Abstract
The mechanisms of estrogen receptor (ER)-α activity can be categorized into those involving direct (classical) or indirect (nonclassical) DNA binding. Although various mouse models have demonstrated the importance of ERα in bone, the specific gene expression patterns affected by these modes of ERα action are unknown. In this report, the gene expression patterns of ERα-deficient (ERKO) mice and nonclassical ER knock-in (NERKI) mice, which can function only by nonclassical means, were analyzed. Three-month-old mice were ovariectomized and implanted with estrogen pellets for 1 month to normalize estrogen levels. Microarray analysis of flushed cortical bone revealed 28% (210 of 763) of the genes differentially expressed in ERKO mice were altered in NERKI mice, suggesting estrogen response element-dependent regulation of these genes in bone. Pathway analysis revealed alterations in genes involved in focal adhesion and extracellular matrix interactions. However, the majority of genes regulated in ERKO mice (72%) were unique (i.e. not altered in NERKI mice), suggesting these are regulated by nonclassical mechanisms. To further explore the pathways affected in ERKO mice, we performed focused quantitative PCR arrays for genes involved in various aspects of bone physiology. Genes involved in bone formation, senescence, apoptosis, and autophagy were significantly regulated. Overall, the majority of the genes regulated by ERα in bone are via nonclassical pathways. However, because NERKI mice display an osteoporotic phenotype, it can be deduced that the minority of the estrogen response element-dependent genes/pathways play critical roles in the regulation of bone physiology. These data demonstrate the importance of classical ERα signaling in regulating bone metabolism.
Bone homeostasis relies on the coordinated actions of osteoblasts, osteocytes, and osteoclasts to regulate the processes of bone formation and turnover. Alterations in these actions, such as observed in estrogen deficiency, alter the relative activities of these cell types, leading to chronic diseases such as osteoporosis (1). The effects of estrogen in various tissues, including bone, are mediated by two related receptors, estrogen receptor (ER)-α and ERβ (2). ERs can modulate gene transcription using a number of signaling pathways. The classical pathway involves direct DNA binding of the ERs to estrogen response elements in the control regions of estrogen target genes. ERs also regulate gene expression indirectly via the nonclassical pathway, which does not involve direct DNA binding, but rather are due to specific protein-protein interactions.
Investigation into the functions of ERs in bone has largely involved the use of specific mouse models in which the ER has been deleted or mutated to address the mechanisms of ER action in bone. For example, the nonclassical ERα knock-in (NERKI) mouse model eliminates classical signaling allowing for investigation of nonclassical ERα signaling alone (3). NERKI mice display an osteoporotic phenotype, demonstrating that loss of classical signaling results in an imbalance between these ERα-mediated modes of action (4–6). Complete loss of ERα expression (ERKO) leads to increased trabecular bone volume and decreased cortical thickness (7). However, the interpretation of the studies may have been hampered by the abnormally high serum sex steroid levels present in these models, which may lead to secondary effects not inherent to bone cells (6, 7). Therefore, identifying the specific gene expression patterns and cellular pathways regulated by ERα in the presence of normal sex steroid levels is essential for the proper interpretation of ERα action in bone.
In this study, we normalized estrogen levels in the ERKO and NERKI mouse models through ovariectomy and estrogen replacement to identify the gene expression patterns regulated by ERα in the presence of normal serum estrogen concentrations. We demonstrate that either complete loss of ERα signaling (ERKO) or disruption of the classical pathway (NERKI) elicit significant alterations of gene expression patterns in bone. The data presented herein describe and define novel signaling pathways regulated by ERα in bones cells in the absence of high estrogen levels and lay the framework for future investigation in modulation of these pathways as potential therapeutic treatments for bone diseases such as osteoporosis.
Materials and Methods
Experimental animals and treatments
Three-month-old C57/BL6 female wild-type (WT; ERα+/+), ERα-deficient (ERα−/−) (8) or NERKI (ERα−/NERKI) mice, which harbors a mutation in the ERα DNA-binding domain that abolishes direct DNA binding (9), were used in this study. All mice were ovariectomized (n = 13 per group) and implanted with a 60-d, slow-release 17β-estradiol pellet (0.015 mg 17β-estradiol per pellet; Innovative Research of America, Sarasota, FL), which achieves a dosage of approximately 10 μg/kg·d (10), to normalize estradiol levels between the groups. Four weeks later, the femurs and tibias were excised, metaphyses discarded and the marrow flushed of its cellular contents. The resulting bone, comprised of the midshaft containing mostly cortical bone, were immediately homogenized in QIAzol (QIAGEN, Valencia, CA) using a Tissue Tearor (BioSpec Products, Inc., Bartlesville, OK) and frozen at −80 C before RNA preparation. All animal studies were conducted in accordance with the principles and procedures outlined in the National Institute of Health Care and Use of Animals under Protocol No. A38108.
Serum 17β-estradiol measurements
Serum 17β-estradiol was measured using a RIA (ultrasensitive estradiol RIA DSL 4800; Beckman Coulter, Brea, CA). The interassay coefficient of variation was 12.2% or less.
RNA preparation and cDNA synthesis
Total RNA was prepared from the flushed cortical bone using RNeasy minicolumns (QIAGEN) as previously described (11), which included an on-column deoxyribonuclease treatment to degrade potential contaminating genomic DNA. We used the WT-Ovation Pico RNA whole transcriptome amplification system (NuGen Technologies Inc., San Carlos, CA) to synthesize large quantities of amplified cDNA (∼6–8 μg), starting with a total RNA input of approximately 50 ng, as previously described (11).
Microarray and gene pathway analysis
Isolated RNA (300 ng) from the flushed cortical bone of WT, ERKO, and NERKI mice (n = 8) was submitted for microarray analysis using the MouseWG-6 version 2.0 Expression BeadChips (Illumina, San Diego, CA). These chips contain 50-mer oligonucleotide probes that profile 45,281 mouse transcripts annotated from the National Center for Biotechnology Information (build 36, release 22; Bethesda, MD), the mouse exonic evidence-based oligonucliotide set and the exemplar protein-coding sequences described in the RIKEN FANTOM2 databases (Saitama, Japan). The preparation of the samples and microarray hybridizations was performed by the Advanced Genomic Technology Center Microarray Shared Resource at the Mayo Clinic as previously described (12). The data were filtered based on a detection P value (P ≤ 0.05, called detected), in which the probe sets not detected in all samples were removed. After this noise filtering, 19,162 probe sets remained. ANOVA statistical modeling was used to categorize differentially expressed genes between of WT, ERKO, and NERKI mice. All genes regulated at P ≤ 0.05, false discovery rate (q) 0.1 or less and fold change less than or equal to or 1.5 or greater were considered statistically significant. For the pathway analysis, probe sets with unknown annotations and therefore unknown identities were excluded from the analysis. Probe sets with known identities were subsequently used for gene ontology analysis using Database for Annotation, Visualization, and Integrated Discovery (DAVID) Bioinformatics Resources, version 6.7 (13, 14). Pathways regulated as a whole (P < 0.05) were considered significant.
Quantitative real-time PCR analysis (QPCR)
To perform pathway-focused QPCR, an expanded set of NuGen-amplified samples from the flushed cortical bone of WT and ERKO mice (n = 13) was used. The QPCR reactions were run in the ABI Prism 7900HT real-time system (Applied Biosystems, Carlsbad, CA) using SYBR Green (QIAGEN) and 5 ng amplified cDNA per reaction well. All primers were designed using Primer Express software, version 3.0 (Applied Biosystems), and the sequences are available upon request. The method for data normalization using multiple reference genes and threshold calculations is as previously described (11).
Statistical analysis
Calculations and statistical analyses were performed using Microsoft Office Excel 2003 (Microsoft Corp., Redmond, WA). The data are presented as the mean ± se. All values of P ≤ 0.05 were considered statistically significant using Student's t test. The QPCR pathway analysis was performed using the Hotelling's T-squared distribution, a multivariate method that ascertains whether multiple means in different groups are equivalent. The Hotelling's T-squared distribution was performed using the Stata Data Analysis and Statistical Software (StataCorp LP, College Station, TX).
Results
Sample preparation, microarray, and pathway analyses
Previous examination of the bone phenotypes of both ERKO (ERα−/−) and the NERKI (ERα−/NERKI), and their associated gene expression patterns, have potentially been confounded by a significant increase in circulating estradiol levels (7, 15). To properly define specific gene expression patterns in the presence of normal systemic estradiol concentrations, we ovariectomized WT, ERKO, and NERKI female mice at 3 months and immediately implanted a slow-release estradiol pellet for an additional month. This serves to normalize estradiol levels among the genotypes and allow for a proper comparison of gene expression patterns in the absence of these abnormally high estradiol levels. Of note, we have previously demonstrated that the dose of estradiol used in this study results in physiological estrogen levels and preservation of bone mass after ovariectomy in C57/BL6 mice (10). In addition, as shown in Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org, serum estradiol levels were now similar in the WT, ERKO, and NERKI ovariectomized and estradiol-replaced mice compared with WT mice.
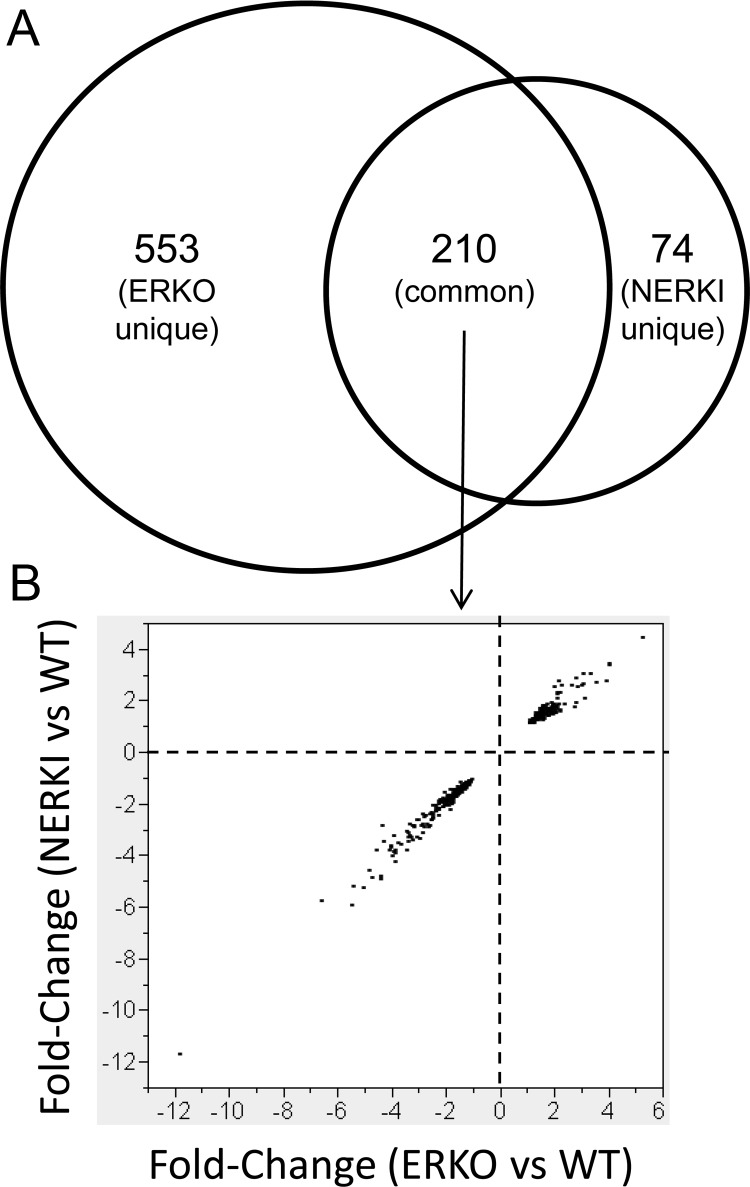
After ovariectomy and estradiol replacement, RNA was prepared from the flushed femurs, comprised of mainly cortical bone, and global gene expression patterns were determined using Illumina microarray technology. The analysis revealed 763 differentially expressed genes in ERKO mice compared with WT, of which 331 (43%) were up-regulated and 432 (57%) were down-regulated (Table 1). Similarly, 284 differentially expressed genes were identified in NERKI mice in which 117 (41%) were up-regulated and 167 (59%) were down-regulated (Table 1). Comparison of the data sets (Fig. 1A) revealed that 28% (210 of 763) of the genes differentially expressed in ERKO mice were also altered in NERKI mice, suggesting estrogen response element (ERE)-dependent regulation of these genes in bone. These 210 ERE-dependent genes in common in the ERKO and NERKI data sets constituted 74% (210 of 284) of the NERKI data set, with a relatively small number of genes [74, ∼10% of the number regulated by the ERKO data set, including the genes common to the ERKO and NERKI data sets (74 of 763)] that were uniquely altered in the NERKI mice (i.e. unaffected in the ERKO mice), suggesting that these genes might be involved in pathways unique to the NERKI receptor (and distinct from the actions of the WT ERα) (Fig. 1A). However, 72% (553 of 763) of the genes regulated in the ERKO data set were not altered in the NERKI mice, thereby not affected by loss of ERE binding and could be considered regulated by nonclassical mechanisms (e.g. protein-protein or membrane pathways). The 210 genes regulated in both genotypes were plotted to determine directionality of the gene expression change, and of interest, all genes were regulated in the same direction (Fig. 1B). The complete list of genes and regulation patterns can be found in Supplemental Tables 1–3.
Table 1.
Microarray gene expression changes in ERKO and NERKI cortical bone, compared with WT mice
| ERKO | NERKI | |
|---|---|---|
| Up-regulated | 331 (43%) | 117 (41%) |
| Down-regulated | 432 (57%) | 167 (59%) |
| Totals | 763 | 284 |
Fig. 1.
Comparison of gene expression patterns between ERKO and NERKI flushed bone samples using microarray analysis. A, The Venn diagram represents the intersection the genes regulated in either ERKO or NERKI bone samples, categorized as ERKO unique, NERKI unique, or common, the latter of which represents genes commonly regulated in the ERKO and NERKI data sets. B, The fold changes of the commonly regulated data set in both ERKO and NERKI samples (both relative to WT) were plotted on the x- and y-axes, respectively. Each dot on the graph represents a gene from the commonly regulated list.
To place the microarray results in the proper cellular context, we performed gene ontology and biological pathway analysis using the DAVID functional annotation tool (13, 14). To increase the power of our analysis, only the annotated genes in the ERKO- and NERKI-regulated data sets were used to compare the groups of genes and pathways regulated compared with WT. This analysis revealed that genes involved in focal adhesion and extracellular matrix-receptor interactions were in common between the ERKO and NERKI data sets (Table 2). Within each of these pathways, nearly identical genes were regulated with similar fold changes and directionality, suggesting that loss of the receptor (as in ERKO) and loss of ERE binding (as in NERKI) yield similar changes in overall cellular pathway regulation. Interestingly, alterations in genes involved in cell cycle regulation were identified only in the ERKO data set (Table 2).
Table 2.
Use of DAVID online annotation software to categorize microarray-regulated genes in into KEGG pathways
| KEGG pathways | Regulated genesa | ERKO vs. WT fold change | NERKI vs. WT fold change |
|---|---|---|---|
| Focal adhesion | Calpain2 (Capn2) | −1.94 | −1.68 |
| Collagen, type II, α1 (Col2a1) | −3.96 | −4.02 | |
| Fibronectin 1 (Fn1) | −2.49 | −2.38 | |
| Protein phosphatase 1, catalytic subunit, β-isoform (Ppp1cb) | 2.83 | 2.39 | |
| Protein phosphatase 1, regulatory subunit 12A (Ppp1r12a) | 1.51 | 1.52 | |
| Integrin binding sialoprotein (Ibsp) | −3.33 | −2.79 | |
| Thrombospondin 1 (Thbs1) | −1.74 | −1.69 | |
| Catenin (cadherin associated protein), β1 (Ctnnb1) | 1.57 | NR | |
| Collagen, type I, α2 (Col1a2) | 1.61 | NR | |
| Myosin, light polypeptide 9, regulatory (Myl9) | −1.76 | NR | |
| ECM receptor interaction | Collagen, type II, α1 (Col2a1) | −3.96 | −4.02 |
| Fibronectin 1 (Fn1) | −2.49 | −2.38 | |
| Integrin binding sialoprotein (Ibsp) | −3.33 | −2.79 | |
| Syndecan 3 (Sdc3) | −1.52 | −1.54 | |
| Thrombospondin 1 (Thbs1) | −1.74 | −1.69 | |
| CD47 antigen (Cd47) | 1.51 | NR | |
| Collagen, type I, α 2 (Col1a2) | 1.61 | NR | |
| Cell cycle | E2F transcription factor 4 (E2f4) | 1.87 | NR |
| RAD21 homolog (Rad21) | 1.77 | NR | |
| Cyclin A2 (Ccna2) | 1.72 | NR | |
| Cyclin E2 (Ccne2) | −1.65 | NR | |
| Cyclin-dependent kinase inhibitor 1C P57 (Cdkn1c) | 2.05 | NR | |
| Inichromosome maintenance deficient 3 (Mcm3) | −1.91 | NR | |
| Cyclin-dependent kinase 4 (Cdk4) | −1.81 | NR |
ECM, Extracellular matrix; NR, not regulated.
Fold change of the given gene compared with WT.
Due to the large overlap between the ERKO and NERKI data sets noted above and because identical pathways were identified between the NERKI vs. ERKO data sets, we chose to focus our subsequent investigation solely on the ERKO data set. Thirty randomly chosen genes from the ERKO data set were analyzed by QPCR in an expanded set of samples (n = 13) to validate the microarray data and regulation of 20 of 30 genes (67%) were confirmed (Table 3). This lower level of independent gene expression confirmation was not unexpected because these samples originate from a primary bone tissue model, which includes confounders between samples such as heterogeneity of the cells harvested and variability between mice.
Table 3.
QPCR confirmation of select, microarray-identified genes in ERKO cortical bone vs. WT control
| Gene symbols | Microarray fold changea | QPCR fold changea | QPCR P value | Validatesb |
|---|---|---|---|---|
| Ear6 | 5.23 | 2.61 | 0.0106 | Yes |
| Epx | 4.00 | 1.51 | 0.0106 | Yes |
| Vpreb3 | 3.91 | 2.88 | 0.0000 | Yes |
| Bach2 | 3.54 | 2.39 | 0.0021 | Yes |
| Prg3 | 3.46 | 2.14 | 0.0014 | Yes |
| Nr2f1 | 3.33 | 0.10 | 0.6235 | |
| Igl-5 | 3.14 | 5.19 | 0.0000 | Yes |
| Myl4 | 3.06 | 1.72 | 0.0019 | Yes |
| Rag1 | 2.77 | 2.77 | 0.0004 | Yes |
| Cd3d | 2.36 | 1.27 | 0.3722 | |
| Lrrc15 | 2.30 | 0.58 | 0.7136 | |
| Jakmip1 | 2.21 | 3.56 | 0.0001 | Yes |
| Sox9 | 2.15 | 1.40 | 0.1776 | |
| Sox4 | 2.14 | 0.70 | 0.2484 | |
| Ccdc3 | 2.17 | 1.59 | 0.0362 | Yes |
| Phex | −2.17 | −1.46 | 0.0919 | |
| Socs2 | −2.25 | −3.07 | 0.0000 | Yes |
| Fn1 | −2.49 | −2.18 | 0.1967 | Yes |
| Gpx3 | −2.49 | −3.51 | 0.0000 | Yes |
| Cyp2e1 | −2.55 | −5.09 | 0.0038 | Yes |
| Hnrph1 | −2.55 | −0.95 | 0.6159 | |
| BSP | −3.33 | −7.10 | 0.0000 | Yes |
| Tmem86a | −3.43 | −3.83 | 0.0848 | |
| Tsc22d1 | −3.85 | −1.86 | 0.0099 | Yes |
| Col8a1 | −3.93 | −7.52 | 0.0000 | Yes |
| Col2a1 | −3.96 | −3.52 | 0.0005 | Yes |
| Fcrls | −4.31 | −9.32 | 0.3506 | |
| Wisp2 | −4.36 | −2.55 | 0.8557 | |
| Panx3 | −5.48 | −5.66 | 0.0000 | Yes |
| Car3 | −5.52 | −2.29 | 0.0094 | Yes |
Ratio of expression in ERKO to WT cortical bone samples; minus sign indicates down-regulation.
Validation of a microarray-identified regulated gene.
QPCR pathway analysis
The microarray analysis provided important information on the global gene expression patterns in ERKO mice compared with WT; however, we sought to examine key pathways with known roles in bone physiology in greater detail using the more sensitive QPCR technique using custom QPCR arrays built in-house. Table 4 lists the genes evaluated for the indicated pathways. We used the Hotelling's T-squared distribution to evaluate whether the genes measured in each pathway changed as a group, therefore suggesting alteration in the activity of the pathway. This analysis, as shown in Table 4, revealed that genes involved in various cell processes (i.e. adhesion, adipogenesis, apoptosis, autophagy, osteoblast differentiation, osteocyte differentiation, proliferation, senescence) as well as in specific cell pathways (i.e. bone morphogenetic protein/TGFβ regulation, hedgehog, nuclear factor-κB (NF-κB), Notch, Wnt) were significantly regulated as a group using the Hotelling's statistical test.
Table 4.
Genes examined in each of the pathways analyzed with the results of the Hotelling's test, measuring changes in all the genes within each pathway as a group
| Pathway | Genes contained within each pathway | Hotelling's P value |
|---|---|---|
| Adhesion moleculesa | Alcam, Icam1, N-cadherin, Vcam1 | 0.0009 |
| Adipogenesisa | Adipogenin, Adiponectin, Adipsin, Fabp4, Cebpα, Cebpβ, Cebpγ, Leptin, Lpl, Perilipin, Pparγ2 | 0.0001 |
| Apoptosisa | Fas, Fasl, Bad, Bax, Bcl2, Bcl-xL, caspase-3, caspase-8 | 0.0068 |
| Autophagy markersa | Atg3, Atg4a, Atg4b, Atg4c, Atg4d, Atg5, Atg7, Atg8, Atg9, Atg10, Atg12, Atg16L1, Atg16L2 | 0.0293 |
| Bmp/Tgfβ-regulated markersa | Areb6, Hes1, Id1, Id2, Smad6, Smad7, Sox4, Tieg | 0.036 |
| Circadian rhythm | Bmal1, Clock, Cry1, Cry2, Csnk1e, Per1, Per2, Per3 | 0.1753 |
| Cytokine markers | Csf1, Ifnγ, Il1ra, Il1β, Tnfα | 0.161 |
| Hedgehog markersa | Gli1, Gli2, Gli3, Ihh, Ptch1, Ptch2, Rab23, Smo | 0.0195 |
| NF-κB targetsa | Ccl2, Ccl5, Cfb, Csf1, Csf2, Mcsf, Cxcl5, Fas, Fasl, Icam1, Ifnγ, Trail, Vcam1, Vegfc | 0.0486 |
| Notch markersa | Hes1, Hey1, Hey2, Heyl, Hoxb4, Jag1, Jag2, NFκB1, NFκB2, Notch1, Notch2, Pax5 | 0.0003 |
| Osteoblast differentiationa | AP, osteocalcin, osteonectin, osteopontin, bone sialoprotein, Col1α1, Col1α2, Runx2, osterix | 0.0015 |
| Osteocyte differentiationa | Dmp1, E11/gp38, Fgf23, Mepe, Phex, Sost | 0.0057 |
| Oxidative stress | Catalase, Foxm1, Gsr, Nos2, Oxsr1, Sod1, Sod2, Sod3 | 0.079 |
| Proliferationa | Cyclin A1, CyclinB1, CyclinB2, CyclinC, CyclinD1, CyclinD2, CyclinE1, CDK2, CDK6, E2F1, Ki67 | 0.0146 |
| Senescencea | Foxo3a, Igfbp2, p15/Cdkn2b, p16/Cdkn2a, p18/Cdkn2c, p19/Cdkn2d, p21/Cdkn1a, p27/Cdkn1b, p57/Cdkn1c, p53, Rb, Pten, Sirt1 | 0.007 |
| Wnt-regulated markersa | Lef1, Tcf7, Axin2, EphrinB4, connexin 43, survivin, versican, Cyr61, Bmp4, Fra1, Id2, Igf1, Igf2, Jag1 | 0.0224 |
These pathways represent Hotelling's P ≤ 0.05 and therefore statistically significant.
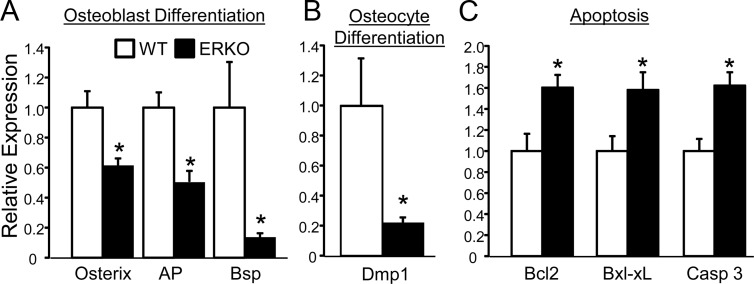
Figure 2 shows the individually significant genes within pathways of high interest (i.e. osteoblast differentiation, osteocyte differentiation, apoptosis). Specifically, the osteoblast differentiation markers osterix, alkaline phosphatase (AP), and bone sialoprotein (Bsp) were down-regulated in ERKO bones (Fig. 2A). The classic osteocytic marker dentin matrix protein 1 was also suppressed in ERKO (Fig. 2B). These data suggest that loss of ERα signaling in the presence of normal circulating estradiol levels leads to suppression of osteoblastic and osteocytic differentiation. Interestingly, genes involved in various aspects of apoptosis were also significantly up-regulated (Fig. 2C). Table 5 lists the individually significant genes within the other pathways with significant Hotelling's P values. Autophagy protein (Atg)-4b, Atg4c, Atg4d, and Atg5 were significantly up-regulated in ERKO bones, suggesting that estrogen deficiency may lead to increased autophagy. Interestingly, the inflammatory marker NF-κB1 was up-regulated in ERKO bones, as has been previously described in the setting of estrogen deficiency (16). Other genes of interest that exhibit enhanced expression in ERKO bones was Pax5, a regulator of B-cell lymphopoiesis (17, 18), and genes involved in cellular senescence.
Fig. 2.
QPCR analysis of individually significant genes (using Student's t test) within statistically significant pathways (using Hotelling's T squared distribution) for genes in the osteoblast differentiation (A), osteocyte differentiation (B), and apoptosis pathways (C) are graphed. The bars represent the fold change between ERKO and WT ± se and statistically significant differences of P ≤ 0.05 (Student's t test) are indicated with an asterisk.
Table 5.
Individually regulated genes within the statistically significant pathways in ERKO compared with WT mice
| Pathway/gene | WT | ERKO | P value |
|---|---|---|---|
| Adhesion molecules | |||
| Vcam1 | 1.00 ± 0.10 | 1.56 ± 0.13 | 0.002 |
| Adipogenesis | |||
| Adipogenin | 1.00 ± 0.22 | 0.21 ± 0.06 | 0.0018 |
| Cebpβ | 1.00 ± 0.13 | 0.56 ± 0.13 | 0.0048 |
| Autophagy markers | |||
| Atg3 | 1.00 ± 0.07 | 0.76 ± 0.054 | 0.012 |
| Atg4b | 1.00 ± 0.16 | 1.46 ± 0.16 | 0.049 |
| Atg4c | 1.00 ± 0.10 | 1.41 ± 0.13 | 0.022 |
| Atg4d | 1.00 ± 0.16 | 1.57 ± 0.11 | 0.0077 |
| Atg5 | 1.00 ± 0.18 | 1.89 ± 0.14 | 0.0006 |
| Atg8 | 1.00 ± 0.07 | 0.75 ± 0.07 | 0.020 |
| NF-κB targets | |||
| Ccl2 | 1.00 ± 0.17 | 0.51 ± 0.09 | 0.019 |
| Csf1 | 1.00 ± 0.19 | 0.42 ± 0.04 | 0.006 |
| Vcam1 | 1.00 ± 0.10 | 1.56 ± 0.13 | 0.002 |
| NFκB1 | 1.00 ± 0.12 | 1.78 ± 0.14 | 0.0003 |
| Notch markers | |||
| Hoxb4 | 1.00 ± 0.19 | 0.51 ± 0.04 | 0.022 |
| NFκB1 | 1.00 ± 0.12 | 1.78 ± 0.14 | 0.0003 |
| Pax5 | 1.00 ± 0.16 | 2.89 ± 0.22 | 3.2 E-7 |
| Proliferation | |||
| CyclinD1 | 1.00 ± 0.15 | 0.34 ± 0.03 | 0.0002 |
| CyclinD2 | 1.00 ± 0.15 | 0.62 ± 0.05 | 0.02 |
| Cdk2 | 1.00 ± 0.12 | 1.49 ± 0.08 | 0.002 |
| Cdk6 | 1.00 ± 0.12 | 1.36 ± 0.12 | 0.048 |
| Senescence | |||
| p16/Cdkn2a | 1.00 ± 0.58 | 0.09 ± 0.03 | 0.005 |
| p19/Cdkn2d | 1.00 ± 0.31 | 2.13 ± 0.43 | 0.03 |
| p21/Cdkn1a | 1.00 ± 0.10 | 0.73 ± 0.08 | 0.049 |
| Rb | 1.00 ± 0.05 | 1.87 ± 0.21 | 0.0006 |
| Pten | 1.00 ± 0.04 | 1.41 ± 0.11 | 0.002 |
| Sirt1 | 1.00 ± 0.07 | 1.59 ± 0.13 | 0.0005 |
| Wnt-regulated markers | |||
| Survivin | 1.00 ± 0.05 | 1.24 ± 0.06 | 0.009 |
| Cyr61 | 1.00 ± 0.23 | 0.40 ± 0.05 | 0.017 |
Data are presented as mean ± se and P values.
Discussion
It is well established that estrogens regulate numerous aspects of bone homeostasis and that estrogen deficiency is associated with increased bone turnover, leading to bone loss. This is evident in women after menopause in which declining estrogen levels are the major determinant of postmenopausal osteoporosis (19). Similarly in rodents, loss of circulating estrogens after ovariectomy leads to significant bone loss. However, estrogen deficiency caused by loss-of-function mutations in ERα can also have adverse skeletal effects, as in the case of a man containing a truncated, and therefore nonfunctional, ERα who had reduced bone mass (20). The examination of the bone phenotype of global ERα mutants in mice has been hampered by high systemic estrogen levels, as is in the case of ERα-null mutants, which exhibit nearly 10-fold increased circulating estradiol levels (7). Therefore, examination of the gene expression profile from ERα-null mice has been problematic. In this study we examined the gene expression profile in bone of ERα-null mice (ERKO) in which no ERα variants are expressed (8). Systemic estradiol levels were normalized through ovariectomy and replacement with a physiological dose of estradiol, allowing for the first time direct examination of alterations in gene expression in the absence of high circulating estrogens. We also examined the gene expression profile of bone in mice harboring a mutation in ERα that cannot directly binding DNA (NERKI) and therefore can signal only through nonclassical mechanisms. These mice also exhibit altered sex steroid levels (6); thus, estrogen levels were also normalized in this model. Overall, we found significant alterations in gene expression profiles in both ERKO and NERKI mice, further delineating the importance of classical vs. nonclassical signaling in bone.
Expression of NERKI leads to an imbalance between classical and non-classical ERα pathways. For example, loss of ERE binding in NERKI-expressing cells reduces expression of genes in the classical pathway, whereas nonclassically regulated genes (i.e. AP1 dependent) may be unchanged or possibly even overexpressed. We found that a majority of genes affected in NERKI bones are also affected in ERKO bones, most likely due to the disruption of binding to EREs, which are contained in the promoters and/or enhancers of the gene in question (a primary effect). Another possibility is a secondary effect attributed to the loss of expression of specific transcription factors (which are ERE dependent) that would affect downstream genes either negatively or positively, depending on whether that factor is an activator or repressor of gene expression. Interestingly, unique (compared with ERKO) alterations in the expression of only 74 genes were observed in the NERKI dataset, suggesting at least limited independent functions of the NERKI receptor. Indeed, Jakacka et al. (3) suggested such a possibility because they found a more severe reproductive phenotype in NERKI heterozygotes (ERα+/NERKI) in comparison with ERα−/+ mice and proposed that the NERKI receptor alters the relative balance between classical and nonclassical ERα signaling. Our study used the ERα−/NERKI mouse, which does not contain a WT allele of ERα, so the effect described herein is solely due to NERKI. It is possible that NERKI causes aberrant expression of genes in the nonclassical pathway, which then adversely impact bone metabolism, although examination of the genes uniquely regulated in the NERKI mice did not provide any obvious candidate pathways mediating this effect (Supplemental Table 2).
One previous study used a model similar to our NERKI (ERα−/NERKI) model to investigate classical vs. nonclassical ERα-dependent gene expression patterns in the uterus (21). Their model, termed KIKO, carried the identical mutation as in our mice. They found altered responses in the uterus after ovariectomy and estrogen treatment, and similar to our findings they reported profound gene expression differences among the WT, ERKO, and KIKO genotypes. An important note is that the ERα-deficient allele used in that study (22) was subsequently found to express an ERα splice variant (23), therefore not representing a true null-allele. Our study uses an ERα-null allele through deletion of exon 3 that was shown to not express any ERα variants (8), and therefore, our data represent the influence of NERKI alone.
It is important to place our findings in the context of the skeletal phenotype of the NERKI (ERα−/NERKI) mice we have previously reported (4–6). In these studies compared with WT mice, the NERKI mice do have reduced bone mass in both females and males. The skeletal changes in the NERKI mice could be due to several reasons, including: 1) the loss of the regulation of genes governed by ERE signaling or 2) the modulation of novel genes not normally regulated by the WT ERα. Of the genes differentially regulated in the NERKI data set (284), 210 (74%) were also differentially regulated in the ERKO data set (consistent with these being ERE regulated genes); only 74 (26%) were genes that were unique to the NERKI data set. In this context, the skeletal phenotype we previously described for the NERKI mice (4–6) could result from either of these alterations in gene expression, although quantitatively, loss of the ERE-regulated genes appears to be more important.
The classic bone marker genes osterix, alkaline phosphatase, and bone sialoprotein were significantly down-regulated in the ERα-deficient ERKO model. These findings suggest that in vivo, ERα signaling is important for osteoblast differentiation, in contrast to the relatively conflicting data on estrogen regulation of osteoblast differentiation in in vitro models (1).
It is of interest that we observed increases in select genes involved in cellular senescence, including p19 (Cdkn2d), Rb, Pten, and Sirt1 (Table 5). Several lines of evidence demonstrate the relationship between ER status, estrogen levels, and cellular senescence. For example, Tuttle et al. (24) found that the senescence-associated gene, YPEL3, was induced by the removal of estrogen from MCF-7 cells, and this triggered cellular senescence. However, it should be noted that we also observed the down-regulation of several senescence-associated genes, such as p16 (Cdkn2a) and p21 (Cdkn1a). Therefore, it remains unclear how ERα deficiency contributes to senescence in bone and provides the impetus for future investigation.
Our experimental evidence demonstrates a number of genes involved in autophagy are increased in ERKO bones (Table 5). Autophagy is a cell survival mechanism occurring in response to cell stress, such as nutrient deprivation or hypoxia, in which damaged organelles and misfolded proteins are degraded in newly assembled autophagosomes to maintain cellular homeostasis (25). Autophagosome formation is catalyzed by a family of Atg proteins that are often up-regulated after cellular insults (26). Atg4 is an important regulator of this process that is critical for autophagic processes. Interestingly, we demonstrate that Atg4b, Atg4c, and Atg4d are up-regulated in ERα-deficient bones, suggesting the induction of autophagy and that ERα deficiency may represent a form of cell stress. We also observed the up-regulation of Atg5, another protein involved in the assembly and function of the autophagosome, which has been reported to be involved in the induction of apoptosis. Yousefi et al. (27) demonstrated that under certain cellular conditions, a truncated form of Atg5 can enter mitochondria in which it associates with Bcl-xL, an inhibitor of apoptosis, and inhibits its function leading to cytochrome c release, activation of caspase-3, and apoptosis.
We observed increased expression of both Bcl-xL and caspase-3 in ERKO bones, suggesting a link between ERα deficiency, autophagy, and apoptosis. The induction of Bcl-xL may represent a feedback mechanism by the cell to blunt the apoptotic signal triggered by Atg5. However, elevation of caspase-3 suggests that apoptotic processes are occurring after ERα deficiency. Indeed, previous reports have demonstrated that estrogen deficiency leads to osteoblastic apoptosis in bone (16). Further studies are needed to understand the connection between ERα deficiency and the induction of autophagy and/or apoptosis and whether increased autophagosome formation occurs in ERα-deficient bones.
We observed an increase in the NFκB1 subunit (Table 5), a member of the NFκB dimer which is an important mediator of inflammatory processes. It is well accepted that estrogen deficiency leads to increases in proinflammatory cytokines, increased bone resorption and bone loss (28, 29). Harada and colleagues found that nobiletin, a polymethoxy flavonoid that suppresses inflammation, restored bone bass following ovariectomy in mice, partially through the inhibition of NF-κB signaling (30). Furthermore, inhibition of inhibitor of κB kinase in osteoblasts leads to increase bone formation and preservation of bone after ovariectomy (31). Therefore, our data are consistent with these reports and suggest the loss of ERα leads to increases in inflammation in bone.
We observed a significant increase in paired box protein 5 (Pax5) expression (Table 5), a transcription factor involved in B-cell lymphopoiesis (17, 18), in ERKO mice, suggesting a relationship between ERα status and the hematopoietic niche in which osteoblasts reside. Pax5−/− mice are devoid of mature B cells (32) and are also severely osteopenic, with a greater than 60% reduction in bone mass due to increases in osteoclastogenesis (33). Interestingly, osteoblastic precursors from the bone marrow were 10-fold higher in Pax5-deficient mice, demonstrating Pax5 deficiency leads to increases in osteoblastic precursors in a possibly compensatory mechanism in response to increases in osteoclasts (33). We observed decreased bone marker gene expression with increased Pax5 expression. Kanematsu and Masuzawa (34, 35) demonstrated that estrogen deficiency in mice is associated with an increase in the number of bone marrow B220+ B cells. This supports our observation that ERα deficiency leads to increased Pax5 levels, which may drive B cell lymphopoiesis in bone. Overall, our data suggest a possible link between ERα status and Pax5 levels, although further studies are necessary to characterize this connection.
We recognize that our study has some limitations because the tissue source for the microarray and QPCR data are homogenates of whole, flushed bone. This leads to several cell types contributing to the overall gene expression patterns. Although the samples are enriched for osteoblastic cells, we cannot completely discriminate between gene expression patterns in these cells among the other cell types present. Therefore, our data represent the true physiological environment that exists in vivo, and as such also represent a major strength of this study. Additionally, the mouse model used represents a complete deletion of ERα (8), compared with only partial deletions that may occur in Cre-lox mouse models. We controlled the circulating estrogen levels through ovariectomy and estrogen replacement to rectify the high sex steroid levels present in the global ERKO and NERKI mouse models (6, 7). These experimental modifications allow for a robust examination of alterations in gene expression patterns in the ER models because they exist in vivo in the absence of high circulating estrogen levels.
In summary, we have demonstrated that ERα deficiency alters the gene expression profile in bone, including suppression of classic bone marker genes (i.e. alkaline phosphatase, osterix, and bone sialoprotein), consistent with an important role for ERα in regulating osteoblast differentiation in vivo. Loss of direct ERE binding (as in NERKI mice) affected many of the same genes as complete ERα deficiency, delineating a set of genes ultimately dependent on ERE binding. Although comparison of ERKO- and NERKI-regulated gene sets demonstrate that a majority of genes regulated in ERKO are via nonclassical pathways in bone (e.g. not affected in NERKI), it can be deduced that those ERE-dependent genes/pathways, although a minority of the ERα-regulated genes, play a critical role in the regulation of bone physiology because NERKI mice display an osteoporotic phenotype (5, 6). Overall, these data demonstrate the importance of classical ERα signaling in regulating bone metabolism and have expanded our understanding of estrogen-dependent pathways in bone.
Supplementary Material
Acknowledgments
We thank James M. Peterson for the QPCR data analyses.
This work was supported by Grant P01-AG004875 from the National Institutes of Health (to D.G.M. and S.K.) and by the Mayo Kogod Aging Center.
Disclosure Summary: All authors have no conflicts of interest.
Footnotes
- AP
- Alkaline phosphatase
- Bsp
- bone sialoprotein
- DAVID
- Database for Annotation, Visualization, and Integrated Discovery
- ER
- estrogen receptor
- ERE
- estrogen response element
- ERKO
- ERα-deficient
- NERKI
- nonclassical ER knock-in
- NF-κB
- nuclear factor-κB
- Pax5
- paired box protein 5
- QPCR
- quantitative real-time PCR analysis
- WT
- wild type.
References
- 1. Khosla S, Oursler MJ, Monroe DG. 15 May 2012. Estrogen and the skeleton. Trends Endocrinol Metab 10.1016/j.tem.2012.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Monroe DG, Spelsberg TC, Khosla S. 2006. Sex steroid effects on bone metabolism. In: Seibel MJ, Robins SP, Bilezikian JP, eds. Dynamics of bone and cartilage metabolism. San Diego: Academic Press; 327–343 [Google Scholar]
- 3. Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL. 2002. An estrogen receptor (ER) α deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol 16:2188–2201 [DOI] [PubMed] [Google Scholar]
- 4. Syed FA, Fraser DG, Monroe DG, Khosla S. 2011. Distinct effects of loss of classical estrogen receptor signaling versus complete deletion of estrogen receptor α on bone. Bone 49:208–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Syed FA, Fraser DG, Spelsberg TC, Rosen CJ, Krust A, Chambon P, Jameson JL, Khosla S. 2007. Effects of loss of classical estrogen response element signaling on bone in male mice. Endocrinology 148:1902–1910 [DOI] [PubMed] [Google Scholar]
- 6. Syed FA, Mödder UI, Fraser DG, Spelsberg TC, Rosen CJ, Krust A, Chambon P, Jameson JL, Khosla S. 2005. Skeletal effects of estrogen are mediated by opposing actions of classical and nonclassical estrogen receptor pathways. J Bone Miner Res 20:1992–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sims NA, Dupont S, Krust A, Clement-Lacroix P, Minet D, Resche-Rigon M, Gaillard-Kelly M, Baron R. 2002. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors beta in bone remodeling in females but not in males. Bone 30:18–25 [DOI] [PubMed] [Google Scholar]
- 8. Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. 2000. Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 127:4277–4291 [DOI] [PubMed] [Google Scholar]
- 9. Jakacka M, Ito M, Weiss J, Chien PY, Gehm BD, Jameson JL. 2001. Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J Biol Chem 276:13615–13621 [DOI] [PubMed] [Google Scholar]
- 10. Modder UI, Riggs BL, Spelsberg TC, Fraser DG, Atkinson EJ, Arnold R, Khosla S. 2004. Dose-response of estrogen on bone versus the uterus in ovariectomized mice. Eur J Endocrinol 151:503–510 [DOI] [PubMed] [Google Scholar]
- 11. Mödder UI, Roforth MM, Hoey K, McCready LK, Peterson JM, Monroe DG, Oursler MJ, Khosla S. 2011. Effects of estrogen on osteoprogenitor cells and cytokines/bone-regulatory factors in postmenopausal women. Bone 49:202–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mödder UI, Oursler MJ, Khosla S, Monroe DG. 2011. Wnt10b activates the Wnt, notch, and NFκB pathways in U2OS osteosarcoma cells. J Cell Biochem 112:1392–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang da W, Sherman BT, Lempicki RA. 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57 [DOI] [PubMed] [Google Scholar]
- 14. Huang da W, Sherman BT, Lempicki RA. 2009. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Syed F, Khosla S. 2005. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun 328:688–696 [DOI] [PubMed] [Google Scholar]
- 16. Jilka RL, Weinstein RS, Parfitt AM, Manolagas SC. 2007. Quantifying osteoblast and osteocyte apoptosis: challenges and rewards. J Bone Miner Res 22:1492–1501 [DOI] [PubMed] [Google Scholar]
- 17. Adams B, Dörfler P, Aguzzi A, Kozmik Z, Urbánek P, Maurer-Fogy I, Busslinger M. 1992. Pax-5 encodes the transcription factor BSAP and is expressed in B lymphocytes, the developing CNS, and adult testis. Genes Dev 6:1589–1607 [DOI] [PubMed] [Google Scholar]
- 18. Horowitz MC, Bothwell AL, Hesslein DG, Pflugh DL, Schatz DG. 2005. B cells and osteoblast and osteoclast development. Immunol Rev 208:141–153 [DOI] [PubMed] [Google Scholar]
- 19. Khosla S, Melton LJ, 3rd, Riggs BL. 2011. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed? J Bone Miner Res 26:441–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. 1994. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331:1056–1061 [DOI] [PubMed] [Google Scholar]
- 21. Hewitt SC, O'Brien JE, Jameson JL, Kissling GE, Korach KS. 2009. Selective disruption of ERα DNA-binding activity alters uterine responsiveness to estradiol. Mol Endocrinol 23:2111–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. 1993. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90:11162–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Flouriot G, Brand H, Denger S, Metivier R, Kos M, Reid G, Sonntag-Buck V, Gannon F. 2000. Identification of a new isoform of the human estrogen receptor-α (hER-α) that is encoded by distinct transcripts and that is able to repress hER-α activation function 1. EMBO J 19:4688–4700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tuttle R, Miller KR, Maiorano JN, Termuhlen PM, Gao Y, Berberich SJ. 2012. Novel senescence associated gene, YPEL3, is repressed by estrogen in ER+ mammary tumor cells and required for tamoxifen-induced cellular senescence. Int J Cancer 130:2291–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hocking LJ, Whitehouse C, Helfrich MH. 2012. Autophagy: a new player in skeletal maintenance? J Bone Miner Res 27:1439–1447 [DOI] [PubMed] [Google Scholar]
- 26. Kaminskyy V, Zhivotovsky B. 2012. Proteases in autophagy. Biochim Biophys Acta 1824:44–50 [DOI] [PubMed] [Google Scholar]
- 27. Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon HU. 2006. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol 8:1124–1132 [DOI] [PubMed] [Google Scholar]
- 28. Kawaguchi H, Pilbeam CC, Vargas SJ, Morse EE, Lorenzo JA, Raisz LG. 1995. Ovariectomy enhances and estrogen replacement inhibits the activity of bone marrow factors that stimulate prostaglandin production in cultured mouse calvariae. J Clin Invest 96:539–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miyaura C, Kusano K, Masuzawa T, Chaki O, Onoe Y, Aoyagi M, Sasaki T, Tamura T, Koishihara Y, Ohsugi Y, et al. 1995. Endogenous bone-resorbing factors in estrogen deficiency: cooperative effects of IL-1 and IL-6. J Bone Miner Res 10:1365–1373 [DOI] [PubMed] [Google Scholar]
- 30. Harada S, Tominari T, Matsumoto C, Hirata M, Takita M, Inada M, Miyaura C. 2011. Nobiletin, a polymethoxy flavonoid, suppresses bone resorption by inhibiting NFκB-dependent prostaglandin E synthesis in osteoblasts and prevents bone loss due to estrogen deficiency. J Pharmacol Sci 115:89–93 [DOI] [PubMed] [Google Scholar]
- 31. Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, Guan K, Krebsbach PH, Wang CY. 2009. Inhibition of osteoblastic bone formation by nuclear factor-κB. Nat Med 15:682–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Urbánek P, Wang ZQ, Fetka I, Wagner EF, Busslinger M. 1994. Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5/BSAP. Cell 79:901–912 [DOI] [PubMed] [Google Scholar]
- 33. Horowitz MC, Xi Y, Pflugh DL, Hesslein DG, Schatz DG, Lorenzo JA, Bothwell AL. 2004. Pax5-deficient mice exhibit early onset osteopenia with increased osteoclast progenitors. J Immunol 173:6583–6591 [DOI] [PubMed] [Google Scholar]
- 34. Kanematsu M, Sato T, Takai H, Watanabe K, Ikeda K, Yamada Y. 2000. Prostaglandin E2 induces expression of receptor activator of nuclear factor-κB ligand/osteoprotegrin ligand on pre-B cells: implications for accelerated osteoclastogenesis in estrogen deficiency. J Bone Miner Res 15:1321–1329 [DOI] [PubMed] [Google Scholar]
- 35. Masuzawa T, Miyaura C, Onoe Y, Kusano K, Ohta H, Nozawa S, Suda T. 1994. Estrogen deficiency stimulates B lymphopoiesis in mouse bone marrow. J Clin Invest 94:1090–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.