Abstract
Purpose
Laparoscopic gastrectomy is a widely accepted surgical technique. Recently, robotic gastrectomy has been developed, as an alternative minimally invasive surgical technique. This study aimed to evaluate the question of whether robotic gastrectomy is feasible and safe for the treatment of gastric cancer, due to its learning curve.
Materials and Methods
We retrospectively reviewed the prospectively collected data of 100 consecutive robotic gastrectomy patients, from November 2008 to March 2011, and compared them to 282 conventional laparoscopy patients during the same period. The robotic gastrectomy patients were divided into 20 initial cases; and all subsequent cases; and we compared the clinicopathological features, operating times, and surgical outcomes between the three groups.
Results
The initial 20 robotic gastrectomy cases were defined as the initial group, due to the learning curve. The initial group had a longer average operating time (242.25±74.54 minutes vs. 192.56±39.56 minutes, P>0.001), and hospital stay (14.40±24.93 days vs. 8.66±5.39 days, P=0.001) than the experienced group. The length of hospital stay was no different between the experienced group, and the laproscopic gastrectomy group (8.66±5.39 days vs. 8.11±4.10 days, P=0.001). The average blood loss was significantly less for the robotic gastrectomy groups, than for the laparoscopic gastrectomy group (93.25±84.59 ml vs. 173.45±145.19 ml, P<0.001), but the complication rates were no different.
Conclusions
Our study shows that robotic gastrectomy is a safe and feasible procedure, especially after the 20 initial cases, and provides a satisfactory postoperative outcome.
Keywords: Robotic, Laparoscopy, Compared, Gastrectomy, Learning curve
Introduction
Gastric cancer is one of the most common malignancies in Korea.(1) Gastroscopy, which occurs routinely in the national health care system, has found many early gastric cancers (EGC). Because the 5-year survival rate of EGC patients exceeds 90%, surgeons' interests focus on patients' postoperative recovery and quality of life.(1,2) Therefore, after laparoscopic gastrectomy (LG) for EGC was rapidly adopted in Korea and Japan, many studies reported that LG is comparable to open gastrectomy, including its oncological outcomes.(3) In these studies, LG could lead to earlier discharge, less wound pain, and less pulmonary complications than open surgery.(4,5) However, laparoscopic surgery has disadvantages, including limitations of motion, amplified hand tremors and two-dimensional images.(6) To overcome these limitations, novel techniques such as robot-assisted surgery have been introduced.
Robotic surgery was introduced by Cadière and colleagues in 1997.(7) The first robot-assisted surgery was a cholecystectomy, but now, robotic surgery is applied to many surgical types, including surgeries of the intra-abdominal organs, neck and prostate.(8) Robotic surgery has been shown to overcome the limitations of the laparoscopic approach. Wristed instruments that allow seven degrees of freedom, tremor filtering, the ability to scale motions, and stereoscopic vision improve a surgeon's dexterity when performing fine manipulations of tissue in a closed, fixed operating field.
Few studies have compared robotic gastrectomy (RG) with LG, but those that have compared the two techniques reported that RG could be a feasible alternative to LG.(6,9-13) However, they did not consider the learning curve of RG. Previous studies reported that 50~100 cases are needed to overcome the learning curve of LG,(14,15) but the learning curve for robot-assisted surgery is generally much shorter. Because the operating procedure of RG is similar to that of LG, the laparoscopic surgeon could easily adapt to RG. Approximately 10~20 cases are sufficient to achieve stabilized operating times.(16,17) Therefore, we compared RG with LG regard to the postoperative outcome.
Materials and Methods
From November 2008, RG was introduced in the Ajou University Hospital (Suwon, Korea). After 517 cases of LG were performed, we prospectively collected patients' demographic data (e.g., sex, age, underlying disease), operating data (e.g., operative time, bleeding, anastomosis type), and post-operative data (e.g., pathology, discharge date, morbidity). Five hundreds seventeen cases of LG were done by one surgeon, and all RG also done by same surgeon. Total 382 cases were enrolled in this study and RG cases were 100. The patients who underwent RG were divided into an initial 20 cases and all subsequent cases.
We defined "underlying disease" as "disease that could affect general anesthesia," and "operating time" as "the time from the initial incision to skin closure." We counted blood loss that suction volume minus irrigation volume. A complication was defined as "an event that delays the normal discharge date".
From November 2008 to March 2011, we reviewed gastric cancer patients who underwent minimally invasive surgeries (RG or LG). Patients whose pre-operative staging was 'T1 or 2' and 'N0 or 1' (American Joint Committee on Cancer [AJCC] 6th edition) were indicated for minimally invasive surgery. RG was selected if patients wanted this type of surgery, regardless of its cost. Combine operation which associated with stomach operation case was included (cholecystectomy or splenectomy) but other combine operation cases were excluded. Open conversion cases or palliative surgery cases were excluded, and there was no conversion to open surgery in robot-assisted cases. We reviewed the operative data and early operative outcomes and analyzed these factors retrospectively.
1. Operating technique (LG and RG)
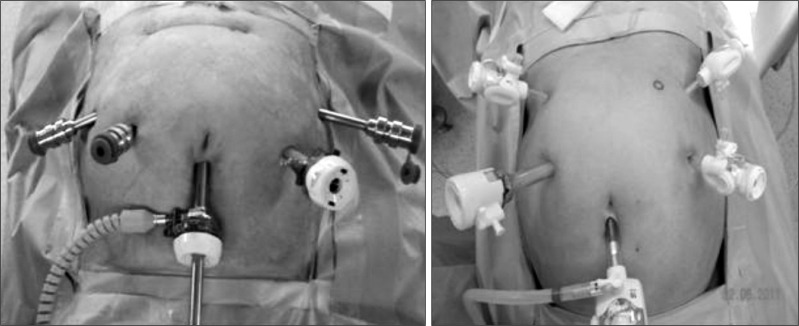
Both operation types used a total of five trocars. The first trocar was inserted in the infraumbilical area (a 10 mm trocar in LG and a 12 mm trocar in RG) using the closed method and made pneumoperitoneum and intracoporeal pressure was increased up to 12 mmHg by CO2 gas. The scope (a single-lens in LG and a dual-lens in RG) was inserted through this trocar. An additional four trocars were placed under direct visualization. In LG, two 5-mm trocars were placed on the left side, and one 5-mm trocar was placed on the upper right side. A 12-mm trocar was placed on the right central side. In RG, two 8-mm trocars were placed on the right side, and one 8-mm trocar was placed on the outer left side. A 12-mm trocar was placed on the central left side (Fig. 1). In LG, the operator works on the patient's right side and uses the right side trocars. Two assistants stand on the left side and use the left side trocars or scope. In RG, Cadiere forceps (Intuitive Surgical Inc., Sunnyvale, CA, USA) were introduced through the right upper 8-mm trocar, and a Harmonic scalpel (Ethicon EndoSurgery Inc., Cincinnati, OH, USA) was introduced through the right central 8-mm trocar. Maryland bipolar forceps (Intuitive Surgical Inc.) were inserted through the left outer 8-mm trocar, and the remaining left central trocar was used by the assistant. Initially, the liver was retracted using a V-shaped method (before the robot was docked in the robot case),(18) and the greater omentum was resected using the Harmonic scalpel in both operation types. After the division and ligation of the left gastroepiploic vessels at the root, dissection around the lymph nodes was performed toward the pylorus. The right gastroepiploic vessels were divided and ligated at the root. After the lesser omentum of the upper duodenum was resected, the right gastric vessels were identified from the hepatic artery and ligated at the root. Then, the duodenum was transected 1~2 cm distal to the pyloric ring using a laparoscopic stapling device. In LG, the operator transected the duodenum, but the assistant performed this procedure in RG. The lymph nodes were dissected until the root of the liver hilum, common hepatic artery and splenic artery were exposed. After the division and ligation of the left gastric vessels at the root, dissection around the lymph nodes was performed. In the total gastrectomy cases, the lymph nodes around the splenic artery or splenic hilum were harvested, and the short gastric artery was ligated. After the vagus nerve was ligated, the esophagus was transected. Resection and anastomosis were similar for LG and RG. In subtotal gastrectomy cases, after the lymph nodes along the lesser curvature were removed, the stomach was transected using a laparoscopic surgical stapling device. In extracorporeal anastomosis, an incision of approximately 5 cm was made at the upper abdomen for a mini-laparotomy. In intracoporeal anastomosis, gastroduodenostomy (linear stapler technique) or gastrojejunostomy or esophagojejunostomy was performed using a laparoscopic stapler device and reinforced by a suture or completely robot-sewn anastomosis, as reported previously.(19,20)
Fig. 1.
Trocar insertion in a robotic gastrectomy case (right) and laparoscopic case (left). The robotic case had lower trocar sites than the laparoscopic case.
2. Postoperative management
Patients with no morbidity were sent to the general ward after their operations. Unless otherwise indicated, such as cases of obstruction, patients were managed using a standardized postoperative clinical pathway (CP) with no nasogastric tube insertion and one closed suction drain. In subtotal gastrectomy, patients were given sips of water on postoperative day 2, a liquid diet on postoperative day 3, and a soft diet on postoperative day 4. The drain was removed on postoperative day 5 if the patient could tolerate the procedure and the drain was clear. In total gastrectomy cases, the diet build-up course was delayed by one day. Discharge from the hospital was recommended on postoperative day 6. Postoperative management was same regardless on surgical method.
3. Statistical analysis
We compared the surgical outcomes among the initial 20 RG cases, the 80 subsequent RG cases and the LG cases during the same period.
Statistical analysis was performed using the PASW Statics 18 program (IBM Co., Armonk, NY, USA). An independent Student's t-test was used for continuous variables between two groups, and a one-way ANOVA test with post-hoc analysis by least significant difference was used for continuous variables among three groups. A chi-squared test was used for the categorical variables. For all tests, P-values less than 0.05 were interpreted as statistically significant.
Results
RG patient characteristics are summarized in Table 1. A total of 382 patients (282 for LG and 100 for RG) were enrolled. In total, 63 male and 37 female patients underwent RG during this period. The average patient age was 53 years old. Seven patients were ultimately diagnosed as stage III by 6th AJCC stomach cancer stage. Seventeen patients in LG and 5 patients in RG were undergone cholecystectomy simultaneously. Two patients in RG were undergone splenectomy.
Table 1.
Patient characteristics of robotic gastrectomy cases
Values are presented as mean±standard deviation or number. BMI = body mass index; AJCC = American Joint Committee on Cancer. *Other co-morbidity includes renal disease, liver disease.
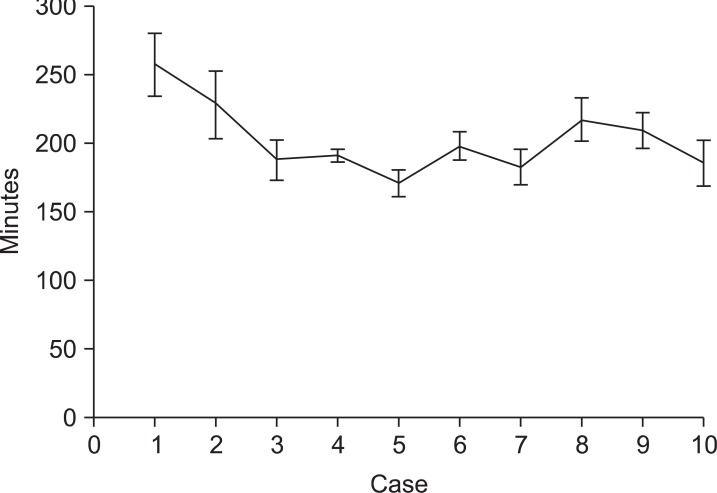
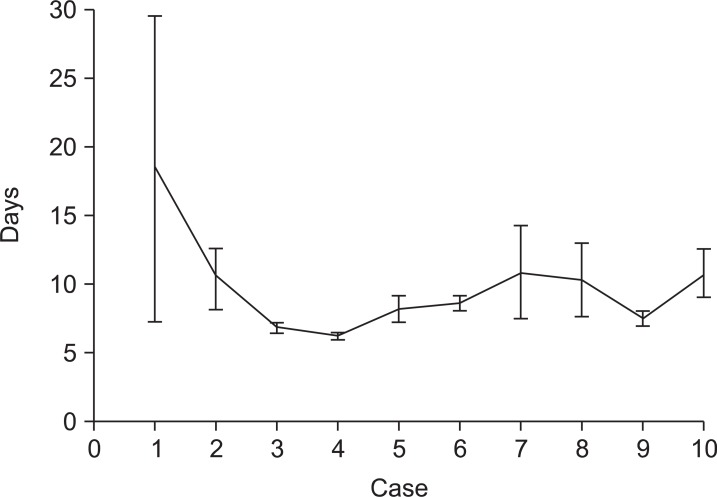
For every 10 cases of RG, we reviewed the average operating time and hospital stay (Fig. 2, 3). The operating time was shorter and similar after 20 RG cases. The standard deviation was also similar for the subsequent 80 cases. The initial 10 cases had longer hospital stays than the other groups because one case of postoperative complications occurred. Except for the initial cases, the other groups had similar hospital stays. The first 20 cases were grouped into the initial group, and the subsequent 80 cases were grouped into the experienced group.
Fig. 2.
The mean operating times of 10 sequential patients from the initial cases of robotic gastrectomy (error bars represent standard error).
Fig. 3.
The mean lengths of hospital stay of 10 sequential patients from the initial cases of robotic gastrectomy (error bars represent standard error).
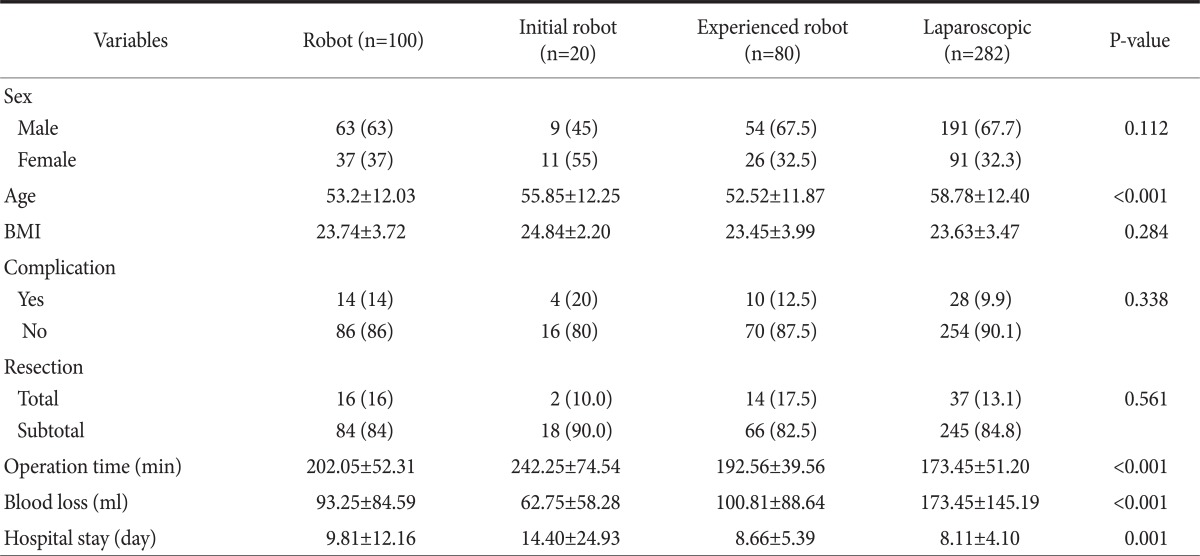
The average age of the patients in the LG group was higher than that of the RG group, but there was no difference in average age between the initial and experienced groups (P=0.280). There was also no difference in complication rates among the three groups. All group had some total gastrectomy cases but there is no difference in complication rate between total gastrectomy and subtotal gastrectomy. The average operating time, blood loss and hospital stay showed statistically significant differences among the initial, experienced and laparoscopy groups in a one-way ANOVA analysis. Therefore, a post-hoc test was performed among these groups (Table 2). The average operating time was shorter in the laparoscopy group compared to the initial group (P<0.001), and the average operating time of the experienced group was shorter than that of the initial group (P<0.001). However, the experienced group had a longer average operating time than the laparoscopy group (P=0.006) (Fig. 4A). There was less blood loss in the RG groups than in the LG group (P<0.001 for initial, P=0.001 for experienced), but no differences were found between the initial and experienced groups (P=0.501) (Fig. 4B). The initial group had a longer average hospital stay than did the experienced (P=0.003) and laparoscopy (P<0.001) groups; however, no difference was found in hospital stay times between the experienced and laparoscopy groups (P=0.849) (Fig. 4C).
Table 2.
Re-grouping of robot-assisted surgery characteristics
Values are presented as number (%) or mean±standard deviation. Blood loss, operation time, and hospital stay time showed statistically significant differences. P-value was from a one-way ANOVA among the initial, experienced and laparoscopic groups. BMI = body mass index.
Fig. 4.
(A) Comparison of the operating time among initial robotic, experienced robotic and laparoscopic gastrectpmy groups. (B) Comparison of the blood loss during the operation among the initial robotic, experienced robotic and laparoscopic gastrectomy groups. (C) Comparison of the length of hospital stay among the initial robotic, experienced robotic and laparoscopic gastrectomy groups. *Means P<0.05 by Tukey's honestly significant difference post-hoc test. Error bars represent standard error.
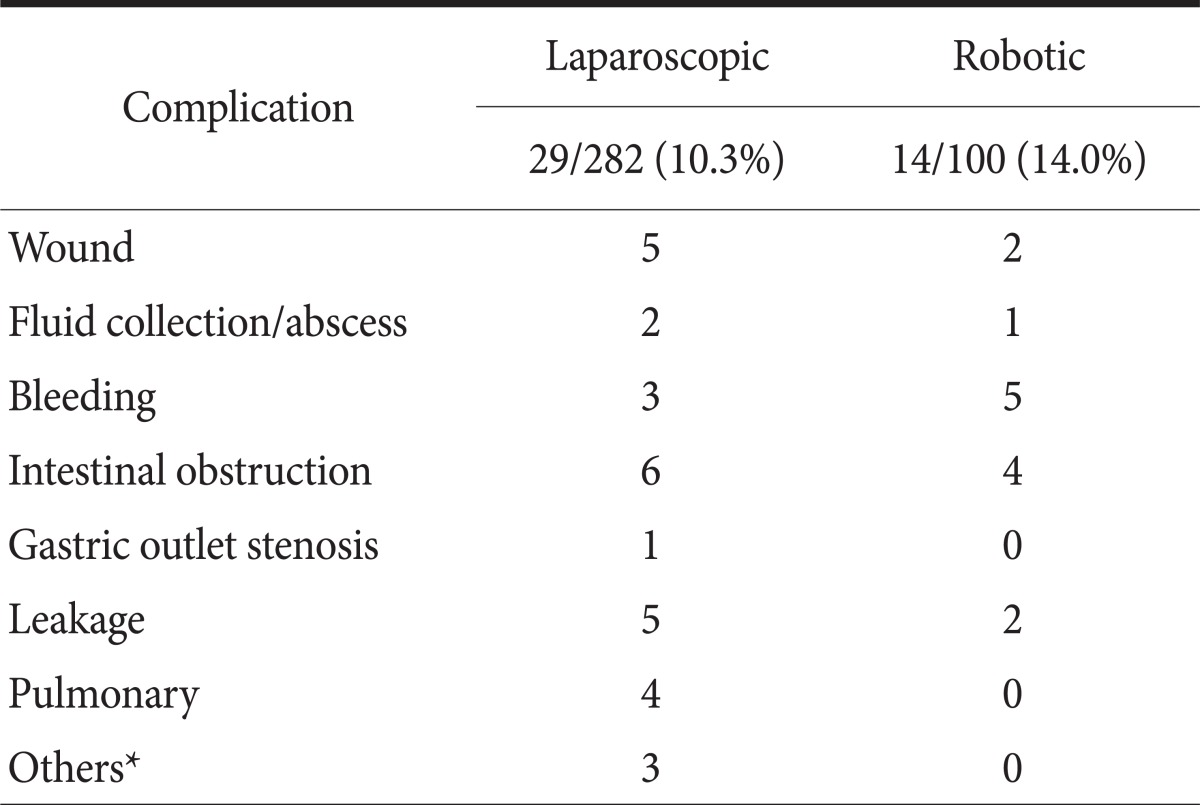
In total, 10.3% (29/282) of the LG group and 14.0% (14/100) of the RG group experienced complications (Table 3). Five RG cases required re-operation because of leakage and obstruction. These operations were performed using laparoscopy or laparotomy. No mortality occurred.
Table 3.
Surgical complications
Fluid collection was diagnosed by computed tomography scan. Iatrogenic colon perforation occurred during laparoscopic surgery, probably due to heat injury. *Pseudomembranous colitis, delirium, iatrogenic colon perforation.
Discussion
In this study, we found RG to be a feasible alternative to LG with regard to early operative outcomes. For an experienced laparoscopy surgeon, after the initial 20 cases of RG, the surgical outcomes were similar to those of LG and could even be considered better than LG when considering the decreased blood loss.
Because of the learning curve of moving to LG from laparotomy, 50~100 cases were needed to stabilize the operating procedure and operating time because of the issues of handling the device and becoming accustomed to the scope view.(14,15) For anastomosis, a learning curve was also present when moving from extracorporeal to intracoporeal anastomosis. By contrast, the learning curve when moving to robot gastrectomy from laparoscopy was much shorter,(16,17) because the operating procedure and scope view were similar to those used in laparoscopy and because anastomosis could be performed by laparoscopic devices (with the exception of robot-sewn anastomosis). The first assistant still used a laparoscopic device (via one port), so he or she did not need to learn a new procedure. The sense of touch of the robotic devices and mastering the movement of the scope were the main problems. Our center usually uses a flexible scope for LG, but the robot only had a rigid scope. However, the three-dimensional images and scaled motion of the robot arm provided a better surgical field. Therefore, 20 cases were sufficient to overcome these problems for the experienced laparoscopic surgeon in our cases.
RG took an average of 202.05±52.31 minutes. This operating time is shorter than other reported operating times (231.3±43.2 minutes by Song et al.(9)), but it was longer than laparoscopy operating times (173.45±51.20 minutes) in our study. The initial robot group had the longest average operating time because the operations proceeded very carefully and because the operator was not familiar with the docking procedure. The experienced group had a shorter average operating time than the initial group, but this time was still significantly longer than that of the LG group. Unfortunately we did not collect docking time data initially, so actual docking time cannot be calculated. But we have known that initial docking time was much longer than these days. Song et al.(9) reported that additional time for docking or a mini-laparotomy contributed to the longer operating time in RG, and the mean additional time was 63.3 minutes. Therefore, even in the experienced group, the operating time was longer than that of LG.(9) In particular, the docking time was much shorter than for the initial group, but it still contributed to the longer overall time. To decrease this additional time, intracoporeal anastomosis was considered because it does not require a mini-laparotomy and because it creates a smaller wound. Vertical or transverse incisions were made in the supra-umbilicus area in extracorporeal anastomosis, but infra-umbilicus incisions were made in intracoporeal anastomosis. Small and infra-umbilical wounds create less pain, and patients were more satisfied under these conditions. In intracoporeal anastomosis, reinforced or anastomosis sutures were sometimes needed. In the suture technique, handling was made much easier by scaled motion of robot arm, and completely robot-sewn anastomosis was possible.(20) In our center, recent robot-assisted surgeries have used intracoporeal anastomosis. Further study is needed to decrease the operating time with this type of anastomosis.
RG led to less bleeding than LG. LG has limits of motion that make bleeding possible, especially during the dissection of lymph nodes #6, #14, #7, #8, and #9.(6) Most bleeding occurred due to limitations of motion and sight. However, the scaled motion of the robot arm and the three-dimensional images in RG made possible a more precise surgery with less bleeding. Woo et al.(11) has reported that robot-assisted surgeries led to less bleeding and less standard deviation, indicating more consistent surgical procedures. Our study also revealed less standard deviation in RG (84.59 ml vs. 145.19 ml). Although the difference was not significant, the initial group experienced less bleeding than the experienced group. In the initial cases, RG was approached more carefully, so major bleeding did not occur. Moreover, the initial group had fewer total gastrectomy cases (10.0% vs. 17.5%) than the experienced group; dissection of the short gastric artery can lead to bleeding.
RG patients had longer average hospital stays than did LG patients (9.81 days vs. 8.11 days, P=0.042). However, the experienced group had similar hospital stays as the LG group (8.66 days vs. 8.11 days, P=0.522). In the initial group, one patient experienced serious complications due to anastomosis leakage and required re-operation and intensive care. Because she had subtotal gastrectomy and extracorporeal anastomosis, robot procedure might not cause of leakage. She stayed at the hospital for 118 days from the time of the initial operation but was discharged as healed and healthy. Sometimes, patients in the RG group refused to be discharged. They already paid the high operation costs, so additional costs were not an issue. Moreover, in Korea, due to the national health care system, the costs of hospital rooms and medications are not prohibitively expensive. We recommended discharge on postoperative day 6 as part of the CP, but most patients (including LG patients) wanted to stay until they healed "complete." The patients in the RG group wanted to stay longer than those in the LG group.
Our study had some limitations. First, we did not compare the costs of these different types of surgeries. RG is not reimbursed by health insurance in Korea, so its cost is approximately three times higher than that of LG. It could be less cost-effective than LG, but RG does not require a scopist or second assistant; thus, it only requires three people (an operator, a first assistant, and a scrub nurse). Both open surgery and laparoscopic surgery require a second assistant, so RG saves labor costs. Further comparison studies and evaluations are needed to explore this issue. Second, the study was not a randomized controlled trial and prospective analysis. It is difficult to perform a randomized study of this type because RG is very expensive. But indication of surgery and postoperative management was same between LG and RG for decreasing selection bias. Third, oncological outcomes were not obtained because the follow-up period was too short. However, RG had a similar procedure to LG procedures, and no technical problems arose. One study reported that the surgical margin status of robot-assisted surgery was similar to that of laparoscopic or open surgical procedures.(11) We expect that the oncological outcomes would be comparable. Finally, RG cases were rare than LG cases during this time period.
Our RG is a feasible alternative to LG in early postoperative outcomes. However, 20 cases were needed to overcome the learning curve.
Acknowledgments
This work was supported by a grant from Korea Healthcare technology R&D project, Ministry of Health, Welfare & Family Affairs, Korea (1020410).
References
- 1.Shin HR, Jung KW, Won YJ, Kong HJ, Yim SH, Sung J, et al. National cancer incidence for the year 2002 in Korea. Cancer Res Treat. 2007;39:139–149. doi: 10.4143/crt.2007.39.4.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn HS, Lee HJ, Yoo MW, Jeong SH, Park DJ, Kim HH, et al. Changes in clinicopathological features and survival after gastrectomy for gastric cancer over a 20-year period. Br J Surg. 2011;98:255–260. doi: 10.1002/bjs.7310. [DOI] [PubMed] [Google Scholar]
- 3.Kim MC, Kim W, Kim HH, Ryu SW, Ryu SY, Song KY, et al. Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group. Risk factors associated with complication following laparoscopy-assisted gastrectomy for gastric cancer: a large-scale korean multicenter study. Ann Surg Oncol. 2008;15:2692–2700. doi: 10.1245/s10434-008-0075-z. [DOI] [PubMed] [Google Scholar]
- 4.Shehzad K, Mohiuddin K, Nizami S, Sharma H, Khan IM, Memon B, et al. Current status of minimal access surgery for gastric cancer. Surg Oncol. 2007;16:85–98. doi: 10.1016/j.suronc.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N Japanese Laparoscopic Surgery Study Group. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68–72. doi: 10.1097/01.sla.0000225364.03133.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim MC, Heo GU, Jung GJ. Robotic gastrectomy for gastric cancer: surgical techniques and clinical merits. Surg Endosc. 2010;24:610–615. doi: 10.1007/s00464-009-0618-9. [DOI] [PubMed] [Google Scholar]
- 7.Cadière GB, Himpens J, Germay O, Izizaw R, Degueldre M, Vandromme J, et al. Feasibility of robotic laparoscopic surgery: 146 cases. World J Surg. 2001;25:1467–1477. doi: 10.1007/s00268-001-0132-2. [DOI] [PubMed] [Google Scholar]
- 8.Lanfranco AR, Castellanos AE, Desai JP, Meyers WC. Robotic surgery: a current perspective. Ann Surg. 2004;239:14–21. doi: 10.1097/01.sla.0000103020.19595.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song J, Oh SJ, Kang WH, Hyung WJ, Choi SH, Noh SH. Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg. 2009;249:927–932. doi: 10.1097/01.sla.0000351688.64999.73. [DOI] [PubMed] [Google Scholar]
- 10.Pugliese R, Maggioni D, Sansonna F, Ferrari GC, Forgione A, Costanzi A, et al. Outcomes and survival after laparoscopic gastrectomy for adenocarcinoma. Analysis on 65 patients operated on by conventional or robot-assisted minimal access procedures. Eur J Surg Oncol. 2009;35:281–288. doi: 10.1016/j.ejso.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Woo Y, Hyung WJ, Pak KH, Inaba K, Obama K, Choi SH, et al. Robotic gastrectomy as an oncologically sound alternative to laparoscopic resections for the treatment of early-stage gastric cancers. Arch Surg. 2011;146:1086–1092. doi: 10.1001/archsurg.2011.114. [DOI] [PubMed] [Google Scholar]
- 12.Anderson C, Ellenhorn J, Hellan M, Pigazzi A. Pilot series of robot-assisted laparoscopic subtotal gastrectomy with extended lymphadenectomy for gastric cancer. Surg Endosc. 2007;21:1662–1666. doi: 10.1007/s00464-007-9266-0. [DOI] [PubMed] [Google Scholar]
- 13.Patriti A, Ceccarelli G, Bellochi R, Bartoli A, Spaziani A, Di Zitti L, et al. Robot-assisted laparoscopic total and partial gastric resection with D2 lymph node dissection for adenocarcinoma. Surg Endosc. 2008;22:2753–2760. doi: 10.1007/s00464-008-0129-0. [DOI] [PubMed] [Google Scholar]
- 14.Kim MC, Jung GJ, Kim HH. Learning curve of laparoscopy-assisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J Gastroenterol. 2005;11:7508–7511. doi: 10.3748/wjg.v11.i47.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin SH, Kim DY, Kim H, Jeong IH, Kim MW, Cho YK, et al. Multidimensional learning curve in laparoscopy-assisted gastrectomy for early gastric cancer. Surg Endosc. 2007;21:28–33. doi: 10.1007/s00464-005-0634-3. [DOI] [PubMed] [Google Scholar]
- 16.Park SS, Kim MC, Park MS, Hyung WJ. Rapid adaptation of robotic gastrectomy for gastric cancer by experienced laparoscopic surgeons. Surg Endosc. 2012;26:60–67. doi: 10.1007/s00464-011-1828-5. [DOI] [PubMed] [Google Scholar]
- 17.Song J, Kang WH, Oh SJ, Hyung WJ, Choi SH, Noh SH. Role of robotic gastrectomy using da Vinci system compared with laparoscopic gastrectomy: initial experience of 20 consecutive cases. Surg Endosc. 2009;23:1204–1211. doi: 10.1007/s00464-009-0351-4. [DOI] [PubMed] [Google Scholar]
- 18.Oh DK, Hur H, Kim JY, Han SU, Cho YK. V-shaped liver retraction during a laparoscopic gastrectomy for gastric cancer. J Gastric Cancer. 2010;10:133–136. [Google Scholar]
- 19.Song HM, Lee SL, Hur H, Cho YK, Han SU. Linear-shaped gastroduodenostomy in totally laparoscopic distal gastrectomy. J Gastric Cancer. 2010;10:69–74. [Google Scholar]
- 20.Hur H, Kim JY, Cho YK, Han SU. Technical feasibility of robot-sewn anastomosis in robotic surgery for gastric cancer. J Laparoendosc Adv Surg Tech A. 2010;20:693–697. doi: 10.1089/lap.2010.0246. [DOI] [PubMed] [Google Scholar]