Abstract
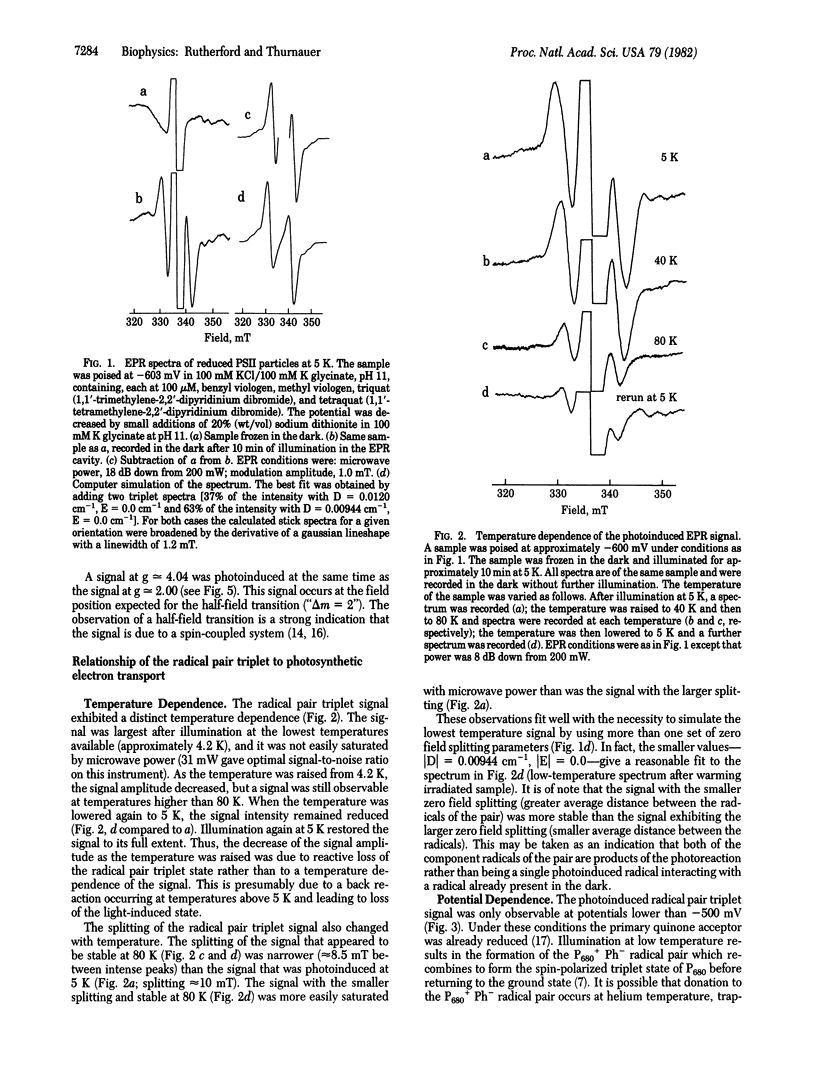
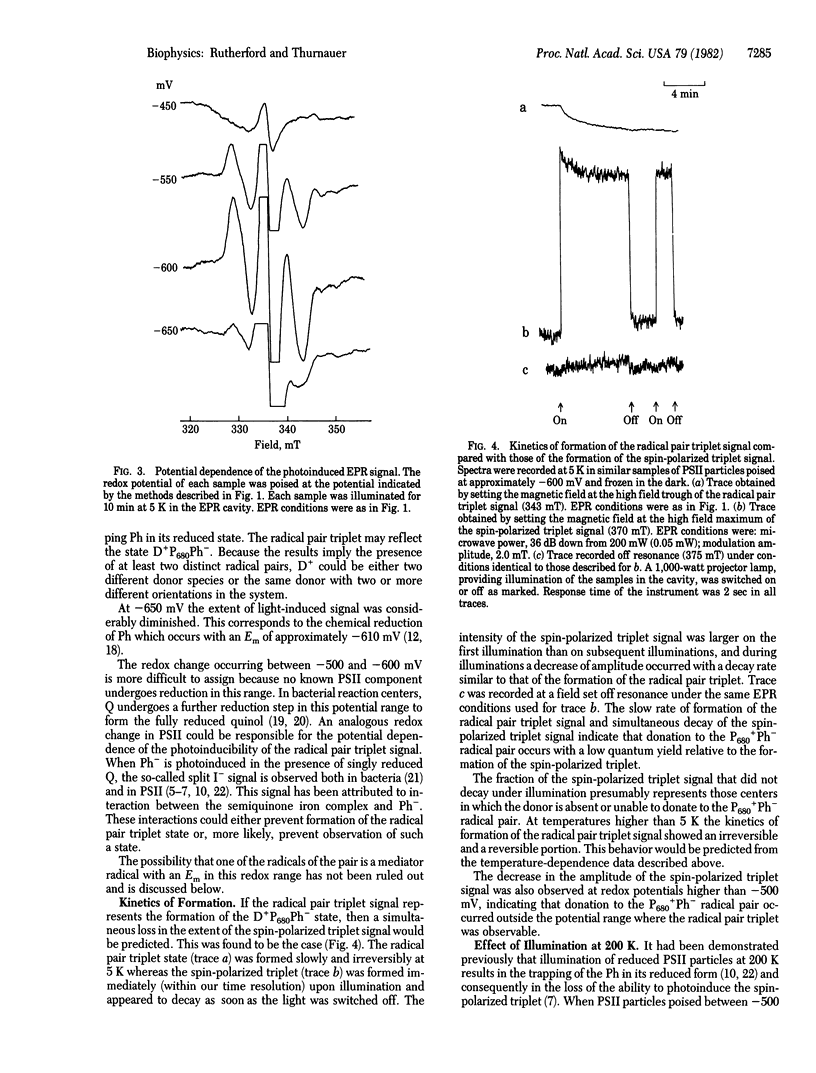
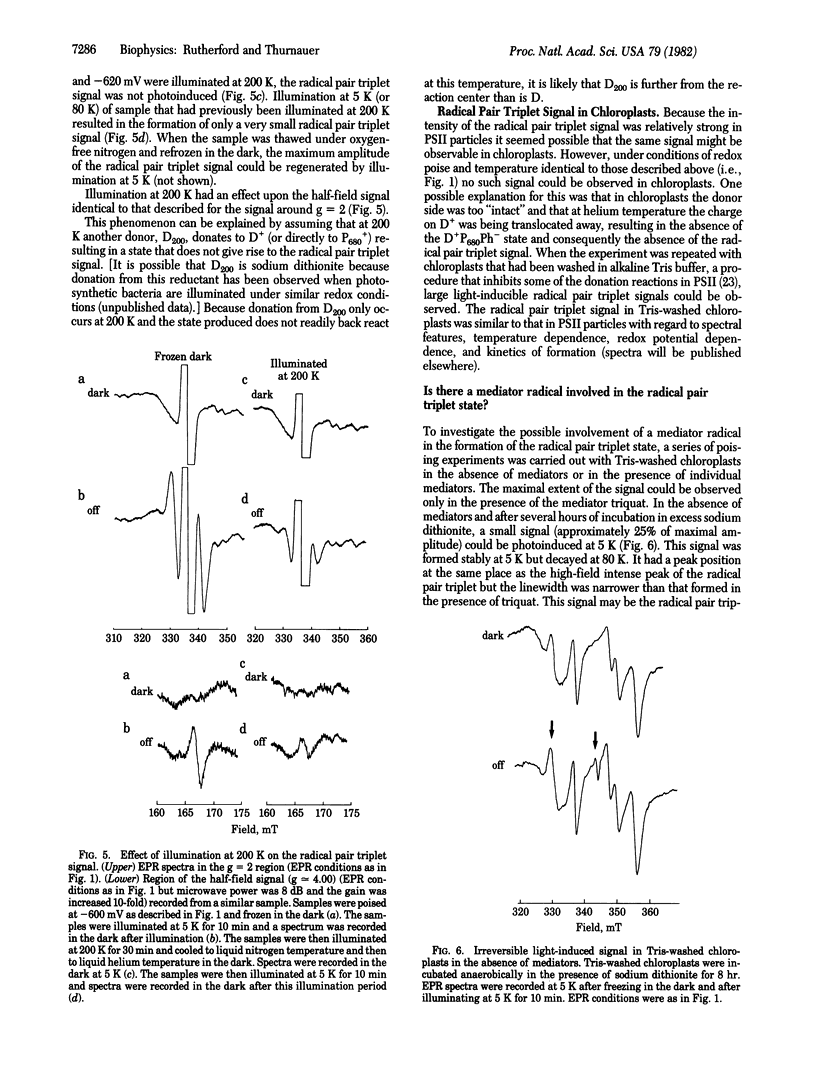
A stable light-induced EPR signal is reported in photosystem II particles and in chloroplasts at 5 K. Characteristic spectral features indicate that the signal arises from dipole-dipole interactions of a radical pair triplet state. From its dependence on potential, its relationship to the spin-polarized triplet state, and the redox state of the pheophytin acceptor (Ph) and because it is present in Tris-washed chloroplasts but not in untreated chloroplasts, we conclude that the signal is formed when the reaction center is in the state D+P680Ph- (P680 is the primary chlorophyll donor and D+ is an oxidized donor to P680). The low-temperature photochemical sequence is thought to occur as follows. (i) Donation from D to the P680+Ph- state occurs at liquid helium temperature in low quantum yield; this reaction is reversible at temperatures above 5 K. (ii) In normal chloroplasts, donation occurs to the D+P680Ph- state, but this does not occur in Tris-washed chloroplasts or in the photosystem II particles at 77 K or lower. (iii) Illumination, at 200 K, of photosystem particles or Tris-washed chloroplasts results in donation to the D+P680Ph- state from another donor. From experiments in the absence of redox mediators and the temperatures dependence of the splitting of the signal, it is suggested that the state D+P680Ph- itself may be the origin of the radical pair triplet signal. The signal has been simulated by assuming the presence of at least two distinct radical pairs that differ slightly in the distance separating the radicals of the pairs. The distance between the radicals of the pair is calculated to be 6-7 Å.
Keywords: photosynthesis, electron paramagnetic resonance, electron transfer, pheophytin, donors
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouges-Bocquet B. Kinetic models for the electron donors of photosystem II of photosynthesis. Biochim Biophys Acta. 1980 Dec;594(2-3):85–103. doi: 10.1016/0304-4173(80)90006-3. [DOI] [PubMed] [Google Scholar]
- Evans W. E. The clinical pharmacist and research. J Clin Pharmacol. 1981 May-Jun;21(5-6):241–244. doi: 10.1002/j.1552-4604.1981.tb02554.x. [DOI] [PubMed] [Google Scholar]
- Inoue Y., Yamashita T., Kobayashi Y., Shibata K. Thermoluminescence changes during inactivation and reactivation of the oxygen-evolving system in isolated chloroplasts. FEBS Lett. 1977 Oct 15;82(2):303–306. doi: 10.1016/0014-5793(77)80607-8. [DOI] [PubMed] [Google Scholar]
- Klimov V. V., Dolan E., Shaw E. R., Ke B. Interaction between the intermediary electron acceptor (pheophytin) and a possible plastoquinone-iron complex in photosystem II reaction centers. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7227–7231. doi: 10.1073/pnas.77.12.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimov V. V., Klevanik A. V., Shuvalov V. A., Kransnovsky A. A. Reduction of pheophytin in the primary light reaction of photosystem II. FEBS Lett. 1977 Oct 15;82(2):183–186. doi: 10.1016/0014-5793(77)80580-2. [DOI] [PubMed] [Google Scholar]
- Mullet J. E., Arntzen C. J. Identification of a 32-34-kilodalton polypeptide as a herbicide receptor protein in photosystem II. Biochim Biophys Acta. 1981 Apr 13;635(2):236–248. doi: 10.1016/0005-2728(81)90023-2. [DOI] [PubMed] [Google Scholar]
- Rutherford A. W., Evans M. C. Direct measurement of the redox potential of the primary and secondary quinone electron acceptors in Rhodopseudomonas sphaeroides (wild-type) by EPR spectrometry. FEBS Lett. 1980 Feb 11;110(2):257–261. doi: 10.1016/0014-5793(80)80086-x. [DOI] [PubMed] [Google Scholar]
- Rutherford A. W., Heathcote P., Evans M. C. Electron-paramagnetic-resonance measurements of the electron-transfer components of the reaction centre of Rhodopseudomonas viridis. Oxidation--reduction potentials and interactions of the electron acceptors. Biochem J. 1979 Aug 15;182(2):515–523. doi: 10.1042/bj1820515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford A. W., Paterson D. R., Mullet J. E. A light-induced spin-polarized triplet detected by EPR in photosystem II reaction centers. Biochim Biophys Acta. 1981 Apr 13;635(2):205–214. doi: 10.1016/0005-2728(81)90020-7. [DOI] [PubMed] [Google Scholar]
- Tiede D. M., Prince R. C., Reed G. H., Dutton P. L. EPR properties of the electron carrier intermediate between the reaction center bacteriochlorophylls and the primary acceptor in Chromatium vinosum. FEBS Lett. 1976 Jun 15;65(3):301–304. doi: 10.1016/0014-5793(76)80134-2. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Butler W. L. Photooxidation by photosystem II of tris-washed chloroplasts. Plant Physiol. 1969 Sep;44(9):1342–1346. doi: 10.1104/pp.44.9.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gorkom H. J. Identification of the reduced primary electron acceptor of photosystem II as a bound semiquinone anion. Biochim Biophys Acta. 1974 Jun 28;347(3):439–442. doi: 10.1016/0005-2728(74)90081-4. [DOI] [PubMed] [Google Scholar]


