Abstract
The discovery that kisspeptin was critical for normal fertility in humans ushered in a new chapter in our understanding of the control of GnRH secretion. In this paper, we will review recent data on the similarities and differences across several mammalian species in the role of kisspeptin in reproductive neuroendocrinology. In all mammals examined to date, there is strong evidence that kisspeptin plays a key role in the onset of puberty and is necessary for both tonic and surge secretion of GnRH in adults, although kisspeptin-independent systems are also apparent in these studies. Similarly, two groups of kisspeptin neurons, one in the arcuate nucleus (ARC) and the other more rostrally, have been identified in all mammals, although the latter is concentrated in a limited area in rodents and more scattered in other species. Estrogen has divergent actions on kisspeptin expression in these two regions across these species, stimulating it the latter and inhibiting expression in the former. There is also strong evidence that the rostral population participates in the GnRH surge, whereas the ARC population contributes to steroid-negative feedback. There may be species differences in the role of these two populations in puberty, with the ARC cells important in rats, sheep, and monkeys, whereas both have been implicated in mice. ARC kisspeptin neurons also appear to participate in the GnRH surge in sheep and guinea pigs, whereas the data on this possibility in rodents are contradictory. Similarly, both populations are sexually dimorphic in sheep and humans, whereas most data in rodents indicate that this occurs only in the rostral population. The functional consequences of these species differences remain to be fully elucidated but are likely to have significance for understanding normal neuroendocrine control of reproduction as well as for use of kisspeptin agonists/antagonists as a therapeutic tool.
After the initial reports that inactivating mutations of the kisspeptin receptor produced hypogonadotropic hypogonadism (1, 2), it was quickly established that kisspeptins are potent stimulators of GnRH in a number of mammals, including rodents (3, 4), sheep (5), monkeys (6), and humans (7). Since then, a wealth of information has been developed on the roles of kisspeptin in control of reproductive function and several excellent reviews focused on different aspects of this topic have recently been published (8–14). Most of these reviews have relied largely on studies in mice and rats, but one emphasized species comparisons (15). However, in light of the new studies, including data from several additional species, which have appeared since that comparative review, it is appropriate to revisit this topic. Thus, this minireview will focus on comparative aspects of kisspeptin mammalian reproductive physiology, first describing aspects that appear to be common across virtually all species and then considering species differences.
Roles common to all species
There is now strong evidence that kisspeptin plays a key role in three aspects of GnRH/LH secretion in all species studied to date, including the following: 1) an increase in kisspeptin at puberty drives onset of fertility, and in adults kisspeptin is critical for 2) tonic episodic secretion of GnRH and LH that occurs in both males and females and is controlled by the negative feedback actions of gonadal steroids, and 3) the estrogen-induced preovulatory GnRH/LH surge at the end of the follicular phase of the ovarian cycle.
Puberty
Based on the lack of pubertal development in humans (1, 2, 10) and mice (2) with genetic disruption of kisspeptin and/or Kiss1r, the first postulated role for kisspeptin signaling was in the onset of reproductive function at puberty. Modest increases in the magnitude of the response to exogenous kisspeptin during pubertal development have been observed in mice (16), rats (17, 18), and monkeys (19), but changes in Kiss1r mRNA are not consistently observed in these species (16, 17, 20–22). Moreover, kisspeptin can clearly increase LH secretion before puberty in all species examined (6, 16–19, 23), so the general consensus is that increased kisspeptin release is the proximate signal for the onset of puberty.
This hypothesis is now supported by evidence that 1) cellular expression of Kiss1 mRNA and/or kisspeptin protein increases during pubertal development in mice (16, 24), rats (17, 25, 26), sheep (27, 28), and monkeys (22), with a parallel increase in kisspeptin-containing close contacts onto GnRH neurons (24, 28); 2) kisspeptin release increases during puberty in the median eminence (ME) (29) and mediobasal hypothalamus (MBH) (30) of female monkeys; 3) exogenous kisspeptin (31) and an activating KISS1R mutation (32) induces precocious puberty in rats and humans; and 4) a Kiss1r antagonist delayed vaginal opening in rats (33). Although there is thus strong evidence that kisspeptin plays an important role in onset of puberty, it should be noted that several lines of mice with genetic knockouts (KOs) that disrupt kisspeptin signaling do show vaginal opening, although it is delayed (34–36).
Tonic GnRH/LH secretion
The primary lines of evidence supporting a critical role for kisspeptin in maintaining tonic GnRH secretion comes from studies of KO of the kisspeptin or the Kiss1r gene in mice (34–36) and antagonists to the Kiss1r in a number of species (11). The gonadal atrophy and minimal development of reproductive tracts in male and female KO mice (34–36) provides indirect biological evidence that kisspeptin signaling is necessary for tonic gonadotropin secretion. Interestingly, LH concentrations in these mice are not consistently less than their wild-type (WT) controls, whereas FSH levels are usually significantly lower (34). This apparent discrepancy between LH and FSH secretion may reflect differences in assays because LH levels were often undetectable (35, 36). Alternatively, because the residual secretion of both LH and FSH is abolished by acyline (36), there may be low amplitude GnRH pulses in these KO mice, as appears to be the case in humans (1), which could favor LH over FSH release. The abnormally low gonadal steroid secretion in these KO mice may also be a contributing factor to the absence of a difference in LH levels between them and WT; one would expect tonic LH secretion to be elevated in WT mice if steroid levels were adjusted to those seen in the KO animals. Indeed, the absence of a postcastration rise in LH concentrations in them (36, 37) indicates the important role for kisspeptin signaling to tonic LH secretion.
Similar support comes from studies using the Kiss1r antagonist, p234 (11). This antagonist blocks the postcastration rise in LH secretion in mice and rats, inhibits episodic LH secretion in ovariectomized (OVX) ewes, and inhibits GnRH secretion in prepubertal and pubertal Rhesus monkeys (19, 38). Although these studies demonstrate that kisspeptin signaling is critical for normal tonic GnRH and LH secretion, they also provide evidence for kisspeptin-independent release of GnRH and LH. Thus, the Kiss1r antagonist does not inhibit LH concentrations in gonadally intact rats and mice (38), and most lines of KO mice show some evidence of gonadal activity (34) that can be inhibited by a GnRH receptor antagonist (36). On the other hand, immunoneutralization of kisspeptin disrupted ovarian cycles in rats (39). Thus, kisspeptin-independent GnRH secretion is insufficient for normal reproductive function, but it could account for some of the variability in reproductive phenotypes in patients with mutations in kisspeptin or its receptor (10, 15).
The preovulatory GnRH/LH surge
Work using immunoneutralization of kisspeptin was the first to demonstrate that this peptide was necessary for the preovulatory (39) and estradiol (E2)-induced (40) LH surge in rats, observations that have recently been confirmed using Kiss1r antagonists in rats (33) and sheep (41). Evidence for activation of a rostral populations of kisspeptin neurons at the time of the LH surge in rats (8, 40, 42), mice (15, 43), sheep (44, 45), and hamsters (46), and a reflex-ovulator (47) have led to the general acceptance that these neurons are involved in the preovulatory GnRH surge (possible species differences in the role of kisspeptin cell populations is discussed below). On the other hand, studies of KO mice have led to conflicting results, with one report that the estrogen-induced GnRH surge is still evident (37), whereas another reported complete ablation (43). Even in those studies reporting disruption of the surge, there is some evidence for kisspeptin-independent release that ranged from minimal in rats (33, 39, 40) to approximately 50% in sheep (41).
In summary, in those species studied to date, there is strong evidence that kisspeptin plays a critical role in the onset of puberty, driving the preovulatory GnRH/LH surge and maintaining tonic GnRH/LH secretion. However, kisspeptin-independent systems are also evident in these studies. Such systems may provide an explanation for the recent observation that genetic ablation of kisspeptin- or Kiss1r-containing neurons does not delay puberty or block ovulation (48). Because similar ablations in adult mice completely disrupted ovarian cycles (48), these data suggest that compensatory mechanisms are activated in development that are not available in adults. One possibility is that these mice become highly sensitive to kisspeptin so that the few kisspeptin neurons that survive the toxin (48) are sufficient for fertility. Alternatively, elimination of the normal efferents from these neurons early in development may allow other stimulatory inputs to their target neurons to replace them, producing compensation not seen with targeted disruption of a single peptide. Although there is general agreement on the role of kisspeptin in these three processes across species, some differences among species become evident when one examines the following: 1) the role of the two predominant hypothalamic kisspeptin populations, 2) sexual differentiation of these populations, and 3) whether kisspeptin is permissive or necessary for episodic GnRH secretion.
Role of kisspeptin subpopulations: species similarities
In every mammalian species examined to date, there are two major hypothalamic populations of kisspeptin cells, a rostral one in the preoptic area (POA) and a caudal one in the arcuate nucleus (ARC) (12). In rodents, the rostral population is located in the anteroventral periventricular nucleus (AVPV) and adjacent periventricular regions, which are collectively referred to as the rostral periventricular area of the third ventricle (RP3V). In sheep and primates, kisspeptin cells are located at similar rostral-caudal levels of the POA but are more scattered and less numerous than in the rodent. In sheep, rostral kisspeptin-immunoreactive (ir) cells are limited to the medial POA at the anterior-posterior level of, and just caudal to, the organum vasculosum of the lamina terminalis (44). They are scattered near the top of the third ventricle slightly off midline in rostral sections and extend ventrally to near the base of the ventricle in more caudal sections. In female rhesus monkeys (49) and women (50), rostral kisspeptin neurons are found in similar areas but are more periventricular and ventral in location. However, this kisspeptin population was not detected in men (50) or castrated male monkeys (51). The ARC (infundibular nucleus in humans) appears to be the most consistent population of kisspeptin cells across species, with the greatest number of cells detected by either mRNA or peptide. The other major consistent observation across all these species is that E2 stimulates kisspeptin expression in the rostral population, whereas it (and other steroids) inhibit expression in the ARC (8, 9, 12, 15). These data have important implications for the physiological roles of these two populations (see below), but it should be noted that there are more ARC, than rostral, kisspeptin neurons, even when circulating E2 levels are maximal (12).
A major difference between the two populations is the colocalization in the caudal, but not the rostral, set of two additional neuropeptides, neurokinin B and dynorphin, each of which has been strongly implicated in control of reproductive neuroendocrine function (52, 53). Although the precise percentages vary, a large majority of ARC kisspeptin cells colocalize both neurokinin B (NKB) and dynorphin in the ARC of mice (90% NKB, 92% dynorphin) (54), rats (97% NKB) (55), sheep (80% NKB, 94% dynorphin) (56), goats (99% NKB, 78% dynorphin) (57), rhesus monkeys (60% NKB) (58), and humans (77% NKB) (50). In addition to their colocalization of NKB and dynorphin, a majority of ARC kisspeptin cells have also been reported to colocalize galanin in the mouse (65%) (59), although a similar study (60) reported this percentage to be less [12.5% by immunocytochemistry (ICC); 42% by in situ hybridization (ISH)]. The colocalization of all three peptides in a single population, has led to this kisspeptin cell group being termed kisspeptin/neurokinin B/dynorphin (KNDy) cells (61). Interestingly, the colocalization of KNDy peptides may be a unique feature of the mammalian kisspeptin system because the kiss2 neurons in the periventricular hypothalamus of zebrafish (62), the cells that are probably functionally homologous to those expressing kiss1 mRNA in mammals (63), show no colocalization of tachykinin-encoding mRNAs (64).
The colocalization of peptides in KNDy cells has allowed for the use of multiple-label ICC to trace neuronal projections specifically arising from this population. Studies in rats (55, 65), monkeys (58), and humans (50) have revealed a fairly consistent picture showing a majority of KNDy cell projections to adjacent regions of the middle and caudal hypothalamus, including the ME, with fewer projections rostrally to the preoptic and periventricular regions. Despite the fewer number of rostral KNDy projections, there is evidence in mice (60) and sheep (61) that at least some of these fibers directly contact GnRH cell bodies. In the ME, there is electron microscopic evidence in sheep (61), goats (66), and rats (67) for direct membrane contacts between KNDy and GnRH axons, although a majority of these contacts appear to be located in the internal, nonneurohemal zone, and kisspeptin cells in either the ARC or RP3V do not appear to have direct access to the portal vessels (68). Finally, a common feature of KNDy cells is the presence of intraarcuate projections to other KNDy cells in sheep (69) and rats (65, 70), efferents that comprise a reciprocal circuit within this population. KNDy-KNDy connections are presumed to play a key role in the synchronization of this caudal population, perhaps contributing to the bursting patterns of multiunit electrophysiological activity observed in the arcuate nucleus of rats (71), goats (57, 72), and monkeys (73) that correlate with individual GnRH/LH pulses.
In rodents, the rostral POA population has also been shown to colocalize other neuropeptides and neurotransmitters, specifically galanin, met-enkephalin, neurotensin, and tyrosine hydroxylase (as a marker for dopamine), although the percentage of colocalization varies between species and studies, and there are limited data in nonrodent species. Using colocalization of kisspeptin and thyroid hormone, RP3V kisspeptin cells in the mouse have been shown to directly contact GnRH neurons (74). In the sheep, there are fewer kisspeptin-kisspeptin contacts among this rostral population than for the ARC KNDy cells (61), but the presence of reciprocal connections among the rostral kisspeptin population has not been examined in other species.
Finally, it should be noted that there are a number of kisspeptin cell populations in the mammalian brain beyond those discussed above in the RP3V and ARC. These include kisspeptin cells of the medial amygdala, which have been demonstrated thus far in the mouse and rat (75); the dorsomedial hypothalamus in mouse (24), sheep (76), horse (77), and guinea pig (78); and the bed nucleus of the stria terminalis in mouse (3) and monkey (49). Although there are some species differences in the presence of kisspeptin cells in these areas [e.g. dorsomedial hypothalamus cells are not detected in the rat (79) or hamster (80)], for many species, the full distribution has yet to be reported. In addition to the amygdala, Kiss1 mRNA has been detected in other areas outside the hypothalamus by RT-PCR. These include the dentate gyrus of the hippocampal formation in which it is regulated by neuronal activity as well as increased following castration (81). The presence of Kiss1 expression in these areas likely portends the importance of this peptide in functions that extend beyond its role in reproduction, but consideration of these roles is beyond the scope of this review.
Role of kisspeptin subpopulations: species differences
Although kisspeptin is clearly important for onset of fertility at puberty, and tonic and surge secretion of GnRH thereafter, in all those species examined to date, species differences do become evident when one examines the role of different kisspeptin cell populations in these processes.
Puberty
The vast majority of data on which kisspeptin neuronal population is responsible for puberty comes from studies of changes in either Kiss1 mRNA levels using ISH or RT-PCR and kisspeptin protein estimated with ICC. Although these indices do not always agree, even in the same report (82), some fairly consistent patterns do emerge. In monkeys (PCR) (22), sheep (ISH, ICC) (27, 28), and rats (ICC, ISH, PCR) (25, 26, 83, 84), kisspeptin expression generally increases in the ARC during puberty, although these increases were not always statistically significant (26, 84). In contrast to these species, ARC Kiss1 mRNA levels do not change during puberty in mice, based on ISH (16) and RT-PCR (82). Studies using ICC in mice all report a dense network of kisspeptin-ir fibers, that increases qualitatively during puberty (24, 82, 85, 86), but few if any visible soma; the source of these fibers is unknown, but because they are positively correlated with E2 (82, 85), it has been suggested that they arise from kisspeptin neurons in the RP3V (8, 85, 86). However, dual-ICC studies using kisspeptin and NKB as markers for ARC kisspeptin neurons in a number of species (51–56), including mice (58), provide strong evidence that many of these fibers arise from local KNDy neurons, at least in adults. Thus, it is likely that both kisspeptin cell populations contribute to the increase in fiber density during puberty in mice.
The opposite pattern emerges in rostral kisspeptin populations: consistent increases are observed in mice (ISH, PCR, ICC) (24, 82, 85, 86), with fewer consistent data in other species. In rats, increases have been observed using ISH (26, 83) and ICC (25), but mRNA levels assessed by RT-PCR are variable (25), and kisspeptin-ir cells are often undetectable, unless rats are pretreated with colchicine (83, 84). An increase in kisspeptin cells with age is observed in the POA of ewes using ISH, but these cell numbers do not correlate with the pubertal increase in LH pulse frequency (27), and few, if any cells, are detected by ICC in pre- and postpubertal ewe lambs (28). There is no information on pubertal changes in rostral kisspeptin cell number in rhesus monkeys, but in light of earlier data that rostral lesions or knife cuts either had no effect on (87, 88) or advanced (89) the onset of puberty, it is unlikely that they are critical in this species.
There are two important caveats to all these studies of kisspeptin expression. First, it must be kept in mind that any changes in ovary-intact animals may be influenced by endogenous steroids, particularly because E2 stimulates kisspeptin expression in the rostral population and inhibits it in the ARC. Indeed, there is strong evidence in mice that peripubertal increases in E2 are largely responsible for the increase in RP3V kisspeptin cell number in both females (85) and males (90). Conversely, the negative feedback of gonadal steroids is likely to limit any increase in ARC kisspeptin expression to a brief window that may be easily missed.
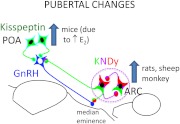
The second major caveat is that all these studies are only correlative in nature. Two studies have tested the functional significance of these changes (beyond the early work in monkeys), and both point to an important role of the ARC kisspeptin population for puberty onset in mice. First, early vaginal opening occurs in mice in which estrogen receptor (ER)-α has been deleted from kisspeptin neurons (86); this correlates with an increase in Kiss1 mRNA in the ARC, whereas kisspeptin expression in the RP3V remains low in these mice. It should be noted, however, that these mice fail to ovulate, probably because of the absence of ER-α in RP3V kisspeptin neurons. Second, ablation of kisspeptin neurons at 20 d of age, which was postulated to selectively lesion the ARC kisspeptin population, completely disrupts ovarian cyclicity (48). Finally, it is interesting to note that the control of ARC kisspeptin cell numbers appear to reflect underlying mechanisms of puberty because low E2 levels inhibit kisspeptin expression, and LH secretion, in juvenile rats (25) and sheep (28), whereas developmental changes in kisspeptin are steroid independent in male monkeys (22). In summary (Fig. 1), there is strong evidence in rats, sheep, and monkeys that increases in ARC kisspeptin neural activity are important for the onset of puberty, whereas data in mice implicate both ARC and RP3V populations in this process. It should be noted, however, that the increase in kisspeptin expression in the RP3V of mice is driven by increasing levels of E2 (85), so it is likely to be a consequence rather than a cause of puberty in females.
Fig. 1.
Schematic drawing illustrating species differences in the roles of the two major hypothalamic kisspeptin populations, located in POA and ARC, respectively, in the processes underlying puberty.
There also appear to be species differences in the role of these kisspeptin populations in seasonal changes in reproduction. Most mammals show annual fluctuations in fertility so that the young are born when environmental conditions favor their survival. The mechanisms underlying the transition between nonbreeding and breeding seasons parallel those at the onset of puberty but are controlled by external day length acting through pineal secretion of melatonin (91). These neuroendocrine mechanisms have been extensively studied in three species: Syrian and Siberian hamsters (91) and sheep (92), and there are major differences among these three species. Syrian hamsters (93) and sheep (94, 95) are similar in that ARC kisspeptin cell number increases in the breeding season but differ in that the number of rostral kisspeptin cells also increase in the former (96) but not the latter (93, 94). In contrast, in Siberian hamsters, the ARC population decreases, whereas the rostral population increases, in the breeding season (80, 97). Moreover, there may be fundamental species differences in the role of kisspeptin because this peptide can overcome the inhibitory effects of photoperiod in the nonbreeding season in sheep (98) and Syrian hamsters (93) but not in Siberian hamsters (99). Thus, increases in ARC kisspeptin expression likely drive the onset of fertility in sheep and Syrian hamsters, whereas the changes seen in Siberian hamsters are more likely driven largely by seasonal differences in testosterone, which inhibits expression in the ARC and stimulate it in the AVPV (100).
Control of tonic and surge secretion of GnRH in adults
Evidence of the differential effects of E2 on expression of kisspeptin in the RP3V and ARC of rodents (8, 9, 12, 14, 15) led to the early hypothesis that ARC kisspeptin neurons mediate E2 negative feedback, whereas those in the RP3V are responsible for the positive-feedback action of E2 (101). This hypothesis set the foundation for much of the subsequent work in rodents, and there is now compelling evidence that kisspeptin neurons in the RP3V mediate E2-positive feedback in these species. Briefly (for more detailed discussion, see Refs. 8 and 9), these data include the following: early work that the RP3V is the site of positive feedback (102, 103); that most RP3V kisspeptin cells contain ERα (12); that E2 increases kisspeptin mRNA and protein expression in this area (8, 9, 12, 14, 15); and that a high percentage of RP3V kisspeptin neurons are activated, based on Fos expression, at the time of the LH surge (40, 42, 43). Evidence that ARC kisspeptin neurons mediate steroid-negative feedback is less compelling because it relies largely on the steroid-induced inhibition of kisspeptin expression in this area (8, 12, 14, 15). Nevertheless, this hypothesis for the role of these two kisspeptin populations is now generally accepted and provides a useful context for examining data in other species.
There is now evidence, mostly from kisspeptin expression studies, in several species that ARC kisspeptin neurons are involved in steroid negative feedback. Thus, gonadectomy increases, and steroid replacement inhibits ARC kisspeptin expression in guinea pigs (78), sheep (94, 104), monkeys (105–108), and musk shrew (47); increases in kisspeptin expression in the infundibular nucleus also occur in postmenopausal women (106). In the ewe, this ARC population is selectively activated by E2 withdrawal because OVX increases Fos expression in ARC, but not POA, kisspeptin neurons (45, 109). Thus, data in all species examined to date support a role for ARC kisspeptin neurons in steroid-negative feedback.
There is much less information in these species on the kisspeptin neurons involved in the preovulatory surge, beyond the evidence that E2 stimulates kisspeptin expression in the POA (8, 11, 15). However, a high level of Fos colocalization with rostral kisspeptin neurons at the time of the preovulatory surge has been observed in sheep (44, 45), the Syrian hamster (46), and the musk shrew (47). Interestingly, Fos studies also implicate ARC kisspeptin neurons in the GnRH/LH surge in ewes: a surge-inducing dose of exogenous E2 induced Fos in this population 1 h later (109), and more than 50% of them contain Fos during the LH surge at the end of the follicular phase (45). An increase in Fos in ARC kisspeptin neurons was not observed during the surge in another study (44), most likely for technical reasons (45). Although exogenous E2 consistently inhibits ARC kisspeptin expression (94, 104), some studies (109, 110), but not others (44), observed increases in the kisspeptin expression in the more posterior portions of the ARC late in the ovine follicular phase.
The evidence in sheep that both populations participate in the GnRH/LH surge raises the interesting question of whether this occurs in other species. This could simply reflect a species differences in sites of positive feedback because E2 acts in the MBH, not the POA, to induce the GnRH surge in sheep (111). On the other hand, although ARC kisspeptin neurons do not express Fos during the coitus-induced LH surge in the musk shrew (46), data in rodents are limited and contradictory. Thus, one group found a dramatic increase in Fos expression in ARC kisspeptin neurons of the rat on the afternoon of proestrus (39), whereas another group found no increase at this time (42). The only other data available in rats (there are no relevant data in mice) used an OVX + E2 model and found high Fos expression in the ARC population (40–60%) in the presence of both low (no surge) and high (surge) E2 treatments (40). Although E2 inhibits kisspeptin expression in the ARC, there are more kisspeptin cells in the ARC than in the RP3V on proestrus (42), and it is possible for this steroid to have both negative and positive effects on these neurons because the latter occurs via the classical estrogen response element, whereas the former does not (112, 113). There is only fragmentary evidence on the role of these kisspeptin neurons in the preovulatory surge in other species. In guinea pigs, expression data in response to E2 treatment suggest that the positive feedback actions may occur in both the POA and ARC populations (78), whereas in monkeys one report suggest an increase in Kiss1 mRNA/cell late in the follicular phase in both ARC and POA (49), but the former was not evident when mRNA was assessed by RT-PCR (108). It should also be noted that if kisspeptin neurons are required for the preovulatory surge in guinea pigs and monkeys, the ARC population is most likely involved because knife cuts around the MBH do not block the positive-feedback action of E2 in these species (114, 115).
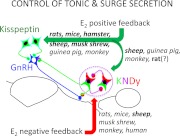
In summary (Fig. 2), there is strong evidence in several species that rostral kisspeptin neurons are involved in the surge, whereas the ARC population participates in steroid-negative feedback. It is interesting to note that the rostral population is concentrated in the RP3V in rodents but not other mammals. This may reflect the coupling of the surge mechanism with the circadian clock in the suprachiasmatic nucleus that occurs only in rodents with short 4- to 5-d estrous cycles (9). In sheep, and possibly guinea pigs and monkeys, the ARC population also appears to contribute to the surge; whether this occurs in rodents remains an open question.
Fig. 2.
Schematic drawing illustrating species differences in the role of the two major hypothalamic kisspeptin populations in the control of tonic and surge secretion of GnRH/LH. Italics indicate evidence based on mRNA expression; bold indicate evidence based on Fos studies.
Role of ARC kisspeptin neurons in pulsatile GnRH secretion
One important inference from studies implicating ARC kisspeptin neurons in the negative-feedback actions of steroids is that they are likely the source of kisspeptin needed for episodic GnRH secretion. As noted above, these cells contain NKB and dynorphin and form an extensive interconnected network (61) that is likely capable of firing synchronously. These characteristics have led to the hypothesis that they play an important role in synchronizing GnRH release during episodic secretion (54, 61). There is evidence in monkeys for episodic kisspeptin release (29, 30), but it is unclear whether kisspeptin is permissive for, or drives, episodic GnRH secretion. Indirect evidence for the latter comes from reports that continuous administration of kisspeptin is unable to maintain elevated LH secretion. The strongest evidence for such desenstization comes from work in juvenile (6, 116) and adult male monkeys (117) in which LH levels return to baseline within 12 h of the start of kisspeptin infusion.
A similar phenomenon, with LH taking 1–2 d to reach baseline, has been observed in gonadally intact rats (118, 119) and anestrous sheep (98), although negative feedback effects of increasing E2 levels have been implicated in sheep (120) and could occur in rats. In contrast, data from hamsters is conflicting; clear desensitization to continuous kisspeptin administration is seen in Siberian hamsters (99) but not their Syrian (93) counterparts. The story in humans is also somewhat confusing because twice-daily injections of kisspeptin-54 causes desensitization within 24 h in women with hypothalamic amenorrhea (121, 122), whereas continuous administration of kisspeptin-10 to normal men was able to maintain elevated LH concentrations for at least 24 h (123). Results of these studies are difficult to compare because of methodological differences (e.g. different kisspeptin preparations and doses) so whether this represents a true sex differences is unclear at this time. Thus, there is strong evidence in most species that episodic kisspeptin release is needed for sustained GnRH and LH secretion. On the other hand, the recent report that kisspeptin infusion for 12 h to infertile patients can restore pulsatile LH secretion (124) suggests that it may be permissive under some circumstances, but this treatment may have caused an overall increase in LH levels that made pulses easier to detect in these patients.
Four different groups (54, 61, 125, 126) have proposed similar roles for the three KNDy neuropeptides in control of episodic GnRH release, which includes the following: 1) kisspeptin is the output signal that drives GnRH release, 2) stimulatory actions of NKB on KNDy neurons triggers kisspeptin release at the onset of each GnRH pulse, and 3) dynorphin release shortly thereafter terminates the kisspeptin (and hence GnRH) pulse. Detailed consideration of this model is beyond the scope of this review, but two aspects of it are particularly relevant: 1) is kisspeptin simply the output signal and 2) does kisspeptin mediate the actions of NKB? The former is supported by the ability of exogenous kisspeptin to increase LH secretion without altering the bursts of multiunit electrophysiological activity (72, 127) that are associated with LH pulses and are thought to originate from KNDy neurons (72), and by data on Kiss1r expression. Although Kiss1r is found in POA GnRH neurons in mice (20), rats (128), and sheep (41, 109), its expression in the ARC is species dependent because it is found there in rats (21) and sheep (41) but not in mice (20). Moreover, although Kiss1r is found in the ovine ARC, it does not colocalize with KNDy neurons (41), so most data suggest KNDy neurons are not directly responsive to kisspeptin. Although there is thus considerable support for the proposal that kisspeptin is simply the output signal, reports that a Kiss1r antagonist (129) and agonist (130) can alter the timing of LH pulses in rats and men, respectively, argue against this proposal.
Several lines of evidence across a number of species support the hypothesis that the stimulatory actions of NKB are mediated by kisspeptin. First, the endogenous NKB receptor, NK3R, is found in KNDy neurons in rats (131), mice (54, 132), and sheep (133), whereas either low level (131), or no (132, 133), NK3R expression occurs in GnRH neurons in these species. Second, senktide, an NK3R agonist, induced Fos in KNDy neurons in the rat (134), and NKB and senktide depolarize KNDy, but not GnRH, neurons in slice preparations (132). Finally, pharmacological (135) or genetic (136) disruption of kisspeptin signaling blocks the stimulatory actions of senktide in moneys and mice, respectively. One interesting species difference has emerged from these studies: although senktide (or NKB) produces stimulatory effects in sheep (28, 137) and monkeys (58, 135), in rodents stimulatory (134, 136), inhibitory (54, 138, 139), and no effects (54, 140) have been reported. In general, these agonists appear to be inhibitory when endogenous LH secretion is high and either stimulatory or without effect when endogenous secretion is low, although this correlation is not always seen. Interestingly, Kinsey-Jones et al. (138) recently reported that an antagonist to the κ-opioid receptor blocks the ability of senktide to inhibit episodic LH secretion in OVX rats (138). Because dynorphin is the endogenous ligand for κ receptors, these data suggest that the inhibitory actions of senktide are mediated by dynorphin, so both stimulatory and inhibitory actions of NKB in rats may occur via KNDy neurons. In summary, it is clear that kisspeptin is needed for episodic GnRH secretion, and most evidence suggests that it normally drives GnRH pulses and that it mediates the stimulatory actions of NKB necessary for fertility in humans (10, 52).
Sex differences
It was noted early on that female rodents have many more kisspeptin-expressing neurons in the RP3V than males (8, 14, 141), a difference that correlates with the ability of females, but not males, to respond to the positive feedback action of E2. In mice, females have approximately 10 times as many kisspeptin-expressing cells than males in the RP3V (ISH, ICC), whereas no sex differences have been observed in the ARC kisspeptin population (24, 82, 142). In rats, a similar sex difference in the RP3V is seen with ISH (26, 143) and ICC if colchicine is given (40, 84, 144, 145), but data on sex differences in the ARC are conflicting. In early work using both ISH and ICC, no differences were observed between females and males (40, 144), whereas some (26, 84, 146), but not all (145), more recent work reported slightly more kisspeptin cell bodies and fibers in the ARC of female than male rats. In sheep (28, 147) and humans (50), both the rostral and ARC kisspeptin populations show marked sexual dimorphism, with more cells in females than in males. Interestingly, one species, the Siberian hamster, does not show any obvious sexual dimorphism in either the AVPV or ARC kisspeptin population (80, 97).
It should be kept in mind that differences in expression in normal adults could be caused by feedback from different endogenous steroids, which might account for some of the conflicting data on ARC kisspeptin expression in rats. Studies using gonadectomy and steroid replacement to normalize hormonal milieu between males and females have clearly ruled out this possibility for the sexual dimorphism of the RP3V kisspeptin neurons in rats (84, 143, 144) and mice (148, 149) and ARC kisspeptin neurons in sheep (28). On the other hand, no sex differences were observed in the ARC kisspeptin population in gonadectomized rats with similar steroid treatments (84, 143, 144). From these data it was inferred that the sexual dimorphism of kisspeptin neurons in the RP3V in rodents and ARC in sheep was most likely due to the organizing actions of androgen during development. This hypothesis was supported by neonatal castration of male (143), and androgen treatment of female (144), rats and genetic manipulations in mice (148, 149). In contrast, androgen treatment of developing ewes during d 30–90 of fetal life (gestation is 147 d long in this species) did not alter the number of ARC kisspeptin neurons in adults (147), although it did decrease the number of NKB-ir and dynorphin-ir ARC neurons to male levels (147). Whether this reflects experimental factors (e.g. contribution of later pre- and postnatal exposure to androgens) or species differences between rodents and sheep remains to be resolved.
There may also be sex differences in several aspects of kisspeptin signaling beyond these differences in kisspeptin expression. For example, three studies have reported that the E2-induced increase in RP3V kisspeptin expression seen in female rats is lost in males (40, 143, 145). Interestingly, this effect of E2 is clearly evident in male mice (82, 90, 150), so there may be species differences in this sexual dimorphism. However, there is also one report that E2 can increase RP3V kisspeptin cell numbers in male rats (144) and small, but not significant, increases were observed in the other studies (40, 143, 145), so this may be a quantitative, not a qualitative, sex difference in rats. It has also been suggested based on KO studies in mice (148, 149) that this response to E2 is dependent on exposure to E2 during postnatal development. If so, this is also species dependent because E2 is able to stimulate RP3V kisspeptin expression in neonatally gonadectomized rats (144). An interesting sexual dimorphism was recently reported in prepubertal mice: females respond to gonadectomy with a robust increase in LH levels and ARC kisspeptin cell numbers, but neither of these variables increased in castrated males (142). There may also be species differences in this phenomenon because gonadectomy increases both LH concentrations and ARC kisspeptin cell numbers in male and female prepubertal sheep (28); likewise, gonadectomy increases LH secretion in male and female rats before puberty (151), although the effects on kisspeptin expression have yet to be determined. Finally, there appears to be a sexual dimorphism in response to kisspeptin-10 in humans that has not been reported in other species, with women showing no response to doses of this peptide that are 30-fold higher than effective doses in men (152).
Conclusions
When the physiological roles of kisspeptin are examined across species, it is important to remember that most inferences depend largely on changes in kisspeptin mRNA expression. Thus, care must be taken when inferring functional roles from these data because mRNA levels do not always correlate with protein levels, and function does not always follow expression. Nevertheless, some useful generalizations do emerge, with more similarities than differences across species. In all species examined to date, kisspeptin is critical for onset of puberty and normal tonic and surge secretion in adults. They also almost all have rostral (POA) and caudal (ARC) hypothalamic subpopulations, with E2 stimulating expression in the former and inhibiting it in the latter. The rostral population is sexually dimorphic and participates in the GnRH surge, whereas the ARC has been implicated in puberty onset and mediates steroid negative feedback. Although it is clear that subtle differences between rats and mice are sometimes overlooked, major species differences exist between rodents and other species. The one obvious species difference in the rostral kisspeptin population is the concentration of these neurons in the RP3V in most rodents but not other species. This most likely reflects the coupling of the surge mechanism with the circadian clock in the suprachiasmatic nucleus that occurs only in rodents with short 4- to 5-d estrous cycles. To test this possibility, it would be interesting to determine the distribution of kisspeptin cells in species closely related to mice and rats but with more prolonged estrous cycles and ovulatory events that are not tightly gated by a circadian clock such as the Octodon degus (153). More species differences are evident in the ARC subpopulation, which appears to be involved in surge secretion and sexually dimorphic in some species and not others. It is tempting to speculate that these may also relate to the genesis of the preovulatory GnRH surge. Perhaps ARC kisspeptin neurons have replaced the biological clock in the suprachiasmatic nucleus as the important signal that stimulates the rostral subpopulation of kisspeptin neurons. This suggestion is consistent with differences in the site of E2-positive feedback and evidence in sheep (109) that the ARC population is activated shortly after a surge-inducing injection of E2, whereas the POA population is not. Interestingly, a reflex ovulator, the musk shrew, resembles mice and rats, with a concentrated population of rostral kisspeptin neurons that are the only ones activated at the time of the surge (47), but it is not known at this time whether this applies to other reflex ovulators, like the rabbit and cat.
This review of the literature also identifies some gaps in our current knowledge. First, because it is clear that kisspeptin neurons play an important role in the onset of puberty, the factors responsible for their activation at the appropriate time become of particular interest. As noted above, initial work on this question suggests that the control of ARC kisspeptin cell numbers parallel underlying mechanisms of puberty because low concentrations of E2 inhibit both kisspeptin expression and LH secretion in juvenile rats (25) and sheep (28), whereas changes in kisspeptin expression appear to be steroid independent in juvenile male monkeys (22). There is also some work on the role of nutrition and leptin in the regulation of kisspeptin expression (13), but the information on this is largely limited to rodents at this time. A second obvious gap is the role of kisspeptin in nonhypothalamic areas. The presence of kisspeptin in areas (e.g. medial amygdala and bed nucleus of the stria terminalis) important for the pheromonal control of sexual behavior (154) and LH secretion (155) raises the possibility that kisspeptin may contribute to these functions. Similarly, its presence in the hippocampus (80, 156) suggests a possible role in memory, whereas its general distribution in limbic structures points to possible influences on emotional status. Clearly these roles remain completely speculative and await testing in future work.
Finally, it is worth considering the value of comparative studies in expanding our understanding the kisspeptin neurons and their roles. First, it is likely that species differences in kisspeptin system organization reflect the distinct ways in which reproductive neuroendocrine function is regulated under normal circumstances. Thus, circadian timing of the surge, which may have adaptive value in rodents, may be supplanted in other species by other factors that regulate timing of the surge, which are of greater physiological importance. Thus, comparative data may help reveal the diversity of ways by which different species have solved the same reproductive problem, e.g. timing of the preovulatory GnRH/LH surge. In addition, specific differences in the organization of the primate kisspeptin system may have important implications not only for assessing the efficacy of kisspeptin agonists/antagonists in the treatment of reproductive disorders but also for identification of their precise mechanisms of action. Therefore, as we gain a fuller understanding of kisspeptin neurons, including their molecular and cellular physiology, comparative data will be increasingly valuable to ensure that the clinical translation of such knowledge is used to its best advantage.
Acknowledgments
This work was supported by National Institutes of Health Grants R01HD33916 and P01 HD44232.
Present address for M.N.L.: Department of Neurobiology and Anatomical Sciences, University of Mississippi Medical Center, Jackson, Mississippi 39216.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ARC
- Arcuate nucleus
- AVPV
- anteroventral periventricular nucleus
- E2
- estradiol
- ER
- estrogen receptor
- ICC
- immunocytochemistry
- ir
- immunoreactive
- ISH
- in situ hybridization
- KNDy
- kisspeptin/neurokinin B/dynorphin
- KO
- knockout
- MBH
- mediobasal hypothalamus
- ME
- median eminence
- NKB
- neurokinin B
- OVX
- ovariectomized
- POA
- preoptic area
- RP3V
- rostral periventricular area of the third ventricle
- WT
- wild type.
References
- 1. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 2. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. 2003. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. 2004. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 [DOI] [PubMed] [Google Scholar]
- 4. Navarro VM, Castellano JM, Fernández-Fernández R, Tovar S, Roa J, Mayen A, Nogueiras R, Vazquez MJ, Barreiro ML, Magni P, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. 2005. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology 146:156–163 [DOI] [PubMed] [Google Scholar]
- 5. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. 2005. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Plant TM, Ramaswamy S, Dipietro MJ. 2006. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology 147:1007–1013 [DOI] [PubMed] [Google Scholar]
- 7. Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. 2005. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab 90:6609–6615 [DOI] [PubMed] [Google Scholar]
- 8. García-Galiano D, Pinilla L, Tena-Sempere M. 2012. Sex steroids and the control of the Kiss1 system: developmental roles and major regulatory actions. J Neuroendocrinol 24:22–33 [DOI] [PubMed] [Google Scholar]
- 9. Khan AR, Kauffman AS. 2012. The role of kisspeptin and RFamide-related peptide-3 neurones in the circadian-timed preovulatory luteinising hormone surge. J Neuroendocrinol 24:131–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Silveira LG, Tusset C, Latronico AC. 2010. Impact of mutations in kisspeptin and neurokinin B signaling pathways on human reproduction. Brain Res 1364:72–80 [DOI] [PubMed] [Google Scholar]
- 11. Millar RP, Roseweir AK, Tello JA, Anderson RA, George JT, Morgan K, Pawson AJ. 2010. Kisspeptin antagonists: unraveling the role of kisspeptin in reproductive physiology. Brain Res 1364:81–99 [DOI] [PubMed] [Google Scholar]
- 12. Lehman MN, Merkley CM, Coolen LM, Goodman RL. 2010. Anatomy of the kisspeptin neural network in mammals. Brain Res 1364:90–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Castellano JM, Bentsen AH, Mikkelsen JD, Tena-Sempere M. 2010. Kisspeptins: bridging energy homeostasis and reproduction. Brain Res 1364:129–138 [DOI] [PubMed] [Google Scholar]
- 14. Tsukamura H, Homma T, Tomikawa J, Uenoyama Y, Maeda K. 2010. Sexual differentiation of kisspeptin neurons responsible for sex difference in gonadotropin release in rats. Ann NY Acad Sci 1200:95–103 [DOI] [PubMed] [Google Scholar]
- 15. Oakley AE, Clifton DK, Steiner RA. 2009. Kisspeptin signaling in the brain. Endocr Rev 30:713–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. 2005. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Navarro VM, Castellano JM, Fernández-Fernández R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. 2004. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 145:4565–4574 [DOI] [PubMed] [Google Scholar]
- 18. Castellano JM, Navarro VM, Fernández-Fernández R, Castaño JP, Malagón MM, Aguilar E, Dieguez C, Magni P, Pinilla L, Tena-Sempere M. 2006. Ontogeny and mechanisms of action for the stimulatory effect of kisspeptin on gonadotropin-releasing hormone system of the rat. Mol Cell Endocrinol 257–258:75–83 [DOI] [PubMed] [Google Scholar]
- 19. Guerriero KA, Keen KL, Millar RP, Terasawa E. 2012. Developmental changes in GnRH release in response to kisspeptin agonist and antagonist in female rhesus monkeys (Macaca mulatta): implication for the mechanism of puberty. Endocrinology 153:825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herbison AE, de Tassigny X, Doran J, Colledge WH. 2010. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology 151:312–321 [DOI] [PubMed] [Google Scholar]
- 21. Knox AM, Li XF, Kinsey-Jones JS, Wilkinson ES, Wu XQ, Cheng YS, Milligan SR, Lightman SL, O'Byrne KT. 2009. Neonatal lipopolysaccharide exposure delays puberty and alters hypothalamic Kiss1 and Kiss1r mRNA expression in the female rat. J Neuroendocrinol 21:683–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. 2005. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA 102:2129–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Redmond JS, Macedo GG, Velez IC, Caraty A, Williams GL, Amstalden M. 2011. Kisspeptin activates the hypothalamic-adenohypophyseal-gonadal axis in prepubertal ewe lambs. Reproduction 141:541–548 [DOI] [PubMed] [Google Scholar]
- 24. Clarkson J, Herbison AE. 2006. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takase K, Uenoyama Y, Inoue N, Matsui H, Yamada S, Shimizu M, Homma T, Tomikawa J, Kanda S, Matsumoto H, Oka Y, Tsukamura H, Maeda KI. 2009. Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. J Neuroendocrinol 21:527–537 [DOI] [PubMed] [Google Scholar]
- 26. Takumi K, Iijima N, Ozawa H. 2011. Developmental changes in the expression of kisspeptin mRNA in rat hypothalamus. J Mol Neurosci 43:138–145 [DOI] [PubMed] [Google Scholar]
- 27. Redmond JS, Baez-Sandoval GM, Spell KM, Spencer TE, Lents CA, Williams GL, Amstalden M. 2011. Developmental changes in hypothalamic Kiss1 expression during activation of the pulsatile release of luteinising hormone in maturing ewe lambs. J Neuroendocrinol 23:815–822 [DOI] [PubMed] [Google Scholar]
- 28. Nestor CC, Briscoe AM, Davis SM, Valent M, Goodman RL, Hileman SM. 2012. Evidence of a role for kisspeptin and neurokinin B in puberty of female sheep. Endocrinology 153:2756–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. 2008. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 149:4151–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guerriero KA, Keen KL, Terasawa E. 2012. Developmental increase in kisspeptin-54 release in vivo is independent of the pubertal increase in estradiol in female rhesus monkeys (Macaca mulatta). Endocrinology 153:1887–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Navarro VM, Fernández-Fernández R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. 2004. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol 561:379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teles MG, Bianco SD, Brito VN, Trarbach EB, Kuohung W, Xu S, Seminara SB, Mendonca BB, Kaiser UB, Latronico AC. 2008. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med 358:709–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pineda R, Garcia-Galiano D, Roseweir A, Romero M, Sanchez-Garrido MA, Ruiz-Pino F, Morgan K, Pinilla L, Millar RP, Tena-Sempere M. 2010. Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology 151:722–730 [DOI] [PubMed] [Google Scholar]
- 34. Colledge WH. 2009. Transgenic mouse models to study Gpr54/kisspeptin physiology. Peptides 30:34–41 [DOI] [PubMed] [Google Scholar]
- 35. Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. 2007. Kiss1minus]/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology 148:4927–4936 [DOI] [PubMed] [Google Scholar]
- 36. Chan YM, Broder-Fingert S, Wong KM, Seminara SB. 2009. Kisspeptin/Gpr54-independent gonadotrophin-releasing hormone activity in Kiss1 and Gpr54 mutant mice. J Neuroendocrinol 21:1015–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA. 2007. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci 27:12088–12095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. 2009. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci 29:3920–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. 2005. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 146:4431–4436 [DOI] [PubMed] [Google Scholar]
- 40. Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. 2007. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 53:367–378 [DOI] [PubMed] [Google Scholar]
- 41. Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, Clarke IJ. 2011. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology 152:1001–1012 [DOI] [PubMed] [Google Scholar]
- 42. Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. 2006. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 26:6687–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. 2008. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 28:8691–8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hoffman GE, Le WW, Franceschini I, Caraty A, Advis JP. 2011. Expression of fos and in vivo median eminence release of LHRH identifies an active role for preoptic area kisspeptin neurons in synchronized surges of LH and LHRH in the ewe. Endocrinology 152:214–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Merkley CM, Porter KL, Coolen LM, Hileman SM, Billings HJ, Drews S, Goodman RL, Lehman MN. 2012. KNDy (kisspeptin/neurokinin B/dynorphin) neurons are activated during both pulsatile and surge secretion of LH in the ewe. Endocrinology, Submitted [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Williams WP, 3rd, Jarjisian SG, Mikkelsen JD, Kriegsfeld LJ. 2011. Circadian control of kisspeptin and a gated GnRH response mediate the preovulatory luteinizing hormone surge. Endocrinology 152:595–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Inoue N, Sasagawa K, Ikai K, Sasaki Y, Tomikawa J, Oishi S, Fujii N, Uenoyama Y, Ohmori Y, Yamamoto N, Hondo E, Maeda K, Tsukamura H. 2011. Kisspeptin neurons mediate reflex ovulation in the musk shrew (Suncus murinus). Proc Natl Acad Sci USA 108:17527–17532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mayer C, Boehm U. 2011. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci 14:704–710 [DOI] [PubMed] [Google Scholar]
- 49. Smith JT, Shahab M, Pereira A, Pau KY, Clarke IJ. 2010. Hypothalamic expression of KISS1 and gonadotropin inhibitory hormone genes during the menstrual cycle of a non-human primate. Biol Reprod 83:568–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Kallo I. 2010. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci 31:1984–1998 [DOI] [PubMed] [Google Scholar]
- 51. Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. 2008. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology 149:4387–4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. 2009. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet 41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. 2004. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology 145:2959–2967 [DOI] [PubMed] [Google Scholar]
- 54. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. 2009. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. 2011. Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J Neuroendocrinol 23:52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Alain Caraty A, Ciofi P, Clarke IJ. 2007. Kisspeptin neurons in the arcuate nucleus of the ewe also express dynorphin A and neurokinin B. Endocrinology 148:5752–5760 [DOI] [PubMed] [Google Scholar]
- 57. Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. 2010. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 30:3124–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. 2010. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 151:4494–4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Porteous R, Petersen SL, Yeo SH, Bhattarai JP, Ciofi P, de Tassigny XD, Colledge WH, Caraty A, Herbison AE. 2011. Kisspeptin neurons co-express met-enkephalin and galanin in the rostral periventricular region of the female mouse hypothalamus. J Comp Neurol 519:3456–3469 [DOI] [PubMed] [Google Scholar]
- 60. Kalló I, Vida B, Deli L, Molnár CS, Hrabovszky E, Caraty A, Ciofi P, Coen CW, Liposits Z. 2012. Co-localisation of kisspeptin with galanin or neurokinin B in afferents to mouse GnRH neurones. J Neuroendocrinol 24:464–476 [DOI] [PubMed] [Google Scholar]
- 61. Lehman MN, Coolen LM, Goodman RL. 2010. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 151:3479–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Servili A, Le Page Y, Leprince J, Caraty A, Escobar S, Parhar IS, Seong JY, Vaudry H, Kah O. 2011. Organization of two independent kisspeptin systems derived from evolutionary-ancient kiss genes in the brain of zebrafish. Endocrinology 152:1527–1540 [DOI] [PubMed] [Google Scholar]
- 63. Tena-Sempere M, Felip A, Gómez A, Zanuy S, Carrillo M. 2012. Comparative insights of the kisspeptin/kisspeptin receptor system: lessons from non-mammalian vertebrates. Gen Comp Endocrinol 175:234–243 [DOI] [PubMed] [Google Scholar]
- 64. Ogawa S, Ramadasan PN, Goschorska M, Anantharajah A, We Ng K, Parhar IS. 2012. Cloning and expression of tachykinins and their association with kisspeptins in the brains of zebrafish. J Comp Neurol 520:2991–3012 [DOI] [PubMed] [Google Scholar]
- 65. Burke MC, Letts PA, Krajewski SJ, Rance NE. 2006. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol 498:712–726 [DOI] [PubMed] [Google Scholar]
- 66. Matsuyama S, Ohkura S, Mogi K, Wakabayashi Y, Mori Y, Tsukamura H, Maeda K, Ichikawa M, Okamura H. 2011. Morphological evidence for direct interaction between kisspeptin and gonadotropin-releasing hormone neurons at the median eminence of the male goat: an immunoelectron microscopic study. Neuroendocrinology 94:323–332 [DOI] [PubMed] [Google Scholar]
- 67. Uenoyama Y, Inoue N, Pheng V, Homma T, Takase K, Yamada S, Ajiki K, Ichikawa M, Okamura H, Maeda KI, Tsukamura H. 2011. Ultrastructural evidence of kisspeptin-gonadotrophin-releasing hormone (GnRH) interaction in the median eminence of female rats: implication of axo-axonal regulation of GnRH release. J Neuroendocrinol 23:863–870 [DOI] [PubMed] [Google Scholar]
- 68. Yeo SH, Herbison AE. 2011. Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology 152:2387–2399 [DOI] [PubMed] [Google Scholar]
- 69. Foradori CD, Amstalden M, Goodman RL, Lehman MN. 2006. Colocalisation of dynorphin A and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol 18:534–541 [DOI] [PubMed] [Google Scholar]
- 70. Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. 2010. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience 166:680–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nishihara M, Sano A, Kimura F. 1994. Cessation of the electrical activity of gonadotropin-releasing hormone pulse generator during the steroid-induced surge of luteinizing hormone in the rat. Neuroendocrinology 59:513–519 [DOI] [PubMed] [Google Scholar]
- 72. Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda KI, Okamura H. 2009. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol 21:813–821 [DOI] [PubMed] [Google Scholar]
- 73. O'Byrne KT, Knobil E. 1993. Electrophysiological approaches to gonadotrophin releasing hormone pulse generator activity in the rhesus monkey. Hum Reprod 8(Suppl 2):37–40 [DOI] [PubMed] [Google Scholar]
- 74. Clarkson J, Herbison AE. 2011. Dual phenotype kisspeptin-dopamine neurones of the rostral periventricular area of the third ventricle project to gonadotrophin-releasing hormone neurones. J Neuroendocrinol 23:293–301 [DOI] [PubMed] [Google Scholar]
- 75. Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. 2011. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology 152:2020–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. 2006. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett 401:225–230 [DOI] [PubMed] [Google Scholar]
- 77. Decourt C, Tillet Y, Caraty A, Franceschini I, Briant C. 2008. Kisspeptin immunoreactive neurons in the equine hypothalamus: interactions with GnRH neuronal system. J Chem Neuroanat 36:131–137 [DOI] [PubMed] [Google Scholar]
- 78. Bosch MA, Xue C, Ronnelkleiv OK. 2012. Kisspeptin expression in guinea pig hypothalamus: effects of 17β-estradiol. J Comp Neurol 520:2143–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Desroziers E, Mikkelsen J, Simonneaux V, Keller M, Tillet Y, Caraty A, Franceschini I. 2010. Mapping of kisspeptin fibres in the brain of the pro-oestrous rat. J Neuroendocrinol 22:1101–1112 [DOI] [PubMed] [Google Scholar]
- 80. Greives TJ, Mason AO, Scotti MA, Levine J, Ketterson ED, Kriegsfeld LJ, Demas GE. 2007. Environmental control of kisspeptin: Implications for seasonal reproduction. Endocrinology 148:1158–1166 [DOI] [PubMed] [Google Scholar]
- 81. Arai AC, Orwig N. 2008. Factors that regulate KiSS1 gene expression in the hippocampus. Brain Res 1243:10–18 [DOI] [PubMed] [Google Scholar]
- 82. Gill JC, Wang O, Kakar S, Martinelli E, Carroll RS, Kaiser UB. 2010. Reproductive hormone-dependent and -independent contributions to developmental changes in kisspeptin in GnRH-deficient hypogonadal mice. PLoS One 5:e11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bentsen AH, Ansel L, Simonneaux V, Tena-Sempere M, Juul A, Mikkelsen JD. 2010. Maturation of kisspeptinergic neurons coincides with puberty onset in male rats. Peptides 31:275–283 [DOI] [PubMed] [Google Scholar]
- 84. Desroziers E, Mikkelsen JD, Duittoz A, Franceschini I. 2012. Kisspeptin-immunoreactivity changes in a sex- and hypothalamic-region specific manner across rat postnatal development. J Neuroendocrinol 24:1154–1165 [DOI] [PubMed] [Google Scholar]
- 85. Clarkson J, Boon WC, Simpson ER, Herbison AE. 2009. Postnatal development of an estradiol-kisspeptin positive feedback mechanism implicated in puberty onset. Endocrinology 150:3214–3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE. 2010. Timing and completion of puberty in female mice depend on estrogen receptor α-signaling in kisspeptin neurons. Proc Natl Acad Sci USA 107:22693–22698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. van der Werff ten Bosch JJ, Dierschke DJ, Terasawa E, Slob AK. 1983. Anterior hypothalamic lesions and pubertal development in female rhesus monkeys. Behav Brain Res 7:321–330 [DOI] [PubMed] [Google Scholar]
- 88. Krey LC, Hess DL, Butler WR, Espinosa-Campos J, Lu KH, Piva F, Plant TM, Knobil E. 1981. Medial basal hypothalamic disconnection and the onset of puberty in the female rhesus monkey. Endocrinology 108:1944–1948 [DOI] [PubMed] [Google Scholar]
- 89. Terasawa E, Noonan JJ, Nass TE, Loose MD. 1984. Posterior hypothalamic lesions advance the onset of puberty in the female rhesus monkey. Endocrinology 115:2241–2250 [DOI] [PubMed] [Google Scholar]
- 90. Clarkson J, Shamas S, Mallinson S, Herbison AE. 2012. Gonadal steroid induction of kisspeptin peptide expression in the rostral periventricular area of the third ventricle during postnatal development in the male mouse. J Neuroendocrinol 24:907–915 [DOI] [PubMed] [Google Scholar]
- 91. Revel FG, Masson-Pévet M, Pévet P, Mikkelsen JD, Simonneaux V. 2009. Melatonin controls seasonal breeding by a network of hypothalamic targets. Neuroendocrinology 90:1–14 [DOI] [PubMed] [Google Scholar]
- 92. Goodman RL, Jansen HT, Billings HJ, Coolen LM, Lehman MN. 2010. Neural systems mediating seasonal breeding in the ewe. J Neuroendocrinol 22:674–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Revel FG, Saboureau M, Masson-Pévet M, Pévet P, Mikkelsen JD, Simonneaux V. 2006. Kisspeptin mediates the photoperiodic control of reproduction in hamsters. Curr Biol 16:1730–1735 [DOI] [PubMed] [Google Scholar]
- 94. Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. 2008. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology 149:5770–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chalivoix S, Bagnolini A, Caraty A, Cognié J, Malpaux B, Dufourny L. 2010. Effects of photoperiod on kisspeptin neuronal populations of the ewe diencephalon in connection with reproductive function. J Neuroendocrinol 22:110–118 [DOI] [PubMed] [Google Scholar]
- 96. Revel FG, Ansel L, Klosen P, Saboureau M, Pévet P, Mikkelsen JD, Simonneaux V. 2007. Kisspeptin: a key link to seasonal breeding. Rev Endocr Metab Disord 8:57–65 [DOI] [PubMed] [Google Scholar]
- 97. Mason AO, Greives TJ, Scotti MA, Levine J, Frommeyer S, Ketterson ED, Demas GE, Kriegsfeld LJ. 2007. Suppression of kisspeptin expression and gonadotropic axis sensitivity following exposure to inhibitory day lengths in female Siberian hamsters. Horm Behav 52:492–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Caraty A, Smith JT, Lomet D, Ben Saïd S, Morrissey A, Cognie J, Doughton B, Baril G, Briant C, Clarke IJ. 2007. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology 148:5258–5267 [DOI] [PubMed] [Google Scholar]
- 99. Greives TJ, Kriegsfeld LJ, Demas GE. 2008. Exogenous kisspeptin does not alter photoperiod-induced gonadal regression in Siberian hamsters (Phodopus sungorus). Gen Comp Endocrinol 156:552–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Greives TJ, Humber SA, Goldstein AN, Scotti MA, Demas GE, Kriegsfeld LJ. 2008. Photoperiod and testosterone interact to drive seasonal changes in kisspeptin expression in Siberian hamsters (Phodopus sungorus). J Neuroendocrinol 20:1339–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Dungan HM, Clifton DK, Steiner RA. 2006. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology 147:1154–1158 [DOI] [PubMed] [Google Scholar]
- 102. Levine JE. 1997. New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biol Reprod 56:293–302 [DOI] [PubMed] [Google Scholar]
- 103. Herbison AE. 2008. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev 57:277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Smith JT, Clay CM, Caraty A, Clarke IJ. 2007. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 148:1150–1157 [DOI] [PubMed] [Google Scholar]
- 105. Shibata M, Friedman RL, Ramaswamy S, Plant TM. 2007. Evidence that down regulation of hypothalamic KiSS-1 expression is involved in the negative feedback action of testosterone to regulate luteinising hormone secretion in the adult male rhesus monkey (Macaca mulatta). J Neuroendocrinol 19:432–438 [DOI] [PubMed] [Google Scholar]
- 106. Rometo AM, Krajewski SJ, Voytko ML, Rance NE. 2007. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 92:2744–2750 [DOI] [PubMed] [Google Scholar]
- 107. Kim W, Jessen HM, Auger AP, Terasawa E. 2009. Postmenopausal increase in KiSS-1, GPR54, and luteinizing hormone releasing hormone (LHRH-1) mRNA in the basal hypothalamus of female rhesus monkeys. Peptides 30:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Eghlidi DH, Haley GE, Noriega NC, Kohama SG, Urbanski HF. 2010. Influence of age and 17β-estradiol on kisspeptin, neurokinin B, and prodynorphin gene expression in the arcuate-median eminence of female rhesus macaques. Endocrinology 151:3783–3794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Smith JT, Li Q, Pereira A, Clarke IJ. 2009. Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology 150:5530–5538 [DOI] [PubMed] [Google Scholar]
- 110. Estrada KM, Clay CM, Pompolo S, Smith JT, Clarke IJ. 2006. Elevated KiSS-1 expression in the arcuate nucleus prior to the cyclic preovulatory gonadotrophin-releasing hormone/lutenising hormone surge in the ewe suggests a stimulatory role for kisspeptin in oestrogen-positive feedback. J Neuroendocrinol 18:806–809 [DOI] [PubMed] [Google Scholar]
- 111. Caraty A, Fabre-Nys C, Delaleu B, Locatelli A, Bruneau G, Karsch FJ, Herbison A. 1998. Evidence that the mediobasal hypothalamus is the primary site of action of estradiol in inducing the preovulatory gonadotropin releasing hormone surge in the ewe. Endocrinology 139:1752–1760 [DOI] [PubMed] [Google Scholar]
- 112. Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL. 2007. Nonclassical estrogen receptor α signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci USA 104:8173–8177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA. 2009. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci 29:9390–9395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. King JC, Ronsheim P, Liu E, Powers L, Slonimski M, Rubin BS. 1998. Fos expression in luteinizing hormone-releasing hormone neurons of guinea pigs, with knife cuts separating the preoptic area and the hypothalamus, demonstrating luteinizing hormone surges. Biol Reprod 58:323–329 [DOI] [PubMed] [Google Scholar]
- 115. Krey LC, Butler WR, Knobil E. 1975. Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey. I. Gonadotropin secretion. Endocrinology 96:1073–1087 [DOI] [PubMed] [Google Scholar]
- 116. Seminara SB, Dipietro MJ, Ramaswamy S, Crowley WF, Jr, Plant TM. 2006. Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology 147:2122–2126 [DOI] [PubMed] [Google Scholar]
- 117. Ramaswamy S, Seminara SB, Pohl CR, DiPietro MJ, Crowley WF, Jr, Plant TM. 2007. Effect of continuous intravenous administration of human metastin 45–54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult male rhesus monkey (Macaca mulatta). Endocrinology 148:3364–3370 [DOI] [PubMed] [Google Scholar]
- 118. Thompson EL, Amber V, Stamp GW, Patterson M, Curtis AE, Cooke JH, Appleby GF, Dhillo WS, Ghatei MA, Bloom SR, Murphy KG. 2009. Kisspeptin-54 at high doses acutely induces testicular degeneration in adult male rats via central mechanisms. Br J Pharmacol 156:609–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Roa J, Vigo E, García-Galiano D, Castellano JM, Navarro VM, Pineda R, Diéguez C, Aguilar E, Pinilla L, Tena-Sempere M. 2008. Desensitization of gonadotropin responses to kisspeptin in the female rat: analyses of LH and FSH secretion at different developmental and metabolic states. Am J Physiol Endocrinol Metab 294:E1088–E1096 [DOI] [PubMed] [Google Scholar]
- 120. Sébert ME, Lomet D, Saïd SB, Monget P, Briant C, Scaramuzzi RJ, Caraty A. 2010. Insights into the mechanism by which kisspeptin stimulates a preovulatory LH surge and ovulation in seasonally acyclic ewes: potential role of estradiol. Domest Anim Endocrinol 38:289–298 [DOI] [PubMed] [Google Scholar]
- 121. Jayasena CN, Nijher GM, Chaudhri OB, Murphy KG, Ranger A, Lim A, Patel D, Mehta A, Todd C, Ramachandran R, Salem V, Stamp GW, Donaldson M, Ghatei MA, Bloom SR, Dhillo WS. 2009. Subcutaneous injection of kisspeptin-54 acutely stimulates gonadotropin secretion in women with hypothalamic amenorrhea, but chronic administration causes tachyphylaxis. J Clin Endocrinol Metab 94:4315–4323 [DOI] [PubMed] [Google Scholar]
- 122. Jayasena CN, Nijher GM, Abbara A, Murphy KG, Lim A, Patel D, Mehta A, Todd C, Donaldson M, Trew GH, Ghatei MA, Bloom SR, Dhillo WS. 2010. Twice-weekly administration of kisspeptin-54 for 8 weeks stimulates release of reproductive hormones in women with hypothalamic amenorrhea. Clin Pharmacol Ther 88:840–847 [DOI] [PubMed] [Google Scholar]
- 123. George JT, Veldhuis JD, Roseweir AK, Newton CL, Faccenda E, Millar RP, Anderson RA. 2011. Kisspeptin-10 is a potent stimulator of LH and increases pulse frequency in men. J Clin Endocrinol Metab 96:E1228–E1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Young J, George JT, Tello JA, Francou B, Bouligand J, Guiochon-Mantel A, Brailly-Tabard S, Anderson RA, Millar RP. 24 February 2012. Kisspeptin restores pulsatile LH secretion in patients with neurokinin B signaling deficiencies: physiological, pathophysiological and therapeutic implications. Neuroendocrinology 10.1159/000336376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Maeda K, Ohkura S, Uenoyama Y, Wakabayashi Y, Oka Y, Tsukamura H, Okamura H. 2010. Neurobiological mechanisms underlying GnRH pulse generation by the hypothalamus. Brain Res 1364:103–115 [DOI] [PubMed] [Google Scholar]
- 126. Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. 2010. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res 1364:116–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kinsey-Jones JS, Li XF, Luckman SM, O'Byrne KT. 2008. Effects of kisspeptin-10 on the electrophysiological manifestation of gonadotropin-releasing hormone pulse generator activity in the female rat. Endocrinology 149:1004–1008 [DOI] [PubMed] [Google Scholar]
- 128. Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. 2004. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of Kiss-1 mRNA in the male rat. Neuroendocrinology 80:264–272 [DOI] [PubMed] [Google Scholar]
- 129. Li XF, Kinsey-Jones JS, Cheng Y, Knox AM, Lin Y, Petrou NA, Roseweir A, Lightman SL, Milligan SR, Millar RP, O'Byrne KT. 2009. Kisspeptin signaling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS One 4:e8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Chan YM, Butler JP, Pinnell NE, Pralong FP, Crowley WF, Jr, Ren C, Chan KK, Seminara SB. 2011. Kisspeptin resets the hypothalamic GnRH clock in men. J Clin Endocrinol Metab 96:E908–E915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. 2005. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol 489:372–386 [DOI] [PubMed] [Google Scholar]
- 132. Navarro VM, Gottsch ML, Wu M, García-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA. 2011. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology 152:4265–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN. 2010. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: Colocalization in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol 22:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. 2011. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab 300:E202–E210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ramaswamy S, Seminara SB, Plant TM. 2011. Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology 94:237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. García-Galiano D, van Ingen Schenau D, Leon S, Krajnc-Franken MA, Manfredi-Lozano M, Romero-Ruiz A, Navarro VM, Gaytan F, van Noort PI, Pinilla L, Blomenröhr M, Tena-Sempere M. 2012. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology 153:316–328 [DOI] [PubMed] [Google Scholar]
- 137. Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, McManus CJ, Nestor CC, Jacobs BH, Goodman RL. 2010. Neurokinin B acts via the neurokinin 3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology 151:3836–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kinsey-Jones JS, Grachev P, Li XF, Lin YS, Milligan SR, Lightman SL, O'Byrne KT. 2012. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology 153:307–315 [DOI] [PubMed] [Google Scholar]
- 139. Sandoval-Guzmán T, Rance NE. 2004. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res 1026:307–312 [DOI] [PubMed] [Google Scholar]
- 140. Corander MP, Challis BG, Thompson EL, Jovanovic Z, Loraine Tung YC, Rimmington D, Huhtanieme IT, Murphy KG, Kemal Topaloglu AK, Yeo GS, O'Rahilly S, Dhillo WS, Semple RK, Coll AP. 2010. A study of the effects of neurokinin B (NKB) upon gonadotrophin release in male rodents. J Neuroendocrinol 22:181–187 [DOI] [PubMed] [Google Scholar]
- 141. Kauffman AS. 2010. Gonadal and nongonadal regulation of sex differences in hypothalamic Kiss1 neurones. J Neuroendocrinol 22:682–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. 2009. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab 297:E1212–E221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. 2007. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 148:1774–1783 [DOI] [PubMed] [Google Scholar]
- 144. Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, Maeda K, Tsukamura H. 2009. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod 81:1216–1225 [DOI] [PubMed] [Google Scholar]
- 145. Xu Z, Kaga S, Mochiduki A, Tsubomizu J, Adachi S, Sakai T, Inoue K, Adachi AA. 2012. Immunocytochemical localization of kisspeptin neurons in the rat forebrain with special reference to sexual dimorphism and interaction with GnRH neurons. Endocr J 59:161–171 [DOI] [PubMed] [Google Scholar]
- 146. Iijima N, Takumi K, Sawai N, Ozawa H. 2011. An immunohistochemical study on the expressional dynamics of kisspeptin neurons relevant to GnRH neurons using a newly developed anti-kisspeptin antibody. J Mol Neurosci 43:146–154 [DOI] [PubMed] [Google Scholar]
- 147. Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. 2010. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology 151:301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. González-Martínez D, De Mees C, Douhard Q, Szpirer C, Bakker J. 2008. Absence of gonadotropin-releasing hormone 1 and Kiss1 activation in α-fetoprotein knockout mice: prenatal estrogens defeminize the potential to show preovulatory luteinizing hormone surges. Endocrinology 149:2333–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Bakker J, Pierman S, González-Martínez D. 2010. Effects of aromatase mutation (ArKO) on the sexual differentiation of kisspeptin neuronal numbers and their activation by same versus opposite sex urinary pheromones. Horm Behav 57:390–395 [DOI] [PubMed] [Google Scholar]
- 150. Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. 2005. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146:2976–2984 [DOI] [PubMed] [Google Scholar]
- 151. Rager K, Zarzycki J, Eichner M, Gupta D. 1975. Effects of experimental bilateral cryptorchidism and castration on the plasma gonadotropins in male rats during sexual development. Res Exp Med (Berl) 165:55–59 [DOI] [PubMed] [Google Scholar]
- 152. Jayasena CN, Nijher GM, Comninos AN, Abbara A, Januszewki A, Vaal ML, Sriskandarajah L, Murphy KG, Farzad Z, Ghatei MA, Bloom SR, Dhillo WS. 2011. The effects of kisspeptin-10 on reproductive hormone release show sexual dimorphism in humans. J Clin Endocrinol Metab 96:E1963–E1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Mahoney MM, Rossi BV, Hagenauer MH, Lee TM. 2011. Characterization of the estrous cycle in Octodon degus. Biol Reprod 84:664–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Veening JG, Coolen LM. 1998. Neural activation following sexual behavior in the male and female rat brain. Behav Brain Res 92:181–193 [DOI] [PubMed] [Google Scholar]
- 155. Beltramino C, Taleisnik S. 1978. Facilitatory and inhibitory effects of electrochemical stimulation of the amygdala on the release of luteinizing hormone. Brain Res 144:95–107 [DOI] [PubMed] [Google Scholar]
- 156. Arai AC, Xia YF, Suzuki E, Kessler M, Civelli O, Nothacker HP. 2005. Cancer metastasis-suppressing peptide metastin upregulates excitatory synaptic transmission in hippocampal dentate granule cells. J Neurophysiol 94:3648–3652 [DOI] [PubMed] [Google Scholar]