Abstract
Purpose
To report the initial clinical experience with single-incision laparoscopic gastric wedge resection for submucosal tumors.
Materials and Methods
The medical records of 10 patients who underwent single-incision laparoscopic gastric wedge resection between July 2009 and March 2011 were reviewed retrospectively. The demographic data, clinicopathologic and surgical outcomes were assessed.
Results
The mean tumor size was 2.5 cm (range, 1.2~5.0 cm), and the tumors were mostly located on the anterior wall (4/10) or along the greater curvature (4/10), of the stomach. Nine of ten procedures were performed successfully, without the use of additional trocars, or conversion to laparotomy. One patient underwent conversion to multiport laparoscopic surgery, to get simultaneous cholecystectomy safely. The mean operating time was 66.5 minutes (range, 24~132 minutes), and the mean postoperative hospital stay was 5 days (range, 4~7 days). No serious perioperative complications were observed. Of the 10 submucosal tumors, the final pathologic report revealed 5 gastrointestinal stromal tumors, 4 schwannomas, and 1 heterotopic pancreas.
Conclusions
Single-incision laparoscopic gastric wedge resection for gastric submucosal tumors is feasible and safe, when performed by experienced laparoscopic surgeons. This technique provides favorable cosmetic results, and also short hospital stay and low morbidity, in carefully selected candidates.
Keywords: Stomach neoplasms; Gastrointestinal stromal tumors; Gastrectomy; Surgical procedures, minimally invasive
Introduction
Laparoscopic surgery is a well-established alternative to open surgery in various abdominal conditions. In general, the benefits of laparoscopy in terms of postoperative pain, recovery, and cosmetic results are widely recognized.
Many surgeons have attempted to reduce the invasiveness of traditional laparoscopic surgery and to achieve better cosmetic results. Recently, two revolutionary techniques were developed: natural orifice transluminal endoscopic surgery (NOTES),(1-4) in which transabdominal incisions are completely avoided, and single-incision laparoscopic surgery (SILS),(5-9) in which laparoscopic procedures are performed through a single umbilical incision. NOTES may be the final frontier of minimally invasive surgery; however, it requires a transition from laparoscopic surgical skills to endoscopic surgical skills and further development of incomplete technology. As a bridge between NOTES and traditional laparoscopic surgery, SILS came into the spotlight because it minimizes invasiveness by reducing the number of incisions, thereby also reducing the degree of postoperative pain.(10)
Our institution began performing SILS for gastric submucosal tumors (SMTs) in July 2009, and we report the technical pitfalls and results of our initial experience of single-incision laparoscopic gastric wedge resection for SMTs in 10 patients.
Materials and Methods
Ten patients underwent single-incision laparoscopic gastric wedge resection for gastric SMTs between July 2009 and March 2011 at our institution. The operations were performed by two surgeons experienced in conventional laparoscopic surgery; each surgeon had performed as many as 200 laparoscopic gastric resections before this challenging procedure. The procedure was offered to patients eligible for laparoscopic gastric wedge resection; those who had tumors 2 to 5 cm in size or rapidly increasing during the follow up period, especially if the tumor was located on the anterior wall or along the greater curvature of the stomach. Since fine needle aspiration or core needle biopsy is considered to carry a risk of tumor dissemination, it is rarely performed before surgical resection in our hospital. The medical records of these patients were reviewed retrospectively for demographic data, diagnostic modalities, operative procedures, clinicopathological findings, and follow-up. Operating time and specific tumor location in the stomach were also recorded.
1. Surgical technique
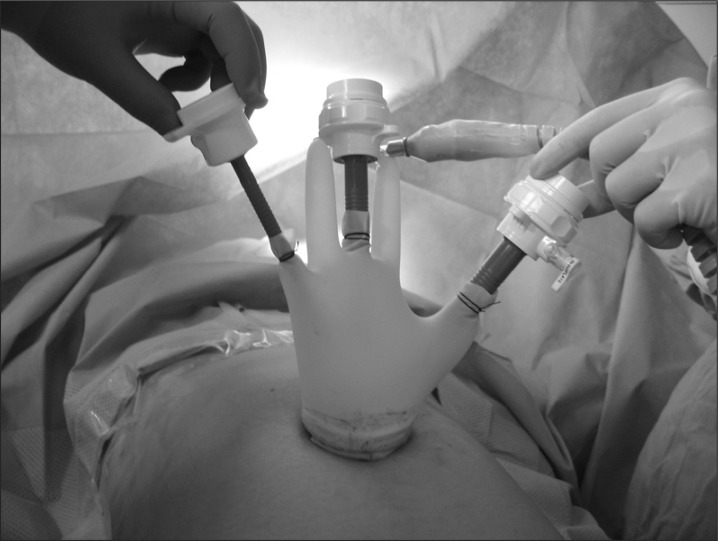
A 3 to 4 cm-long single vertical incision was made in the umbilicus, and access to the peritoneal cavity was achieved via an open technique. Then, the two surgeons used different types of platforms to introduce laparoscopic instruments into the abdominal cavity through a single incision. One surgeon used a homemade single-port device composed of a small wound protractor (Alexis®, Applied Medical, Rancho Santa Margarita, CA, USA) and a surgical glove for 4 patients (Fig. 1), and the other used the commercially available OCTO-port (DalimSurgNet, Seoul, Korea) or SILS™ port (Covidien, Mansfield, MA, USA) for the remaining 6 patients. A rigid 10 mm laparoscope of 30 degrees and conventional laparoscopic instruments with additional articulating instruments were used to optimize the range of motion.
Fig. 1.
Home-made single-port device was made from small wound protractor and a surgical glove with conventional laparoscopic trocars.
Each step in the single-incision laparoscopic procedure was similar to that in the conventional laparoscopic procedure. Tumor location was detected mostly by indirect palpation with laparoscopic instruments, but intraoperative endoscopic assistance was necessary in 2 patients when small endoluminal tumors were not detected by laparoscopic exploration. During the endoscopic procedures, proximal jejunum below the Treiz ligament was gently clamped with laparoscopic instruments to minimize the gas insufflation into the small bowel. After identifying the lesions, the greater omentum and/or short gastric vessels were divided from the greater curvature with ultrasonic coagulating shears, as per requirement. The tumor was elevated by retracting the normal gastric wall near the tumor with a articulating laparoscopic grasper or by pulling the tagging suture on the gastric wall near the tumor. Partial resection of the stomach including the tumor lesion was carried out using multiple endoscopic linear staplers, securing the gross negative margin. After meticulous hemostasis was achieved along the stapled line, the specimen was placed in a laparoscopic specimen bag and extracted through the umbilicus. Finally, the umbilical wound was closed with an absorbable suture and the umbilicus was restored to its physiological position.
Results
The mean age at presentation was 54.5 years (range, 44~79), and the patients consisted of 6 men and 4 women. Most patients (80%) were asymptomatic at presentation, with the tumors found incidentally during regular checkups. Other initial symptoms included regurgitation and dyspepsia. No metastatic disease was observed during the initial visit.
The diagnostic tools used most often to characterize the tumor before surgery were esophagogastroduodenoscopy (EGD) and computed tomography. All 10 patients underwent both, and 9 out of 10 also underwent additional endoscopic ultrasonography to delineate the anatomic layer of the tumor origin. All tumors were detected by EGD, and the mean tumor size measured by EGD was 2.5 cm (range, 1.2~5.0 cm). According to the EGD findings, all tumors were located in the body of the stomach (4 at angle, 4 in the lower body, and the other 2 in the mid-body). Among the 10 gastric SMTs located in the body of stomach, 4 tumors were localized on the anterior wall, 2 were localized on the posterior wall, and 4 were localized along the greater curvature. Eight of 10 patients underwent routine intraluminal endoscopic biopsy, but all failed to obtain significant microscopic diagnosis other than chronic gastritis.
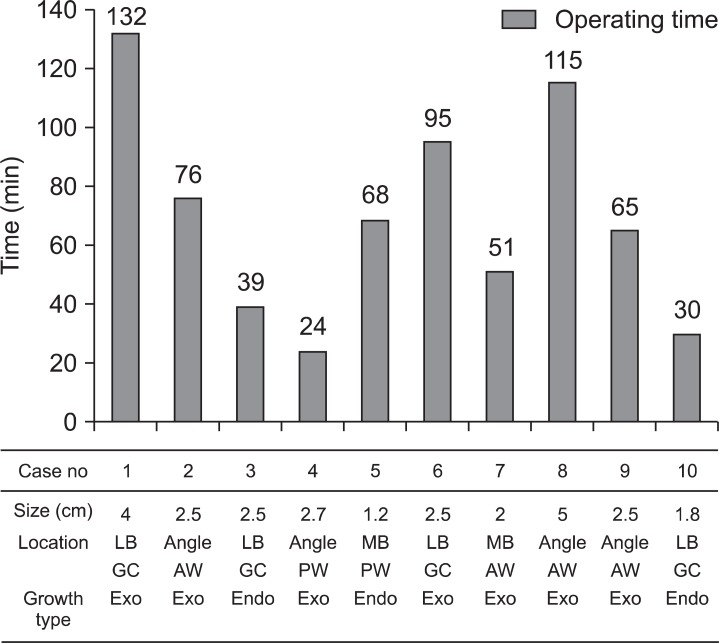
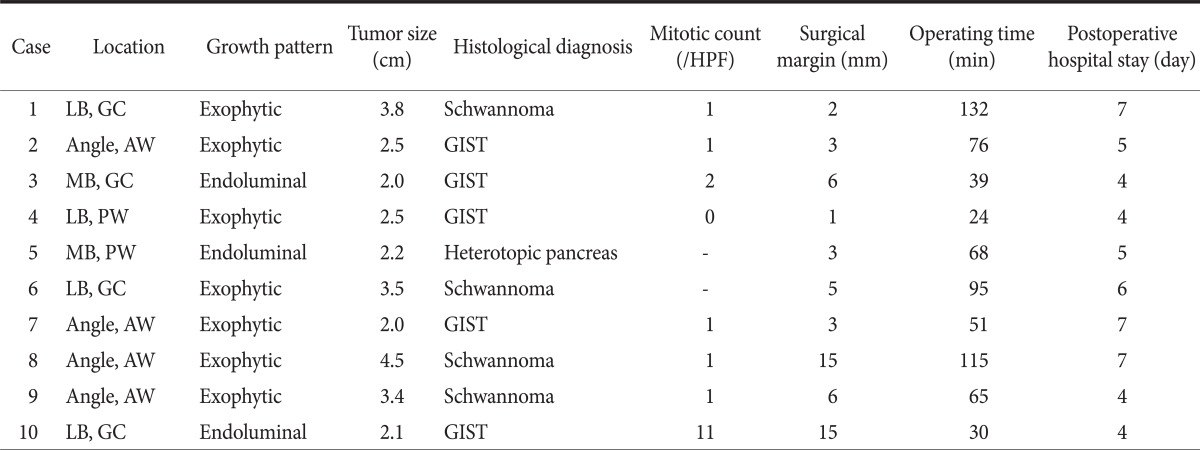
Single-incision laparoscopic gastric wedge resection was successfully completed in 9 patients without conversion to conventional laparoscopic surgery. In 1 patient, moderate hemorrhagic event occurred during cholelcystectomy after the successful gastric resection, and 2 additional trocars were needed to safely perform simultaneous cholecystectomy for comorbid gall bladder stones. All tumors were resected via an extraluminal approach. The mean operating time was 66.5 minutes (range, 24~132 minutes). The operating time varied a lot according to the tumor size and location (Fig. 2). The estimated blood loss during the operation was minimal in all patients. The mean postoperative hospital stay was 5 days (range, 4~7 days). The postoperative course was uneventful, with no postoperative complications in the follow-up period. The final pathology confirmed 5 gastrointestinal stromal tumors, 4 schwannomas, and 1 heterotopic pancreas. Eight specimens showed ≤5 mitoses in 50 high-power fields, although 1 case showed 11 mitoses per 50 high-power fields. The mean distance from the tumor to the resection margin was 0.4 cm (range, 0.1~1.5 cm). Nine patients were followed regularly, and the mean follow-up duration was 13 months (range, 2~25 months), during which time no recurrence or metastases were observed (Table 1). The mean follow-up duration for 5 gastrointestinal stromal tumor (GIST) patients was 15 months (range, 12~25 months).
Fig. 2.
Operating time and tumor characteristics. The operating time varied according to tumor size and location, but generally tended to decline with the accumulation of the surgeon's experience. HPF = high power field; LB = lower body; MB = mid-body; GC = greater curvature; AW = anterior wall; PW = posterior wall; exo = exophytic; endo = endoluminal.
Table 1.
Patients, tumor characteristics, and perioperative outcomes
HPF = high power field; LB = lower body; GC = greater curvature; AW = anterior wall; GIST = gastrointestinal stromal tumor; MB = mid-body; PW = posterior wall.
Discussion
Gastric SMTs encompass both neoplastic and nonneoplastic lesions of various etiologies, which can be either benign or potentially malignant. Although endoscopy can accurately identify these lesions, it is difficult to arrive at a preoperative histological diagnosis through routine intraluminal endoscopic biopsy. Preoperative fine-needle aspiration biopsy and core needle biopsy under endoscopic ultrasonographic guidance might be useful diagnostic tools, but are not applicable in all cases and can also lead to tumor cell dissemination.(11) Furthermore, even with needle biopsy specimen, it remains difficult to predict whether the tumor poses malignant potential to metastasize to distant organs.(12) Thus, without clear evidence of benign features, SMTs should be considered GISTs, which accounts for the majority of gastric SMTs, and should be resected to arrive at a definite pathological diagnosis and ensure removal of the lesion.
In recent years, laparoscopic wedge resection has been regarded as safe and technically feasible with favorable oncologic outcomes when performed by skilled surgeons.(11,13-16) Generally accepted indications for laparoscopic management of gastric SMTs include tumors between 2 and 5 cm in diameter, rapid increase in tumor size during endoscopic surveillance, and presence of symptoms. Incidentally discovered SMTs less than 2 cm in diameter are usually followed up by endoscopy or computed tomography every 6 months to 12 months. For patients with rapidly growing tumors suspected to possess malignant potential, surgical resection is strongly recommended regardless of tumor size.(11,14,16-18) Of the patients for whom laparoscopic wedge resection is indicated, those who have suitable tumor locations and characteristics can undergo a SILS approach.
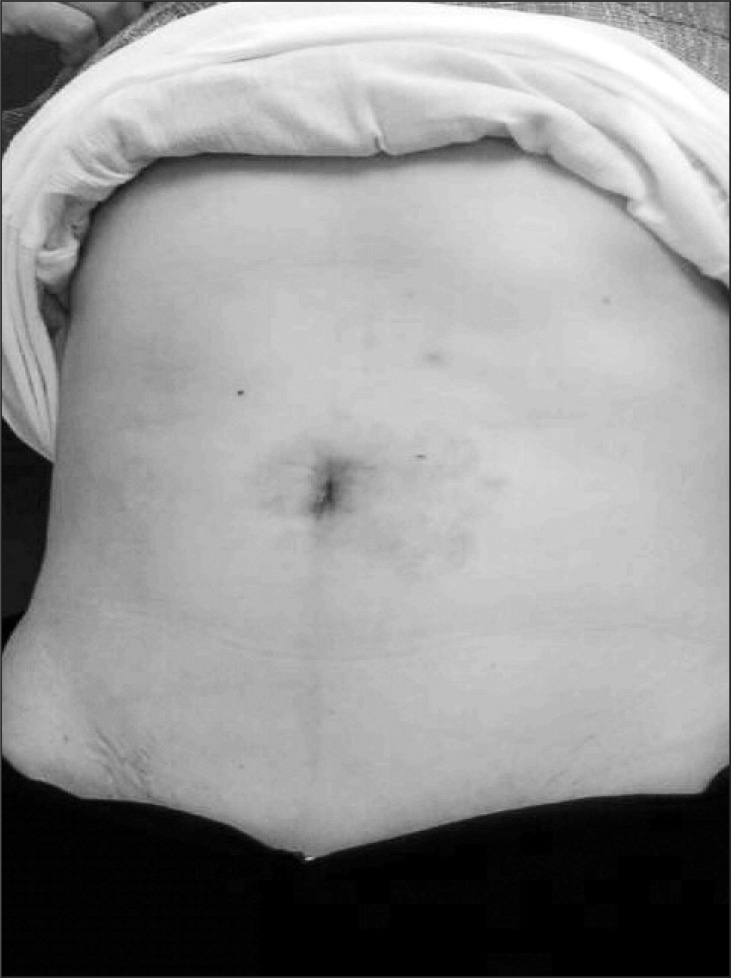
The perioperative results in our patients were thought to be comparable to those in the previously reported laparoscopic series.(15,16) The cosmetic outcomes after SILS were excellent as the only single incision made stayed hidden inside the umbilicus (Fig. 3). Generally, the length of postoperative hospital stay is considered as a parameter of invasiveness of surgery.(19) The mean hospital stay after SILS gastric wedge resection was 5 days, which is acceptable and even shorter than that seen after conventional laparoscopic surgery. These results suggest that SILS procedure can provide patients with the following benefits: better cosmesis, less postoperative pain, fewer postoperative morbidities, and shorter convalescence. This also satisfies a growing demand for less-invasive surgical procedures. Moreover, SILS can be performed with conventional laparoscopic instruments and skills, with little modification.
Fig. 3.
Photograph of the abdomen taken 1 month after the operation shows excellent cosmesis with minimal scar.
The most challenging point in SILS as well as conventional laparoscopic surgery is confirming the precise location of intraluminal tumors and determining the appropriate resection line. Because excessive resection may result in deformity of the stomach, and consequent gastric stasis, detecting the precise location of the tumor during surgery is crucial. Several methods have been used to demarcate the tumor, such as laparoscopic ultrasonography, endoscopic intraluminal marking with dye, diaphanoscopy, and magnetic marking clips,(20,21) but all these methods are cumbersome, often expensive and still do not elucidate the exact location of the tumor. Recent reports have described various combined laparoscopic and endoscopic surgical techniques involving the simultaneous use of two procedures.(22,23) Although they showed some promising results, all these methods were used and evaluated in conventional multiport laparoscopic surgeries. The use of these methods is expected to be far more challenging during single-incision surgery and needs further evaluation to be generalized in clinical practice.
In addition, SILS has some technical challenges. Insertion of several instruments, along with the laparoscope, into the abdominal cavity through a single incision markedly decreases the range of motion and results in conflict between instruments. This conflict makes surgical dissection much more difficult compared to conventional multiport laparoscopic surgery. It is also difficult to keep an ideal view due to consistent clash of camera with operating instruments, so that expert camera operator experienced in handling flexible or 30 degree laparoscope is essential. The use of articulating instruments (laparoscopic graspers, shears, and staplers) could improve the performance with less interference, and the recently introduced pre-bent instruments further facilitate the SILS procedure with lower time requirement and better maneuverability.(24) Adopting right-angle light cable adaptor which makes light cable run parallel with laparoscope and a long-shaft camera would further reduce external collisions.(25) Nevertheless, a learning curve for these instruments is expected before their practical use, and further development of instrumentation is required for the widespread use of this new technique in more complicated procedures. It should be noted that SILS can be easily converted into conventional laparoscopic surgery by adding a few extra ports. Some investigators introduced 2~3 mm instruments, which serves as atraumatic retractors to secure a better surgical view and still leaves no scar after surgery(9); this could be an alternative solution for better surgical performance during SILS.
A small extension of the transumbilical incision can be considered for difficult-to-access tumors. Transumbilical incision with slight extension in SILS can be used to exteriorize the lesion for resection when the tumor is hard to access or manipulate in the abdominal cavity; this would further facilitate SILS in many cases.
This study has several limitations. The number of patients included in the present study was small, and the comparative data between SILS and conventional laparoscopic approach were not presented. Additional prospective randomized controlled trials are needed to examine its feasibility and safety. The participating two surgeons utilized different types of platform to perform single-incisional wedge resection in the present study; this would have influenced the surgical performance and serve as a limitation of the present study.
In conclusion, gastric wedge resection using SILS for gastric SMTs is safe and feasible when performed by experienced laparoscopic surgeons. This scar-free abdominal procedure is a promising alternative method for the treatment of gastric SMTs, especially those located at the anterior wall or along the greater curvature of the stomach. However, careful selection of patients based on tumor location and a standardized method for tumor localization should precede the widespread use of this new technique. Although the immediate benefit appears to be cosmetic, potential benefits and disadvantages require further evaluation. Prospective randomized studies comparing SILS and conventional laparoscopic surgery for gastric wedge resection are necessary to confirm the benefits of this new technique.
References
- 1.Allori AC, Leitman IM, Heitman E. Natural orifice transluminal endoscopic surgery: lessons learned from the laparoscopic revolution. Arch Surg. 2008;143:333–334. doi: 10.1001/archsurg.143.4.333. [DOI] [PubMed] [Google Scholar]
- 2.de la Fuente SG, Demaria EJ, Reynolds JD, Portenier DD, Pryor AD. New developments in surgery: Natural Orifice Transluminal Endoscopic Surgery (NOTES) Arch Surg. 2007;142:295–297. doi: 10.1001/archsurg.142.3.295. [DOI] [PubMed] [Google Scholar]
- 3.Flora ED, Wilson TG, Martin IJ, O'Rourke NA, Maddern GJ. A review of natural orifice translumenal endoscopic surgery (NOTES) for intra-abdominal surgery: experimental models, techniques, and applicability to the clinical setting. Ann Surg. 2008;247:583–602. doi: 10.1097/SLA.0b013e3181656ce9. [DOI] [PubMed] [Google Scholar]
- 4.Bessler M, Stevens PD, Milone L, Parikh M, Fowler D. Transvaginal laparoscopically assisted endoscopic cholecystectomy: a hybrid approach to natural orifice surgery. Gastrointest Endosc. 2007;66:1243–1245. doi: 10.1016/j.gie.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Hong TH, You YK, Lee KH. Transumbilical single-port laparoscopic cholecystectomy: scarless cholecystectomy. Surg Endosc. 2009;23:1393–1397. doi: 10.1007/s00464-008-0252-y. [DOI] [PubMed] [Google Scholar]
- 6.Reavis KM, Hinojosa MW, Smith BR, Nguyen NT. Single-laparoscopic incision transabdominal surgery sleeve gastrectomy. Obes Surg. 2008;18:1492–1494. doi: 10.1007/s11695-008-9649-x. [DOI] [PubMed] [Google Scholar]
- 7.Navarra G, Pozza E, Occhionorelli S, Carcoforo P, Donini I. One-wound laparoscopic cholecystectomy. Br J Surg. 1997;84:695. [PubMed] [Google Scholar]
- 8.Podolsky ER, Curcillo PG., 2nd Single port access (SPA) surgery--a 24-month experience. J Gastrointest Surg. 2010;14:759–767. doi: 10.1007/s11605-009-1081-6. [DOI] [PubMed] [Google Scholar]
- 9.Omori T, Oyama T, Akamatsu H, Tori M, Ueshima S, Nishida T. Transumbilical single-incision laparoscopic distal gastrectomy for early gastric cancer. Surg Endosc. 2011;25:2400–2404. doi: 10.1007/s00464-010-1563-3. [DOI] [PubMed] [Google Scholar]
- 10.Lee YS, Kim JH, Moon EJ, Kim JJ, Lee KH, Oh SJ, et al. Comparative study on surgical outcomes and operative costs of transumbilical single-port laparoscopic appendectomy versus conventional laparoscopic appendectomy in adult patients. Surg Laparosc Endosc Percutan Tech. 2009;19:493–496. doi: 10.1097/SLE.0b013e3181c15493. [DOI] [PubMed] [Google Scholar]
- 11.Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, et al. GIST consensus meeting panelists. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005;16:566–578. doi: 10.1093/annonc/mdi127. [DOI] [PubMed] [Google Scholar]
- 12.Kinoshita K, Isozaki K, Tsutsui S, Kitamura S, Hiraoka S, Watabe K, et al. Endoscopic ultrasonography-guided fine needle aspiration biopsy in follow-up patients with gastrointestinal stromal tumours. Eur J Gastroenterol Hepatol. 2003;15:1189–1193. doi: 10.1097/00042737-200311000-00007. [DOI] [PubMed] [Google Scholar]
- 13.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otani Y, Furukawa T, Yoshida M, Saikawa Y, Wada N, Ueda M, et al. Operative indications for relatively small (2-5 cm) gastrointestinal stromal tumor of the stomach based on analysis of 60 operated cases. Surgery. 2006;139:484–492. doi: 10.1016/j.surg.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Huguet KL, Rush RM, Jr, Tessier DJ, Schlinkert RT, Hinder RA, Grinberg GG, et al. Laparoscopic gastric gastrointestinal stromal tumor resection: the mayo clinic experience. Arch Surg. 2008;143:587–590. doi: 10.1001/archsurg.143.6.587. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki A, Koeda K, Obuchi T, Nakajima J, Nishizuka S, Terashima M, et al. Tailored laparoscopic resection for suspected gastric gastrointestinal stromal tumors. Surgery. 2010;147:516–520. doi: 10.1016/j.surg.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 17.Ryu KJ, Jung SR, Choi JS, Jang YJ, Kim JH, Park SS, et al. Laparoscopic resection of small gastric submucosal tumors. Surg Endosc. 2011;25:271–277. doi: 10.1007/s00464-010-1173-0. [DOI] [PubMed] [Google Scholar]
- 18.Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8(Suppl 2):S1–S41. doi: 10.6004/jnccn.2010.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002;131:S306–S311. doi: 10.1067/msy.2002.120115. [DOI] [PubMed] [Google Scholar]
- 20.Santambrogio R, Montorsi M, Schubert L, Pisani Ceretti A, Costa M, Moroni E, et al. Laparoscopic ultrasound-guided resection of gastric submucosal tumors. Surg Endosc. 2006;20:1305–1307. doi: 10.1007/s00464-005-0600-0. [DOI] [PubMed] [Google Scholar]
- 21.Patrzyk M, Schreiber A, Heidecke CD, Glitsch A. Laser-supported diaphanoscopy: an innovative technique for locating gastric stromal tumors in gastroscopic-laparoscopic rendezvous: a case series. Endoscopy. 2009;41:1090–1094. doi: 10.1055/s-0029-1215321. [DOI] [PubMed] [Google Scholar]
- 22.Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, et al. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729–1735. doi: 10.1007/s00464-007-9696-8. [DOI] [PubMed] [Google Scholar]
- 23.Tanabe K, Urabe Y, Tokumoto N, Suzuki T, Yamamoto H, Oka S, et al. A new method for intraluminal gastrointestinal stromal tumor resection using laparoscopic seromuscular dissection technique. Dig Surg. 2010;27:461–465. doi: 10.1159/000320458. [DOI] [PubMed] [Google Scholar]
- 24.Stolzenburg JU, Kallidonis P, Oh MA, Ghulam N, Do M, Haefner T, et al. Comparative assessment of laparoscopic single-site surgery instruments to conventional laparoscopic in laboratory setting. J Endourol. 2010;24:239–245. doi: 10.1089/end.2009.0296. [DOI] [PubMed] [Google Scholar]
- 25.Ragupathi M, Nieto J, Haas EM. Pearls and pitfalls in SILS colectomy. Surg Laparosc Endosc Percutan Tech. 2012;22:183–188. doi: 10.1097/SLE.0b013e31824e2814. [DOI] [PubMed] [Google Scholar]