lombardi G, Galdiero M, Auriemma RS, Pivonello R, Colao A. 2006. Acromegaly and the cardiovascular system. Neuroendocrinology 83:211–217 [DOI] [PubMed] [Google Scholar]
- 50. Wollert KC, Drexler H. 2001. The role of interleukin-6 in the failing heart. Heart Fail Rev 6:95–103 [DOI] [PubMed] [Google Scholar]
- 51. Ichikawa KI, Hidai C, Okuda C, Kimata SI, Matsuoka R, Hosoda S, Quertermous T, Kawana M. 1996. Endogenous endothelin-1 mediates cardiac hypertrophy and switching of myosin heavy chain gene expression in rat ventricular myocardium. J Am Coll Cardiol 27:1286–1291 [DOI] [PubMed] [Google Scholar]
- 52. Fujio Y, Kunisada K, Hirota H, Yamauchi-Takihara K, Kishimoto T. 1997. Signals through gp130 upregulate bcl-x gene expression via STAT1-binding cis-element in cardiac myocytes. J Clin Invest 99:2898–2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Latif N, Khan MA, Birks E, O'Farrell A, Westbrook J, Dunn MJ, Yacoub MH. 2000. Upregulation of the Bcl-2 family of proteins in end stage heart failure. J Am Coll Cardiol 35:1769–1777 [DOI] [PubMed] [Google Scholar]
- 54. Negoro S, Oh H, Tone E, Kunisada K, Fujio Y, Walsh K, Kishimoto T, Yamauchi-Takihara K. 2001. Glycoprotein 130 regulates cardiac myocyte survival in doxorubicin-induced apoptosis through phosphatidylinositol 3-kinase/Akt phosphorylation and Bcl-xL/caspase-3 interaction. Circulation 103:555–561 [DOI] [PubMed] [Google Scholar]
- 55. Yamamura T, Otani H, Nakao Y, Hattori R, Osako M, Imamura H. 2001. IGF-I differentially regulates Bcl-xL and Bax and confers myocardial protection in the rat heart. Am J Physiol Heart Circ Physiol 280:H1191–H1200 [DOI] [PubMed] [Google Scholar]
- 56. Lee KK, Tang MK, Yew DT, Chow PH, Yee SP, Schneider C, Brancolini C. 1999. gas2 is a multifunctional gene involved in the regulation of apoptosis and chondrogenesis in the developing mouse limb. Dev Biol 207:14–25 [DOI] [PubMed] [Google Scholar]
- 57. Sgorbissa A, Benetti R, Marzinotto S, Schneider C, Brancolini C. 1999. Caspase-3 and caspase-7 but not caspase-6 cleave Gas2 in vitro: implications for microfilament reorganization during apoptosis. J Cell Sci 112(Pt 23):4475–4482 [DOI] [PubMed] [Google Scholar]
- 58. Hadoke PW, Iqbal J, Walker BR. 2009. Therapeutic manipulation of glucocorticoid metabolism in cardiovascular disease. Br J Pharmacol 156:689–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sainte-Marie Y, Nguyen Dinh Cat A, Perrier R, Mangin L, Soukaseum C, Peuchmaur M, Tronche F, Farman N, Escoubet B, Benitah JP, Jaisser F. 2007. Conditional glucocorticoid receptor expression in the heart induces atrio-ventricular block. FASEB J 21:3133–3141 [DOI] [PubMed] [Google Scholar]
- 60. Levitan ES, Hershman KM, Sherman TG, Takimoto K. 1996. Dexamethasone and stress upregulate Kv1.5 K+ channel gene expression in rat ventricular myocytes. Neuropharmacology 35:1001–1006 [PubMed] [Google Scholar]
- 61. Narayanan N, Yang C, Xu A. 2004. Dexamethasone treatment improves sarcoplasmic reticulum function and contractile performance in aged myocardium. Mol Cell Biochem 266:31–36 [DOI] [PubMed] [Google Scholar]
- 62. Wang L, Feng ZP, Duff HJ. 1999. Glucocorticoid regulation of cardiac K+ currents and L-type Ca2+ current in neonatal mice. Circ Res 85:168–173 [DOI] [PubMed] [Google Scholar]
- 63. Mallat Z, Tedgui A, Fontaliran F, Frank R, Durigon M, Fontaine G. 1996. Evidence of apoptosis in arrhythmogenic right ventricular dysplasia. N Engl J Med 335:1190–1196 [DOI] [PubMed] [Google Scholar]
- 64. Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, Schmidt U, Semigran MJ, Dec GW, Khaw BA. 1996. Apoptosis in myocytes in end-stage heart failure. N Engl J Med 335:1182–1189 [DOI] [PubMed] [Google Scholar]
- 65. Chung T, Grossman A, Clark AJ. 2010. Adrenal insufficiency. In: Jameson JL, Degroot LJ, eds. Endocrinology. Philadelphia: Saunders Elsevier; 1853–1863 [Google Scholar]
- 66. van den Akker EL, Koper JW, van Rossum EF, Dekker MJ, Russcher H, de Jong FH, Uitterlinden AG, Hofman A, Pols HA, Witteman JC, Lamberts SW. 2008. Glucocorticoid receptor gene and risk of cardiovascular disease. Arch Intern Med 168:33–39 [DOI] [PubMed] [Google Scholar]
- 67. Rocha R, Williams GH. 2002. Rationale for the use of aldosterone antagonists in congestive heart failure. Drugs 62:723–731 [DOI] [PubMed] [Google Scholar]
- 68. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. 2003. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 348:1309–1321 [DOI] [PubMed] [Google Scholar]
- 69. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. 1999. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 341:709–717 [DOI] [PubMed] [Google Scholar]
- 70. Rossier MF, Python M, Maturana AD. 2010. Contribution of mineralocorticoid and glucocorticoid receptors to the chronotropic and hypertrophic actions of aldosterone in neonatal rat ventricular myocytes. Endocrinology 151:2777–2787 [DOI] [PubMed] [Google Scholar]
- 71. De Silva DS, Wilson RM, Hutchinson C, Ip PC, Garcia AG, Lancel S, Ito M, Pimentel DR, Sam F. 2009. Fenofibrate inhibits aldosterone-induced apoptosis in adult rat ventricular myocytes via stress-activated kinase-dependent mechanisms. Am J Physiol Heart Circ Physiol 296:H1983–H1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rousseau MF, Gurné O, Duprez D, Van Mieghem W, Robert A, Ahn S, Galanti L, Ketelslegers JM. 2002. Beneficial neurohormonal profile of spironolactone in severe congestive heart failure: results from the RALES neurohormonal substudy. J Am Coll Cardiol 40:1596–1601 [DOI] [PubMed] [Google Scholar]
- 73. Silvestre JS, Robert V, Heymes C, Aupetit-Faisant B, Mouas C, Moalic JM, Swynghedauw B, Delcayre C. 1998. Myocardial production of aldosterone and corticosterone in the rat. Physiological regulation. J Biol Chem 273:4883–4891 [DOI] [PubMed] [Google Scholar]
- 74. Wu J, Bresnick EH. 2007. Glucocorticoid and growth factor synergism requirement for Notch4 chromatin domain activation. Mol Cell Biol 27:2411–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Periyasamy S, Sánchez ER. 2002. Antagonism of glucocorticoid receptor transactivity and cell growth inhibition by transforming growth factor-beta through AP-1-mediated transcriptional repression. Int J Biochem Cell Biol 34:1571–1585 [DOI] [PubMed] [Google Scholar]
- 76. Galliher-Beckley AJ, Williams JG, Collins JB, Cidlowski JA. 2008. Glycogen synthase kinase 3beta-mediated serine phosphorylation of the human glucocorticoid receptor redirects gene expression profiles. Mol Cell Biol 28:7309–7322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lonard DM, O'Malley BW. 2005. Expanding functional diversity of the coactivators. Trends Biochem Sci 30:126–132 [DOI] [PubMed] [Google Scholar]
- 78. Rosenfeld MG, Glass CK. 2001. Coregulator codes of transcriptional regulation by nuclear receptors. J Biol Chem 276:36865–36868 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.