Abstract
Copper (Cu), iron (Fe), and thyroid hormone (TH) deficiencies produce similar defects in late brain development including hypomyelination of axons and impaired synapse formation and function, suggesting that these micronutrient deficiencies share a common mechanism contributing to these derangements. We previously demonstrated that fetal/neonatal Cu and Fe deficiencies lower circulating TH concentrations in neonatal rats. Fe deficiency also reduces whole-brain T3 content, suggesting impaired TH action in the developing Fe-deficient brain. We hypothesized that fetal/neonatal Cu and Fe deficiencies will produce mild or moderate TH deficiencies and will impair TH-responsive gene expression in the neonatal cerebral cortex and hippocampus. To test this hypothesis, we rendered pregnant Sprague Dawley rats Cu-, Fe-, or TH-deficient from early gestation through postnatal d 10 (P10). Mild and moderate TH deficiencies were induced by 1 and 3 ppm propylthiouracil treatment, respectively. Cu deficiency did not significantly alter serum or tissue TH concentrations or TH-responsive brain mRNA expression. Fe deficiency significantly lowered P10 serum total T3 (45%), serum total T4 (52%), whole brain T3 (14%), and hippocampal T3 (18%) concentrations, producing a mild TH deficiency similar to 1 ppm propylthiouracil treatment. Fe deficiency lowered Pvalb, Enpp6, and Mbp mRNA levels in the P10 hippocampus. Fe deficiency also altered Hairless, Dbm, and Dio2 mRNA levels in the P10 cerebral cortex. These results suggest that some of the brain defects associated with Fe deficiency may be mediated through altered thyroidal status and the concomitant alterations in TH-responsive gene transcription.
Globally, billions of people suffer from insufficient dietary intake of micronutrients such as zinc, vitamin A, selenium, copper (Cu), iron (Fe), and iodine (1). Micronutrient deficiencies are most prevalent in developing countries and often coexist due to consumption of diets lacking nutrient diversity (1). Given their profound impact on child development and prosperity, it is critical to understand how micronutrients interact during fetal and infant neurodevelopment.
Interactions between Fe and iodine/thyroid hormones (THs) have serious implications for human health. Approximately 1.9 billion people have insufficient iodine intake and 1.6 billion people suffer from anemia or Fe deficiency (2, 3). Data from Zimmermann et al. (4–6) indicate that 20–44% of West and North African children suffer from both deficiencies. Treatment of Fe deficiency in these children improves the efficacy of iodine supplementation, reducing thyroid volume and goiter prevalence (4, 6) and increasing serum total T4 concentrations, indicating an Fe-specific effect on thyroidal status (6). Additional cross-sectional studies demonstrate correlations between Fe and thyroidal parameters (reviewed in Ref. 7). Interestingly, a recent study in pregnant Swiss women shows that low maternal body Fe stores are correlated with increased serum thyroid stimulating hormone (TSH) and reduced total T4 (TT4) concentrations, suggesting Fe deficiency may compound the deleterious effects of developmental iodine deficiency (8).
Studies in FeD rodents have provided additional data to support the hypothesis that Fe is important for TH synthesis and/or metabolism. Fe deficiency in adolescent or adult rats decreases circulating TT4 and total T3 concentrations (9–12), and free T4 and T3 concentrations (11), plasma and pituitary TSH concentrations (9, 10), hepatic T4 deiodination (10, 11), and thyroid peroxidase activity (12). Cu deficiency lowers plasma Fe levels, circulating TT4 and total T3 (TT3) concentrations, and peripheral conversion of T4 to T3 (13, 14).
Cu, Fe, and iodine/TH deficiencies have their most profound effects on early life brain development and ultimately on adult intellectual outcome (15, 16). Interestingly, Cu, Fe, and iodine/TH deficiencies result in similar defects in rodent hippocampal and cerebral cortical development including aberrant myelination, blunted neuronal maturation, impaired synapse formation and function, altered neurotransmission, and changes in tissue energy metabolism (15–17). The hippocampal and cerebral cortical developmental defects associated with these deficiencies persist into adulthood and result in permanent behavioral abnormalities including impaired learning, memory, and sensorimotor function (15–20).
We recently demonstrated that fetal/neonatal Cu and Fe deficiencies decrease neonatal serum TT4 and TT3 concentrations (18, 21). Neonatal FeD rats also have reduced whole-brain T3 content with concomitant alterations in whole brain mRNA expression for some TH-responsive genes (21). However, the expression of several additional Fe- or TH-responsive genes was not significantly altered in Fe- or TH-deficient whole brains. A potential explanation for this observation is that, when analyzing whole brains, Fe- or TH-dependent changes in mRNA expression in discrete brain regions are diluted by unaffected brain regions. Therefore, we hypothesized that Cu and Fe deficiencies will impair TH-responsive gene expression in the neonatal cerebral cortex and/or hippocampus, two brain regions that are sensitive to Cu, Fe, and TH insufficiencies during development. In addition, we hypothesized that Cu and Fe deficiencies will disrupt the thyroid axis to a similar extent as a mild or moderate TH deficiency. In this study we show that fetal/neonatal Fe deficiency decreases circulating and brain TH concentrations, producing a mild TH-deficient state in neonatal rats. We also demonstrate that fetal/neonatal Fe deficiency impairs hippocampal or cerebral cortical mRNA expression for several TH-responsive genes.
Materials and Methods
Animals and diets
Sperm-positive Sprague Dawley female rats were purchased from Charles River Laboratories (Wilmington, MA). At gestational day (E) 2, 25 sperm-positive rats were randomly assigned to one of five groups: control, copper-deficient (CuD), iron-deficient (FeD), 1 ppm (1 mg/liter) 6-propyl-2-thiouracil (PTU; Sigma-Aldrich; St. Louis, MO) treatment, and 3 ppm (3 mg/liter) PTU treatment (n = 5 dams per group). The PTU groups are denoted as 1 ppm PTU and 3 ppm PTU. Beginning at E2, CuD and FeD dams were fed a semipurified diet (Harlan Laboratories, Madison, WI) deficient in Cu or Fe, respectively (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). The Fe content of the FeD diet was increased compared with our previous study (21) to produce a less severe Fe deficiency. Control and PTU dams were fed a Cu- and Fe-adequate diet (Supplemental Table 1). Dams in the control, CuD, and FeD groups drank deionized water. Beginning at E6, 1 and 3 ppm PTU dams were offered deionized water containing either 1 or 3 ppm PTU. Day of birth was designated as postnatal day (P) 0, and at P2 all litters were culled to 10 pups. At P10, male pups were killed to evaluate Cu and Fe biomarkers, serum and brain TH concentrations, and brain mRNA expression. Dams were killed at P11 to evaluate metal and TH status.
Animals were given free access to diet and drinking water throughout the study and were housed at constant temperature and humidity on a 12-h light, 12-h dark cycle. All animal studies were conducted in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The local Institutional Animal Care and Use Committee approved these procedures.
Sample collection
At P10, one or two male pups per litter were killed (seven to 10 total per group). From each pup, trunk blood was collected after decapitation and kept on ice until centrifuged to collect serum. Serum was stored at −80 C until analyzed. A 5-μl blood sample was removed to determine hemoglobin concentrations. A blood sample was also drawn from the trunk into heparanized microhematocrit tubes. A portion of liver was removed, rinsed with deionized water, weighed, and processed for metal analyses. Brains were removed and bisected at the midline. Five half-brains for metal analyses were rinsed with deionized water and weighed. Seven to 10 half-brains for TH analysis were weighed and flash frozen in liquid nitrogen. A second cohort of one or two P10 male pups per litter was killed (eight to10 total per group). Serum was collected as described above. Hippocampi and cerebral cortices were dissected, removed, and placed immediately into an RNAlater RNA stabilization reagent (QIAGEN, Valencia, CA). Finally, a third cohort of two P10 male pups per litter (10 total per group) was killed and intracardially perfused with PBS to remove blood from the brains. Hippocampi and cerebral cortices were dissected, removed, and flash frozen in liquid nitrogen for tissue TH measurement.
Metal and biochemical analyses
Cu, Fe, Zn, hemoglobin, and ceruloplasmin measurements were performed as described (21). Microhematocrit tubes were centrifuged and hematocrit was measured using a standard microcapillary reader.
Serum and brain hormone measurements
Serum TT4 and TT3 concentrations were measured using RIA kits (Siemens Medical Solutions Diagnostics, Los Angeles, CA) modified for rodent use as described (21). The minimum detectable concentration (MDC) for T4 (1.7 ng/ml) and T3 (7.6 ng/dl) RIAs was calculated statistically as 3 sds above the zero calibrator (n = 10). Samples with T4 or T3 concentrations below the MDC were set to the MDC for statistical purposes. The serum TSH concentrations were measured using a rodent TSH ELISA kit (Endocrine Technologies, Newark, CA). The serum was diluted 1:2 in the provided sample diluent for the TSH measurements.
THs were extracted from half brains, hippocampi, and cerebral cortices as described (21). For hippocampi, the following modifications were used due to the small size of this brain region. Hippocampi were homogenized for 30 sec at approximately 20,000 rpm with a PowerGen 125 homogenizer (Fisher Scientific, Pittsburgh, PA). The volume of 125I-T4 tracer was reduced to 50 μl. All steps of hippocampal TH extractions were carried out in 1.5-ml polypropylene screw-cap tubes. Each lyophilized hippocampal sample was resuspended in 250 μl hormone-stripped rat serum.
Brain mRNA analysis
Total RNA was extracted from brain subregions using QIAGEN RNeasy midi (hippocampi) or maxi (cerebral cortices) kits. The optional on-column deoxyribonuclease digestion was performed to remove genomic DNA. RNA integrity and purity was established spectrophotometrically and by RNA gels. cDNA was synthesized from 2 μg (hippocampi) or 3 μg (cerebral cortices) total RNA using SuperScript III first-strand synthesis supermix and random hexamers (Invitrogen, Carlsbad, CA). Quantitative real-time PCR (qPCR) was performed using Rotor-Gene SYBR Green PCR kits (QIAGEN) and a Corbett RotorGene RG-3000 (QIAGEN). Primer pairs for the assayed genes are outlined in Supplemental Table 2. PCR reactions were performed on cDNA equivalent to 80 ng total RNA according to the manufacturer's protocol except that a final volume of 12.5 μl was used. Quantification cycle values were determined in the log-linear amplification phase. Relative mRNA levels were calculated relative to internal hippocampal or cerebral cortical cDNA samples from a P10 control rat pup.
Statistical analysis
One-way ANOVA was used for making statistical comparisons between treatment groups. Bartlett's test was used to assess homogeneity of variances. When variances were equal across groups, a Tukey's post hoc test was used. When variances were unequal, data were ln transformed and a Tukey's test was used. When transformation did not normalize the variances, Scheffé's post hoc test was used on the untransformed data. Statistical outliers were determined by Grubbs' test (http://www.graphpad.com/quickcalcs/Grubbs1.cfm). All data are presented as mean ± sem. Linear regressions were performed to compare serum TT4 concentrations with hippocampal or cerebral cortical mRNA levels for individual pups. Statistical analyses and data graphing were carried out using Prism (GraphPad Software, La Jolla, CA) or Kaleidagraph (Synergy Software, Reading, PA) software. An α = 0.05 was chosen to define significant differences. Cu deficiency did not impact serum or brain TH concentrations or TH-responsive gene expression and thus was excluded from statistical analyses. The 3-ppm PTU group served as a positive hypothyroid control and was excluded from ANOVA analyses but was included in linear regressions and figures for comparison.
Results
Evaluating dam and pup Cu and Fe status
Cu deficiency was confirmed in CuD dams as measured by a 99% reduction in serum ceruloplasmin activity, compared with controls (Table 1). Fe deficiency anemia was confirmed in FeD dams as measured by significant reductions in serum Fe (98% lower than controls) and hemoglobin (21% lower than controls) levels (Table 1). TH deficiency was confirmed in 3-ppm PTU dams as determined by a 77% reduction in serum TT4 (Table 1). Serum TT3 concentrations were not altered in dams of any treatment (Table 1). Litter sizes from each treatment group were similar (data not shown).
Table 1.
Cu, Fe, and TH status of rat dams
| Characteristic | Control | CuD | FeD | 1 ppm PTU | 3 ppm PTU |
|---|---|---|---|---|---|
| Body weight (g) | 342 ± 5.4a | 329 ± 9.5a | 337 ± 13.4a | 325 ± 7.2a | 341 ± 7.9a |
| Hemoglobin (g/liter) | 172 ± 1.5a | 158 ± 4.3a | 136 ± 5.8b | 167 ± 4.5a | 161 ± 0.9a |
| Ceruloplasmin (U/liter) | 203 ± 18.3a | 0.54 ± 0.14b | 190 ± 11.3a | 208 ± 27.0a | 168 ± 27.1a |
| Serum Fe (μg/ml) | 5.37 ± 0.47a | 2.68 ± 0.66b | 0.97 ± 0.20c | 4.81 ± 0.09a | 5.02 ± 0.39a |
| Serum TT4 (ng/ml) | 37.0 ± 2.37a, b | 43.3 ± 3.05a | 38.5 ± 5.74a, b | 27.6 ± 3.62b | 8.51 ± 1.40c |
| Serum TT3 (ng/dl) | 37.3 ± 2.76a | 41.2 ± 3.01a | 40.7 ± 4.33a | 40.2 ± 5.19a | 36.5 ± 5.83a |
Data are presented as the mean ± sem (n = 5). Within a specific row, groups not sharing a common superscript are significantly different by one-way ANOVA and Tukey's or Scheffé's multiple comparison test (P < 0.05).
In P10 pups (Table 2), blood hemoglobin, hematocrit, serum Fe concentrations, and serum ceruloplasmin activity were lower in both CuD and FeD pups compared with controls. Cu deficiency lowered liver and brain Cu levels and Fe deficiency lowered liver and brain Fe levels. In keeping with our previous studies using this CuD diet, liver and brain Fe levels were not significantly altered compared with controls (18, 21). Brain Cu content was higher in FeD pups, as reported previously (21). The 1- and 3-ppm PTU treatment did not significantly alter Fe or Cu status. Together these data demonstrate that Fe and Cu deficiencies were generated in P10 pups nursing on dams fed nutritionally restricted diets.
Table 2.
Characteristics of P10 rat pups after fetal and neonatal Cu deficiency, Fe deficiency, or PTU treatment
| Characteristic | Control | CuD | FeD | 1 ppm PTU | 3 ppm PTU |
|---|---|---|---|---|---|
| Body weight (g) | 26.5 ± 0.88a | 25.0 ± 0.81a | 19.8 ± 1.06b | 24.3 ± 1.15a | 25.7 ± 0.74a |
| Hemoglobin (g/liter) | 118 ± 2.16a | 96.4 ± 2.16b | 58.2 ± 4.02c | 116 ± 2.99a | 123 ± 4.88a |
| Hematocrit (%) | 40.4 ± 1.01a | 30.7 ± 1.94b | 17.8 ± 1.02c | 37.9 ± 1.19a | 40.0 ± 1.62a |
| Ceruloplasmin (U/liter) | 46.2 ± 3.01a | 4.91 ± 0.55b | 27.5 ± 3.55c | 36.0 ± 1.87a,c | 51.7 ± 10.5a |
| Serum Fe (μg/ml) | 1.52 ± 0.15a | 0.82 ± 0.08b | 0.31 ± 0.04b | 1.90 ± 0.20a | 1.65 ± 0.08a |
| Liver Cu (μg/g) | 49.9 ± 2.02a | 1.93 ± 0.10b | 103 ± 9.68c | 46.4 ± 5.67a | 42.6 ± 2.82a |
| Liver Fe (μg/g) | 45.0 ± 6.40a | 45.0 ± 8.35a | 21.7 ± 2.85a | 48.8 ± 8.16a | 35.4 ± 2.43a |
| Liver Zn (μg/g) | 76.8 ± 4.06a | 65.5 ± 5.75a | 54.7 ± 4.63b | 72.5 ± 3.42a | 72.2 ± 5.73a |
| Brain Cu (μg/g) | 0.93 ± 0.02a | 0.51 ± 0.02b | 1.18 ± 0.03c | 0.96 ± 0.02a | 0.86 ± 0.02a |
| Brain Fe (μg/g) | 7.65 ± 1.63a | 5.40 ± 0.39a | 3.37 ± 0.08b | 6.02 ± 0.41a | 5.85 ± 0.16a |
| Brain Zn (μg/g) | 9.02 ± 0.27a | 8.86 ± 0.33a | 8.88 ± 0.08a | 8.57 ± 0.28a | 8.45 ± 0.27a |
Data are presented as the mean ± sem (n = 4–10). Within a specific row, groups not sharing a common superscript are significantly different by one-way ANOVA and Tukey's or Scheffé's multiple comparison test (P < 0.05).
Effects of Cu and Fe deficiency on thyroidal status
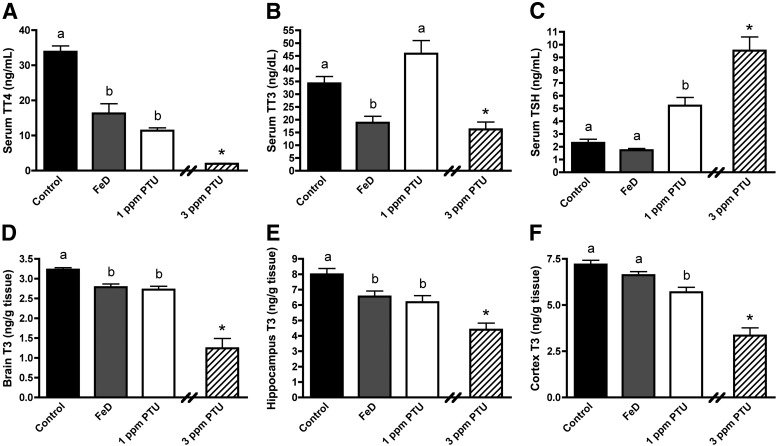
We previously reported that Cu and Fe deficiencies during fetal/neonatal development reduce neonatal circulating TH concentrations (21). In this study, Cu deficiency did not significantly alter P10 circulating hormone concentrations (data not shown). In keeping with our hypothesis, Fe deficiency reduced P10 serum TT4 by 52% and serum TT3 by 45% (Fig. 1, A and B). Fe deficiency did not alter serum TSH concentrations (Fig. 1C). Serum TT4 was 66% lower and serum TT3 was 34% higher in 1-ppm PTU P10 pups (Fig. 1, A and B). Serum TSH concentrations were 2.3 times higher in 1-ppm PTU pups, compared with controls (Fig. 1C). Both serum TT4 (93%) and TT3 (57%) were lower in 3-ppm PTU pups, compared with controls (Fig. 1, A and B). Serum TSH concentrations were 4.1 times higher in 3-ppm PTU pups (Fig. 1C), compared with controls, indicating these pups are TH-deficient and are an appropriate hypothyroid control group. Serum TT4 concentrations for six of nine 3-ppm PTU samples fell below the MDC. Serum TT3 concentrations for three of nine 3-ppm PTU samples and one out of 12 FeD samples fell below the MDC.
Fig. 1.
Fetal and neonatal Fe deficiency reduces serum and brain TH levels. A, Serum TT4. B, Serum TT3. C, Serum TSH. Serum was harvested from P10 male pups (n = 8–10). D, Whole-brain T3. E, Hippocampal T3. F, Cerebral cortical T3. Half brains (n = 6–9), hippocampi (n = 9–10), and cerebral cortices (n = 9) were harvested from P10 male pups. Data are presented as the mean ± sem. Groups not sharing a common superscript are significantly different by one-way ANOVA and Tukey's or Scheffé's multiple comparison test (P < 0.05). The 3-ppm PTU group served as a positive hypothyroid control. Asterisks indicate a statistical difference between 3 ppm PTU and control.
Given the reduction in circulating TH concentrations in FeD pups, brain subregions that are dependent on TH for development and function may be susceptible to altered tissue TH concentrations. T3 content was measured in P10 half-brains, hippocampi, and cerebral cortices. Brain T3 content was not significantly lower in CuD pups (data not shown). P10 whole-brain T3 content was 14% lower in FeD pups, 16% lower in 1-ppm PTU pups, and 62% lower in 3-ppm PTU pups (Fig. 1D). P10 hippocampus T3 content was 18% lower in FeD pups, 23% lower in 1-ppm PTU pups, and 45% lower in 3-ppm PTU pups (Fig. 1E). P10 cerebral cortex T3 content was not statistically lower in FeD pups but was 21% lower in 1-ppm PTU pups, and 53% lower in 3-ppm PTU pups (Fig. 1F).
Effect of Fe deficiency and PTU treatment on hippocampal and cerebral cortical mRNA expression
In our previous study, several known TH-responsive genes were not altered in Fe- or TH-deficient whole brains (21). This led us to hypothesize that the effect of Fe deficiency on TH-responsive gene expression will be more pronounced in brain subregions such as the cerebral cortex or hippocampus. To test this hypothesis, qPCR was performed for several genes, including some that are known Fe- or TH-responsive genes in the developing brain.
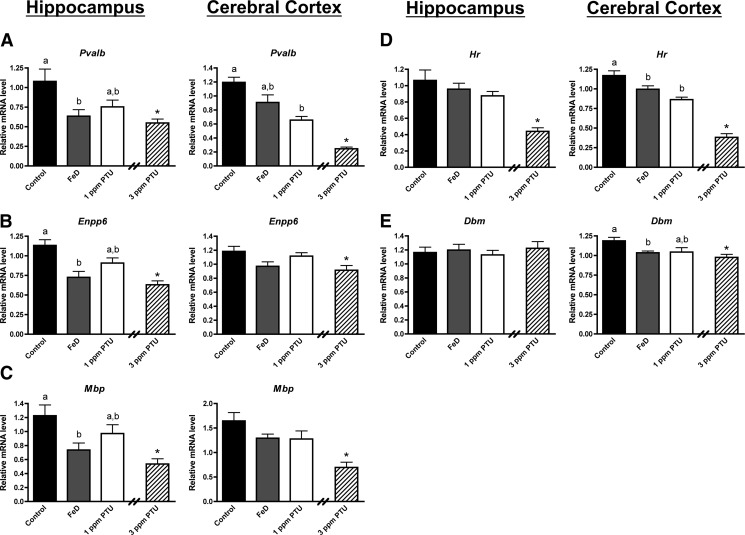
Figure 2 shows qPCR data for genes with mRNA expression that was altered by both FeD and 1 or 3 ppm PTU in the P10 hippocampus or cerebral cortex. Pvalb, Enpp6, and Mbp mRNA expression levels were significantly lower in the P10 FeD hippocampus compared with controls (Fig. 2, A–C). In contrast the mRNA expression of Pvalb, Enpp6, and Mbp was not significantly altered in the FeD cerebral cortex. These data are similar to changes in the T3 contents observed in the hippocampus and cerebral cortex (Fig. 1). Interestingly, Hr and Dbm mRNA expression levels were significantly reduced in the P10 FeD cerebral cortex but not the hippocampus (Fig. 2, D and E). The hippocampal and cerebral cortical mRNA levels of Pvalb, Enpp6, Mbp, Hr, and Dbm were reduced to a similar extent in the FeD and 1-ppm PTU pups. One part per million of PTU significantly lowered cerebral cortical Pvalb and Hr mRNA levels compared with controls. The mRNA expression of Pvalb, Enpp6, Mbp, and Hr demonstrated a dose response to 1 and 3 ppm PTU treatment in the hippocampus and cerebral cortex, indicating TH-dependent regulation. Together these data suggest that altered thyroidal status may contribute to aberrant gene expression in the neonatal FeD brain.
Fig. 2.
Fe deficiency impairs TH-dependent gene expression for some genes in the neonatal hippocampus or cerebral cortex. Hippocampi or cerebral cortices were harvested from P10 male pups (n = 8–10), total RNA was extracted, and cDNA was synthesized. Quantitative real-time PCR was performed for several TH-responsive genes. A, Parvalbumin (Pvalb). B, Ectonucleotide pyrophosphatase/phosphodiesterase 6 (Enpp6). C, Myelin basic protein (Mbp). D, Hairless (Hr). E, Dopamine β-monooxygenase (Dbm). Relative mRNA levels are calculated relative to an internal control cDNA sample. Data are presented as mean ± sem. Groups not sharing a common superscript are significantly different by one-way ANOVA and Tukey's or Scheffé's multiple comparison test (P < 0.05). The 3-ppm PTU group served as a positive hypothyroid control. Asterisks indicate statistical difference between 3 ppm PTU and control.
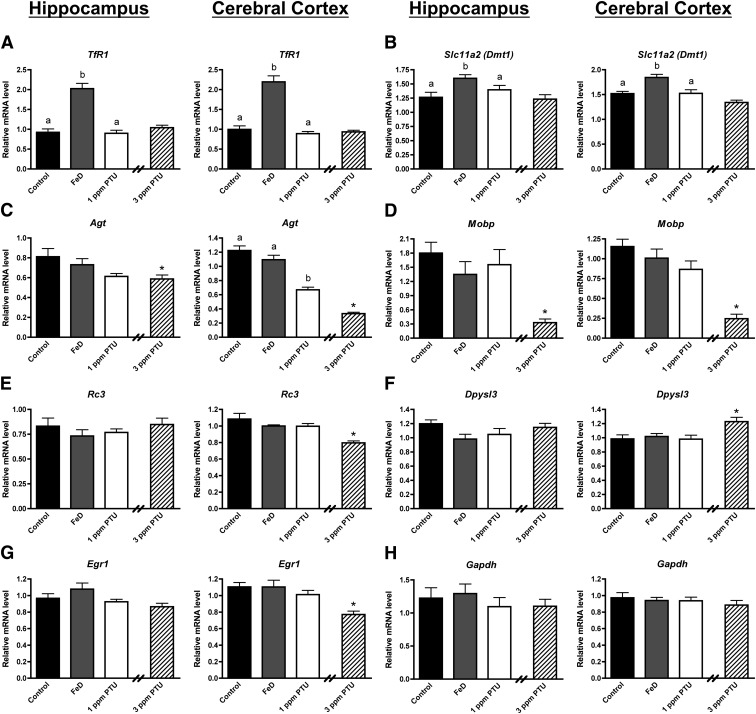
This study also revealed several genes that are selectively responsive to Fe or TH. The TfR1 and Dmt1 genes code for proteins involved in tissue Fe uptake (16). Fe response element regulated TfR1 and Dmt1 expression is increased in neonatal FeD brains (22, 23) and therefore served as Fe-responsive controls. Fe deficiency, but not 1 or 3 ppm PTU treatment, increased TfR1 and Dmt1 mRNA levels in the P10 hippocampus and cerebral cortex (Fig. 3, A and B). Agt and Mobp mRNA levels were significantly reduced in the 1- and/or 3-ppm PTU hippocampus and cerebral cortex but not in the FeD hippocampus and cerebral cortex (Fig. 3, C and D). Interestingly, the TH-responsive genes Rc3, Dpysl3, and Egr1 were significantly altered only in the 3-ppm PTU cerebral cortex (Fig. 3, E–G), suggesting differential responsivity of these genes to altered T3 levels in the cerebral cortex compared with the hippocampus. Fe and TH deficiencies did not significantly alter Bdnf IV, Bdnf VI, and total Bdnf mRNA levels in the P10 hippocampus or cerebral cortex (Supplemental Fig. 1). Gapdh mRNA expression was measured as a negative control and was not altered in FeD or PTU brain regions (Fig. 3H).
Fig. 3.
The expression of some genes is Fe-responsive, TH-responsive, or neither in the neonatal hippocampus and cerebral cortex. Hippocampi or cerebral cortices were harvested from P10 male pups (n = 8–10), total RNA was extracted, and cDNA was synthesized. Quantitative real-time PCR was performed for several Fe-responsive, TH-responsive, or negative control genes. A, Transferrin receptor 1 (TfR1). B, Divalent metal transporter 1 (Dmt1). C, Angiotensinogen (Agt). D, Myelin-associated oligodendrocyte basic protein (Mobp). E, Neurogranin (Rc3). F, Dihydropyrimidinase-like 3 (Dpysl3). G, Early growth response factor 1 (Egr1). H, Glyceraldehyde 3-phosphate dehydrogenase (Gapdh). Relative mRNA levels are calculated relative to an internal control cDNA sample. Groups not sharing a common superscript are significantly different by one-way ANOVA and Tukey's or Scheffé's multiple comparison test (P < 0.05). The 3-ppm PTU group served as a positive hypothyroid control. Asterisks indicate statistical difference between 3 ppm PTU and control.
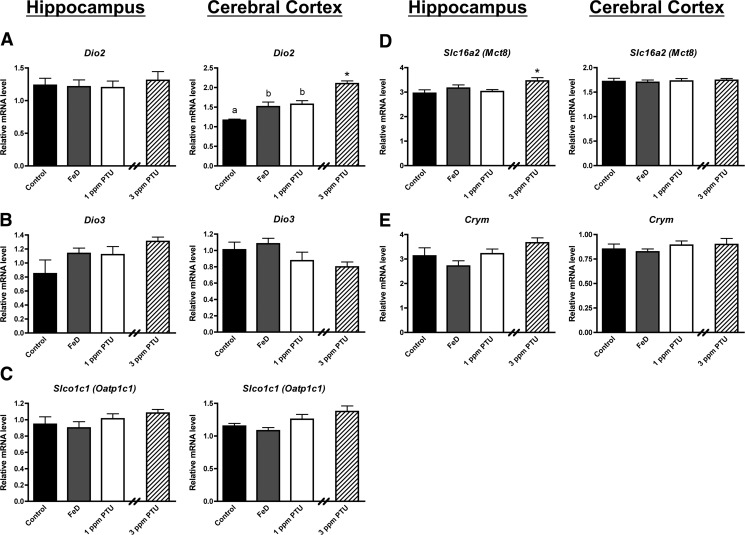
Finally, we examined mRNA levels for several genes involved in maintaining brain TH homeostasis (Fig. 4). Interestingly, Dio2 mRNA expression was significantly increased in P10 FeD, 1-ppm PTU, and 3-ppm PTU cerebral cortex but not the hippocampus (Fig. 4A). These data may explain the larger reduction in hippocampal T3 compared with cerebral cortical T3 for FeD pups (Fig. 1, D and E). Mct8 mRNA expression was significantly increased only in the 3-ppm PTU hippocampus (Fig. 4D). We did not observe significant changes in Dio3, Oatp1c1, or Crym mRNA expression in either brain region for any treatment groups (Fig. 4, B, C, and E).
Fig. 4.
The neonatal cerebral cortex, but not hippocampus senses mild TH deficiencies and up-regulates compensatory mechanisms. Hippocampi or cerebral cortices were harvested from P10 male pups (n = 8–10), total RNA was extracted, and cDNA was synthesized. Quantitative real-time PCR was performed for several genes involved in maintaining tissue TH homeostasis. A, Type 2 deiodinase (Dio2). B, Type 3 deiodinase (Dio3). C, Organic anion transporting polypeptide 1c1 (Oatp1c1). D, Monocarboxylate transporter 8 (Mct8). E, μ-Crystallin (Crym). Relative mRNA levels are calculated relative to an internal control cDNA sample. Data are presented as the mean ± sem. Groups not sharing a common superscript are significantly different by one-way ANOVA and Tukey's or Scheffé's multiple comparison test (P < 0.05). The 3-ppm PTU group served as a positive hypothyroid control. Asterisks indicate statistical difference between 3 ppm PTU and control.
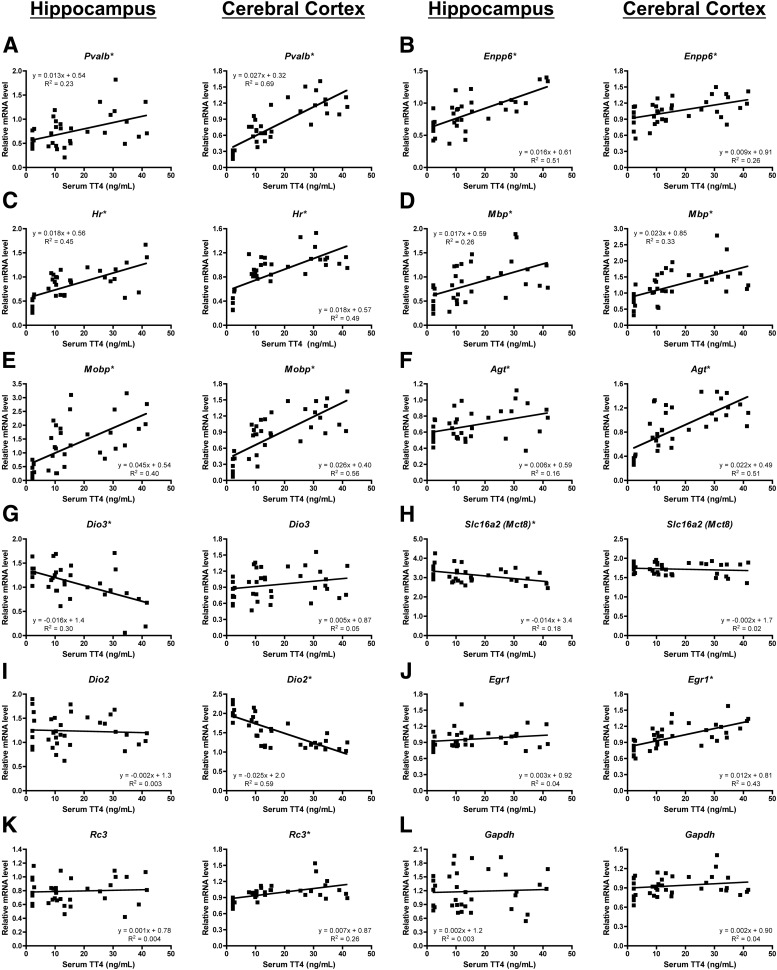
To further assess brain region differences in gene TH responsivity, we performed linear regression between serum TT4 and cerebral cortex or hippocampus mRNA levels (Fig. 5 and Supplemental Fig. 2). The relationships between Pvalb, Enpp6, Hr, Mbp, Mobp, and Agt mRNA levels and serum TT4 concentrations had slopes that were significantly different from zero in both the cerebral cortex and the hippocampus. Correlation coefficients (R2 values) demonstrate regional differences in relationship strengths, with Pvalb, Mobp, and Agt having stronger relationships in the cerebral cortex than the hippocampus and Enpp6 having a stronger relationship in the hippocampus than the cerebral cortex. Hr and Mbp had similar R2 values in the cerebral cortex and hippocampus. Only two genes, Dio3 and Mct8, had correlations with slopes that significantly deviated from zero in the hippocampus but not the cerebral cortex. In contrast, Dio2, Egr1, Rc3, Oatp1c1, Dpysl3, and Dbm had relationships with slopes that were significantly different from zero in the cerebral cortex but not the hippocampus. Together these data reveal gene- and brain region-specific TH sensitivities in the developing rat brain.
Fig. 5.
Serum TT4 and brain mRNA correlations demonstrate regional differences in TH responsivity. Linear regression scatter plots demonstrating the relationship between serum total T4 concentrations and either hippocampus or cerebral cortex mRNA levels in individual rat pups are shown for several genes. A, Parvalbumin (Pvalb). B, Ectonucleotide pyrophosphatase/phosphodiesterase 6 (Enpp6). C, Hairless (Hr). D, Myelin basic protein (Mbp). E, Myelin-associated oligodendrocyte basic protein (Mobp). F, Angiotensinogen (Agt). G, Type 3 deiodinase (Dio3). H, Monocarboxylate transporter 8 (Mct8). I, Type 2 deiodinase (Dio2). J, Early growth response factor 1 (Egr1). K, Neurogranin (Rc3). L, Glyceraldehyde 3-phosphate dehydrogenase (Gapdh). The slope, y-intercept, and Pearson correlation R2 values are shown for each linear regression analysis. Asterisks indicate relationships with slopes that significantly deviate from zero (P < 0.05).
Discussion
Fe deficiency affects greater than 1 billion people worldwide, having its most profound impact on fetal/infant neurodevelopment and the long-term cognitive and behavioral outcome of affected children (3, 24). In rodents, fetal/neonatal Fe and TH deficiencies lead to similar brain developmental defects including aberrant myelination, blunted neuronal maturation and neurotransmission, and impaired synapse formation and function (16, 17). Brain developmental impairments associated with these deficiencies often persist into adulthood and result in permanent behavioral abnormalities including impaired learning and memory (16, 20). Whether impaired thyroid function contributes to the derangements in brain development associated with Fe deficiency is unknown.
We recently demonstrated that Fe deficiency reduces both circulating and whole-brain TH concentrations in the neonatal rodent (21). In the current study, we confirm these findings and also demonstrate that Fe deficiency lowers neonatal brain T3 concentrations in a brain region-specific manner, more severely impacting the hippocampus compared with the cerebral cortex. We show that both Fe and mild or moderate TH deficiencies alter the mRNA expression of several TH-responsive genes, including Pvalb, Enpp6, Mbp, Hr, Dbm, and Dio2 in the neonatal cerebral cortex or hippocampus. These data suggest that some aspects of mammalian brain development may be partially impaired by reduced TH content in the FeD brain.
Fe and TH deficiencies were previously shown to reduce Pvalb mRNA expression in the neonatal whole brain (21, 23) or cerebral cortex (25). Here we also show that moderate TH insufficiency impairs Pvalb mRNA expression in the neonatal hippocampus. Fe deficiency significantly reduced Pvalb mRNA expression in the P10 hippocampus but not the cerebral cortex, correlating with the observed brain region T3 contents. These data suggest that Pvalb mRNA expression may be altered in the FeD neonatal hippocampus due to altered tissue T3 content. Parvalbumin is expressed in inhibitory GABAergic (γ-aminobutyric acid) interneurons and has been implicated in synaptic calcium signaling, synaptic plasticity, and memory formation in the developing rodent brain (26–28). Therefore, TH deficiency may contribute to some of the defects in hippocampal neuronal signaling and the associated deficits in learning and memory observed in FeD rats.
Mild and moderate TH deficiency was previously shown to reduce Enpp6 mRNA expression in the neonatal hippocampus (25). Our data confirm these findings in the hippocampus and also demonstrate that Enpp6 is TH-responsive in the neonatal cerebral cortex. In accordance with our tissue T3 data, Fe deficiency significantly reduced Enpp6 mRNA levels in the hippocampus but not the cerebral cortex. Mbp mRNA expression is down-regulated throughout the brain including the cerebral cortex and hippocampus of developing hypothyroid rats (25, 29) and in P21 FeD rat whole brains (23). Fe deficiency reduced Mbp mRNA expression in the hippocampus and cerebral cortex. The magnitude of this reduction was similar to the 1-ppm PTU group and was statistically significant only in the hippocampus. Myelin basic protein (Mbp) is one of the most highly enriched proteins in the myelin sheath surrounding central nervous system neurons, and thus, Mbp expression is a good indicator of myelination status (30). Ectonucleotide pyrophosphatase/phosphodiesterase 6 (Enpp6) may also be important for myelination of neuronal axons because it is involved in choline metabolism and is highly expressed in differentiating rat oligodendrocytes and the myelin fraction of mouse brain homogenates (30–32). Our Mbp and Enpp6 expression data suggest that secondary TH deficiency may contribute to the well-established hypomyelination in developing FeD brains.
Fe deficiency altered the mRNA expression of three genes, Hr, Dbm, and Dio2, in the cerebral cortex but not the hippocampus despite a nonsignificant decrease in cerebral cortex T3 content in FeD pups. Interestingly, the 1-ppm PTU treatment also altered the expression of these three genes in the cerebral cortex but not the hippocampus, suggesting that TH sensitivity and responsiveness of Hr, Dbm, and Dio2 mRNA expression is brain region specific in the P10 rat brain. Noradrenergic synaptic efficacy (33) and ligand binding to the norepinephrine transporter (34) are impaired in the FeD hippocampus and locus ceruleus, respectively. Our data suggest that conversion of dopamine to norepinephrine may be impaired in the FeD cerebral cortex due to reduced Dbm mRNA expression and that reduced brain T3 levels may contribute to this phenomenon.
Hairless is a well-characterized TH-responsive gene in the developing rodent brain, coding for a corepressor involved in transcriptional repression of TH-responsive genes (35). Type II deiodinase catalyzes the conversion of T4 to T3 and thus has an important role in maintaining brain TH homeostasis. The elevated Dio2 mRNA expression in the FeD cerebral cortex suggests that this brain region is sensing the altered thyroidal status and is attempting to maintain adequate T3 concentrations. This result may explain the differential effect of Fe deficiency on hippocampal and cerebral cortical T3 concentrations. Together the altered cerebral cortical Hr and Dio2 mRNA expression provides support that TH action is impaired in the developing FeD rodent brain.
Bdnf expression initiates from one of several different promoters resulting in 11 different gene products (36). In accordance with previous work in our laboratory with a different model of nutritional Fe deficiency (37), Bdnf IV and Bdnf VI [formerly Bdnf III and IV, respectively (36)] expression was reduced in the FeD hippocampus. However, in this study, the reduction in Bdnf expression was not statistically significant. In severe models of TH deficiency, Bdnf TH responsivity is regulated in a promoter-, age-, and brain region-specific fashion (reviewed in Ref. 17). In agreement with our mRNA data, Lasley et al. (38) recently demonstrated that fetal/neonatal 1, 2, and 3 ppm PTU treatment does not alter brain-derived neurotrophic factor (Bdnf) protein expression in the neonatal hippocampus, cerebral cortex, or cerebellum.
Cu deficiency lowered P12 and P24 serum TH concentrations in our previous studies (18, 21) but did not significantly alter P10 serum TH concentrations in this study. Although the same CuD diet was used, differences in the severity of Cu deficiency, anemia, and secondary Fe deficiency may explain the lack of significant impact on serum TH concentrations. All P10 CuD pups in this study, compared with no P12 CuD pups in the previous study (21), had measurable levels of ceruloplasmin activity, suggesting a less severe Cu deficiency. Cu deficiency lowered P10 hemoglobin and serum Fe levels by 18 and 46% (Table 2), compared with 23 and 56% in P12 pups from the previous study, suggesting less severe anemia and secondary Fe deficiency. Additionally, rat age (P10 vs. P12) may contribute to the severity of Cu deficiency, secondary Fe deficiency, and the accompanying effects on serum TH concentrations.
Unlike FeD neonatal pups, serum TT3 concentrations were not reduced in 1-ppm PTU pups. It is likely that 1-ppm PTU pups maintain normal serum TT3 concentrations through peripheral T4-to-T3 conversion, suggesting that peripheral T4-to-T3 conversion may be impaired in neonatal FeD rats. Supporting this hypothesis, hepatic T4-to-T3 conversion is impaired in adolescent or adult FeD rodents (10, 11). In addition, Fe deficiency may alter circulating TH concentrations through impaired thyroidal T4 and T3 synthesis as activity of thyroid peroxidase, an Fe-containing enzyme, is reduced in adolescent FeD rats (12).
Interestingly, Fe deficiency did not alter circulating TSH concentrations, despite decreased serum and brain TH concentrations. These data could indicate that fetal/neonatal Fe deficiency is associated with nonthyroidal illness, which is characterized by decreased circulating T3 concentrations and normal or low circulating TSH concentrations. However, our TSH data could also indicate that Fe deficiency directly impairs TRH and/or TSH synthesis or secretion, leading to a secondary or tertiary hypothyroid state. Previous studies show that Fe deficiency in weanling rats reduces plasma TSH concentrations (9, 10) and pituitary TSH content (9). Exogenous TRH administration did not completely normalize plasma TSH concentrations in FeD rats but did induce an increase in plasma TSH concentrations similar to Fe-sufficient controls. These data suggest Fe deficiency decreases hypothalamic TRH production and/or release. Further research is warranted to determine the mechanism(s) by which Fe deficiency reduces circulating and tissue TH concentrations.
In our previous study, the effects of Fe and Cu deficiencies on neonatal thyroidal status were compared with severe TH deficiency (10 ppm PTU), making it difficult to determine the degree of thyroidal impairment. We now have directly compared circulating and brain TH concentrations in FeD neonatal rats to mild (1 ppm PTU) and moderate (3 ppm PTU) thyroidal insults. Fetal/neonatal Fe deficiency reduced P10 serum TT4 concentrations and whole brain and hippocampal T3 content to a similar extent as 1 ppm PTU. Interestingly, Fe deficiency did not significantly alter cerebral cortical T3 content, suggesting a brain region-specific difference in TH metabolism. As discussed above, Dio2 mRNA expression was significantly increased in the FeD cerebral cortex, which may explain the difference in T3 content of these brain regions. Together the TH data indicate that fetal/neonatal Fe deficiency produces a mild TH deficiency in the neonatal circulation and brain.
Demonstrating that the developing FeD brain experiences a TH insufficiency comparable with 1 ppm PTU treatment provides a basis for developing hypotheses about the contribution of reduced TH levels to impairments in the developing FeD brain. The fetal/neonatal 1 ppm PTU treatment model is associated with reduced cerebral cortex and hippocampus Bdnf protein expression in adult offspring (38), aberrant neonatal cerebral cortex and hippocampus gene expression [(25, 39) and current study], reduced neonatal corpus callosum cellular density (40), and impaired long-term potentiation in the dentate gyrus of adult offspring (41). Fe deficiency is also associated with altered neonatal brain gene expression [(21–23) and current study], reduced hippocampus BDNF mRNA and protein expression in adult offspring (42), and impaired hippocampal long-term potentiation in adult offspring (43). Thus, it will be important to directly evaluate the contribution of altered thyroidal status to the brain developmental defects associated with Fe deficiency through TH repletion studies.
Previous work demonstrated that TH regulates brain gene expression in a gene-, age-, and brain region-specific fashion (reviewed in Ref.17). Our data provide further support for a model of TH-regulated brain gene expression in which individual genes have unique, brain region-specific sensitivities to changes in TH levels. Dbm, Rc3, Dpysl3, Egr1, and Dio2 mRNA expression was significantly altered in the cerebral cortex but not the hippocampus of 3-ppm PTU pups, indicating a brain region-specific TH-responsivity for these genes at this developmental stage. In addition, Pvalb, Hr, and Agt mRNA expression was significantly altered in both the 1- and 3-ppm PTU cerebral cortex but only the 3-ppm PTU hippocampus, indicating a brain region-specific difference in TH sensitivity for these TH-responsive genes. Our data correlating mRNA expression levels to serum TT4 concentrations also provide support for brain region-specific differences in TH responsivity. Dio2, Oatp1c1, Rc3, Egr1, Dpysl3, and Dbm mRNA levels were significantly correlated with serum TT4 concentrations in the cerebral cortex but not the hippocampus. Alternatively, the only genes with mRNA levels significantly correlated with serum TT4 concentrations in the hippocampus but not the cerebral cortex were Dio3 and Mct8. These data indicate an increased TH responsivity in the P10 cerebral cortex compared with the hippocampus, in keeping with previous findings (25).
Brain T3 can be acquired from two different sources: transport into the brain from the circulation or local conversion from T4. Morte et al. (44) recently demonstrated that some TH-responsive genes are differentially dependent on the source of T3, requiring locally generated T3 for regulation in the developing mouse cerebral cortex. Interestingly, Dio2 mRNA was increased in the FeD, 1-ppm PTU, and 3-ppm PTU cerebral cortex but not the hippocampus, and mRNA for the T3 transporter, Mct8, was increased in the 3-ppm PTU hippocampus but not cerebral cortex. These data suggest that a difference in the source of available T3 may explain the apparent increase in cerebral cortical TH responsivity compared with the hippocampus. Although the molecular mechanisms controlling the brain region-specific differences in TH responsivity are complex and not fully understood, one possible explanation is differential TH receptor and cofactor expression and/or recruitment to gene promoters. In addition, mRNA levels for the intracellular T3 binding protein, Crym, are strikingly high in the hippocampus compared with the rest of the rodent brain (Supplemental Fig. 3). Differential cytosolic T3 binding could contribute to differences in T3 responsivity of brain subregions by regulating T3 entry into the nucleus and/or altering T3 interaction with TH receptors. In addition, brain region-specific differences in developmental timing including cellular differentiation and formation of cell-cell connections could also contribute to differences in TH responsivity.
A major implication of these data is that even mild perturbations to thyroidal status can aberrantly impact the developing mammalian brain. In humans, thyroidal perturbations could be due to naturally occurring goitrogens (e.g. thiocyanates, isoflavanoids), anthropogenic thyroid disrupting chemicals (e.g. bisphenol A, polychlorinated biphenyl, perfluorinated compounds), or micronutrient deficiencies (e.g. iodine, Fe, selenium, vitamin A, zinc). In developing countries millions of people are deficient in not one, but multiple micronutrients due to eating a diet lacking nutrient diversity (1). In addition, people living in developing countries are often exposed to thyroid disrupting chemicals, such as thiocyanate precursors in cassava, on a daily basis. Individually, dietary goitrogens, environmental chemicals, and micronutrient deficiencies may exert only minor effects on the thyroid axis. However, in combination, these effects may be exacerbated, resulting in a more severe insult to the thyroid axis and more severe developmental impairments. Given their profound impact on child development and prosperity, it is important to understand how micronutrient deficiencies and other thyroid disruptors interact to affect the thyroid axis during fetal and infant development.
Supplementary Material
Acknowledgments
We thank the members of the Anderson and Prohaska laboratories for their invaluable assistance with tissue collection and selected assays. In particular, we thank Margaret Broderius and Kevin Viken. The Duluth Medical Research Institute core facilities were used for quantitative PCR experiments.
This work was supported by Grants 5R03HD055423-02, R01HD039708-08, 2R01HD29421-16, and F31-NS063667 from the National Institutes of Health, the University of Minnesota Brain Barriers Research Center, and a gift from 3M Corp. T.W.B. received financial support from the University of Minnesota Lyle and Sharon Bighley Graduate Fellowship and the University of Minnesota Doctoral Dissertation Fellowship. J.A.A. received financial support from the University of Minnesota Duluth Chemistry Summer Undergraduate Research Program and the University of Minnesota Undergraduate Research Opportunities Program.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- Bdnf
- Brain-derived neurotrophic factor
- CuD
- copper-deficient
- E
- gestational day
- Enpp6
- ectonucleotide pyrophosphatase/phosphodiesterase 6
- FeD
- iron-deficient
- Mbp
- myelin basic protein
- MDC
- minimum detectable concentration
- P
- postnatal day
- PTU
- 6-propyl-2-thiouracil
- qPCR
- quantitative real-time PCR
- TH
- thyroid hormone
- TSH
- thyroid stimulating hormone
- TT4
- total T4
- TT3
- total T3.
References
- 1. Benton D. 2008. Micronutrient status, cognition and behavioral problems in childhood. Eur J Nutr 47(Suppl 3):38–50 [DOI] [PubMed] [Google Scholar]
- 2. de Benoist B, McLean E, Andersson M, Rogers L. 2008. Iodine deficiency in 2007: global progress since 2003. Food Nutr Bull 29:195–202 [DOI] [PubMed] [Google Scholar]
- 3. McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. 2009. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr 12:444–454 [DOI] [PubMed] [Google Scholar]
- 4. Hess SY, Zimmermann MB, Adou P, Torresani T, Hurrell RF. 2002. Treatment of iron deficiency in goitrous children improves the efficacy of iodized salt in Cote d'Ivoire. Am J Clin Nutr 75:743–748 [DOI] [PubMed] [Google Scholar]
- 5. Zimmermann M, Adou P, Torresani T, Zeder C, Hurrell R. 2000. Persistence of goiter despite oral iodine supplementation in goitrous children with iron deficiency anemia in Cote d'Ivoire. Am J Clin Nutr 71:88–93 [DOI] [PubMed] [Google Scholar]
- 6. Zimmermann MB, Zeder C, Chaouki N, Torresani T, Saad A, Hurrell RF. 2002. Addition of microencapsulated iron to iodized salt improves the efficacy of iodine in goitrous, iron-deficient children: a randomized, double-blind, controlled trial. Eur J Endocrinol 147:747–753 [DOI] [PubMed] [Google Scholar]
- 7. Hess SY. 2010. The impact of common micronutrient deficiencies on iodine and thyroid metabolism: the evidence from human studies. Best Pract Res Clin Endocrinol Metab 24:117–132 [DOI] [PubMed] [Google Scholar]
- 8. Zimmermann MB, Burgi H, Hurrell RF. 2007. Iron deficiency predicts poor maternal thyroid status during pregnancy. J Clin Endocrinol Metab 92:3436–3440 [DOI] [PubMed] [Google Scholar]
- 9. Tang F, Wong TM, Loh TT. 1988. Effects of cold exposure or TRH on the serum TSH levels in the iron-deficient rat. Horm Metab Res 20:616–619 [DOI] [PubMed] [Google Scholar]
- 10. Beard J, Tobin B, Green W. 1989. Evidence for thyroid hormone deficiency in iron-deficient anemic rats. J Nutr 119:772–778 [DOI] [PubMed] [Google Scholar]
- 11. Brigham DE, Beard JL. 1995. Effect of thyroid hormone replacement in iron-deficient rats. Am J Physiol 269:R1140–R1147 [DOI] [PubMed] [Google Scholar]
- 12. Hess SY, Zimmermann MB, Arnold M, Langhans W, Hurrell RF. 2002. Iron deficiency anemia reduces thyroid peroxidase activity in rats. J Nutr 132:1951–1955 [DOI] [PubMed] [Google Scholar]
- 13. Prohaska JR, Gybina AA. 2005. Rat brain iron concentration is lower following perinatal copper deficiency. J Neurochem 93:698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Olin KL, Walter RM, Keen CL. 1994. Copper deficiency affects selenoglutathione peroxidase and selenodeiodinase activities and antioxidant defense in weanling rats. Am J Clin Nutr 59:654–658 [DOI] [PubMed] [Google Scholar]
- 15. Johnson WT. 2005. Copper and brain function. In: Lieberman HR, Kanarek RB, Prasad C, eds. Nutritional neuroscience. Boca Raton, FL: Taylor and Francis Group; 289–305 [Google Scholar]
- 16. Fretham SJ, Carlson ES, Georgieff MK. 2011. The role of iron in learning and memory. Adv Nutr 2:112–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson GW, Mariash CN. 2002. Molecular aspects of thyroid hormone-regulated behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, eds. Hormones, brain and behavior. San Diego: Academic Press; 539–566 [Google Scholar]
- 18. Bastian TW, Lassi KC, Anderson GW, Prohaska JR. 2011. Maternal iron supplementation attenuates the impact of perinatal copper deficiency but does not eliminate hypotriiodothyroninemia nor impaired sensorimotor development. J Nutr Biochem 22:1084–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Railey AM, Micheli TL, Wanschura PB, Flinn JM. 2010. Alterations in fear response and spatial memory in pre- and post-natal zinc supplemented rats: remediation by copper. Physiol Behav 100:95–100 [DOI] [PubMed] [Google Scholar]
- 20. Gilbert ME, Sui L. 2006. Dose-dependent reductions in spatial learning and synaptic function in the dentate gyrus of adult rats following developmental thyroid hormone insufficiency. Brain Res 1069:10–22 [DOI] [PubMed] [Google Scholar]
- 21. Bastian TW, Prohaska JR, Georgieff MK, Anderson GW. 2010. Perinatal iron and copper deficiencies alter neonatal rat circulating and brain thyroid hormone concentrations. Endocrinology 151:4055–4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carlson ES, Stead JD, Neal CR, Petryk A, Georgieff MK. 2007. Perinatal iron deficiency results in altered developmental expression of genes mediating energy metabolism and neuronal morphogenesis in hippocampus. Hippocampus 17:679–691 [DOI] [PubMed] [Google Scholar]
- 23. Clardy SL, Wang X, Zhao W, Liu W, Chase GA, Beard JL, True Felt B, Connor JR. 2006. Acute and chronic effects of developmental iron deficiency on mRNA expression patterns in the brain. J Neural Transm Suppl 173–196 [DOI] [PubMed] [Google Scholar]
- 24. Lozoff B, Georgieff MK. 2006. Iron deficiency and brain development. Semin Pediatr Neurol 13:158–165 [DOI] [PubMed] [Google Scholar]
- 25. Royland JE, Parker JS, Gilbert ME. 2008. A genomic analysis of subclinical hypothyroidism in hippocampus and neocortex of the developing rat brain. J Neuroendocrinol 20:1319–1338 [DOI] [PubMed] [Google Scholar]
- 26. Jiang M, Swann JW. 2005. A role for L-type calcium channels in the maturation of parvalbumin-containing hippocampal interneurons. Neuroscience 135:839–850 [DOI] [PubMed] [Google Scholar]
- 27. Caillard O, Moreno H, Schwaller B, Llano I, Celio MR, Marty A. 2000. Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity. Proc Natl Acad Sci USA 97:13372–13377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, Rozov A, Whittington MA, Traub RD, Rawlins JN, Monyer H. 2007. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron 53:591–604 [DOI] [PubMed] [Google Scholar]
- 29. Ibarrola N, Rodríguez-Peña A. 1997. Hypothyroidism coordinately and transiently affects myelin protein gene expression in most rat brain regions during postnatal development. Brain Res 752:285–293 [DOI] [PubMed] [Google Scholar]
- 30. Jahn O, Tenzer S, Werner HB. 2009. Myelin proteomics: molecular anatomy of an insulating sheath. Mol Neurobiol 40:55–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sakagami H, Aoki J, Natori Y, Nishikawa K, Kakehi Y, Natori Y, Arai H. 2005. Biochemical and molecular characterization of a novel choline-specific glycerophosphodiester phosphodiesterase belonging to the nucleotide pyrophosphatase/phosphodiesterase family. J Biol Chem 280:23084–23093 [DOI] [PubMed] [Google Scholar]
- 32. Dugas JC, Tai YC, Speed TP, Ngai J, Barres BA. 2006. Functional genomic analysis of oligodendrocyte differentiation. J Neurosci 26:10967–10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McEchron MD, Goletiani CJ, Alexander DN. 2010. Perinatal nutritional iron deficiency impairs noradrenergic-mediated synaptic efficacy in the CA1 area of rat hippocampus. J Nutr 140:642–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burhans MS, Dailey C, Beard Z, Wiesinger J, Murray-Kolb L, Jones BC, Beard JL. 2005. Iron deficiency: differential effects on monoamine transporters. Nutr Neurosci 8:31–38 [DOI] [PubMed] [Google Scholar]
- 35. Potter GB, Zarach JM, Sisk JM, Thompson CC. 2002. The thyroid hormone-regulated corepressor hairless associates with histone deacetylases in neonatal rat brain. Mol Endocrinol 16:2547–2560 [DOI] [PubMed] [Google Scholar]
- 36. Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. 2007. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res 85:525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tran PV, Carlson ES, Fretham SJ, Georgieff MK. 2008. Early-life iron deficiency anemia alters neurotrophic factor expression and hippocampal neuron differentiation in male rats. J Nutr 138:2495–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lasley SM, Gilbert ME. 2011. Developmental thyroid hormone insufficiency reduces expression of brain-derived neurotrophic factor (BDNF) in adults but not in neonates. Neurotoxicol Teratol 33:464–472 [DOI] [PubMed] [Google Scholar]
- 39. Sharlin DS, Gilbert ME, Taylor MA, Ferguson DC, Zoeller RT. 2010. The nature of the compensatory response to low thyroid hormone in the developing brain. J Neuroendocrinol 22:153–165 [DOI] [PubMed] [Google Scholar]
- 40. Sharlin DS, Tighe D, Gilbert ME, Zoeller RT. 2008. The balance between oligodendrocyte and astrocyte production in major white matter tracts is linearly related to serum total thyroxine. Endocrinology 149:2527–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gilbert ME. 2011. Impact of low-level thyroid hormone disruption induced by propylthiouracil on brain development and function. Toxicol Sci 124:432–445 [DOI] [PubMed] [Google Scholar]
- 42. Tran PV, Fretham SJ, Carlson ES, Georgieff MK. 2009. Long-term reduction of hippocampal brain-derived neurotrophic factor activity after fetal-neonatal iron deficiency in adult rats. Pediatr Res 65:493–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jorgenson LA, Sun M, O'Connor M, Georgieff MK. 2005. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus 15:1094–1102 [DOI] [PubMed] [Google Scholar]
- 44. Morte B, Ceballos A, Diez D, Grijota-Martínez C, Dumitrescu AM, Di Cosmo C, Galton VA, Refetoff S, Bernal J. 2010. Thyroid hormone-regulated mouse cerebral cortex genes are differentially dependent on the source of the hormone: a study in monocarboxylate transporter-8- and deiodinase-2-deficient mice. Endocrinology 151:2381–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.