Abstract
Endometriosis is the estrogen-dependent growth of endometrial tissue outside the uterus. Endometriosis has an effect on the eutopic endometrium; however, the nature of the cellular or molecular signal from the lesion to the uterus is unknown. Here we demonstrate that cells migrate from endometriosis to eutopic endometrium. Experimental endometriosis was established by transplanting endometrial tissue from green fluorescent protein (GFP) mice to the peritoneal cavity of DS-Red mice. Immunofluorescence (IF) identified cells from the ectopic lesions in the uterus. The eutopic endometrial cells were sorted by fluorescence activated cell sorting, and the GFP+/DS-Red− population was characterized using microarray analysis. The results of cell sorting as well as the array results were confirmed by quantitative PCR and IF. GFP+/DS-red−/Cd45− cells were identified in the eutopic endometrium of mice with experimental endometriois (∼1.8%) and not in controls. Global gene expression profiling of these cells showed absence of leukocyte and increased expression of pan-epithelial markers in the uterine GFP+ cells. Moreover, GFP+ cells showed up-regulation of Wnt7A expression and 17 other genes associated with the Wingless pathway. Several genes that are associated with epithelial-to-mesenchymal transition were also highly differentially expressed in GFP+ cells. IF confirmed the presence of the GFP+/CD45−/Wnt7a+/cytokeritin+ cells in the endometrium of endometriotic animals, and not in controls. Cells from endometriotic lesions are capable of migrating to the eutopic endometrium. The ectopic expression of Wnt7A suggests a possible mechanism by which ectopic lesions affect the eutopic endometrium and interfere with embryo implantation and fertility.
Endometriosis is defined as the extrauterine growth of endometrial tissue, most commonly on the peritoneal and visceral surfaces of the pelvis, and containing both glandular and stromal components (1–4). Endometriosis occurs in approximately 10% of reproductive-aged women and is a common cause of pelvic pain and infertility. The growth of endometriosis is regulated by estrogen.
Postulated theories concerning the histological origin of endometriosis aim to explain the ability of endometrial tissue to develop ectopically; however, no consensus has yet been reached with regard to a single theory. Sampson (5) first proposed that retrograde flow of endometrial tissue fragments passes through the fallopian tubes during menstruation into the abdominal cavity, followed by implantation and development on peritoneal surfaces. Sampson's hypothesis is supported by evidence from the lesions locations, animal transplant models, and the exclusivity of the diseases in primates and not in nonmenstruating species (6). Another theory, coelomic metaplasia, holds that the genesis of endometriotic lesions within the peritoneal cavity is a result of mesothelial differentiation into endometrium-like tissue (7). Finally, the embryonic rest theory, suggests that primitive cells of Müllerian origin could be the cause of the diseases (8–10). However, cases of distant ectopic foci outside of the pelvis (e.g. brain, lungs, etc.) cannot be accounted for by any of the postulated theories (11–13). Sampson therefore hypothesized that endometriosis can form in ectopic sites via lymphovascular spread (5, 10, 14). Alternatively, studies from our laboratory and others suggest that stem cells from the bone marrow and/or progenitor cells from the basal layer of the endometrium contribute to the development of the disease (10, 15–18).
In metastatic cancer a bidirectional communication between the primary and metastatic tumor has been demonstrated (19). Kim et al. (19) were able to show that in certain types of metastatic cancer, the secondary tumor is also capable shedding circulating cells into the blood circulation. These circulating cancer cells selectively migrate and engraft the original tumor and in doing so contribute to the progression of the diseases. Despite the definition of endometriosis as a benign condition, the disease has many characteristics in common with malignancy: formation at local and distant sites, tissue invasion, and a chronic inflammatory state (20–22). Moreover, in comparison with healthy women, endometriotic patients often have angiogenic, endocrine, and immunological abnormalities (20, 23, 24). There has been no previous description of a similar bidirectional communication between the ectopic and the eutopic endometrium. The possibility that ectopic endometrial lesions affect the eutopic endometrium, through a similar cellular exchange between the two sites, suggests a potential mechanism to explain infertility in women with endometriosis.
The association between endometriosis and infertility among women with endometriosis is well established; however, the pathophysiology is not fully characterized. Mechanistic explanations by which endometriosis impairs fertility, include physical distortion of pelvic anatomy, endocrine and paracrine changes in peritoneal fluid, aberrant gene regulation in the endometrium during implantation, and alteration of inflammatory responses and/or autoimmune influences (25). Specifically the eutopic endometrium is altered in women with endometriosis, diminishing endometrial receptivity and embryo implantation (25–31). The mechanistic link between the presence of ectopic endometrium and the reduction in endometrial receptivity is still unknown. Although estrogen is the primary hormone regulating both endometriosis and endometrium, women with endometriosis have normal serum estradiol levels.
In this study we demonstrate the presence of a cell population that migrated from the endometriotic lesion into the uterus; these cells produced factors capable of altering uterine receptivity.
Materials and Methods
Animal model
All the animal procedures were conducted under an approved protocol of the Yale Institutional Animal Care and Use Committee. The endometriosis mouse model was achieved using two transgenic strains of mice. Sixteen DsRed mice [Tg(CAG-DsRed*MST)1Nagy/J; Jackson Laboratories, Bar Harbor, ME] were used as hosts and four green fluorescent protein (GFP) mice [B6.129(ICR)-Tg(CAG-ECFP)CK6Nagy/J; Jackson Laboratories] were used as donors. The model of induced endometriosis in the mouse was achieved as previously described (32). In the experimental group (n = 8), each host was transplanted with one uterine horn from a donor. One centimeter of the uterine horn was divided into three equal pieces and the serosal surface sutured to the peritoneum. The control group consisted of an equal number of animals as the experimental group, in which a sham surgery was conducted, leaving only sutures on the peritoneal wall. Three months after the surgery, the uteri of the DSRed mice were removed and analyzed. All animals were euthanized in the estrous phase. Finally, both the spleen and the femur of the host were also harvested for further assessment.
Two transgenic strains were used to eliminate confounding of a high level of autofluorescence in the spectral wavelength range of 488 nm that was observed in the uterus of normal mice. Furthermore, confirmation for the quality of the sorting procedure could be monitored in real time analysis due to the differential emission signal of GFP and red fluorescent protein (RFP) and confirmed by PCR. Briefly, the GFP uterine donors were euthanized by a cervical dislocation and their uterine horns were removed and placed in PBS. Each uterus horn was opened in the midline to expose endometrium. Open uteri were sutured to the peritoneum of the host mice after a vertical midline incision. After the procedure the peritoneal incision was sutured, and the skin was stapled.
Cell sorting
After 3 months recipient animals were killed and the uterine horns, femur, and spleen of the control and endometriosis animals were removed, finely minced, and subsequently digested with a solution of Hanks' balanced salt solution (Life Technologies, Inc., Invitrogen, Carlsbad, CA) containing HEPES (25 mm), collagenase B (1 mg/mL; Roche Diagnostics, Indianapolis, IN), and deoxyribonuclease I (0.1 mg/ml; Sigma-Aldrich, St. Louis, MO) for 60–90 min at 37 C. Bone marrow samples were flushed with PBS. All samples were filtered using 70-μm mesh. Sorting was performed on a fluorescence-activated cell sorting (FACS) Vantage SE instrument (BD Biosciences, Franklin Lakes, NJ) using the corresponding excitation wavelengths for GFP and RFP, 488 and 558 nm, respectively. Data were and analyzed using the software FlowJo (FlowJo, Ashland OR).
DNA and RNA extraction from sorted cells
Total RNA and DNA from sorted cells were isolated using Trizol (Invitrogen), according to the manufacturer's protocol. PCR using 10 μg of the extracted DNA was carried out using the primers listed in the Table 1. The, PCR products were separated by 1.5% agarose gel electrophoresis and visualized by ethidium bromide staining to assess the expression of GFP and RFP in the sorted samples.
Table 1.
PCR Primers
| Genes | Primers | Cycles, n |
|---|---|---|
| β−actin | Forward, GTTGACATCCGTAAAGACCTC | 42 |
| β−actin | Reverse, CTTGATCTTCATGGTGCTAGG | 42 |
| DsRed | Forward, CCCATGGTCTTCTTCTGCAT | 42 |
| DsRed | Reverse, AAGGTGTACGTGAAGCACCC | 42 |
| EGFP1 | Forward, AAG TTC ATC TGC ACC ACC G | 42 |
| EGFP1 | Reverse, TCC TTG AAG AAG ATG GTG CG | 42 |
Global gene expression profiling and statistical analysis
Fifty nanograms of purified mRNA were amplified using the Ovation Pico WTA System (Nugen, San Carlos, CA). This kit employs the use of 3′(polydeoxythymidine) and random primers to amplify the entire transcriptome in a uniform manner. Gene expression was analyzed in triplicates using Affimetrix Mouse Exon Arrays 1.0 ST (Affimetrix, Santa Clara, CA). The Partek Genomics Suite (Partek, Singapore) software was used to process the microarray data. Statistical analysis was first made by two-sample t test comparisons between the conditions/groups, followed by false discovery rate (FDR) to obtain an adjusted P value accounting for multiple testing. The MetaCore software (GeneGo, St. Joseph, MI) was used for pathway and Go analyses. P < 0.01 and ±1.5 fold change expression were considered statistically significant.
Paraffin-embedded and formaldehyde-fixed tissue immunofluorescence
One uterine horn was harvested from every animal in the study. Tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Three-micrometer tissue sections were mounted on slides followed by 10 min boiling in sodium citrate (pH 6) for antigen retrieval and blocking using donkey serum (Invitrogen). Representative slides were selected from multiple regions of the uterus to assess consistency. Primary antibodies for immunofluorescence staining were monoclonal rabbit anti-GFP antibody (Rockland, Gilbertsville, PA) in 1:200 dilution, rat anti-Cd45 (Abcam, Cambridge, MA) in 1:50 dilution, rabbit anti-Wnt7a (Invitrogen) in 1:25 dilution, and a rabbit antibovine wide-side spectra of cytokeratins (Dako, Carpinteria, CA) in 1:100. Secondary antibodies consisted of donkey antirabbit Alexa 488 (Invitrogen), Alexa donkey antirabbit 568 (Invitrogen), and donkey antigoat Alexa 633 (Invitrogen), all in the 1:500 dilutions. Inmunoreaction with amplification but without primary and/or secondary antibodies were performed as controls. All the visualizations of the slides were done with an NLO confocal microscope (Carl Zeiss, New York, NY) and the ZEN software (Carl Zeiss).
Results
Presence of ectopic GFP+ cells in the host GFP− uterus
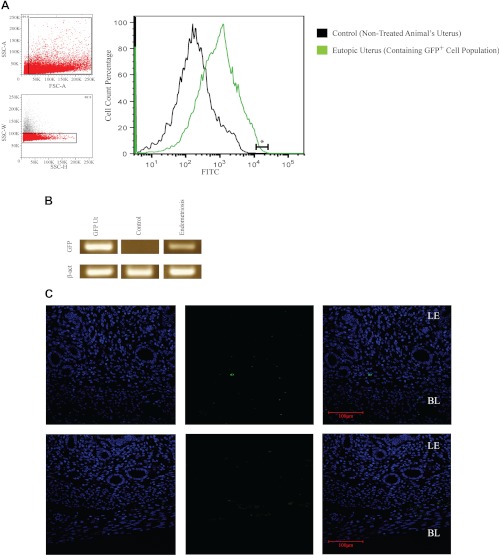
To determine whether ectopic endometrial lesions have cells that migrate to the uterus, we used the endometriosis mouse model as described above. An endometriotic group (n = 8) was transplanted with endometrial tissue from GFP transgenic donors, and a sham surgery was performed on control group (n = 8). After 3 months the animals were killed, and their uteri were harvested, processed, and sorted for GFP-expressing cells using FACS. On average, 1.8% of the total cellular populations of the endometriotic group's uteri were GFP+ (Fig. 1A). In the group in which uterine tissue was transplanted, sites of transplantation were still discernible. A thorough examination of the abdominal cavity did not show any signs spread of the ectopic transplant to other sites or evidence of hyperplasia in any of the ectopic lesions. PCR for the presence of GFP and DSRed confirmed the absence of GFP in the control group and its presence in the uteri of mice in the endometriosis group (Fig. 1B). GFP expression was not observed in the hosts' bone marrow or spleen. GFP protein expression was noted in stromal cells of the uterus (Fig. 1C). Interestingly, GFP-expressing cells in the sample from the experimental group were mainly localized to the basal layer, often next to blood vessels, and never in the epithelial lining of the lumen or within the glands. Cell counts confirmed that approximately 2% of cells expressed GFP, confirming the results of the FACS analysis.
Fig. 1.
Uteri of endometriotic mice contain GFP+ cells from the ectopic lesions. A, FACS analysis of uterine tissue from mice with GFP+ endometriosis contains GFP+ cells. The two small panels on the left represent the gates for the sorted population of GFP+ cells: noncellular debris (upper panel) and single cells (lower panel). The large diagram represents the percentages of GFP+ cells (fluorescein isothiocyanate channel), in which the green plot represents endometriotic samples and the black plot the control. The linear segment labeled with an asterisk shows the percentage of GFP+ cells that were collected from the sample (1.8%). B, PCR confirms the expression of GFP in uteri collected from animals with GFP+ endometriosis. The GFP amplification product was observed only in the uteri of animals with endometriosis and not the control group. β-Actin was used as a loading control. C, Immunofluorescence was used to detect GFP protein expression in the mouse uterus. GFP expression was detected in stromal cells of the animals with GFP+ endometriosis and not in controls. In all the samples from the experimental group (n = 8), GFP-expressing cells were located in the basalis layer. BL, Basalis, LE, luminal epithelium. From left to right: GFP (green channel), 4′,6′-diamino-2-phenylindole (blue channel), and a merged channel. Scale bar, 100 μm.
Cells in the uterus that originated in ectopic lesion have a distinct gene expression profile compared with the eutopic endometrium
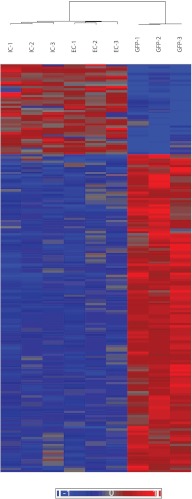
GFP+ cells from the uteri of mice with endometriosis were isolated by FACS and subjected to global gene expression analysis using microarray. To observe the distinct transcriptome of those ectopic cells that relocated to the uterus, the gene expression profile of GFP+ cells were compared with two groups of endometrial cells. The first comparison, an external comparison (EC), compared gene expression of the GFP+ cells that migrated to the uterus with the gene expression of uterine cells from the control group. The second comparison, or internal comparison (IC), compared the migrated GFP+ cells with the surrounding GFP− uterine cells in the same animal (Fig. 2). Statistical analysis showed a high clustering level (relative similarity) of expressed genes in the profiles of the two control groups (EC and IC), and a distinct gene expression profile of the GFP+ group. Although there were discernible differences in gene expression between normal control endometrial cells and GFP− endometrial cells from animals with endometriosis, the GFP+ cells were clearly distinct from either group.
Fig. 2.
GFP+ cells show distinct gene expression when compared with control and the gene expression heat map of GFP+ cells. Microarray data from GFP+ cells were compared with uterine cells from control group [external control (EC) 1–3], a second comparison was conducted against GFP− sorted cells from the mice with GFP+ endometriosis [internal control (IC) 1–3]. Each group contained three samples. Global gene expression proofing of the external and internal control cells were remarkably similar. These two controls clustered together. Both controls were markedly different from the GFP+ cells in the endometrium that arose from the endometriosis (P < 0.05).
Next, the results from the two comparisons were further analyzed for genes with differential expression of ±1.5-fold change and P < 0.05. From the two comparisons, the Δ EC-IC less than 1 for all the differential gene was used to compose a single list of 3479 differential genes to identify the distinct transcriptome profile of GFP+ cells. To characterize those cells, we identified a group of 38 statistically significant pathways that were differentially regulated in GFP+ cells, with the Wingless-related mouse mammary tumor virus (MMTV) superfamily and cellular adhesion pathways that were significantly activated in the GFP+ cells. These two pathways include 21 individual genes that were either significantly up-regulated or down-regulated (Table 2). Interestingly, these genes are associated with the endometrial epithelium during uterine development and in epithelial-to-mesenchymal transition. Specifically, Wnt7a is known to be secreted by the glandular epithelium and to be involved in endometriosis.
Table 2.
Summary of differential gene expression of GFP+ cells analysis in comparisons with external and internal controls (EC and IC, respectively)
| Gene symbol | EC | IC | Gene description |
|---|---|---|---|
| Wnt7a | 2.52058 | 3.21196 | Wingless-related MMTV integration site 7A |
| Snai1 | 2.14918 | 2.09986 | Snail 1 |
| Fzd8 | 2.19816 | 2.66954 | Frizzled 8 |
| Wnt3a | 2.01367 | 1.94325 | Wingless-related MMTV integration site 3A |
| Wnt2 | 1.9299 | 1.98037 | Wingless-related MMTV integration site 2 |
| Snai3 | 1.91185 | 1.97103 | Snail 3 |
| Wnt8a | 1.86916 | 1.71388 | Wingless-related MMTV integration site 8A |
| Wnt10a | 1.86071 | 1.97899 | Wingless related MMTV integration site 10a |
| Fzd10 | 1.84805 | 2.03044 | Frizzled homolog 10 |
| Wnt9a | 1.77514 | 1.84285 | Wingless-type MMTV integration site 9A |
| Wif1 | 1.76827 | 1.84473 | Wnt inhibitory factor 1 |
| Wnt3a | 1.73157 | 1.78647 | Wingless-related MMTV integration site 3A |
| Wnt10b | 1.72677 | 1.70997 | Wingless related MMTV integration site 10b |
| Wnt9b | 1.71303 | 1.80598 | Wingless-type MMTV integration site 9B |
| Wnt1 | 1.69791 | 1.74659 | Wingless-related MMTV integration site 1 |
| Dkk1 | 1.68243 | 1.81371 | Dickkopf homolog 1 |
| Wnt6 | 1.64788 | 1.931 | Wingless-related MMTV integration site 6 |
| Fzd5 | 1.57186 | 1.73073 | Frizzled 5 |
| Wnt8b | 1.51593 | 1.69694 | Wingless related MMTV integration site 8b |
| Gsc | 1.57094 | 1.73831 | Goosecoid homeobox |
| Zeb2 | −2.90415 | −3.8155 | Zinc finger E-box binding homeobox 2 |
Cells from ectopic lesions can localize to the uterus
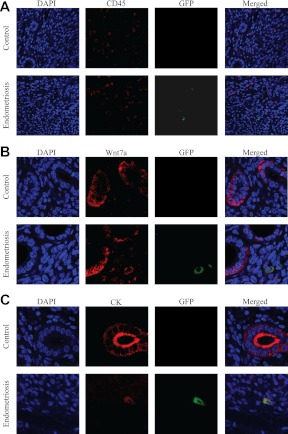
Analysis of the microarray data showed no significant differential expression of Ptprc, the gene that encodes for the hematopoietic pan-marker Cd45, within the GFP+ cellular population. These data suggest that the migratory cells are not leukocytes. To confirm that the GFP+ populations were not hematopoietic cells, one uterine horn was harvested from each animal in the study and was subjected to immunohistological assessment of Cd45 and GFP. GFP-expressing cells in the eutopic endometrium were observed in all of the uterine samples from the experimental group. The GFP+ cells did not coexpress Cd45 (Fig. 3A). Morphologically, GFP+ cells appeared fibroblastic with large cytosol to nucleus ratio, whereas GFP−/Cd45+ cells resembled immune cells, as expected. No GFP-positive staining was observed in the endometrium of samples from the control group. Both groups contained Cd45+ cells as expected. Of note, similar results were observed in all the paraffinized tissue samples in the experimental group (n = 8). Finally, no significant difference in the Cd45 staining was observed between the two groups. These results confirmed the observation that cells from ectopic endometrial lesions can relocate to the uterus, and they suggest that they are not immune cells.
Fig. 3.
GFP+ cells express Wnt7a and cytokeratins and do not express CD45. GFP-expressing cells have characteristics of epithelial cells but localize to the stroma. A, As expected, GFP−/Cd45+ cells (leukocytes) were observed in both the endometriosis and control groups. GFP+/Cd45− cells were found only in the basal stroma of the endometriotic group and never in the control. Blue [4′,6′-diamino-2-phenylindole (DAPI)], red (CD45), and green (GFP). B, Wnt7a is known to be expressed in endometrial epithelial cells. GFP−/Wnt7a+ cells were observed in both groups and were always located in the glandular epithelium. GFP+/Wnt7a+ were found only in the basal stroma of the endometriotic group and never in the controls. Blue (DAPI), red (Wnt7a), green (GFP) are shown. C, Cytokeratin was used as a marker of epithelial cells. GFP−/cytokeratin+ cells were observed in both groups and were always located to the glandular epithelium. GFP+/cytokeratin K+ cells were found only in the basal stroma of the endometriosis group and never in the controls. Blue (DAPI), red (CytoK), and green (GFP) are shown. All figures here were obtained from the uterine midsection, after having confirmed similar results in multiple sections from all areas of the uterus.
GFP+ cells secrete Wnt7a and express cytokeratins
Among the most up-regulated genes expressed in GFP+ cells was Wnt7a, a member of the Wingless-type MMTV integration site family. Wnt7a is known not only for its involvement in formation of the anterior-posterior axis of the female reproductive tract but also for its role in adult uterine function (33, 34). Wnt7a is normally secreted by the endometrial epithelium. This production of Wnt7a was unexpectedly localized to GFP+ cells located primarily in the basal stroma. Colocalization of Wnt7a and GFP is shown in Fig. 3B. Wnt7a is robustly expressed in GFP+ cells that are localized in the stroma and primarily in the basal stroma (Fig. 3B).
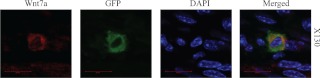
Not only was the expression of Wnt7a noted in the GFP+ population, but also data from the microarray analysis revealed that the GFP+ cell population had significantly enhanced expression of pan-epithelial markers. We next determined whether those GFP+/Cd45− cells located in the basal stroma have characteristics of epithelial lineages. Tissue samples were costained for GFP and a wide spectrum of cytokeratins. Cytokeratins are intermediate filaments, component of the cytoplasmic cytoskeleton, and used as epithelial pan-markers. In both groups, robust cytokeratin staining was observed in the glandular and luminal epithelium, as expected. Interestingly, in the endometriotic group, GFP+ cells that were located in the stroma demonstrated robust cytokeratin staining (Fig. 3C). Again, no GFP staining was observed in the control group. The GFP+ population of cells that migrated to the uterus expressed epithelial markers. A high-power view of cells expressing both GFP and Wnt7a is shown in Fig. 4.
Fig. 4.
Immunofluorescencent images of GFP-positive cells that express Wnt7a. Higher magnification (×130) immunohistochemical fluorescent picture of a double-positive cell, originating from of an ectopic lesion, and expressing cytoplasmic GFP and Wnt7a in the basalis layer of the endometrium. Panels from left to right are as follows: Wnt7a (red channel), GFP (green channel), 4′,6′-diamino-2-phenylindole (blue channel), and a merged channel. Scale bar, 30 μm.
Discussion
In this study, we demonstrated that experimental endometriosis was capable of shedding cells that migrate selectively to and populate the uterus. This cellular migration was selective to the endometrium and not to other organs. Other tissues that were analyzed included the liver, spleen, and bone marrow, in which no GFP signal was found. This observation implies that these migrating cells have a special tropism for endometrial tissue. The localization to the uterus is unlikely to be the result of direct migration through the fallopian tubes, i.e. the reverse of the suspected route of migration of endometrial cells that form endometriosis in humans. In rodents, unlike primates, the endometrium is isolated from the peritoneal cavity by a membrane that encloses the ovaries and fallopian tubes. However, the possibility of tubal transport cannot be ruled out, and future studies will address this issue. In our model, the GFP+ cells were localized near blood vessels at the base of the endometrium and not integrated into the surface epithelium. Therefore, we speculate that those cells migrated to the endometrium through the circulation.
Previously our laboratory demonstrated that mesenchymal stem cells from the bone marrow contribute to the endometrium and to the ectopic lesions through the circulatory system (15–16). In addition, Schwab and colleagues (44) characterized a population of endometrial progenitor cells, which were located in perivascular areas of the endometrium. The possibility of invasion of a stem cell population from the transplants in the peritoneal cavity to the uterus cannot be excluded and may also contribute to the population of stem/progenitor cells in this organ (37). The signal that recruits these cells to the uterus is still unknown and currently under investigation.
The increased expression of Wnt7a in endometrial cells from ectopic lesions agrees with previously reported data from studies in human endometriosis patients (33, 34). However, Wnt7a expression is normally localized to the epithelium. Appropriate expression of Wnt7a has crucial role in the development and maintenance of fertility in the murine endometrium, likely by signaling between the epithelium and the stroma (35, 36). Increased expression of Wnt7a could affect endometrial receptivity. Further ectopic Wnt7a expression outside of the gland likely distorts the epithelial-stromal polarity required for normal fertility.
Although epithelial-lineage origin of GFP+ cells is suggested by the expression array, those results also point toward a possible mechanism by which these features were adopted. The activation of Wnt signaling pathways in those cells, together with the up-regulation of Snail1, Snail3, Goosecoid, and the down-regulation of Zeb2, are associated with of epithelial-to-mesenchymal transition (EMT) (37, 38). This is a process by which epithelial cells lose their polarity and are converted to a mesenchymal phenotype. EMT and the reverse transition from a mesenchymal to an epithelial phenotype are crucial in embryogenesis, fibrosis, and tumor metastasis (39). The expression of cytokeratins in those cells that migrated into the uterus from the ectopic lesions further supports this hypothesis.
The Wnt-signaling pathway is crucial to estrogen-mediated uterine growth (40) and implantation in mice (41, 42). Moreover, high serum 17β-estradiol and/or progesterone concentrations may advance endometrial development after ovarian stimulation, altering the implantation window and possibly decreasing pregnancy rate, after ovarian stimulation during in vitro fertilization treatments (43). Liu et al. (43) sampled the endometrium of those women and looked for aberrant gene expression to trace possible explanation for their subfertility. Interestingly, they found similar up-regulated expression of Dkk1 in those endometrial samples and therefore hypothesized that the aberrant activation of the Wnt pathway disturbs synchronized endometrial development in the implantation window. Therefore, it is possible that migrating cells from ectopic lesions in the uterus alter the gene expression within the eutopic tissue through Wnt signaling and lead to subfertility in those endometriosis patients.
Taken together our results support the bidirectional movement of cells between eutopic and ectopic endometrial tissue. These cells likely undergo EMT, enabling this migration, and preferentially home to the basalis layer of the endometrium. The ectopic Wnt signaling likely distorts the epithelial-stromal dialog needed for optimal endometrial development and receptivity.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- EC
- External comparison
- EMT
- epithelial-to-mesenchymal transition
- FACS
- fluorescence-activated cell sorting
- FDR
- false discovery rate
- GFP
- green fluorescent protein
- IC
- internal comparison
- MMTV
- mouse mammary tumor virus
- RFP
- red fluorescent protein (also shows up as DSRed).
References
- 1. Nardo LG, Jones CJP, 2009. Endometrial changes in women with endometriosis. In: Garcia-Velasco Botros Rizk, eds. Endometriosis: current management and future trends. New Delhi, India: Jaypee Brothers Medical Publishers (P) Ltd.; 21–28 [Google Scholar]
- 2. Berkley KJ, Rapkin AJ, Papka RE. 2005. The pains of endometriosis. Science 308:1587–1589 [DOI] [PubMed] [Google Scholar]
- 3. Bulun SE. 2009. Endometriosis. N Engl J Med 360:268–279 [DOI] [PubMed] [Google Scholar]
- 4. Falcone T, Lebovic DI. 2011. Clinical management of endometriosis. Obstet Gynecol 118:691–705 [DOI] [PubMed] [Google Scholar]
- 5. Sampson JA. 1927. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 14:422–469 [Google Scholar]
- 6. D'Hooghe TM, Debrock S. 2002. Endometriosis, retrograde menstruation and peritoneal inflammation in women and in baboons. Hum Reprod Update 8:84–88 [DOI] [PubMed] [Google Scholar]
- 7. Ferguson BR, Bennington JL, Haber SL. 1969. llerian pelvic lymph node glandular inclusions: evidence for histogenesis by mullerian metaplasia of coelomic epithelium. Obstet Gynecol 33:617–625 [PubMed] [Google Scholar]
- 8. Von Recklinghausen F. 1896. Adenomyomas and cystadenomas of the wall of the uterus and tube: their origin as remnants of the wolffian body. Wien Klin Wochenschr 8:530 [Google Scholar]
- 9. Russell WW. 1899. Aberrant portions of the Mullerian duct found in an ovary. Bull Johns Hopkins Hosp 10:10 [Google Scholar]
- 10. Sasson IE, Taylor HS. 2008. Stem cells and the pathogenesis of endometriosis. Ann NY Acad Sci 1127:106–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sarma D, Iyengar P, Marotta TR, terBrugge KG, Gentili F, Halliday W. 2004. Cerebellar endometriosis. AJR Am J Roentgenol 182:1543–1546 [DOI] [PubMed] [Google Scholar]
- 12. Cassina PC, Hauser M, Kacl G, Imthurn B, Schröder S, Weder W. 1997. Catamenial hemoptysis. Diagnosis with MRI. Chest 111:1447–1450 [DOI] [PubMed] [Google Scholar]
- 13. Van Schil PE, Vercauteren SR, Vermeire PA, Nackaerts YH, Van Marck EA. 1996. Catamenial pneumothorax caused by thoracic endometriosis. Ann Thorac Surg 62:585–586 [PubMed] [Google Scholar]
- 14. Sampson JA. 1927. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol 3:109. [PMC free article] [PubMed] [Google Scholar]
- 15. Taylor HS. 2004. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA 292:81–85 [DOI] [PubMed] [Google Scholar]
- 16. Du H, Taylor HS. 2007. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells 25:2082–2086 [DOI] [PubMed] [Google Scholar]
- 17. Wolff EF, Gao XB, Yao KV, Andrews ZB, Du H, Elsworth JD, Taylor HS. 2011. Endometrial stem cell transplantation restores dopamine production in a Parkinson's disease model. J Cell Mol Med 15:747–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Santamaria X, Massasa EE, Feng Y, Wolff E, Taylor HS. 2011. Derivation of insulin producing cells from human endometrial stromal stem cells and use in the treatment of murine diabetes. Mol Ther 19:2065–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, Massagué J. 2009. Tumor self-seeding by circulating cancer cells. Cell 139:1315–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sundqvist J, Falconer H, Seddighzadeh M, Vodolazkaia A, Fassbender A, Kyama C, Bokor A, Stephansson O, Gemzell-Danielsson K, D'Hooghe TM. 2011. Ovarian cancer-associated polymorphisms in the BNC2 gene among women with endometriosis. Hum Reprod 26:2253–2257 [DOI] [PubMed] [Google Scholar]
- 21. Varma R, Rollason T, Gupta JK, Maher ER. 2004. Endometriosis and the neoplastic process. Reproduction 127:293–304 [DOI] [PubMed] [Google Scholar]
- 22. Nezhat F, Datta MS, Hanson V, Pejovic T, Nezhat C, Nezhat C. 2008. The relationship of endometriosis and ovarian malignancy: a review. Fertil Steril 90:1559–1570 [DOI] [PubMed] [Google Scholar]
- 23. Erzen M, Kovacic J. 1998. Relationship between endometriosis and ovarian cancer. Eur J Gynaecol Oncol 19:553–555 [PubMed] [Google Scholar]
- 24. Modesitt SC, Tortolero-Luna G, Robinson JB, Gershenson DM, Wolf JK. 2002. Ovarian and extraovarian endometriosis-associated cancer. Obstet Gynecol 100:788–795 [DOI] [PubMed] [Google Scholar]
- 25. de Ziegler D, Borghese B, Chapron C. 2010. Endometriosis and infertility: pathophysiology and management. Lancet 376:730–738 [DOI] [PubMed] [Google Scholar]
- 26. Bulun SE, Utsunomiya H, Lin Z, Yin P, Cheng YH, Pavone ME, Tokunaga H, Trukhacheva E, Attar E, Gurates B, Milad MP, Confino E, Su E, Reierstad S, Xue Q. 2009. Steroidogenic factor-1 and endometriosis. Mol Cell Endocrinol 300:104–108 [DOI] [PubMed] [Google Scholar]
- 27. Velarde MC, Aghajanova L, Nezhat CR, Giudice LC. 2009. Increased mitogen-activated protein kinase kinase/extracellularly regulated kinase activity in human endometrial stromal fibroblasts of women with endometriosis reduces 3′,5′cyclic adenosine 5′-monophosphate inhibition of cyclin D1. Endocrinology 150:4701–4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eun Kwon H, Taylor HS. 2004. The role of HOX genes in human implantation. Ann NY Acad Sci 1034:1–18 [DOI] [PubMed] [Google Scholar]
- 29. Lee B, Du H, Taylor HS. 2009. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol Reprod 80:79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taylor HS, Bagot C, Kardana A, Olive D, Arici A. 1999. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod 14:1328–1331 [DOI] [PubMed] [Google Scholar]
- 31. Daftary GS, Taylor HS. 2004. EMX2 gene expression in the female reproductive tract and aberrant expression in the endometrium of patients with endometriosis. J Clin Endocrinol Metab 89:2390–2396 [DOI] [PubMed] [Google Scholar]
- 32. Cummings AM, Metcalf JL. 1995. Induction of endometriosis in mice: a new model sensitive to estrogen. Reprod Toxicol 9:233–238 [DOI] [PubMed] [Google Scholar]
- 33. Gaetje R, Holtrich U, Karn T, Cikrit E, Engels K, Rody A, Kaufmann M. 2007. Characterization of WNT7A expression in human endometrium and endometriotic lesions. Fertil Steril 88:1534–1540 [DOI] [PubMed] [Google Scholar]
- 34. Hayashi K, Yoshioka S, Reardon SN, Rucker EB, Spencer TE, 3rd, DeMayo FJ, Lydon JP, MacLean JA., 2nd 2011. WNTs in the neonatal mouse uterus: potential regulation of endometrial gland development. Biol Reprod 84:308–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xie H, Tranguch S, Jia X, Zhang H, Das SK, Dey SK, Kuo CJ, Wang H. 2008. Inactivation of nuclear Wnt-β-catenin signaling limits blastocyst competency for implantation. Development 135:717–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parr BA, McMahon AP. 1998. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature 395:707–710 [DOI] [PubMed] [Google Scholar]
- 37. Stilley JA, Woods-Marshall R, Sutovsky M, Sutovsky P, Sharpe-Timms KL. 2009. Reduced fecundity in female rats with surgically induced endometriosis and in their daughters: a potential role for tissue inhibitors of metalloproteinase 1. Biol Reprod 80:649–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sethi S, Sarkar FH, Ahmed Q, Bandyopadhyay S, Nahleh ZA, Semaan A, Sakr W, Munkarah A, Ali-Fehmi R. 2011. Molecular markers of epithelial-to-mesenchymal transition are associated with tumor aggressiveness in breast carcinoma. Transl Oncol 4:222–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yao D, Dai C, Peng S. 2011. Mechanism of mesenchymal-epithelial transition and the relationship with metastatic tumor formation. Mol Cancer Res 9:1608–1620 [DOI] [PubMed] [Google Scholar]
- 40. Hou X, Tan Y, Li M, Dey SK, Das SK. 2004. Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol 18:3035–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC. 2003. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology 144:2870–2881 [DOI] [PubMed] [Google Scholar]
- 42. Mohamed OA, Jonnaert M, Labelle-Dumais C, Kuroda K, Clarke HJ, Dufort D. 2005. Uterine Wnt/β-catenin signaling is required for implantation. Proc Natl Acad Sci USA 102:8579–8584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu Y, Kodithuwakku SP, Ng PY, Chai J, Ng EH, Yeung WS, Ho PC, Lee KF. 2010. Excessive ovarian stimulation up-regulates the Wnt-signaling molecule DKK1 in human endometrium and may affect implantation: an in vitro co-culture study. Hum Reprod 25:479–490 [DOI] [PubMed] [Google Scholar]
- 44. Schwab KE, Gargett CE. 2007. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod 22:2903–2911 [DOI] [PubMed] [Google Scholar]