Abstract
Purpose
The use of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography-computed tomography as a routine preoperative modality is increasing for gastric cancer despite controversy with its usefulness in preoperative staging. In this study we aimed to determine the usefulness of preoperative positron emission tomography-computed tomography scans for staging of gastric cancer.
Materials and Methods
We retrospectively analyzed 396 patients' positron emission tomography-computed tomography scans acquired for preoperative staging from January to December 2009.
Results
The sensitivity of positron emission tomography-computed tomography for detecting early gastric cancer was 20.7% and it was 74.2% for advanced gastric cancer. The size of the primary tumor was correlated with sensitivity, and there was a positive correlation between T stage and sensitivity. For regional lymph node metastasis, the sensitivity and specificity of the positron emission tomography-computed tomography were 30.7% and 94.7%, respectively. There was no correlation between T stage and maximum standardized uptake value or between tumor markers and maximum standardized uptake value. Fluorodeoxyglucose uptake was detected by positron emission tomography-computed tomography in 24 lesions other than the primary tumors. Among them, nine cases were found to be malignant, including double primary cancers and metastatic cancers. Only two cases were detected purely by positron emission tomography-computed tomography.
Conclusions
Positron emission tomography-computed tomography could be useful in detecting metastasis or another primary cancer for preoperative staging in gastric cancer patients, but not for T or N staging. More prospective studies are needed to determine whether positron emission tomography-computed tomography scans should be considered a routine preoperative imaging modality.
Keywords: Positron-emission tomography and computed tomography, Cancer staging, Stomach neoplasms
Introduction
Currently, the main processes for treating gastric cancer include surgery, chemotherapy, and radiotherapy.(1,2) Among those treatments, surgery is considered the only curative treatment.(3,4) Surgery requires radical resection with appropriate lymph node resection, and some patients require neoadjuvant chemotherapy.(4,5) Therefore, it is very important that each patient is accurately staged preoperatively to ensure the appropriate treatment program and surgical extent is selected. Computed tomography (CT) and endoscopic ultrasonography are considered the standard imaging modalities in gastric cancer.(6-9) However, endoscopic ultrasonography cannot be used to evaluate the lymph nodes except the perigastric nodes, although it can be used to detect T stage and perigastric lymph nodes.(10) CT scans have sensitivity issues in the detection of small metastatic lymph nodes, and specificity issues in the detection of lymph nodes enlarged due to inflammation.(5) Additionally, a previous study found that 23% of cases that had no distant metastases on preoperative clinical and imaging studies did in fact show distal metastases during surgery.(11) Therefore other alternative, non-invasive modalities are needed in addition to the established standard imaging modalities to facilitate accurate preoperative staging. The use of 18F-2-deoxy-2-fluoro-d-glucose positron emission tomography-computed tomography (PET-CT) as a routine preoperative modality is increasing. Thus, we aimed to determine the usefulness of preoperative PET-CT scans for staging and predicting the prognosis of gastric cancer patients.
Materials and Methods
1. Patients
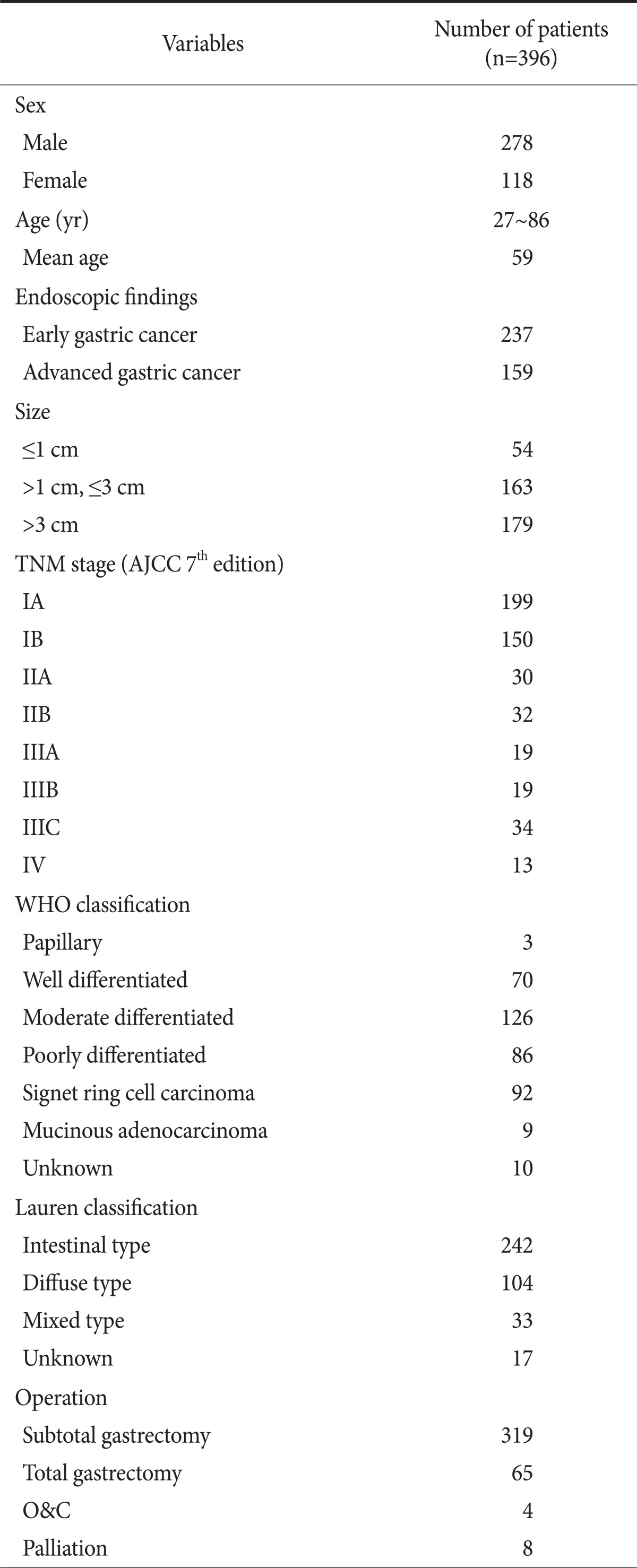
We retrospectively studied 396 patients who underwent PET-CT scanning prior to gastrectomy to treat gastric cancer at Kosin Medical College Department of Surgery from 1 January 2009 to 31 December 2009. Among these 396 patients (278 men, 118 women; age range: 27 to 86; mean age: 59 years), 384 underwent radical subtotal or total gastrectomies, 4 underwent open and closure (O&C) and 8 cases had palliative surgery. One patient who had subtotal gastrectomy and another patient who had palliative surgery had got neoadjuvant therapy. Patients who had other treatment modalities such as endoscopic mucosal resection and endoscopic submucosal dissection would be enrolled in this study only after gastrectomies. TNM staging was done based on specimen pathology determined after surgery. TNM staging was based on the America Joint Committee on Cancer Staging Manual-Seventh Edition.
To determine the utility of PET-CT for gastric cancer staging, we assessed the relationship between T stage and fluorodeoxyglucose (FDG) uptake, the sensitivity and specificity for N staging, the sensitivity for pathologic classification, and the relationships among carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9) and FDG uptake. We used SPSS for Windows (ver. 17.0, SPSS Inc., Chicago, IL, USA) for statistical analysis of the data. We also evaluated the clinical courses of the 24 patients in whom FDG uptake in lesions other than the primary tumor was detected.
2. PET-CT techniques
All patients fasted for at least six hours and were confirmed to have a serum glucose level less than 140 mg/dl before IV injection of FDG. Scanning was performed 60 minutes after FDG administration. Scans were acquired with a PET-CT system (CTI, Knoxville, TN, USA), which consisted of a full-ring positron emission tomography (PET) scanner and a dual-detector-row spiral CT scanner (Somatom Emotion Duo, Biograph, Erlangen, Germany). The CT scan was done from the head to the pelvic floor according to a standard protocol at 130 kVp and 30 mA with a tube rotation time of 0.8 seconds per rotation, a pitch of 6, and a 5 mm section thickness to match the PET section thickness. Immediately after the non-enhanced CT scan, PET was performed in the identical transverse field of view. PET data sets were obtained via interactive reconstruction using an ordered subset expectation maximization algorithm and by application of segmented attenuation correction (two interactions, 28 subsets) to the CT data. Co-registered scans were displayed with the software, which enabled image fusion and analysis.
3. Image analysis
PET-CT scans were interpreted by one nuclear medicine physician, and he defined a positive primary lesion as an abnormally higher FDG uptake compared to the uptake of the surrounding tissues. Positive lymph node metastases were also defined as abnormal FDG uptake in a lymph node higher than normal tissues. Positivity for double primary cancer or distant metastases were defined as significantly higher FDG uptake in the detected lesions compared to the uptake of the surrounding tissue
Results
1. Patient clinicopathologic characteristics
According to the endoscopic findings, there were 237 (59.8%) patients who had early gastric cancer and 159 (40.2%) who had advanced gastric cancer. The endoscopic findings of the advanced gastric cancers revealed 8, 88, 50 and 13 patients with Borrmann type I, II, III and IV, respectively. The primary lesion size was less than 1 cm in 54 patients, between 1 cm and 3 cm in 163 patients, and larger than 3 cm in 179 patients.
World Health Organization (WHO) and Lauren pathologic classifications were used in this study. The WHO system classifies tumors into the following sub-classes: papillary adenocarcinoma, well differentiated tubular adenocarcinoma (TA), moderately differentiated TA, poorly differentiated TA, mucinous adenocarcinoma and signet ring cell type. It also classifies tumors based on pathologic differentiation such as "differentiated adenocarcinoma" and "undifferentiated adenocarcinoma" (Table 1).
Table 1.
Patient clinicopathologic findings
AJCC = American Joint Committee on Cancer; WHO = World Health Organization; O&C = open and closure.
2. Detection of primary tumors
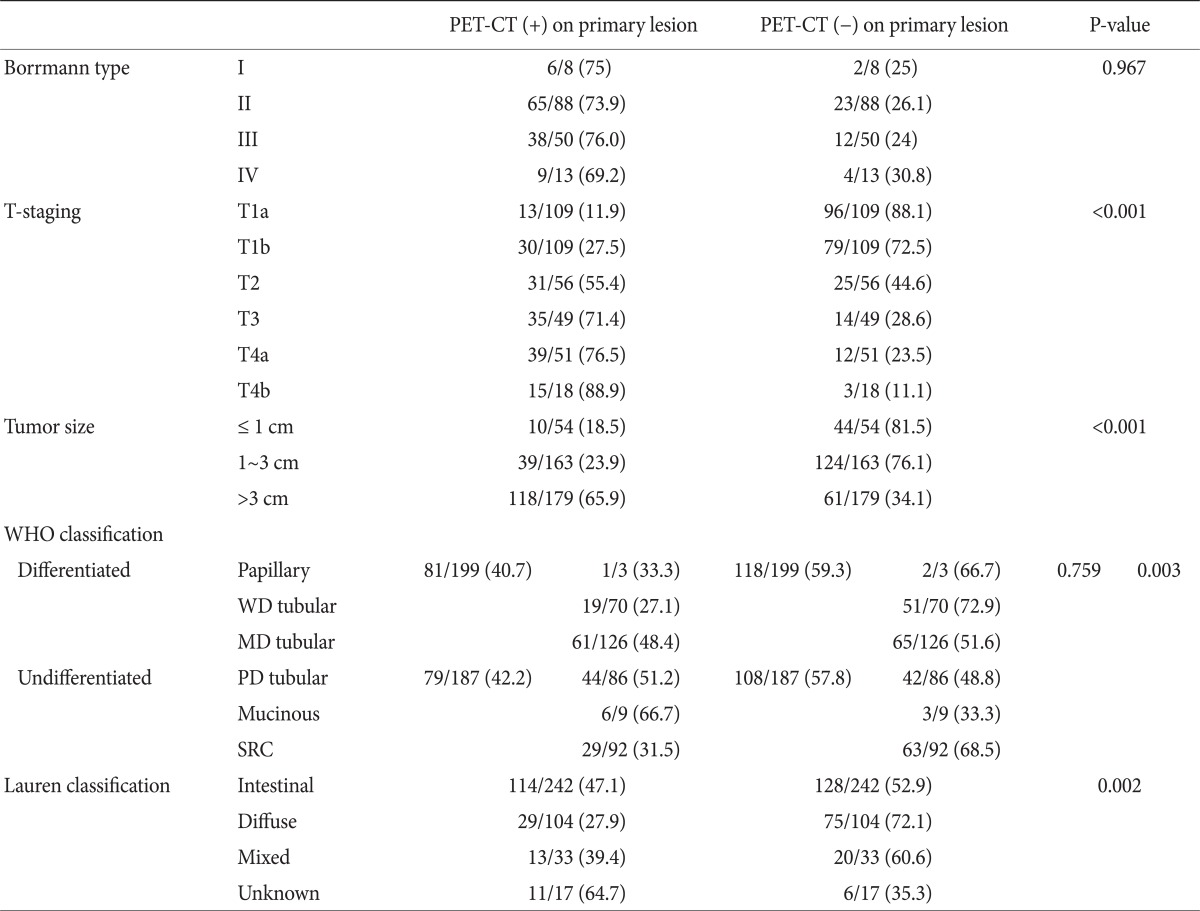
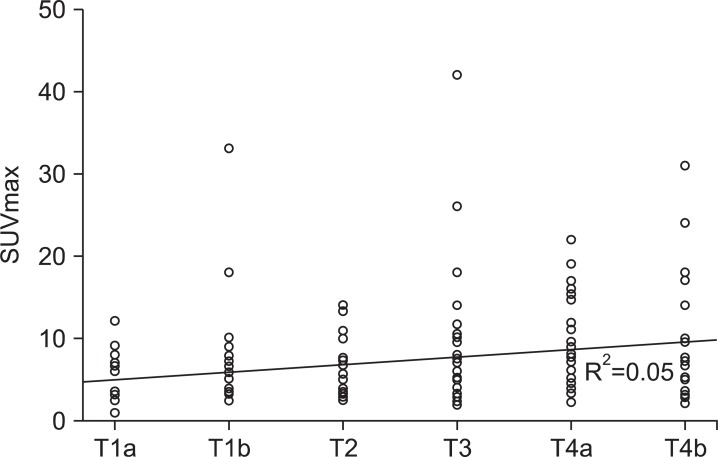
Endoscopic findings indicated early gastric cancer in 237 of the 396 patients and advanced gastric cancer in the remaining 159. PET-CT findings were positive in 49 of the 237 early gastric cancer patients (20.7%) and in 118 of the advanced gastric cancer patients (74.2%), indicating that PET-CT is more sensitive for detecting advanced gastric cancer. We could not find considerable differences between those results and results of T-staging according to pathologic findings. Among the advanced gastric cancers, the sensitivities of positive uptake on PET-CT were 75%, 73.9%, 76% and 69.2% for Borrmann type I, II, III and IV, respectively, and P-value was 0.967 (Table 2). We found that the sensitivity of PET-CT for T staging was 11.9% for T1a (13/109), 27.5% for T1b (30/109), 55.4% for T2 (31/56), 71.4% for T3 (35/49), 76.5% for T4a (39/51) and 88.9% for T4b (16/18), suggesting that the sensitivity of this modality increases with T stage and it had <0.001 of P-value (Table 2). Sensitivities in terms of primary lesion size were 18.5% for lesions less than 1 cm (10/54), 23.9% for those 1~3 cm (39/163) and 65.9% for lesions larger than 3 cm (118/179) (Table 2). P-value was <0.001. These finding indicate that the sensitivity of PET-CT is influenced by the T stage and the size of the primary lesion. Thus, we assessed the statistical relationship between the T stage and maximum standardized uptake value (SUVmax) to define the T stage. We found that as the T stage increased to T4b, SUVmax also increased and the value of R2 was 0.05 (Fig. 1). We determined the relationship between the WHO pathologic classification and uptake on PET-CT and found that 81 of 199 cases were the differentiated type and 79 of 187 were the undifferentiated type (Table 2). In terms of the Lauren pathologic classification, the sensitivity of PET-CT for detecting the intestinal type was 47.1% (114/242), 27.9% (29/103) for the diffuse type, 39.4% (13/33) for the mixed type and 64.7% (11/17) for the unknown type, which included O&C cases. The P-value was 0.002 (Table 2).
Table 2.
Positive FDG uptake on primary lesion for Borrmann type, T-staging, tumor size and histologic type
Values are presented as number (%). FDG = fluorodeoxyglucose; PET-CT = positron emission tomography-computed tomography; WHO = World Health Organization; WD = well differentiated; MD = moderate differentiated; PD = poorly differentiated; SRC = signet ring cell.
Fig. 1.
Correlation between SUVmax and T stage (R2=0.05). SUVmax = maximum standardized uptake value.
3. Detection of metastatic lymph nodes
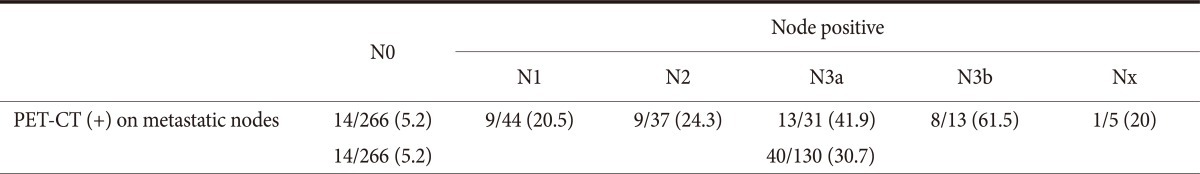
We evaluated the sensitivity of PET-CT for detecting primary lesion uptake according to the N stage and found that 25% of cases were node negative and 75% were node positive. It is thought that most of the node positive cases would be advanced gastric cancer. To understand the utility of PET-CT for N staging, the sensitivity of this modality for detecting the uptake of metastatic nodes was assessed and the results were 5.2% for N0 (14/266) and 30.7% for N1-N3b, Nx (40/130). In cases of O&C and palliation, they were included in Nx staging. The sensitivity for overall node metastasis was 30.7%, the specificity was 94.7%, the positive predictive value was 74.1% and the negative predictive value was 73.7% (Table 3).
Table 3.
Positive FDG uptake in metastatic nodes for each N stage
Values are presented as number (%). Sensitivity: 30.7%. Positive predictive value: 74.1%. Specificity: 94.7%. Negative predictive value: 73.7%. FDG = fluorodeoxyglucose; PET-CT = positron emission tomography-computed tomography.
4. Correlation to tumor markers
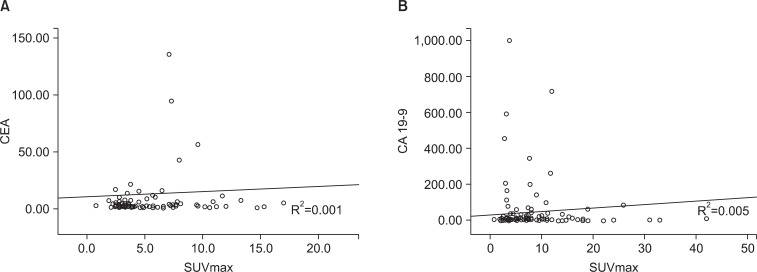
Two of the most used gastric cancer tumor markers are CEA and CA 19-9 and both had a positive correlation with SUVmax. R2 values were 0.001 and 0.005, respectively (Fig. 2).
Fig. 2.
Correlation between tumor markers and SUVmax. (A) Correlation between CEA and SUVmax (R2=0.001). (B) Correlation between CA 19-9 and SUVmax (R2=0.005). CEA = carcinoembryonic antigen; SUVmax = maximum standardized uptake value; CA19-9 = carbohydrate antigen 19-9.
5. Extragastric FDG uptake
Uptake in lesions other than the primary tumor was noted in 24 of the 396 patients. These 24 patients were studied in detail and in order to determine the value of PET-CT for M staging. There were seven cases that showed colon uptake and all had previously undergone routine pre-operative colonoscopy. One patient was determined to have colon cancer based on histologic analysis of the polypectomy specimen, and the patient subsequently underwent a combined operation. Two of the other six cases were determined to be benign polyps after polypectomy. The four remaining cases showed normal findings on colonoscopy and they are still being followed-up.
Five of the twenty-four cases showed liver uptake, and all of these patients underwent a routine pre-operative abdominal CT and liver ultrasonography and also both of them had positive findings. Two cases underwent hepatectomy with gastrectomy, and hepatocellular carcinoma was diagnosed in one while a metastatic nodule was found in the other. Two cases underwent palliative gastrectomy because of liver lesions and one patient had a palliative bypass gastrojejunostomy for cancer peritonei.
There were four cases of uptake in the lungs and all underwent a high resolution chest CT. One case was strongly suspected to be a metastatic nodule and the patient underwent palliative gastrectomy after neoadjuvant chemotherapy. One case had an undetermined pulmonary nodule and therefore underwent bronchoscopy and endoscopic bronchial ultrasonographic biopsy and the nodule was confirmed to be benign. Others were suspected of having latent tuberculosis and therefore underwent gastrectomy with subsequent observation.
Thyroid uptake was noted in two cases, and cancer was already suspected in one of the two patients was due to the presence of a large goiter. The patient underwent fine needle aspiration biopsy, which led to a diagnosis of follicular neoplasm. The patient then underwent a combined thyroidectomy and the neoplasm was found to be follicular carcinoma, which required continued observation and care. The other case underwent thyroid ultrasonography and the result was an indeterminate nodule. This patient underwent fine needle aspiration biopsy, which revealed it to be a benign nodule.
Two cases with gallbladder uptake received combined cholecystectomy and both patients had diagnosis of cholecystitis. Bone uptake was observed in two cases and both patients had a bone scan. One case was strongly suspected to be bone metastasis, so the patient underwent palliative gastrectomy with chemotherapy, while the other case was suspected to be degenerative change. One case showed abdominal wall uptake and was subsequently assessed with CT. During the laparoscopic distal gastrectomy, the suspect lesion was observed, but appeared normal. Thus, the patient was followed-up with PET-CT 6 months later post-operatively and the lesion was found to have disappeared on PET-CT.
One case with a breast uptake underwent breast ultrasonography and mammography, and the result was a category 2 lesion, which was followed up. There was one case that showed cerebellar uptake. The patient's history was taken and transcranial Doppler ultrasonography and 3D angio CT were performed. The uptake was determined to be an old cerebellar infarction.
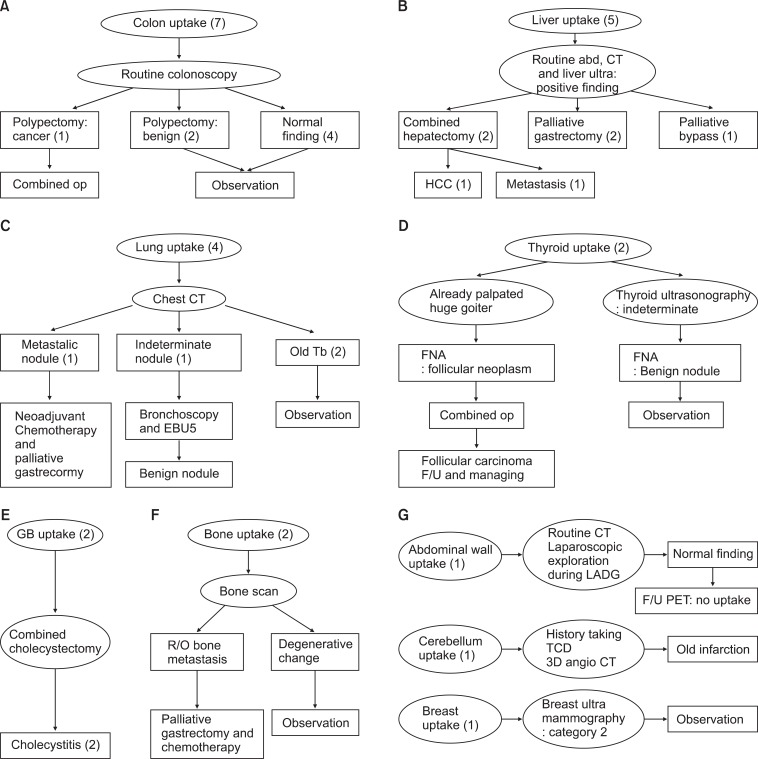
To summarize, abnormal uptake on PET-CT was observed in 24 of 396 patients for M staging and nine cases were found to have malignant lesions, including primary and metastatic lesions. In 12 cases the lesions were found only on PET-CT without routine preoperative studies. As a result, malignancies were detected in two cases only on PET-CT (Fig. 3).
Fig. 3.
Case series of positive FDG uptake in another lesion. (A) Colon. (B) Liver. (C) Lung. (D) Thyroid. (E) Gall bladder. (F) Bone. (G) Other. Four cases in the lung(s), one case in the thyroid, two cases in the gall bladder, two cases in the bone, one case in the breast and one case of cerebellar uptake were observed and progress was evaluated with PET-CT alone. One case of lung uptake and one case of bone uptake were determined to be malignant. op = operation; abd. = abdominal; CT = computed tomography; HCC = hepatocellular carcinoma; Tb = tuberculosis; EBU5 = endobronchial ultrasonography; FNA = fine needle aspiration; F/U = follow-up; GB = gall bladder; R/O = rule out; LADG = laparoscopic assisted distal gastrectomy; PET = positron emission tomography; TCD = transcranial doppler; FDG = fluorodeoxyglucose.
Discussion
The use of PET-CT as a routine pre-operative imaging modality is increasing, but there is controversy over whether it is useful in pre-operative gastric cancer staging.(4,6,12-15) However, there some studies have proven the superiority of PET-CT over conventional CT in terms of detecting metastatic mediastinal lymph nodes in lung cancer and metastatic lymph nodes in esophageal cancer.(16-21) We retrospectively studied the utility of PET-CT for accurately staging and predicting the prognosis of gastric cancer patients.
A study in 1990s reported that the sensitivity of PET-CT in gastric cancer was 93% and the specificity was 100%.(12) However, other studies reported sensitivities of 60%. A later study done to determine the usefulness of PET-CT scans for detecting node metastasis found the specificity to be 95% and sensitivity to be 22-50%.(4,6,12-14) In our study, PET-CT had a sensitivity of 42.2% for gastric cancers; 20.7% and 42.2% for early and advanced cases, respectively, classified based on endoscopic findings. Thus, the sensitivity was higher for more advanced cancer stages.
We investigated the clinical value of PET-CT for T staging, N staging, and M staging according to the America Joint Committee on Cancer Staging Manual, Seventh Edition. In cases of T staging, the sensitivity of PET-CT increased as the stage advanced and the tumor size increased. However there was not a significant correlation between the SUVmax of uptake and T stage (R2=0.05).
Some researchers expected that PET-CT would be very effective for N-staging because even with the limited anatomical resolution, PET scans can be used to find small metastatic nodes according to a patient's metabolic status. Thus, PET-CT enables the clinician to simultaneously assess metabolic change and morphologic change.(3) Nevertheless, our study revealed that PET-CT has 30.3% sensitivity and 94.7% specificity for metastatic nodes in node positive cases. This result is approximately the same as previous studies. The sensitivity is too low to define the N stage, but PET-CT has a high specificity and high positive and negative predictive values (74%, 73.1%), and could be useful for other purposes.
PET-CT had about the same sensitivity for differentiated and undifferentiated adenocarcinomas classified according to WHO definitions, 40.7% (81/199) and 42.2% (79/187), respectively. This result contradicts the results of previous studies, which reported a significantly lower uptake in undifferentiated carcinomas than in differentiated carcinomas.(4,6,13,22,23) This discrepancy might be due to the small number of cases involved in our study. Our study had higher uptake rates in poorly differentiated TAs.
Some studies showed that diffuse types according to the Lauren classification had a lower PET-CT detection rate than intestinal types because diffuse types of gastric cancer have an abundant mucin content that make the cell density lower.(4,6,13,22,24) Our study also showed that PET-CT has a higher sensitivity in intestinal types (Table 1). Additionally, two frequently used tumor markers, CEA and CA 19-9, were not significantly correlated with SUVmax in this study.
To determine the efficacy of PET-CT for M staging, 24 cases that showed FDG uptake in other lesions other than the primary gastric lesions were followed-up. Our institute employs routine pre-operative modalities such as esophagogastroduodenoscopy, endoscopic ultrasonography, colonoscopy, abdominal CT and liver-gall bladder-pancreas ultrasonography. Without those routine studies, 12 cases were found to have other lesions by PET-CT alone, and two of these cases were malignancies.
Our study lacks data to compare PET-CT to established imaging modalities such as abdominal CT and endoscopic ultrasonography. Also, the interpreter already knew the pathologic results of the gastric cancer because patients who had a PET-CT scan underwent endoscopic biopsies before the PET-CT for health insurance purposes. Both of these facts could be limitations of the study.
In this study we determined that PET-CT does not have sufficient sensitivity/specificity to be used as a preoperative imaging modality for T staging or N staging, and PET-CT findings were not correlated with the mostly commonly used tumor markers (CEA, CA 19-9) or WHO pathologic types. However, this modality was useful for detecting metastases or another primary cancer for preoperative staging in gastric cancer patients. Yet, because only 2 of 396 (0.5%) patients experienced this benefit, controversy may still remain with regard to adding PET-CT as a routine imaging modality for preoperative staging. Therefore, more prospective studies are needed to determine whether PET-CT should be considered a routine preoperative imaging modality in gastric cancer patients.
References
- 1.Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56:1–9. doi: 10.1016/s0895-4356(02)00534-6. [DOI] [PubMed] [Google Scholar]
- 2.Kim JP. Surgical results in gastric cancer. Semin Surg Oncol. 1999;17:132–138. doi: 10.1002/(sici)1098-2388(199909)17:2<132::aid-ssu8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.Kim EY, Lee WJ, Choi D, Lee SJ, Choi JY, Kim BT, et al. The value of PET/CT for preoperative staging of advanced gastric cancer: comparison with contrast-enhanced CT. Eur J Radiol. 2011;79:183–188. doi: 10.1016/j.ejrad.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Mochiki E, Kuwano H, Katoh H, Asao T, Oriuchi N, Endo K. Evaluation of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography for gastric cancer. World J Surg. 2004;28:247–253. doi: 10.1007/s00268-003-7191-5. [DOI] [PubMed] [Google Scholar]
- 5.Dassen AE, Lips DJ, Hoekstra CJ, Pruijt JF, Bosscha K. FDG-PET has no definite role in preoperative imaging in gastric cancer. Eur J Surg Oncol. 2009;35:449–455. doi: 10.1016/j.ejso.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Kim SK, Kang KW, Lee JS, Kim HK, Chang HJ, Choi JY, et al. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging. 2006;33:148–155. doi: 10.1007/s00259-005-1887-8. [DOI] [PubMed] [Google Scholar]
- 7.Hiki Y, Shimao J, Yamao Y, Kobayashi N, Kuranami M, Kikuchi S, et al. The concepts, procedures, and problems related in endoscopic laser therapy of early gastric cancer. A retrospective study on early gastric cancer. Surg Endosc. 1989;3:1–6. doi: 10.1007/BF00591306. [DOI] [PubMed] [Google Scholar]
- 8.Ohgami M, Otani Y, Kumai K, Kubota T, Kim YI, Kitajima M. Curative laparoscopic surgery for early gastric cancer: five years experience. World J Surg. 1999;23:187–192. doi: 10.1007/pl00013167. [DOI] [PubMed] [Google Scholar]
- 9.Marczell AP, Rosen HR, Hentschel E. Diagnosis and tactical approach to surgery for early gastric carcinoma: a retrospective analysis of the past 16 years in an Austrian general hospital. Gastroenterol Jpn. 1989;24:732–736. doi: 10.1007/BF02774176. [DOI] [PubMed] [Google Scholar]
- 10.Han EJ, Choi WH, Chung YA, Kim KJ, Maeng LS, Sohn KM, et al. Comparison between FDG uptake and clinicopathologic and immunohistochemical parameters in pre-operative PET/CT scan of primary gastric carcinoma. Nucl Med Mol Imaging. 2009;43:26–34. [Google Scholar]
- 11.Sarela AI, Miner TJ, Karpeh MS, Coit DG, Jaques DP, Brennan MF. Clinical outcomes with laparoscopic stage M1, unresected gastric adenocarcinoma. Ann Surg. 2006;243:189–195. doi: 10.1097/01.sla.0000197382.43208.a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeung HW, Macapinlac H, Karpeh M, Finn RD, Larson SM. Accuracy of FDG-PET in gastric cancer. Preliminary experience. Clin Positron Imaging. 1998;1:213–221. doi: 10.1016/s1095-0397(98)00018-1. [DOI] [PubMed] [Google Scholar]
- 13.Stahl A, Ott K, Weber WA, Becker K, Link T, Siewert JR, et al. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging. 2003;30:288–295. doi: 10.1007/s00259-002-1029-5. [DOI] [PubMed] [Google Scholar]
- 14.Yun M, Lim JS, Noh SH, Hyung WJ, Cheong JH, Bong JK, et al. Lymph node staging of gastric cancer using (18)F-FDG PET: a comparison study with CT. J Nucl Med. 2005;46:1582–1588. [PubMed] [Google Scholar]
- 15.Park MJ, Lee WJ, Lim HK, Park KW, Choi JY, Kim BT. Detecting recurrence of gastric cancer: the value of FDG PET/CT. Abdom Imaging. 2009;34:441–447. doi: 10.1007/s00261-008-9424-4. [DOI] [PubMed] [Google Scholar]
- 16.Lordick F, Ott K, Krause BJ, Weber WA, Becker K, Stein HJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 2007;8:797–805. doi: 10.1016/S1470-2045(07)70244-9. [DOI] [PubMed] [Google Scholar]
- 17.Pieterman RM, van Putten JW, Meuzelaar JJ, Mooyaart EL, Vaalburg W, Koëter GH, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med. 2000;343:254–261. doi: 10.1056/NEJM200007273430404. [DOI] [PubMed] [Google Scholar]
- 18.Flamen P, Lerut A, Van Cutsem E, De Wever W, Peeters M, Stroobants S, et al. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol. 2000;18:3202–3210. doi: 10.1200/JCO.2000.18.18.3202. [DOI] [PubMed] [Google Scholar]
- 19.Valk PE, Pounds TR, Hopkins DM, Haseman MK, Hofer GA, Greiss HB, et al. Staging non-small cell lung cancer by whole-body positron emission tomographic imaging. Ann Thorac Surg. 1995;60:1573–1581. doi: 10.1016/0003-4975(95)00752-0. [DOI] [PubMed] [Google Scholar]
- 20.Vitola JV, Delbeke D, Sandler MP, Campbell MG, Powers TA, Wright JK, et al. Positron emission tomography to stage suspected metastatic colorectal carcinoma to the liver. Am J Surg. 1996;171:21–26. doi: 10.1016/S0002-9610(99)80067-1. [DOI] [PubMed] [Google Scholar]
- 21.Kole AC, Plukker JT, Nieweg OE, Vaalburg W. Positron emission tomography for staging of oesophageal and gastroesophageal malignancy. Br J Cancer. 1998;78:521–527. doi: 10.1038/bjc.1998.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ott K, Fink U, Becker K, Stahl A, Dittler HJ, Busch R, et al. Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial. J Clin Oncol. 2003;21:4604–4610. doi: 10.1200/JCO.2003.06.574. [DOI] [PubMed] [Google Scholar]
- 23.Mukai K, Ishida Y, Okajima K, Isozaki H, Morimoto T, Nishiyama S. Usefulness of preoperative FDG-PET for detection of gastric cancer. Gastric Cancer. 2006;9:192–196. doi: 10.1007/s10120-006-0374-7. [DOI] [PubMed] [Google Scholar]
- 24.Kawamura T, Kusakabe T, Sugino T, Watanabe K, Fukuda T, Nashimoto A, et al. Expression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival. Cancer. 2001;92:634–641. doi: 10.1002/1097-0142(20010801)92:3<634::aid-cncr1364>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]