Abstract
Purpose
The aim of this study was to evaluate chronological change of quality of life after surgery in patients with gastric cancer during one year postoperatively.
Materials and Methods
Quality of life data were obtained from 272 gastric cancer patients who underwent curative gastrectomy between September 2008 and February 2011 at the Kyungpook National University Hospital. The Korean versions of the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core (QLQ) 30 with gastric cancer-specific module, the EORTC QLQ-STO22 were used to assess quality of life. All patients had no evidence of recurrence or metastasis during the first postoperative year. Patients were asked to complete the questionnaire, by themselves preoperatively, 3-, 6-, 9-, and 12-months postoperatively.
Results
Physical functioning score and role functioning score significantly decreased at first 3 months after surgery and the significant differences were noticed until 12 months after surgery. Emotional functioning score started with the lowest score before surgery and significant improvement was shown 6 months after surgery. Most symptom scores and STO-22 scores were highest at 3 months after surgery and gradually decreased, thereafter. Eating restriction, anxiety, taste, body image scores was highest at 3 months after surgery without significant decrease afterwards.
Conclusions
Most scales worsened after surgery and gradually recovered afterwards with some differences in rate of recovery. However the scales did not fully recover by 1 year period. Further follow-up after 1 year would be helpful in determining which scales are permanently damaged and which are just taking longer time to recover.
Keywords: Stomach neoplasms, Quality of life, Gastrectomy
Introduction
The overall survival rate of patients with gastric cancer has increased due to the development of diagnostic tools and mass screening program. Many studies have been performed to evaluate survival itself after gastric cancer surgery rather than subjective life quality. Undoubtedly, it is more or less unavoidable for patients who have undergone gastric cancer surgery to suffer from various gastrointestinal symptoms and malfunctions.(1) However the quality of life (QoL) of patients with gastric cancer after surgery has not been properly evaluated.
There are several studies comparing QoL between different surgical procedures for patients with gastric cancer.(2-7) However, the scores need to be reviewed in longitudinal aspect to understand the chronological change of QoL after gastrectomy comprehensively. Most of the studies reviewing QoL in gastric cancer patients only covered physical and psychological functioning rather than social functioning, and the scores were in fact physician-reported rather than patient-reported.(8) There are a few studies that compared QoL at certain period of time according to surgery performed.(9-12) However, few studies evaluated chronological change of QoL after surgery in patients with gastric cancer. Recently many in form of questionnaire, such as Functional Assessment of Cancer Therapy-General or European Organization for Research and Treatment of Cancer QoL Questionnaire Core 30 (EORTC QLQ-C30) with gastric cancer-specific module, the EORTC QLQ-STO22, have been developed to evaluate QoL.(13-15) In this study, the EORTC QLQ-C30 and the QLQ-STO22 have been applied to gastric cancer patients to evaluate changes of QoL during one year postoperatively.
Materials and Methods
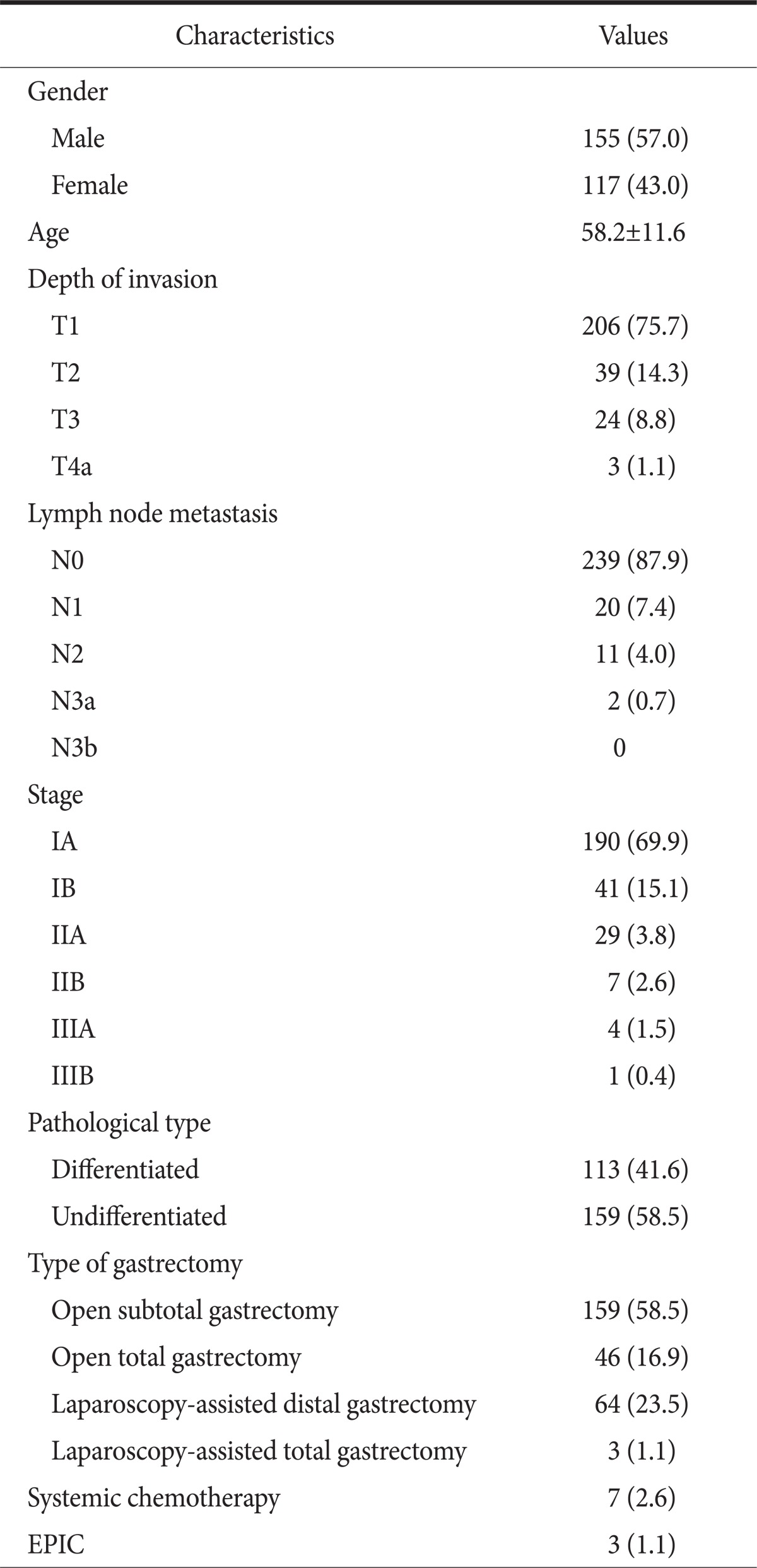
The QoL data obtained from 308 gastric cancer patients who underwent curative gastrectomy between September 2008 and February 2011 at the Kyungpook National University Hospital were analyzed. Thirty-six patients with comorbidities that could affect the QoL were excluded: 15 patients with other combined malignancy, 11 with cardiovascular disease, 6 with chronic respiratory disease, 2 with cerebrovascular disease, and 2 with previous bowel resection operation. Remaining 272 patients were included in this study. Forty six patients underwent total gastrectomy, 159 distal subtotal gastrectomy, 67 laparoscopy-assisted gastrectomy. Sixty-four patients of laparoscopy-assisted gastrectomy had laparoscopy-assisted distal gastrectomy, 3 had laparoscopy-assisted total gastrectomy. One hundred and fifty five were male, 117 female. The mean age was 58.2±11.6 years. Patient demographics are summarized in Table 1.
Table 1.
Characteristics of patients enrolled
Values are presented as number (%) or mean±standard deviation. Stage grouping by 7th edition of the International Union Against Cancer classification. EPIC = early postoperative intraperitoneal chemotherapy.
The Korean versions of the EORTC QLQ-C30 and the EORTC QLQ-STO22, were used to assess QoL. EORTC QLQ-C30 is composed of both multi-item scales and single-item measures. These include five functional scales, three symptom scales, a global health status/QoL scale, and six single items. These 15 scales and items can be grouped in three groups, a global health status/QoL scale, 5 functional scales, and 9 symptom scales. QLQ-STO22 is composed of 5 multi-item scales and 4 single-item measures.
Patients were asked to complete the questionnaire, by themselves preoperatively, 3-, 6-, 9-, and 12-months postoperatively. The answers were then translated into scores from 0 to 100, according to the scoring manual provided by the EORTC. High scores of functioning scales and lower scores of symptom scales represent better QoL.For the global health status/QoL and the five functional scales, a high score represents high QoL, but for the symptom scales, a high score represents low QoL. For the EORTC QLQSTO22, like symptom scales, a high score represents low QoL.(13)
Mean scores of each scale were calculated, and compared in a longitudinal fashion during the first postoperative year. The one way ANOVA was performed to compare scores of each questionnaire of 3-month interval during one year postoperative period. P-value of less than 0.05 was considered statistically significant.
Results
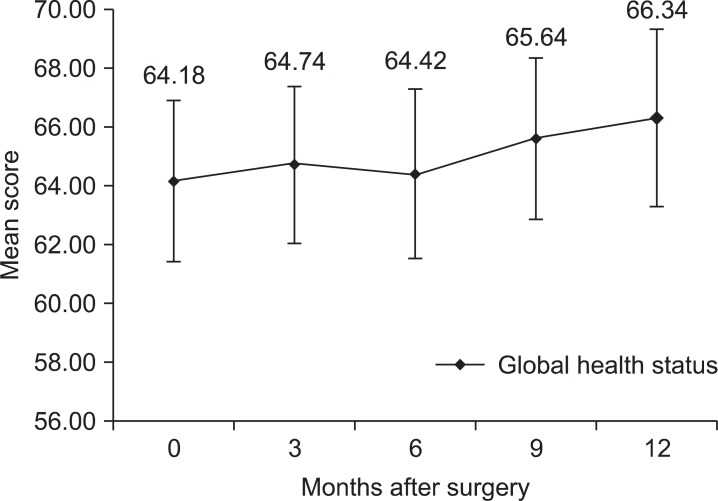
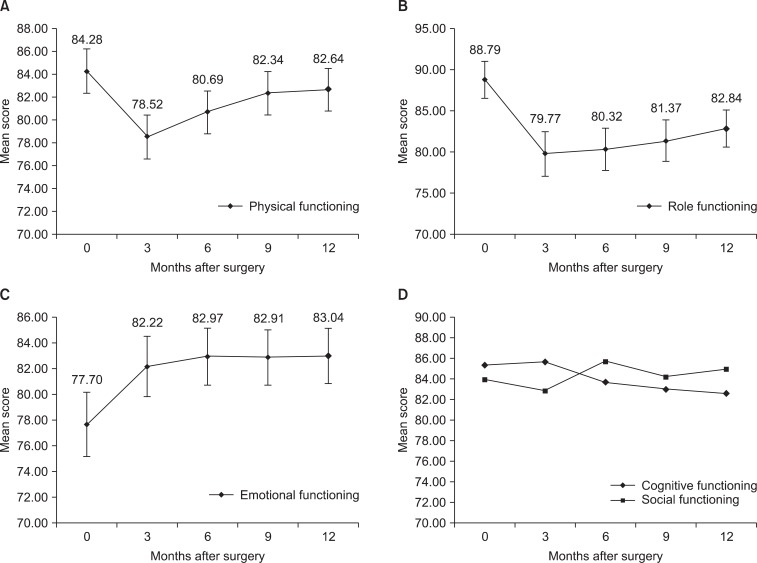
Global health status/QoL showed tendency to improve without statistically significant differences during the first year after surgery (Fig. 1). The functional scales showed tendency to decrease during three months after surgery and gradually increased afterwards. Physical functioning score significantly decreased at first 3 months after surgery (P<0.001). The score gradually increased after 3 months and there was no statistically significant difference compared to preoperative score after 6 months (Fig. 2A). Role functioning score decreased significantly 3 months after surgery (P<0.001) and the significant differences compared to preoperative score were noticed until 12 months after surgery (Fig. 2B). Emotional functioning score started with the lowest score before surgery and steadily improved after surgery. Statistically significant improvement compared to preoperative score was shown 6 months after surgery (P=0.020) (Fig. 2C). Cognitive functioning score and social functioning score showed no statistically significant differences compared to preoperative score during the first year after surgery (Fig. 2D).
Fig. 1.
Changes of mean score of global health status. Error bars represent 95% confidence interval.
Fig. 2.
Changes of mean score of functioning scales. (A) Physical functioning score and (B) role functioning score decreased 3 months after surgery and gradually improved, thereafter. (C) Emotional functioning scale started with lowest score before surgery and improved afterwards. (D) Cognitive and social functioning scores did not show any pattern. Error bars represent 95% confidence interval.
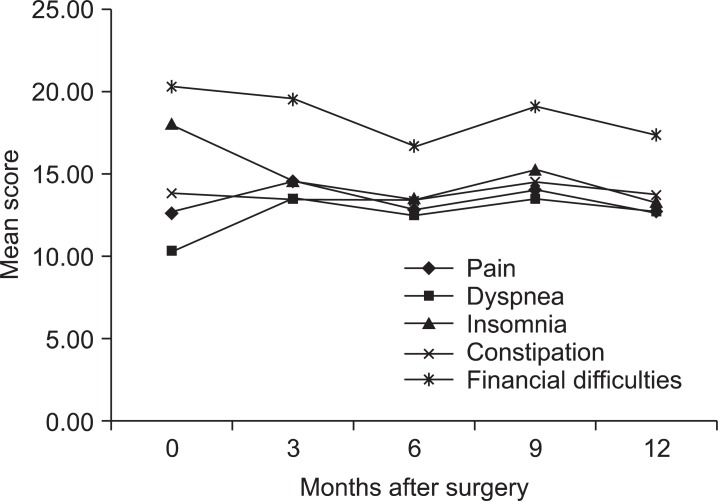
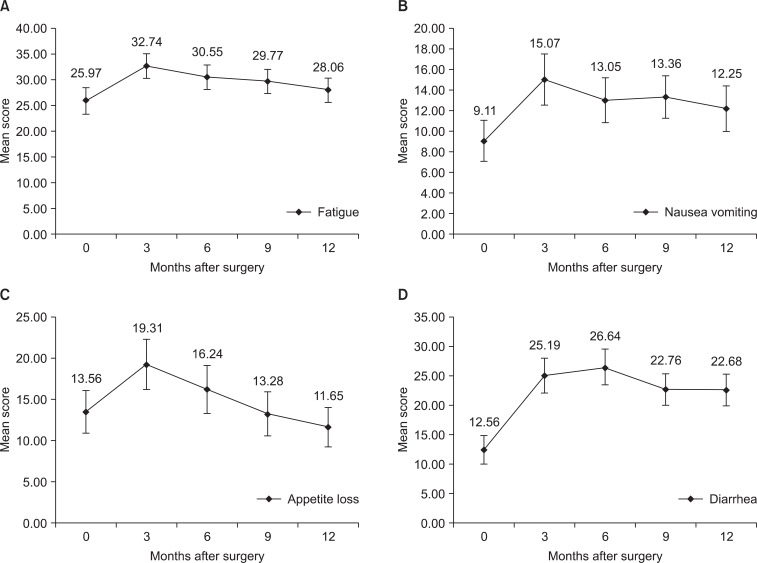
Most symptom scales increased at 3 months after surgery and steadily decreased afterwards. However pain, dyspnea, insomnia, constipation, and financial difficulties did not show statistically significant change compared to preoperative scores during 12 months postoperatively (Fig. 3). Fatigue score increased 3 months after surgery with statistically significant difference (P=0.002). The score showed tendency to decrease after 3 months (Fig. 4A). Likewise nausea and vomiting score increased 3 months after surgery (P=0.002), and showed tendency to decrease afterwards (Fig. 4B). Appetite loss score was highest at 3 months after surgery (P=0.031) and decreased afterward with statistically significant difference compared to the highest score was noticeable by at 9 months after surgery (P=0.020) (Fig. 4C). Diarrhea score increased 3 months after surgery (P<0.001), but did not decrease by 6 months, 9 months, 1 year after surgery (Fig. 4D).
Fig. 3.
Changes of mean score of symptom scales without statistical significance.
Fig. 4.
Changes of mean score of symptom scales with statistical significance. (A) Fatigue, (B) nausea vomiting, and (C) appetite loss scores were highest at 3 months after surgery. (D) Diarrhea score increased after surgery and did not decrease afterwards. Error bars represent 95% confidence interval.
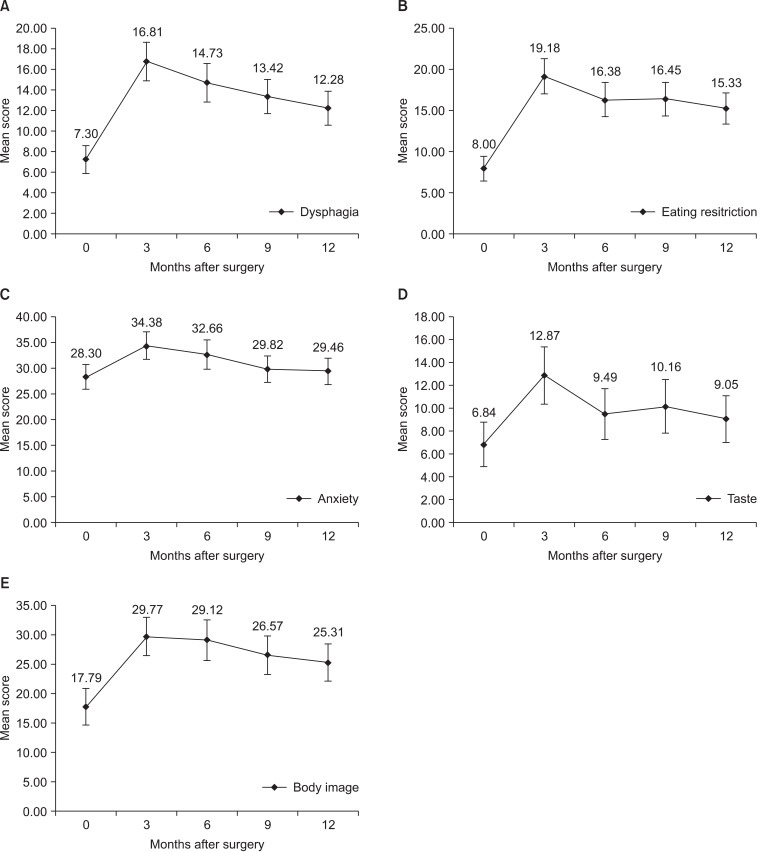
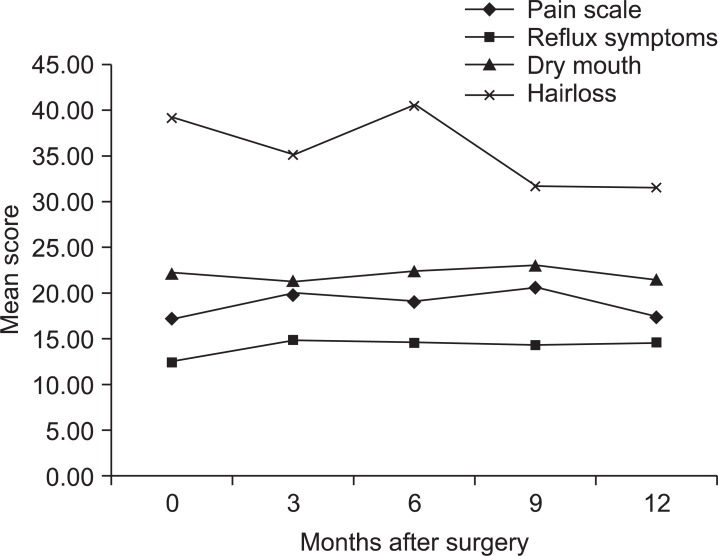
QLQ-STO22 scales showed similar pattern with symptom scales. Most scores were highest at 3 months after surgery and decreased afterwards. Dysphagia score was highest at 3 months after surgery (P<0.001), and gradually decreased afterwards. Statistically significant decrease compared to the highest score was shown by 9 months after surgery (P=0.042) (Fig. 5A). Eating restriction score was highest at 3 months after surgery (P<0.001) without significant decrease afterwards (Fig. 5B). Likewise anxiety (P=0.012), taste (P=0.002), and body image score (P<0.001) increased after surgery without significant decrease afterwards (Fig. 5C~E). However pain, reflux symptom, dry mouth, and hair loss scores had no statistically significant change during one year period (Fig. 6).
Fig. 5.
Changes of mean score of QLQ-STO22 scales with statistical significance. (A) Dysphagia, (B) eating restriction, (C) anxiety, (D) taste, and (E) body image score increased after surgery and gradually decreased afterwards. Error bars represent 95% confidence interval.
Fig. 6.
Changes of mean score of QLQ-STO22 scales without statistical significance.
Discussion
Most functional scales decreased after surgery and gradually improved during the postoperative period, similar to other studies.(16) Physical functioning and role functioning showed lowest score after surgery and they gradually improved afterwards. However they did not fully recover to preoperative level during 1 year postoperatively, which can be understood as the detrimental adverse effect of gastrectomy. Emotional functionining score, unlike scores of other functional scales, was lowest before surgery and steadily improved after surgery. Since the questionnaire was completed after being diagnosed of gastric cancer, depression from the diagnosis probably caused the lowest score. A longitudinal study of QoL after gastrectomy reported that the psychiatric domain was predominantly impaired preoperatively, while the somatic domain postoperatively.(17) Cognitive and social functioning scores showed no statistical difference during 1 year follow-up in this study. In a similar study performed by Kobayashi et al.,(1) both scales showed similar pattern with other functional scales, being lowest after operation, and gradually improving afterwards. However, they measured the score 1 month after operation, which was not measured in this study.
Some symptom scales, such as fatigue, nausea and vomiting, and appetite loss were highest 3 months after surgery and gradually decreased afterwards (Fig. 3). Fatigue and nausea and vomiting were worst at 3 months after surgery and did not fully recover during 1 year progress. Therefore, further follow-up is necessary to confirm if the symptom will recover or not. Appetite loss showed similar pattern to other symptom scales, however, the mean score fully recovered by 9 months after surgery, and showed even better score 12 months after surgery. Regarding the fact that the preoperative score was taken after being diagnosed of gastric cancer, the depression after being diagnosed of cancer could have affected the preoperative score, and the relief from the burden after surgery perhaps improved the symptom at 12 months after surgery. Diarrhea showed slightly different course to other symptom scales. Symptom was highest at 6 months after surgery and did not improve until 12 months after surgery. This can be explained as one of the detrimental adverse effect of gastrectomy, therefore further follow-up should be done to be certain. Other symptom scales did not show statistically significant differences during 1 year. One peculiar result was that pain did not show any statistically significant pattern in this study. Considering the fact that a person had undergone a surgery, it is a logical assumption that the patient suffered some pain after surgery, no matter how minimal. However, the QoL being a measure of subjective feeling, the pain score could have been masked by the pain being lighter than the expectation. Another explanation is that the pain score could have reached maximum point immediately after surgery, and recovered before the first post operative questionnaire was taken at 3 months after surgery. A similar study performed by Kobayashi et al.(1) showed such pattern, where pain score peaked maximum by the time of one month after surgery and rapidly recovered afterwards.
Dysphagia, eating restriction, anxiety, taste, and body image scores of the QLQ-STO22, similarly to symptom scales, increased after surgery and gradually decreased afterwards. Although they did not fully recover by the time of 1 year, the tendency to decrease remained, and with further follow-up of these scales may reveal full recovery.
A limitation of this study is that the time interval from surgery to first time the questionnaire has been completed is too long. Three-month interval was too long to measure some scales, such as pain. Perhaps additional questionnaire at 1 month after surgery might help. Also some scales that did not show statistically significant difference in this study could reveal hidden pattern. Chronological change of QoL may differ according to the type of surgery performed. In this study, there were not enough cases for each type of surgery. So we did not separate each case by the type of surgery to review overall picture. Therefore further study comparing by the type of surgery will be necessary.
Most scales showed similar pattern of worsening after surgery and gradually recovering afterwards. However, there were some differences in rate of recovery. Also, some scales did not recover in 1 year period. Further follow-up after 1 year would be helpful in determining which scales are permanently damaged and which are just taking longer time to recover. It will be helpful to comprehend the pattern, so a patient undergoing a gastrectomy can be properly advised of the life after gastrectomy.
References
- 1.Kobayashi D, Kodera Y, Fujiwara M, Koike M, Nakayama G, Nakao A. Assessment of quality of life after gastrectomy using EORTC QLQ-C30 and STO22. World J Surg. 2011;35:357–364. doi: 10.1007/s00268-010-0860-2. [DOI] [PubMed] [Google Scholar]
- 2.Yu W, Lee CH, Chung HY. Quality of life after curative surgery in patients with gastric cancer: comparison between a subtotal gastrectomy and a total gastrectomy. J Korean Gastric Cancer Assoc. 2001;1:44–49. [Google Scholar]
- 3.Yu W, Chung HY. Quality of life and nutritional outcomes of billroth I and billroth II reconstruction. J Korean Gastric Cancer Assoc. 2002;2:91–95. [Google Scholar]
- 4.Yasuda K, Shiraishi N, Etoh T, Shiromizu A, Inomata M, Kitano S. Long-term quality of life after laparoscopy-assisted distal gastrectomy for gastric cancer. Surg Endosc. 2007;21:2150–2153. doi: 10.1007/s00464-007-9322-9. [DOI] [PubMed] [Google Scholar]
- 5.Adachi Y, Suematsu T, Shiraishi N, Katsuta T, Morimoto A, Kitano S, et al. Quality of life after laparoscopy-assisted Billroth I gastrectomy. Ann Surg. 1999;229:49–54. doi: 10.1097/00000658-199901000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies J, Johnston D, Sue-Ling H, Young S, May J, Griffith J, et al. Total or subtotal gastrectomy for gastric carcinoma? A study of quality of life. World J Surg. 1998;22:1048–1055. doi: 10.1007/s002689900515. [DOI] [PubMed] [Google Scholar]
- 7.Takiguchi S, Yamamoto K, Hirao M, Imamura H, Fujita J, Yano M, et al. Osaka University Clinical Research Group for Gastroenterological Study. A comparison of postoperative quality of life and dysfunction after Billroth I and Roux-en-Y reconstruction following distal gastrectomy for gastric cancer: results from a multi-institutional RCT. Gastric Cancer. 2012;15:198–205. doi: 10.1007/s10120-011-0098-1. [DOI] [PubMed] [Google Scholar]
- 8.Kaptein AA, Morita S, Sakamoto J. Quality of life in gastric cancer. World J Gastroenterol. 2005;11:3189–3196. doi: 10.3748/wjg.v11.i21.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svedlund J, Sullivan M, Liedman B, Lundell L, Sjödin I. Quality of life after gastrectomy for gastric carcinoma: controlled study of reconstructive procedures. World J Surg. 1997;21:422–433. doi: 10.1007/pl00012265. [DOI] [PubMed] [Google Scholar]
- 10.Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–727. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 11.Lee SS, Ryu SW, Kim IH, Sohn SS. Quality of life beyond the early postoperative period after laparoscopy-assisted distal gastrectomy: the level of patient expectation as the essence of quality of life. Gastric Cancer. 2012;15:299–304. doi: 10.1007/s10120-011-0113-6. [DOI] [PubMed] [Google Scholar]
- 12.Munene G, Francis W, Garland SN, Pelletier G, Mack LA, Bathe OF. The quality of life trajectory of resected gastric cancer. J Surg Oncol. 2012;105:337–341. doi: 10.1002/jso.22139. [DOI] [PubMed] [Google Scholar]
- 13.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 14.Vickery CW, Blazeby JM, Conroy T, Arraras J, Sezer O, Koller M, et al. EORTC Quality of Life Group. Development of an EORTC disease-specific quality of life module for use in patients with gastric cancer. Eur J Cancer. 2001;37:966–971. doi: 10.1016/s0959-8049(00)00417-2. [DOI] [PubMed] [Google Scholar]
- 15.Blazeby JM, Conroy T, Bottomley A, Vickery C, Arraras J, Sezer O, et al. European Organisation for Research and Treatment of Cancer Gastrointestinal and Quality of Life Groups. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-STO 22, to assess quality of life in patients with gastric cancer. Eur J Cancer. 2004;40:2260–2268. doi: 10.1016/j.ejca.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 16.Wu CW, Chiou JM, Ko FS, Lo SS, Chen JH, Lui WY, et al. Quality of life after curative gastrectomy for gastric cancer in a randomised controlled trial. Br J Cancer. 2008;98:54–59. doi: 10.1038/sj.bjc.6604097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt-Matthiesen A, Weidmann R, Sänger P. Longitudinal study on quality of life after gastrectomy for gastric cancer and starting points for intensified care. Zentralbl Chir. 2003;128:304–308. doi: 10.1055/s-2003-38794. [DOI] [PubMed] [Google Scholar]