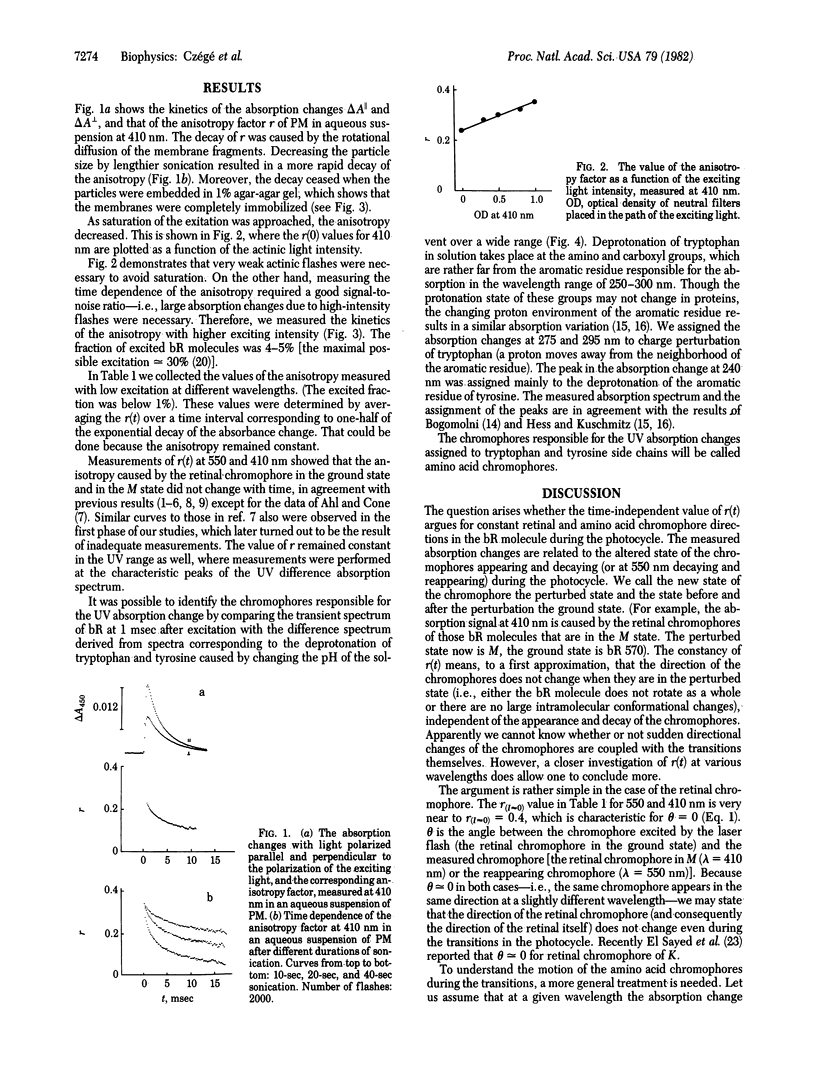
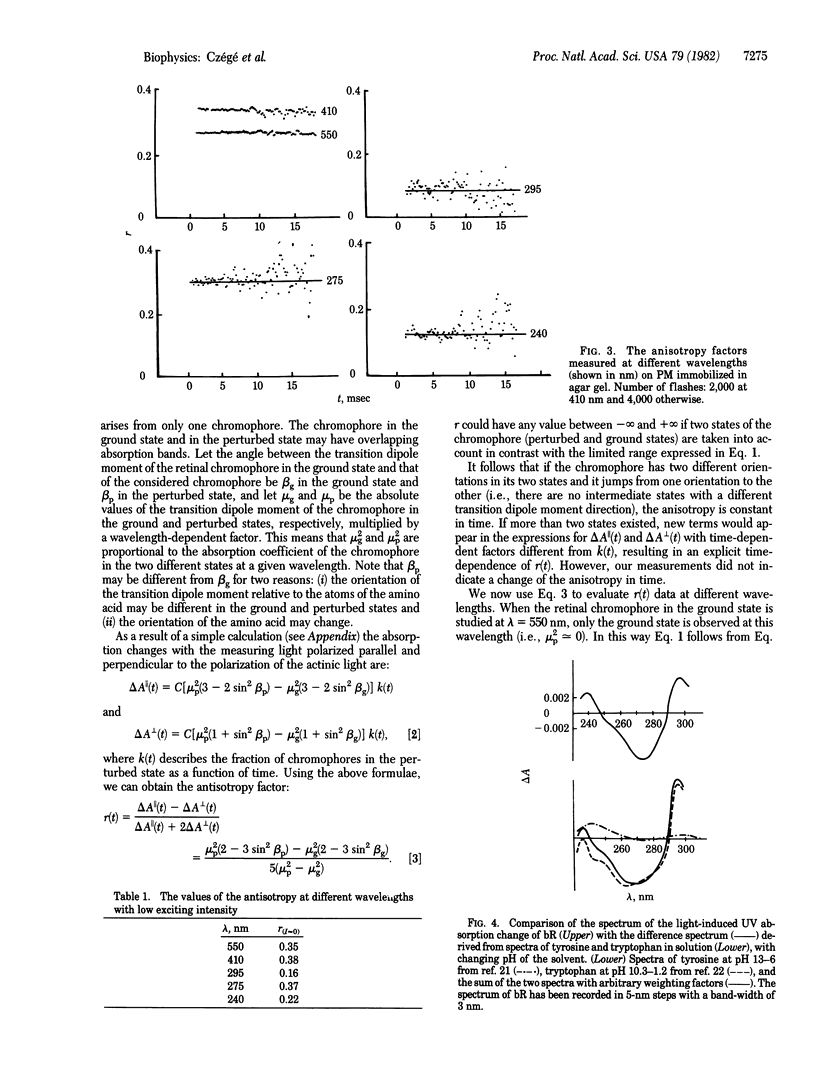
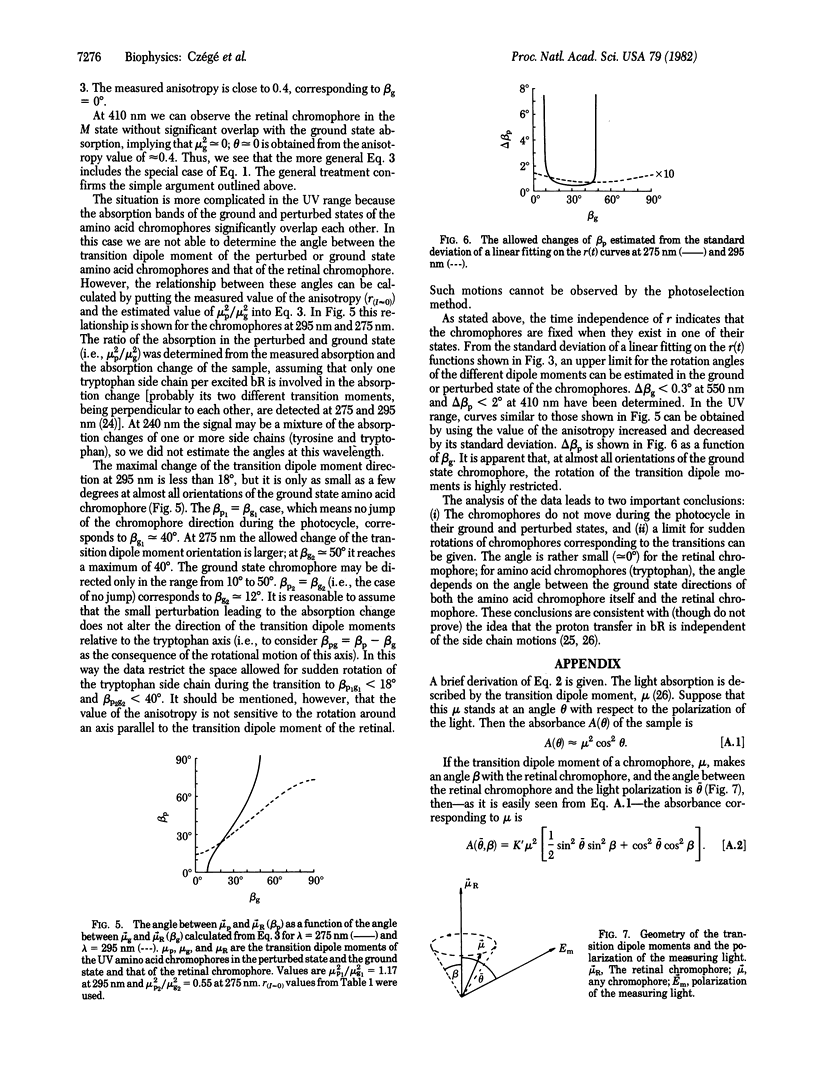
Abstract
Linear dichroism was measured during the photocycle of bacteriorhodopsin. The anisotropy of the sample was produced by the photoselection method. The measurements on purple membrane fragments embedded in agar gel were performed at room temperature with 200 microseconds time resolution at several wavelengths in the 240- to 550-nm spectral region. The induced anisotropy of the retinal chromophore remained constant after the formation of the photocycle intermediate M. The anisotropy was also time independent at the characteristic peaks of the UV absorption change. These experimental data suggest that the direction of the retinal transition dipole moment remains unchanged. Moreover, the affected aromatic protein side chains also do not show any rotational motion when they are in the perturbed or ground states during the photocycle. Our data render it possible to calculate the restricted range of sudden chromophore rotations that might be coupled to the appearance and decay of the M intermediate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becher B., Ebrey T. G. The quantum efficiency for the photochemical conversion of the purple membrane protein. Biophys J. 1977 Feb;17(2):185–191. doi: 10.1016/S0006-3495(77)85636-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B., Tokunaga F., Ebrey T. G. Ultraviolet and visible absorption spectra of the purple membrane protein and the photocycle intermediates. Biochemistry. 1978 Jun 13;17(12):2293–2300. doi: 10.1021/bi00605a006. [DOI] [PubMed] [Google Scholar]
- Belford G. G., Belford R. L., Weber G. Dynamics of fluorescence polarization in macromolecules. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1392–1393. doi: 10.1073/pnas.69.6.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogomolni R. A., Stubbs L., Lanyi J. K. Illumination-dependent changes in the intrinsic fluorescence of bacteriorhodopsin. Biochemistry. 1978 Mar 21;17(6):1037–1041. doi: 10.1021/bi00599a015. [DOI] [PubMed] [Google Scholar]
- Cherry R. J., Heyn M. P., Oesterhelt D. Rotational diffusion and exciton coupling of bacteriorhodopsin in the cell membrane of Halobacterium halobium. FEBS Lett. 1977;78(1):25–30. doi: 10.1016/0014-5793(77)80265-2. [DOI] [PubMed] [Google Scholar]
- El-Sayed M. A., Karvaly B., Fukumoto J. M. Primary step in the bacteriorhodopsin photocycle: photochemistry or excitation transfer? Proc Natl Acad Sci U S A. 1981 Dec;78(12):7512–7516. doi: 10.1073/pnas.78.12.7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillbro T. Flash kinetic study of the last steps in the photoinduced reaction cycle of bacteriorhodopsin. Biochim Biophys Acta. 1978 Oct 11;504(1):175–186. doi: 10.1016/0005-2728(78)90016-6. [DOI] [PubMed] [Google Scholar]
- Hess B., Kuschmitz D. Kinetic interaction between aromatic residues and the retinal chromophore of bacteriorhodopsin during the photocycle. FEBS Lett. 1979 Apr 15;100(2):334–340. doi: 10.1016/0014-5793(79)80364-6. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Cherry R. J., Müller U. Transient and linear dichroism studies on bacteriorhodopsin: determination of the orientation of the 568 nm all-trans retinal chromophore. J Mol Biol. 1977 Dec 15;117(3):607–620. doi: 10.1016/0022-2836(77)90060-2. [DOI] [PubMed] [Google Scholar]
- Korenstein R., Hess B. Immobilization of bacteriorhodopsin and orientation of its transition moment in purple membrane. FEBS Lett. 1978 May 1;89(1):15–20. doi: 10.1016/0014-5793(78)80512-2. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Niederberger W. The photochemical cycle of bacteriorhodopsin. Fed Proc. 1977 May;36(6):1805–1809. [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Razi Naqvi K., Gonzalez-Rodriguez J., Cherry R. J., Chapman D. Spectroscopic technique for studying protein rotation in membranes. Nat New Biol. 1973 Oct 24;245(147):249–251. doi: 10.1038/newbio245249a0. [DOI] [PubMed] [Google Scholar]
- Sherman W. V., Caplan S. R. Chromophore mobility in bacteriorhodopsin. Nature. 1977 Jan 20;265(5591):273–274. doi: 10.1038/265273a0. [DOI] [PubMed] [Google Scholar]
- Sherman W. V., Slifkin M. A., Caplan S. R. Kinetic studies of phototransients in bacteriorhodopsin. Biochim Biophys Acta. 1976 Feb 16;423(2):238–248. doi: 10.1016/0005-2728(76)90182-1. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]


