Abstract
The cardioaccelerator and ventilatory responses to rhythmic exercise in the human are commonly viewed as being mediated predominantly via feedforward ‘central command’ mechanisms, with contributions from locomotor muscle afferents to the sympathetically mediated pressor response. We have assessed the relative contributions of three types of feedback afferents on the cardiorespiratory response to voluntary, rhythmic exercise by inhibiting their normal ‘tonic’ activity in healthy animals and humans and in chronic heart failure. Transient inhibition of the carotid chemoreceptors during moderate intensity exercise reduced muscle sympathetic nerve activity (MSNA) and increased limb vascular conductance and blood flow; and reducing the normal level of respiratory muscle work during heavier intensity exercise increased limb vascular conductance and blood flow. These cardiorespiratory effects were prevented via ganglionic blockade and were enhanced in chronic heart failure and in hypoxia. Blockade of μ opioid sensitive locomotor muscle afferents, with preservation of central motor output via intrathecal fentanyl: (a) reduced the mean arterial blood pressure (MAP), heart rate and ventilatory responses to all steady state exercise intensities; and (b) during sustained high intensity exercise, reduced O2 transport, increased central motor output and end-exercise muscle fatigue and reduced endurance performance. We propose that these three afferent reflexes – probably acting in concert with feedforward central command – contribute significantly to preserving O2 transport to locomotor and to respiratory muscles during exercise. Locomotor muscle afferents also appear to provide feedback concerning the metabolic state of the muscle to influence central motor output, thereby limiting peripheral fatigue development.

Jerome A. Dempsey was born and educated in Canada (Universities of Western Ontario and Alberta) and received his PhD at the University of Wisconsin – Madison. He has been on the faculty at UW Medical School since 1968, Director of the John Rankin Laboratory of Pulmonary Medicine since 1981 and is currently Professor Emeritus. His major interests have included the respiratory physiology, pathophysiology and cardiorespiratory interactions attending exercise, hypoxia, sleep, obesity, COPD and CHF, and the training of pre- and postdoctoral fellows and undergraduates.
On behalf of the 68 pre- and postdoctoral fellows and many undergraduate alumni of the John Rankin Laboratory of Pulmonary Medicine, University of Wisconsin and the dedicated UW faculty who served our laboratory's research and educational missions I humbly accept this distinguished award, named after two of our profession's greatest scientists and innovators. I am especially grateful that several Rankin Laboratory alumni were in attendance at the London meeting for this 17th Bayliss–Starling lecture. Given the theme of our meeting it is relevant to recall Professor Starling's insights from his 1919 lecture delivered at the Royal Army Medical College into the neural mechanisms of blood flow regulation during exercise, beyond those which he had uncovered in the isolated heart (Starling, 1920): ‘The extraordinary powers with which the heart muscle is endowed represent but the central fortress of the system and under normal conditions is protected from coming into play by the activation of the defending positions and outposts provided by the central nervous system and its servants.’
I am especially excited by the fact that two of the previous memorial lecturers were scientific heroes of mine and gentlemen with whom I had the opportunity to interact and to learn from. I refer specifically to Professor John Pappenheimer (1915–2007) (1982 lecturer) whose pioneering work on the physiological significance of the medullary chemoreceptors in the 1960s provided great impetus to our laboratory's research on cerebrospinal fluid pH regulation and chemoreceptor interactions. In addition, Ivan De Burgh Daly (1893–1974) (1966 lecturer) who together with his son Professor Michael De Burgh Daly (1922–2002) conducted two lifetimes of basic research into cardiorespiratory control, provided the basis for our attempts to apply these fundamental principles to problems of respiratory:cardiovascular interactions during exercise in healthy and diseased humans. Finally, I would especially like to recognize the personal influence provided by the late Brian Whipp (1937–2011). Brian and I were contemporaries who would meet almost annually at various meetings to share our findings on some aspect of respiratory control, often following a squash match. We were always highly critical of one another's theories and often agreed to disagree on many points, but I always learned something of significance from Brian's insights (and from his superior skills in squash) and am grateful for these wonderful memories.
Introduction
The primary controllers underlying cardiorespiratory responses to exercise have been the subject of fascination and debate among physiologists for over a century. The hyperpnoea accompanying rhythmic exercise represents the ultimate in homeostatic response owing to its precision in matching the rising CO2 production and protecting alveolar and arterial gases, combined with its efficiency in minimizing the work and energy cost of respiratory muscles over the entire range of mild to maximum intensity exercise (Forster et al. 2012). The large selective increases in locomotor muscle blood flow during exercise require powerful local vasodilator mechanisms contained in active skeletal muscle vascular endothelium combined with simultaneous, sympathetically mediated vasoconstriction of vasculature within both active and inactive organs, so that blood pressure may be maintained along with high perfusion pressures (Andersen & Saltin, 1985; Rowell, 2004). Primary sources of increased sympathetic activity and inhibition of parasympathetic activity, as well as the drive to breathe, are known to include strong feedforward ‘central command’ influences that are activated along central neural pathways in parallel to motor command, as well as feedback from limb muscle afferents with a secondary modulating role for baroreceptors and chemoreceptors. The evidence for a strong contribution from central command mechanisms originating from several potential supra-pontine locations has been demonstrated convincingly in the decorticate cat model of ‘fictive’ locomotion (Eldridge et al. 1981; Waldrop & Iwamoto, 2006) and more recently in the human undergoing voluntary or ‘imagined’ exercise via the use of brain imaging techniques (Fink et al. 1995; Thornton et al. 2001) or the direct recording of local field potentials in a number of cortical nuclei (Green et al. 2007; Basnayake et al. 2012).
We now present evidence accumulating over the past two decades which implicates important feedback contributions to cardioventilatory control during exercise from three sources, namely the carotid chemoreceptors, the respiratory muscle metaboreceptors and the limb locomotor muscle group III–IV afferents. There is already sufficient evidence establishing that each of these reflexes, when stimulated in isolation, is capable of eliciting significant cardioventilatory responses akin to those present in voluntary whole body rhythmic exercise. However, the presence of redundant mechanisms and pathways available to protect the adequacy of gas transport during voluntary rhythmic exercise has confounded our ability to determine whether each of these sensory inputs is an obligatory cardiorespiratory controller. In our experiments we have addressed this question by using voluntary rhythmic exercise of a large muscle mass, which engages all potential feedforward and feedback mechanisms. On this background we superimposed a transient and/or steady state inhibition of the tonic afferent input from the specific receptor site(s) in question in order to determine the relative import of its contribution to cardiorespiratory control.
Carotid chemoreceptor contributions to sympathoexcitation/blood flow distribution
Research over the past decade has revealed a highly multifunctional, interactive and malleable carotid chemoreceptor. The carotid chemoreceptors are now known to respond sensitively to many circulating stimuli beyond oxygen, such as potassium, noradrenaline (norepinephrine), temperature, potassium, adenosine and glucose, and to play a major role in controlling sympathetic nerve activity as well as ventilation (Teppema & Dahan, 2010). They are also capable of upregulating their sensitivity in response to even relatively brief durations of arterial hypoxaemia (Nielsen et al. 1988; Wang et al. 2008) or reduced blood flow and sheer stress (Sun et al. 1999; Ding et al. 2011) and when stimulated, or inhibited, or denervated have also been shown to exert marked hyperadditive effects on medullary chemoreceptor CO2 sensitivity (Dahan et al. 2007; Blain et al. 2010). A role for the carotid chemoreceptors in cardiorespiratory regulation during exercise has previously emphasized the ventilatory response. Most data to date, mainly from chemo-denervated preparations, suggest a potential role in mediating at least a portion of the hyperventilatory response to heavy exercise, i.e. when circulating metabolites have increased in arterial blood, although this contention remains controversial and confounded by the remodelling which occurs following denervation as well as the multiple potential systemic and central stimuli which undergo rapid changes under these conditions (Forster et al. 2012).
We have attempted to evaluate the carotid chemoreceptor's contribution to the increased sympathetic nerve activity and blood flow distribution accompanying exercise. First we examined these questions in the resting and exercising canine by transiently inhibiting the carotid sinus nerve activity using close intra-arterial infusions of hyperoxic saline or dopamine (Bisgard et al. 1979) with the catheter tip positioned at the bifurcation of the common carotid artery (Stickland et al. 2007). This transient inhibition of carotid sinus nerve activity caused immediate increases in limb vascular conductance and blood flow during mild intensity exercise in the healthy canine – but not at rest – and these effects were prevented by ganglionic blockade or carotid body denervation (see Fig. 1; Stickland et al. 2007).
Figure 1. The effects of transient inhibition of carotid chemoreceptor tonic input on hind limb (HL) vascular conductance and blood flow during moderate intensity exercise in the healthy canine.
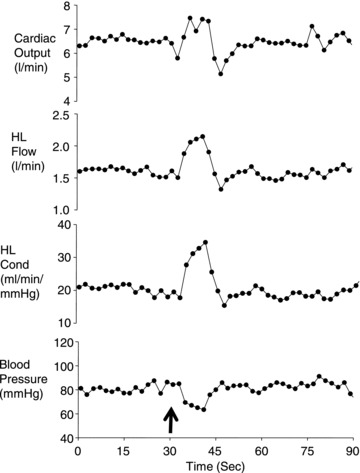
The arrow indicates the time of the close carotid injection of 10 μg kg−1 dopamine. Carotid body denervation or α adrenergic receptor blockade with phentolamine prevented these cardiovascular effects of carotid body inhibition. Figure adapted from Stickland et al. (2007).
In order to determine the relative contribution of the carotid chemoreceptors to the total sympathetic constraint we compared the maximum increase in limb vascular conductance with total systemic α receptor blockade vs. that achieved via carotid chemoreceptor inhibition alone (in the intact animal). As shown in Fig. 2, α blockade elicited a 50% increase in vascular conductance at rest and a 130% increase in conductance in mild intensity exercise, whereas carotid chemoreceptor inhibition by itself accounted for none of the total sympathetic constraint at rest and about one-third of the total increase in vascular conductance during exercise. In the same dogs studied after several weeks of cardiac pacing at a high rate into heart failure, left ventricular ejection fraction fell from 55 to 18% and carotid chemosensitivity increased, as shown by a threefold increase in the ventilatory response to isocapnic hypoxia. The magnitude of the total sympathetic constraint in chronic heart failure (CHF) (vs. healthy) animals on limb vascular conductance increased more than fourfold at rest and during mild exercise. Under these conditions of enhanced chemoreceptor sensitivity in CHF, tonic activity from the chemoreceptors – even at rest – accounted for 20–25% of the enhanced sympathetic constraint of vascular conductance (Stickland et al. 2007).
Figure 2. Effects of α blockade on hindlimb vascular conductance at rest and during mild intensity exercise.
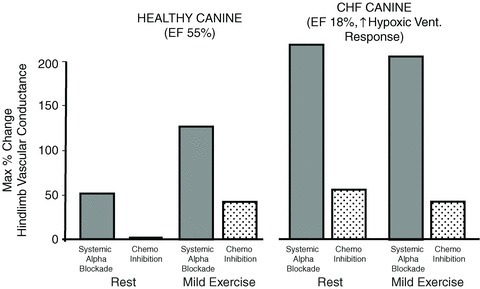
Note in the healthy canine, systemic α blockade increased limb vascular conductance by 50% at rest with no effect of chemoreceptor inhibition, per se, in the unblocked animal. During mild exercise the effect of α blockade on vascular conductance was increased 2.5-fold above that at rest and chemoreceptor inhibition contributed about one-third to the total increase in vascular conductance. The right-hand panel shows the effects of similar experiments in the same animals following 3 weeks of cardiac pacing to produce chronic heart failure (CHF). Note the left ventricular ejection fraction (EF) was reduced from 55% in health to 18% in the CHF animals and that the ventilatory response to isocapnic hypoxia was increased 3-fold in CHF vs. healthy animals. In the CHF animal the sympathetic blockade produced a 4-fold greater increase in limb vascular conductance both at rest and exercise and the influence of transient chemoreceptor inhibition was apparent even under resting conditions. Data obtained from Stickland et al. (2007).
A role for carotid chemoreceptor influences on sympathetic vasoconstriction in the healthy human was demonstrated by reductions in muscle sympathetic nerve activity (MSNA) elicited via transient inhaled hyperoxia during moderate intensity, rhythmic, arm exercise (Stickland et al. 2008; see Fig. 3). More recently a similar experiment in humans showed that transient chemoreceptor inhibition also increased limb vascular conductance and blood flow during mild intensity, single leg, rhythmic exercise (Stickland et al. 2011).
Figure 3. The effects in the healthy human of transient inhibition of the carotid chemoreceptor with 1.0  during rhythmic handgrip exercise (20 r.p.m. at 50% of maximum voluntary contraction).
during rhythmic handgrip exercise (20 r.p.m. at 50% of maximum voluntary contraction).

Ventilation and  were voluntarily controlled via feedback by the subject during the transient hyperoxia intervention. Note ∼15–20 s after
were voluntarily controlled via feedback by the subject during the transient hyperoxia intervention. Note ∼15–20 s after  rose above ∼250 mmHg, MSNA frequency was markedly diminished and then returned to control levels as exercise was continued and normoxic conditions were restored. The reduction in MSNA with transient hyperoxia averaged 35% in 7 subjects. Data obtained from Stickland et al. (2008). Subsequently, transient hyperoxia was also shown to increase limb vascular conductance and blood flow during single leg kick exercise (Stickland et al. 2011).
rose above ∼250 mmHg, MSNA frequency was markedly diminished and then returned to control levels as exercise was continued and normoxic conditions were restored. The reduction in MSNA with transient hyperoxia averaged 35% in 7 subjects. Data obtained from Stickland et al. (2008). Subsequently, transient hyperoxia was also shown to increase limb vascular conductance and blood flow during single leg kick exercise (Stickland et al. 2011).
These findings provide evidence in support of a significant contribution of the tonic sensory input from the carotid chemoreceptors as one mechanism, among other feedback and feedforward mechanisms (see section below regarding respiratory and limb afferents), to provide a significant contribution to sympathetic vasoconstrictor outflow and blood flow distribution to working muscles across a wide range of exercise intensities. We do not yet understand the mechanisms underlying an apparent exercise-induced increase in carotid body sensitivity (Stickland et al. 2007), although a similar synergistic effect of mild intensity exercise on both ventilatory and MSNA responsiveness to acute hypoxia has been previously reported (Weil et al. 1972; Seals et al. 1991). These data also demonstrate, using the CHF animal model, that carotid chemoreceptor hypersensitivity does occur under chronic conditions of reduced shear stress (Ding et al. 2011; Schultz, 2011) and this will elicit an enhanced contribution to sympathetically mediated vascular conductance and locomotor muscle blood flow both at rest and exercise.
Respiratory muscle afferents
We now summarize evidence in support of the contributions of a respiratory muscle metaboreflex to blood flow distribution and exercise performance. First, we know that unmyelinated sensory fibres are abundant in the phrenic nerve (Duron, 1981) and unmyelinated group IV metaboreceptor afferents are activated when the diaphragm is fatigued in the anaesthetized animal (Jammes & Balzamo, 1992; Hill, 2000). Secondly, it has also been demonstrated that pharmacological or electrical stimulation of phrenic afferents in anaesthetized animals elicits vasoconstriction and reduces blood flow in selective vascular beds (Hussain et al. 1991). Thirdly, in the human at rest, fatiguing the diaphragm or expiratory muscles (via voluntary breathing efforts against resistance) will cause a time dependent increase in MSNA (St Croix et al. 2000; Derchak et al. 2002) and a reduction in vascular conductance and blood flow in the resting limb (Sheel et al. 2001, 2002). Furthermore, in the resting or mildly exercising canine, infusing lactic acid into the phrenic artery and diaphragm caused vasoconstriction and reduced blood flow in the contracting limb muscle and this vasoconstrictive effect was prevented via adrenergic blockade (Rodman et al. 2003; see Fig. 4).
Figure 4. Effects of acidification of the diaphragm via lactic acid injection into the phrenic artery during mild intensity exercise in the chronically instrumented canine (n = 7).
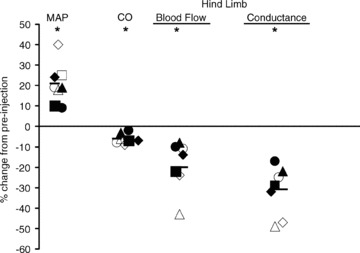
Note that the injection of lactic acid elicited an average increase in MAP of 20% and a decrease in limb vascular conductance of 30% and blood flow of 20%. These vasoconstrictor effects of diaphragm acidification were prevented via sympathetic blockade. Data obtained from Rodman et al. (2003). MAP, mean arterial blood pressure; CO, cardiac output.
Physiological and pathophysiological roles for the respiratory muscle metaboreflex
These types of data point strongly to the existence of a respiratory muscle metaboreflex capable of activating sympathetically mediated vasoconstriction in locomotor muscles, at least under conditions which elicit diaphragm fatigue and/or accumulation of anaerobic metabolites. However, to determine if there is a physiological role for this reflex during exercise it was important to reduce most of the respiratory muscle work normally incurred during exercise. When this was achieved with a unique servo-controlled mechanical ventilator while the subject exercised at >85% of the maximum rate of O2 consumption (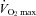 ), we observed (a) that diaphragm fatigue was prevented (Babcock et al. 2002), and (b) that, relative to the control condition, vasodilatation and increased blood flow occurred in the exercising legs (despite a concomitant reduction in stroke volume and cardiac output) (Harms et al. 1997, 1998; see Fig. 5, top). It would follow that these apparent effects of respiratory muscle work on compromising limb blood flow and O2 transport would also affect the rates at which locomotor muscle metabolites accumulated and limb muscle fatigue occurred during exercise (Barclay, 1986; Hogan et al. 1999). This possibility was assessed by measuring the force output of the quadriceps muscle (Qtwitch) in response to supermaximal magnetic stimulation of the femoral nerve before and immediately following cycling exercise (ΔQtwitch). Indeed, unloading the inspiratory muscles: (a) prevented a significant amount of limb fatigue when comparisons of ΔQtwitch were made between control and respiratory muscle unloaded conditions at identical heavy intensity work rates (>80% maximum (max) to exhaustion) for identical exercise durations (Romer et al. 2006; Amann et al. 2007), (b) increased exercise duration to exhaustion (by 14 ± 4%), and (c) significantly reduced perceived magnitude of ‘limb discomfort’ when exercise was carried out at a fixed, high intensity work load (Harms et al. 2000) (see Fig. 6).
), we observed (a) that diaphragm fatigue was prevented (Babcock et al. 2002), and (b) that, relative to the control condition, vasodilatation and increased blood flow occurred in the exercising legs (despite a concomitant reduction in stroke volume and cardiac output) (Harms et al. 1997, 1998; see Fig. 5, top). It would follow that these apparent effects of respiratory muscle work on compromising limb blood flow and O2 transport would also affect the rates at which locomotor muscle metabolites accumulated and limb muscle fatigue occurred during exercise (Barclay, 1986; Hogan et al. 1999). This possibility was assessed by measuring the force output of the quadriceps muscle (Qtwitch) in response to supermaximal magnetic stimulation of the femoral nerve before and immediately following cycling exercise (ΔQtwitch). Indeed, unloading the inspiratory muscles: (a) prevented a significant amount of limb fatigue when comparisons of ΔQtwitch were made between control and respiratory muscle unloaded conditions at identical heavy intensity work rates (>80% maximum (max) to exhaustion) for identical exercise durations (Romer et al. 2006; Amann et al. 2007), (b) increased exercise duration to exhaustion (by 14 ± 4%), and (c) significantly reduced perceived magnitude of ‘limb discomfort’ when exercise was carried out at a fixed, high intensity work load (Harms et al. 2000) (see Fig. 6).
Figure 5. Average changes in cardiac output (total blood flow) and leg blood flow during maximum cycling exercise in fit athletes with superimposed increases and decreases in inspiratory muscle work.
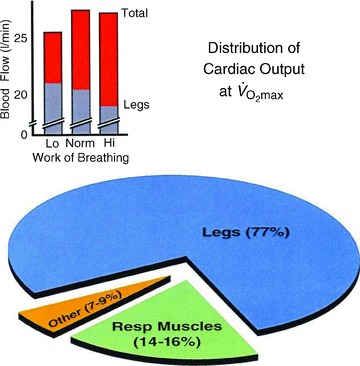
Limb vascular conductance and flow increased with reduced work of breathing and decreased with increased work of breathing. Stroke volume and cardiac output decreased with the reduced work of breathing and remained unchanged with increased work of breathing (also see bracketed note in the text on the quantification of respiratory muscle blood flow during exercise). Data adapted from Harms et al. (1997, 1998).
Figure 6.
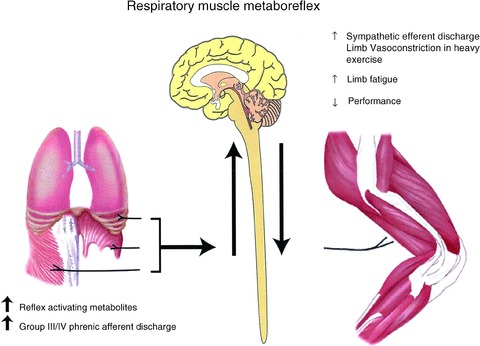
Schematic representation of the proposed respiratory muscle metaboreflex from the diaphragm and expiratory muscles activated by fatiguing contractions of these muscles and eliciting increased sympathetic discharge and limb vasoconstriction in heavy intensity exercise, with consequences to enhancing the rate of development of limb fatigue and reductions in exercise performance (see text). From Dempsey et al. (2006).
The relative importance of the respiratory muscle metaboreflex to blood flow distribution and limb fatigue during exercise was enhanced under conditions of increased ventilatory work and/or reduced blood flow – as occurs upon hypoxic exposure and/or in the presence of compromised cardiac output. For example, in health and during exercise in normoxia, unloading the respiratory muscles at work rates <75% of max had little effect on limb vascular resistance or limb fatigue or blood flow (Wetter et al. 1999; Amann et al. 2007). However, healthy subjects undergoing submaximal exercise in moderate hypoxia (arterial O2 saturation,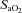 ∼80%) experienced substantial hyperventilation and unloading their inspiratory muscles alleviated the development of limb locomotor muscle fatigue by ∼30% and significantly prolonged exercise performance (Amann et al. 2007). So the increased work of breathing incurred by exercise in hypoxia contributed significantly to the limb fatigue and reduced exercise performance experienced in hypoxic environments.
∼80%) experienced substantial hyperventilation and unloading their inspiratory muscles alleviated the development of limb locomotor muscle fatigue by ∼30% and significantly prolonged exercise performance (Amann et al. 2007). So the increased work of breathing incurred by exercise in hypoxia contributed significantly to the limb fatigue and reduced exercise performance experienced in hypoxic environments.
Further, many CHF patients have an augmented, tachypnoeic ventilatory response to exercise combined with a limited availability of a reduced cardiac output during exercise for respiratory and limb muscle perfusion. Accordingly, when the respiratory muscles in these patients were unloaded via mechanical ventilation during even very mild exercise intensities (<60% peak work rate), limb vascular conductance and blood flow were markedly increased (Miller et al. 2007; Olson et al. 2010; see note below). Similarly in chronic obstructive pulmonary disease (COPD) patients, exercise-induced quadriceps fatigue occurred when cycling exercise to exhaustion was carried out at only 50% of 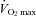 , whereas no limb fatigue occurred under similar conditions in age matched controls. Unloading the inspiratory muscles in these COPD patients relieved a significant amount (but not all) of the limb fatigue induced during submaximal exercise (Amann et al. 2010b). These findings implicated a compromised aerobic capacity of limb locomotor muscles (Maltais et al. 2000) – in addition to reduced O2 transport – as a significant contribution to exercise-induced limb fatigue in these highly sedentary COPD patients.
, whereas no limb fatigue occurred under similar conditions in age matched controls. Unloading the inspiratory muscles in these COPD patients relieved a significant amount (but not all) of the limb fatigue induced during submaximal exercise (Amann et al. 2010b). These findings implicated a compromised aerobic capacity of limb locomotor muscles (Maltais et al. 2000) – in addition to reduced O2 transport – as a significant contribution to exercise-induced limb fatigue in these highly sedentary COPD patients.
[In CHF patients, unloading the respiratory muscles via positive pressure mechanical ventilation – which reduces the left ventricular afterload – also increased stroke volume and cardiac output which accounted for a significant portion of the observed increase in limb blood flow, along with increases in limb vascular conductance (Miller et al. 2007; Olson et al. 2010). This increase in stroke volume contrasts sharply with the reductions in stroke volume experienced in healthy animals (Miller et al. 2007) and humans (Harms et al. 1997) when the negativity of inspiratory pleural pressure is reduced via positive pressure assist during exercise (see Fig. 5).]
Major questions remaining regarding the respiratory muscle metaboreflex
We propose that the evidence to date supports a significant role for respiratory muscle afferents in activating sympathetically mediated vasoconstriction and regulating blood flow distribution during sustained, high intensity exercise, with significant negative consequences for locomotor muscle fatigue and exercise performance. However, several major questions remain. For example, what mechanisms/conditions are required to trigger III–IV muscle afferents from inspiratory and expiratory muscles during exercise? (Also see section on limb afferents below.) Secondly, why, in the face of metaboreceptor induced increases in MSNA during exercise does the limb locomotor muscle vasculature constrict and the diaphragm vasculature dilate? Aacker and Laughlin showed in isolated vessels that noradrenaline-induced vasoconstriction was about twofold more sensitive in gastrocnemius muscle vasculature (in red or white muscle) than in the phrenic artery feed vessels (Aaker & Laughlin, 2002).
Thirdly, we know that altering the work of breathing during exercise will change blood flow to the exercising limb (see above) in the human but the presumption that this also means increased blood flow to respiratory muscles has only been verified to date via microsphere data in the CHF rat model in whom reduced blood flow to the limb musculature during exercise occurred simultaneously with a greater blood flow to the diaphragm (Musch, 1993). The state of the art for measuring blood flow in respiratory muscles does not yet appear to be sufficiently sensitive or specific to test this hypothesis in exercising humans (Vogiatzis et al. 2008) and methods are not available for measuring diaphragmatic flow in humans. However, supramaximal phrenic nerve stimulation has been used in humans to show that the diaphragm fatigues during heavy intensity, sustained running exercise, but does not fatigue when comparable levels of respiratory muscle work are performed via voluntary hyperpnoea in the subject while at rest. We interpreted these contrasting effects of respiratory muscle work between rest and exercise to mean that locomotor muscles do indeed compete to a significant extent, for the available cardiac output during heavy intensity exercise (Babcock et al. 1995). This interpretation is supported by a similar experimental design which showed that the increase in intercostal muscle blood flow accompanying high levels of ventilation was substantially reduced during cycling vs. voluntary, isocapnic hyperpnoea at rest (Vogiatzis et al. 2009; see note below). Finally, in contrast to the sympathoexcitatory effects elicited by activating respiratory muscle afferents, the effects on the control of breathing are contradictory, showing either a stimulatory or inhibitory effect in anaesthetized animals (Road, 1990). When we acidified the diaphragm in the exercising dog we observed a small but consistent slowing of breathing frequency (Rodman et al. 2003).
[The quantification of respiratory muscle blood flow during exercise remains controversial. On the one hand, estimates of ‘trunk and head’ blood flow based on the difference between cardiac output and flow to the arms plus legs (measured with dye dilution via catheterization of the subclavian and femoral veins) suggest that the lumped structure of the head, neck, heart, abdomen viscera, kidney, respiratory muscles and gluteal muscles receives about 20% of the cardiac output (CO) and 15% of the
during maximum (max) upright cycling exercise (Calbet et al. 2007). On the other hand, higher estimates of respiratory muscle blood flow alone during max cycling exercise ranging from 8 to 10% in the untrained, to 14–16% in the well trained (at higher max work rates and minute ventilation,
), were derived based on (a) the measured O2 cost and increased cardiac output required while mimicking the work of breathing (WOB) achieved at max exercise (Anholm et al. 1987; Aaron et al. 1992a,b; Vogiatzis et al. 2008), (b) the reduction in CO obtained when the WOB was reduced via mechanical ventilation during max exercise (Harms et al. 1998; see Fig. 5, bottom pie chart), and (c) the total blood flow (measured directly via microsphere distribution) in all inspiratory and expiratory muscles during max treadmill running in the pony (Manohar, 1986). One problem not yet addressed in any study is the identification of all muscles – in the chest wall, abdomen, upper back and shoulders – which are actually engaged (both dynamically and as fixators) in producing the hyperpnoea accompanying heavy intensity exercise.]
A logical extension of the evidence summarized above is to ask if specific respiratory muscle training will delay the rate of development of respiratory muscle fatigue during sustained exercise, and therefore also delay the activation of respiratory muscle metaboreflexes and blood flow redistribution during exercise? There is some limited evidence to date using adequately controlled studies to suggest that there might indeed be some small but significant effects of respiratory muscle training on reducing exercise-induced diaphragm fatigue (Verges et al. 2007), on alleviating calf muscle fatigue during plantar flexion exercise (McConnell & Lomax, 2006), on attenuating activation of the respiratory muscle reflex (Witt et al. 2007) and on exercise (time trial) performance (Romer et al. 2002). However, direct evidence for a benefit of respiratory muscle training on blood flow distribution during whole body high intensity exercise, i.e. when competition between locomotor and limb muscles for an increased share of cardiac output is at its peak, is not yet available.
Locomotor muscle afferents, exercise hyperpnoea, cardiovascular regulation and exercise performance
Cardioventilatory regulation
When studied in isolation, using muscle stimulation, i.e. without the central command mechanism of voluntary exercise, limb muscle group III–IV afferents clearly have the capability to increase respiratory motor output, ventilation, blood pressure and heart rate in anaesthetized animals or awake humans (Alam & Smirk, 1937, 19387; Asmussen et al. 1943; Coote et al. 1971; McCloskey & Mitchell, 1972; Tibes, 1977; Adams et al. 1984; Forster et al. 2012). Further, in vitro evidence in the isolated brain stem–spinal cord rodent preparation also supports an important role for muscle afferents in ventilatory control by demonstrating that lumbar locomotor networks can rhythmically entrain medullary respiratory neurons (Morin & Viala, 2002). However, over the past 100 plus years most supportive evidence in this regard is counteracted by negative findings which have questioned a clear, obligatory physiological role for these muscle afferents during voluntary, rhythmic exercise in humans. Note the following examples.
There are an abundance of group III–IV afferent endings in limb muscle and they are activated by normal submaximal, rhythmic exercise (Pickar et al. 1994; Adreani et al. 1997) – but countering evidence claims muscle ischaemia (i.e. a mismatch between blood O2 supply and demand), as opposed to a rhythmically contracting muscle with normally increased blood flow, is required to activate cardioventilatory responses via muscle afferents (Freund et al. 1979; Rowell, 2004). Furthermore, even imposed muscle ischaemia has been reported to elevate MAP – but to inhibit ventilation – during recovery from exercise (Dejours et al. 1957; Rowell et al. 1976; Eiken, 1987).
Electrical stimulation of limb muscle contractions in intact humans elicits a cardioventilatory response comparable to that during physiological whole body voluntary exercise – at least over a modest range of increase in
 above resting levels; however, spinal cord lesions do not always prevent these responses (Asmussen et al. 1943; Adams et al. 1984; Forster et al. 2012).
above resting levels; however, spinal cord lesions do not always prevent these responses (Asmussen et al. 1943; Adams et al. 1984; Forster et al. 2012).Blocking opiate sensitive group III–IV afferents via intrathecal opiate agonists or epidural lidocaine (lignocaine) markedly reduced most of the cardioventilatory responses to induced static muscle contrac-tions in anaesthetized cats (Hill & Kaufman, 1990; Meintjes et al. 1995) or limb vascular occlusion in exercising dogs (Pomeroy et al. 1986) or humans (Mitchell et al. 1989; Strange et al. 1993). However, afferent blockade with epidural lidocaine in the human undergoing rhythmic (cycling) exercise has most often shown no effect and occasionally even an increase in ventilatory and/or heart rate responses, along with a small reduction in MAP (Hornbein et al. 1969; Fernandes et al. 1990; Strange et al. 1993; Kjaer et al. 1999; Smith et al. 2003; Amann et al. 2008).
So, the prevailing opinion was that central command, i.e. feedforward, plays the major, obligatory role in determining the ventilatory and cardioaccelerator responses to rhythmic exercise with limb afferents exerting some influence of significance on the sympathetically mediated increase in blood pressure (Kaufman, 2010). The negative findings in these last-mentioned epidural lidocaine experiments were especially damning to a ‘physiological’ or ‘tonic’ role for limb muscle afferents in voluntary, rhythmic exercise because they supposedly prevented the effect of any normally occurring, i.e. tonic, muscle afferent feedback. Apparently, the ‘redundant’ mechanisms remaining after blockade, i.e. primarily central command, were sufficiently dominant to completely overcome the loss of this feedback mechanism. However, an alternative explanation for these negative results is that epidural lidocaine also has a significant effect on efferent motor output (on the ventral horn of the spinal cord) in addition to its effects on sensory input (on the dorsal horn), thereby causing muscle weakness (Hornbein et al. 1969; Strange et al. 1993; Amann et al. 2008). In turn, as shown in experiments using partial muscle paralysis (Galbo et al. 1987), in order to achieve a given force output in the face of muscle weakness, more motor units must be recruited via augmented central command, thereby rendering this experimental approach inappropriate to determine the effects of afferent blockade, per se, on cardioventilatory responses to exercise.
Accordingly, to circumvent this problem we chose to employ intrathecal fentanyl, a μ opioid agonist, as a means of blocking opiate-sensitive limb muscle afferents, an approach which would also spare efferent motor output and muscle strength. Cephalad movement of fentanyl to the CNS is a critical potential confounder but we and others employed several tests to determine that the drug did not move beyond the cervical level following lumbar level injection (Amann et al. 2010a; Gagnon et al. 2012). As summarized in Fig. 7A and B, at all steady state exercise intensities during cycling, this partial blockade of limb muscle afferents caused significant reductions in breathing frequency and ventilation, heart rate and MAP. (These cardiorespiratory effects of afferent blockade were originally revealed – serendipitously – as the byproduct of studies with the quite different aim of exploring the influence of muscle afferents on central motor output (Amann et al. 2008, 2009).) These afferents appear to mediate a highly significant portion of the normal cardioventilatory response as indicated by the observations in steady state exercise that (a) significant hypoventilation (+3 to 8 mmHg mean increases in end-tidal partial pressure of CO2, 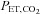 ) persisted in the steady state throughout all exercise intensities despite (presumably) marked systemic and CNS acidosis (and even some hypoxaemia, i.e. minimum
) persisted in the steady state throughout all exercise intensities despite (presumably) marked systemic and CNS acidosis (and even some hypoxaemia, i.e. minimum 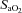 ∼90% at peak work rate) which presented severe levels of central and peripheral chemoreceptor stimulation, and (b) sympathetic efferent outflow remained depressed throughout exercise, as evidenced by a relative hypotension and bradycardia, even in the face of substantial and persistent unloading of the baroreceptors. It also appeared as though the greatest relative effects of afferent blockade on cardioventilatory responses occurred during moderate intensity exercise, as the effect of blockade was diminished near maximal exercise intensities. This may reflect the addition of powerful overriding feedforward and (chemoreceptor) feedback stimuli, commensurate with the onset of heavy intensity, fatiguing exercise. Follow-up studies using intrathecal fentanyl blockade during low intensity single leg kick exercise also showed significant reductions in cardiac output, and limb blood flow (Amann et al. 2011a). We emphasize that these observed cardioventilatory effects of blockade probably represent only a partial blockade of limb afferents, i.e. of only those pathways sensitive to μ opioid receptors (Hill & Kaufman, 1990).
∼90% at peak work rate) which presented severe levels of central and peripheral chemoreceptor stimulation, and (b) sympathetic efferent outflow remained depressed throughout exercise, as evidenced by a relative hypotension and bradycardia, even in the face of substantial and persistent unloading of the baroreceptors. It also appeared as though the greatest relative effects of afferent blockade on cardioventilatory responses occurred during moderate intensity exercise, as the effect of blockade was diminished near maximal exercise intensities. This may reflect the addition of powerful overriding feedforward and (chemoreceptor) feedback stimuli, commensurate with the onset of heavy intensity, fatiguing exercise. Follow-up studies using intrathecal fentanyl blockade during low intensity single leg kick exercise also showed significant reductions in cardiac output, and limb blood flow (Amann et al. 2011a). We emphasize that these observed cardioventilatory effects of blockade probably represent only a partial blockade of limb afferents, i.e. of only those pathways sensitive to μ opioid receptors (Hill & Kaufman, 1990).
Figure 7.
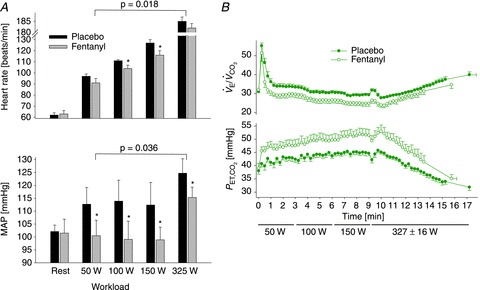
A, group mean effects of blockade of opiate sensitive limb muscle afferents on cardioacceleration and mean arterial blood pressure (MAP) at rest and during the steady state of voluntary rhythmic cycling exercise in healthy humans. Data obtained from Amann et al. (2010a). B, reduced steady state ventilation and CO2 retention resulting from muscle afferent blockade via intrathecal fentanyl in healthy humans at mild to heavy steady state exercise intensities. Note the persistence of the hypoventilatory response, especially during moderate intensity exercise, despite the presence of increased chemoreceptor stimulation. From Amann et al. (2010a).
This demonstration of significant effects of blockade of μ opioid-sensitive muscle afferents across varying intensities of steady-state rhythmic exercise (Fig. 8) confirms our previous findings during the highly variable work rates experienced during time-trial cycling exercise (Amann et al. 2009). Our findings are also consistent with the significant impact of cold blockade of group III–IV muscle afferents on the cardiovascular response to rhythmic electrical stimulation of limb muscles in the anaesthetized dog (Tibes, 1977) and with evidence in humans showing that muscle afferent blockade during rhythmic exercise significantly attenuated ‘baroreceptor resetting’ (Smith et al. 2003). However, these rather limited data to date need to be extended to larger numbers of subjects undergoing different intensities and durations of exercise. In such future assessments of the physiological role of muscle afferent feedback it will be important to recognize (a) the importance of the size of the exercising muscle mass utilized (Rowell & O’Leary, 1990), and (b) the potential importance of drug dosage on the effectiveness of afferent blockade, which remains untested and highly variable among the limited published reports to date (Amann et al. 2010a, 2011a, 2011b; Gagnon et al. 2012).
Figure 8. Effects of afferent blockade from limb locomotor muscles during constant load heavy intensity exercise (80% max).
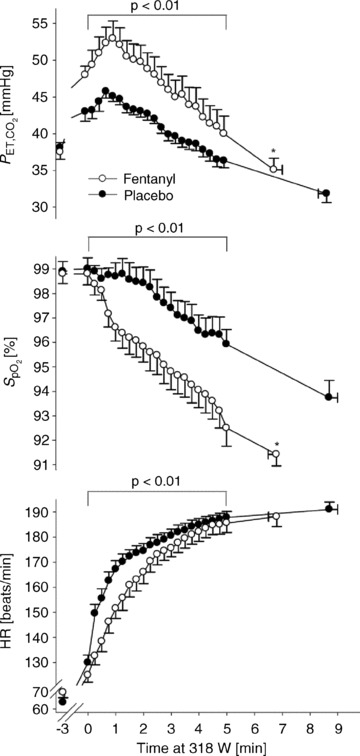
Note the increase in  secondary to a reduction in
secondary to a reduction in 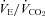 , the accompanying arterial O2 desaturation and the reduced cardioacceleration accompanying afferent blockade during this heavy intensity exercise. Also note the 21% average reduction in exercise time to exhaustion.
, the accompanying arterial O2 desaturation and the reduced cardioacceleration accompanying afferent blockade during this heavy intensity exercise. Also note the 21% average reduction in exercise time to exhaustion.  , pulse arterial blood O2 saturation; HR, heart rate. Data from Amann et al. (2011b).
, pulse arterial blood O2 saturation; HR, heart rate. Data from Amann et al. (2011b).
Interpreting the effects of afferent blockade
Fundamental questions remain regarding the interpretation of these findings of a suppressed cardioventilatory response to rhythmic exercise with blockade of opiate sensitive afferents and also of the strategies used to study the role of limb muscle afferents during physiological exercise. First, our finding that afferent blockade during rhythmic exercise attenuates the cardiorespiratory responses over a wide range of exercise intensities means that III–IV afferents and associated respiratory and/or sympathetic activities may be activated even under conditions where O2 supply to locomotor muscles is clearly adequate relative to metabolic demand (Pickar et al. 1994; Adreani et al. 1997). Similarly, in human patients with myophosphorylase deficiency, increased MSNA accompanies isometric contractions even in the absence of muscle acidification (Vissing et al. 2001). It has also been documented that group III–IV afferents may be activated via accumulation of multiple muscle metabolites even at very moderate exercise intensities (Light et al. 2008) or via increased muscle blood flow and venous distention stimulating group III–IV fibres located in the blood vessel adventitia (Haouzi et al. 1999). The importance of venous distension was demonstrated during induced muscle contractions in anaesthetized animals by showing that impeding the circulation from the venous side alone stimulated ventilation, whereas impeding arterial inflow alone depressed ventilation (Haouzi et al. 2004). These contrasting effects question the physiological relevance of the common use of ‘total’ vascular occlusion techniques as a means of testing the effects of exercise-induced metaboreceptor stimulation (see above).
Secondly, our studies in humans do not distinguish between metabo- and mechanoreflex effects. While discharge of type III mechanoreceptors clearly responds to mechanical distortion of their receptive fields (which usually occurs during the contraction phase of a step cycle), their sensitivity is also heightened by muscle metabolite accumulation (Hayes et al. 2006; Kaufman, 2012).
Thirdly, we question whether the cardiorespiratory effects of blocking spinal afferents represent primarily a direct effect on supraspinal input to the nucleus of the solitary tract and medullary cardiorespiratory neurons?…or are these predominantly interactive effects of muscle afferents on descending ‘central command’ at the level of the mid-brain or hypothalamus or even on descending output at the level of the spinal motor neurons (Garland & Kaufman, 1995; Gandevia, 2001). There is evidence, of both a neuroanatomical and functional nature, that significant interactions do indeed occur between muscle afferents and central command and that thin fibre muscle afferents to the medulla are ‘gated’ by descending inputs from central command. However, whether these interactive effects are multiplicative or even hypoadditive (Waldrop et al. 1986; Degtyarenko & Kaufman, 2002; Plowey et al. 2002) has not yet been assessed under conditions of physiological exercise.
In summary, the accumulating evidence suggests that stimulation of cardioventilatory responses to rhythmic exercise via group III–IV afferents may be critically dependent on the presence of increased muscle blood flow, muscular contractions and/or descending influences from central command. Whether any of these mechanisms operates at a gain sufficient to account for a significant portion of the total (intact) cardiorespiratory response needs to be tested under physiological conditions. Given that these potential mechanisms all require an element of the ‘exercising’ state it may be misleading to use observations obtained at rest during the recovery phase following exercise – with or without vascular occlusion – to evaluate the relative contributions from muscle afferents to exercise-induced cardiorespiratory responses.
Limb afferents, peripheral/central ‘fatigue’ and exercise performance
Finally, we address the complex question of locomotor muscle afferent influences on endurance exercise performance. We based our approach to this question on the concept (Amann et al. 2010a, 2011b; Gandevia, 2001) (a) that the accumulation of muscle metabolites hastens the development of limb locomotor muscle fatigue during exercise (as determined by changes in supramaximal femoral nerve stimulation, ΔQtwitch measured pre- vs. post-exercise), and (b) that these metabolite accumulations provide feedback via muscle afferents which engage sensory systems in the CNS and are responsible in part for the perceptual expression of ‘muscle pressure and tiredness’ leading to inhibition of central motor command (Gandevia et al. 1996; Light et al. 2008; Taylor & Gandevia, 2008). That muscle afferents do indeed inhibit central motor output was clearly shown during a time trial cycling exercise in which experienced competitive cyclists in the presence of muscle afferent blockade voluntarily ‘chose’ much higher force outputs (vs. placebo) over the initial half of a time trial. This ‘strategy’, chosen in the absence of feedback, led to extreme limb fatigue developed over the second half of the trial as manifested via excessive quadriceps fatigue (i.e. increased 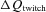 ) at end-exercise (Amann et al. 2009).
) at end-exercise (Amann et al. 2009).
We have now tested the effects of group III–IV muscle afferent blockade during constant load exercise to exhaustion at 85%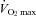 and found that all cyclists reduced their performance time, an average of 21 ± 4% (Amann et al. 2011b). We believe this reduction in performance represents the net effect of two opposing influences of muscle afferents. On the one hand O2 transport was reduced with blockade (vs. placebo) secondary to hypoventilation, arterial hypoxaemia and reduced heart rate and perfusion pressure (Amann et al. 2010a; see Fig. 8). On the other hand, central motor command was increased in the absence of afferent feedback, as indicated indirectly by the greater increase over time in quadriceps EMG during the constant load exercise test (see Fig. 9). This led to a 30% increase >placebo in limb fatigue (ΔQtwitch) as measured at end-exercise even though the exercise duration was shorter in the blocked state vs. placebo. (The significance of this excessive fatigue at end-exercise is noted by the extreme difficulty subjects experienced with post-exercise ambulation.)
and found that all cyclists reduced their performance time, an average of 21 ± 4% (Amann et al. 2011b). We believe this reduction in performance represents the net effect of two opposing influences of muscle afferents. On the one hand O2 transport was reduced with blockade (vs. placebo) secondary to hypoventilation, arterial hypoxaemia and reduced heart rate and perfusion pressure (Amann et al. 2010a; see Fig. 8). On the other hand, central motor command was increased in the absence of afferent feedback, as indicated indirectly by the greater increase over time in quadriceps EMG during the constant load exercise test (see Fig. 9). This led to a 30% increase >placebo in limb fatigue (ΔQtwitch) as measured at end-exercise even though the exercise duration was shorter in the blocked state vs. placebo. (The significance of this excessive fatigue at end-exercise is noted by the extreme difficulty subjects experienced with post-exercise ambulation.)
Figure 9. Effects of afferent blockade on an indirect estimate of ‘central motor command’ (quadriceps EMG) during prolonged heavy exercise to exhaustion.
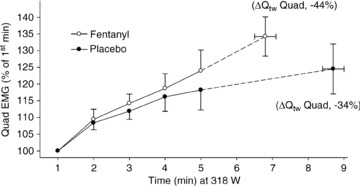
Note the increased EMG during muscle afferent blockade (vs. placebo) during the later stages of the exercise test. Also note the 25% greater limb fatigue (as measured by ΔQtwitch) pre- to post-exercise, despite the shorter exercise time in the presence of locomotor muscle afferent blockade. Data from Amann et al. (2011b).
Our interpretation of this reduced performance plus the excessive muscle fatigue development at end-exercise with feedback blockade is as follows. First, the reduced O2 transport induced by blockade (see Fig. 8) caused a substantially faster rate of development of muscle fatigue at 80% of maximum exercise. Secondly, when an enhanced rate of peripheral fatigue development was previously produced experimentally in subjects with intact feedback (by reducing the inspired O2 fraction (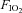 )) or causing pre-exercise specific limb fatigue; Amann et al. 2006) the exercise time was also shortened, but, unlike that in the subjects with limb afferent blockade, the end-exercise ΔQtwitch was identical to that under control conditions. These correlative data point to the role of feedback inhibition (in the intact subject) of central motor output in order to limit exercise duration, thereby preventing ‘excessive’ peripheral muscle fatigue. On the other hand, with afferent blockade and the absence of feedback inhibition from the limbs, the cyclist apparently ‘pushes’ himself to exercise for a longer duration, i.e. beyond the ‘threshold’ level of peripheral fatigue he usually achieves (when intact) at end-exercise.
)) or causing pre-exercise specific limb fatigue; Amann et al. 2006) the exercise time was also shortened, but, unlike that in the subjects with limb afferent blockade, the end-exercise ΔQtwitch was identical to that under control conditions. These correlative data point to the role of feedback inhibition (in the intact subject) of central motor output in order to limit exercise duration, thereby preventing ‘excessive’ peripheral muscle fatigue. On the other hand, with afferent blockade and the absence of feedback inhibition from the limbs, the cyclist apparently ‘pushes’ himself to exercise for a longer duration, i.e. beyond the ‘threshold’ level of peripheral fatigue he usually achieves (when intact) at end-exercise.
This evidence supports the postulate that limb muscle afferents contribute to essential determinants of exercise performance by (a) stimulating optimal cardioventilatory responses to preserve O2 transport to working muscles, and (b) providing feedback of the metabolic status of the locomotor muscles to influence central motor command. We caution that we do not understand the mechanisms or even the specific sites of action in the higher CNS which underlie these afferent links between accumulation of muscle metabolites, afferent feedback and inhibition of central motor command (Gandevia, 2001). Nor do we intend to imply that a host of other influences – originating systemically or in the higher CNS – do not also provide important sources of limitation to exercise performance. Of course they do! (Amann & Secher, 2010; Marcora, 2010.)
Locomotor vs. respiratory muscle metaboreflexes
Finally, it is of interest to contrast the consequences of the respiratory vs. limb muscle afferents on O2 transport and exercise performance. First, it appears as though they may have quite different thresholds of activation because activation of the respiratory muscle afferents and their cardiovascular sequelae (in health) appear to require sustained, heavy intensity, near-fatiguing contractions of the inspiratory and/or expiratory muscles whereas limb muscle afferents appear to be activated along with their cardiorespiratory sequelae during even moderate intensity rhythmic exercise. Furthermore, activating the respiratory muscle afferents precipitates sympathetic vasoconstriction and a selective effect which reduces blood flow and therefore O2 transport to working limb locomotor muscles while local vasodilatation and increased blood flow (apparently) occurs in the respiratory musculature. So the fatiguing diaphragm presumably benefits from activation of its own metaboreflex but vasoconstricted limb muscles do not; the net effect is exacerbation of the rate of limb fatigue development leading to inhibition of central motor command, thereby reducing endurance exercise performance. On the other hand, in the healthy intact subject the cardiorespiratory consequences of activation of limb muscle afferents promotes increased systemic O2 transport to meet metabolic requirements, thereby delaying the development of limb fatigue and preserving exercise performance.
These positive effects of limb muscle afferents in health may not apply to chronic disease states such as CHF and COPD. These extremely sedentary patients have highly untrained, fatiguable locomotor muscles with reduced aerobic capacity (Maltais et al. 2000; Amann et al. 2010b). These conditions are likely to promote a highly sensitive muscle metaboreflex which in turn would contribute to abnormally high levels of tachypnoeic ventilation and ventilatory work as well as exacerbation of sympathetic vasoconstrictor activity during even low intensity exercise. Accordingly, Gagnon et al. (2012) have recently demonstrated that intrathecal fentanyl blockade in (non-hypoxaemic) COPD patients reduced their exercise-induced tachycardia and MAP, as well as their tachypnoea and dead space ventilation. As a result, hyperinflation and dyspnoea were reduced as were perceptions of limb fatigue in the presence of sensory blockade (vs. placebo) when comparisons were made at equal exercise time points. These effects of muscle afferent blockade in COPD patients produced substantial improvements in constant load exercise time to exhaustion and an increase in the magnitude of quadriceps fatigue at end-exercise.
Summary
Accumulating evidence in recent years reveals significant, complementary roles for three types of feedback mechanisms in controlling the cardiorespiratory responses to voluntary, rhythmic exercise in the intact human. We propose the following hypothesis.
Locomotor muscle afferents provide an essential contribution to cardioacceleration, sympathetically mediated vasoconstriction and to hyperpnoea and arterial blood gas homeostasis over most exercise intensities.
Respiratory muscle metaboreflexes activate sympa-thetically mediated, selective vasoconstriction of locomotor muscle vasculature, thereby contributing to blood flow redistribution between respiratory and locomotor muscles during heavy intensity exercise. The exact balance achieved in flow distribution between these two vascular beds (in healthy subjects) remains unknown.
Carotid chemoreceptors contribute significantly to tonic levels of sympathetically mediated vasoconstriction and locomotor muscle blood flow during exercise of all intensities.
The exact stimuli responsible for activating limb muscle afferents or carotid chemoreceptors during mild to moderate intensities of rhythmic exercise remain unknown although there are some promising candidates. Further, the relative contribution of each of these reflex mechanisms to the total cardiorespiratory response and their interactive effects at the level of the central nervous system and/or spinal cord with ongoing central command mechanisms remains to be determined.
Acknowledgments
The original research reported here was supported by the National Heart, Lung, and Blood Institute and the American Heart Association. J.A.D. is especially indebted to many colleagues in the John Rankin Laboratory who contributed to this work, including Elizabeth Aaron, Bruce Johnson, Mark Babcock, Kurt Saupe, Curtis Smith, Mike Stickland, Lee Romer, Barbara Morgan, Claudette St Croix, Alex Derchak, William Sheel, Markus Amann, Tony Jacques, David Pegelow, Kathy Henderson, Marlowe Eldridge, Les Proctor, Josh Sebranek, Peter Hanson, Jordan Miller and Gregory Blain. I welcome this opportunity to acknowledge the final speaker of our London meeting, Professor Bengt Saltin, for more than 50 years of landmark contributions to the biology of exercise. Bengt has not only extended the century-long Scandinavian leadership in this field, he has raised the bar of excellence to new heights. Thanks Bengt, …all of us working in this field have benefited immeasurably from the example you have set with the quality of your science, your selfless mentoring, your service to the profession and public, and your humility.
References
- Aaker A, Laughlin MH. Diaphragm arterioles are less responsive to α1-adrenergic constriction than gastrocnemius arterioles. J Appl Physiol. 2002;92:1808–1816. doi: 10.1152/japplphysiol.01152.2001. [DOI] [PubMed] [Google Scholar]
- Aaron EA, Johnson BD, Seow CK, Dempsey JA. Oxygen cost of exercise hyperpnea: measurement. J Appl Physiol. 1992a;72:1810–1817. doi: 10.1152/jappl.1992.72.5.1810. [DOI] [PubMed] [Google Scholar]
- Aaron EA, Seow KC, Johnson BD, Dempsey JA. Oxygen cost of exercise hyperpnea: implications for performance. J Appl Physiol. 1992b;72:1818–1825. doi: 10.1152/jappl.1992.72.5.1818. [DOI] [PubMed] [Google Scholar]
- Adams L, Frankel H, Garlick J, Guz A, Murphy K, Semple SJ. The role of spinal cord transmission in the ventilatory response to exercise in man. J Physiol. 1984;355:85–97. doi: 10.1113/jphysiol.1984.sp015408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol. 1997;82:1811–1817. doi: 10.1152/jappl.1997.82.6.1811. [DOI] [PubMed] [Google Scholar]
- Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol. 1937;89:372–383. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M, Smirk FH. Unilateral loss of blood pressure raising, pulse accelerating, reflex from voluntary muscle due to a lesion of the spinal cord. Clin Sci. 1938;3:247–258. [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol. 2010a;109:966–976. doi: 10.1152/japplphysiol.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Eldridge MW, Lovering AT, Stickland MK, Pegelow DF, Dempsey JA. Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. J Physiol. 2006;575:937–952. doi: 10.1113/jphysiol.2006.113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Pegelow DF, Jacques AJ, Dempsey JA. Inspiratory muscle work in acute hypoxia influences locomotor muscle fatigue and exercise performance of healthy humans. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2036–R2045. doi: 10.1152/ajpregu.00442.2007. [DOI] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Eldridge MW, Pegelow DF, Dempsey JA. Somatosensory feedback from the limbs exerts inhibitory influences on central neural drive during whole body endurance exercise. J Appl Physiol. 2008;105:1714–1724. doi: 10.1152/japplphysiol.90456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol. 2009;587:271–283. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Regan MS, Kobitary M, Eldridge MW, Boutellier U, Pegelow DF, Dempsey JA. Impact of pulmonary system limitations on locomotor muscle fatigue in patients with COPD. Am J Physiol Regul Integr Comp Physiol. 2010b;299:R314–R324. doi: 10.1152/ajpregu.00183.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol. 2011b;589:3855–3866. doi: 10.1113/jphysiol.2011.209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J Physiol. 2011b;589:5299–5309. doi: 10.1113/jphysiol.2011.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Secher NH. Point: Afferent feedback from fatigued locomotor muscles is an important determinant of endurance exercise performance. J Appl Physiol. 2010;108:452–454. doi: 10.1152/japplphysiol.00976.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anholm JD, Johnson RL, Ramanathan M. Changes in cardiac output during sustained maximal ventilation in humans. J Appl Physiol. 1987;63:181–187. doi: 10.1152/jappl.1987.63.1.181. [DOI] [PubMed] [Google Scholar]
- Asmussen E, Nielsen M, Wieth-Pedersen G. Cortical or reflex control of respiration during muscular work. Acta Physiol Scand. 1943;6:168–175. [Google Scholar]
- Babcock MA, Pegelow DF, Harms CA, Dempsey JA. Effects of respiratory muscle unloading on exercise-induced diaphragm fatigue. J Appl Physiol. 2002;93:201–206. doi: 10.1152/japplphysiol.00612.2001. [DOI] [PubMed] [Google Scholar]
- Babcock MA, Pegelow DF, McClaran SR, Suman OE, Dempsey JA. Contribution of diaphragmatic power output to exercise-induced diaphragm fatigue. J Appl Physiol. 1995;78:1710–1719. doi: 10.1152/jappl.1995.78.5.1710. [DOI] [PubMed] [Google Scholar]
- Barclay JK. A delivery-independent blood flow effect on skeletal muscle fatigue. J Appl Physiol. 1986;61:1084–1090. doi: 10.1152/jappl.1986.61.3.1084. [DOI] [PubMed] [Google Scholar]
- Basnayake SD, Green AL, Paterson DJ. Mapping the central neurocircuitry that integrates the cardiovascular response to exercise in humans. Exp Physiol. 2012;97:29–38. doi: 10.1113/expphysiol.2011.060848. [DOI] [PubMed] [Google Scholar]
- Bisgard GE, Mitchell RA, Herbert DA. Effects of dopamine, norepinephrine and 5-hydroxytryptamine on the carotid body of the dog. Respir Physiol. 1979;37:61–80. doi: 10.1016/0034-5687(79)90092-6. [DOI] [PubMed] [Google Scholar]
- Blain GM, Smith CA, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2. J Physiol. 2010;588:2455–2471. doi: 10.1113/jphysiol.2010.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbet JA, Gonzalez-Alonso J, Helge JW, Sondergaard H, Munch-Andersen T, Boushel R, Saltin B. Cardiac output and leg and arm blood flow during incremental exercise to exhaustion on the cycle ergometer. J Appl Physiol. 2007;103:969–978. doi: 10.1152/japplphysiol.01281.2006. [DOI] [PubMed] [Google Scholar]
- Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol. 1971;215:789–804. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, Nieuwenhuijs D, Teppema L. Plasticity of central chemoreceptors: effect of bilateral carotid body resection on central CO2 sensitivity. PLoS Med. 2007;4:e239. doi: 10.1371/journal.pmed.0040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degtyarenko AM, Kaufman MP. Spinoreticular neurons that receive group III input are inhibited by MLR stimulation. J Appl Physiol. 2002;93:92–98. doi: 10.1152/japplphysiol.00072.2002. [DOI] [PubMed] [Google Scholar]
- Dejours P, Mithoefer JC, Raynaud J. Evidence against the existence of specific ventilatory chemoreceptors in the legs. J Appl Physiol. 1957;10:367–371. doi: 10.1152/jappl.1957.10.3.367. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Romer L, Rodman J, Miller J, Smith C. Consequences of exercise-induced respiratory muscle work. Respir Physiol Neurobiol. 2006;151:242–250. doi: 10.1016/j.resp.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Derchak PA, Sheel AW, Morgan BJ, Dempsey JA. Effects of expiratory muscle work on muscle sympathetic nerve activity. J Appl Physiol. 2002;92:1539–1552. doi: 10.1152/japplphysiol.00790.2001. [DOI] [PubMed] [Google Scholar]
- Ding Y, Li YL, Schultz HD. Role of blood flow in carotid body chemoreflex function in heart failure. J Physiol. 2011;589:245–258. doi: 10.1113/jphysiol.2010.200584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duron B. Regulation of Breathing. New York: Dekker; 1981. Intercostal and diaphragmatic muscle endings and afferents; pp. 473–540. [Google Scholar]
- Eiken O. Responses to dynamic leg exercise in man as influenced by changes in muscle perfusion pressure. Acta Physiol Scand Suppl. 1987;566:1–37. [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Waldrop TG. Exercise hyperpnea and locomotion: parallel activation from the hypothalamus. Science. 1981;211:844–846. doi: 10.1126/science.7466362. [DOI] [PubMed] [Google Scholar]
- Fernandes A, Galbo H, Kjaer M, Mitchell JH, Secher NH, Thomas SN. Cardiovascular and ventilatory responses to dynamic exercise during epidural anaesthesia in man. J Physiol. 1990;420:281–293. doi: 10.1113/jphysiol.1990.sp017912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Adams L, Watson JD, Innes JA, Wuyam B, Kobayashi I, Corfield DR, Murphy K, Jones T, Frackowiak RS, Guz A. Hyperpnoea during and immediately after exercise in man: evidence of motor cortical involvement. J Physiol. 1995;489:663–675. doi: 10.1113/jphysiol.1995.sp021081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster HV, Haouzi P, Dempsey JA. Control of breathing during exercise. Compr Physiol. 2012;2:743–777. doi: 10.1002/cphy.c100045. [DOI] [PubMed] [Google Scholar]
- Freund PR, Rowell LB, Murphy TM, Hobbs SF, Butler SH. Blockade of the pressor response to muscle ischemia by sensory nerve block in man. Am J Physiol Heart Circ Physiol. 1979;237:H433–H439. doi: 10.1152/ajpheart.1979.237.4.H433. [DOI] [PubMed] [Google Scholar]
- Gagnon P, Bussieres JS, Ribeiro F, Gagnon SL, Saey D, Gagne N, Provencher S, Maltais F. Influences of Spinal Anesthesia on Exercise Tolerance in Patients with COPD. Am J Respir Crit Care Med. 2012 doi: 10.1164/rccm.201203-0404OC. (ePub) [DOI] [PubMed] [Google Scholar]
- Galbo H, Kjaer M, Secher NH. Cardiovascular, ventilatory and catecholamine responses to maximal dynamic exercise in partially curarized man. J Physiol. 1987;389:557–568. doi: 10.1113/jphysiol.1987.sp016672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Allen GM, Butler JE, Taylor JL. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol. 1996;490:529–536. doi: 10.1113/jphysiol.1996.sp021164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland SJ, Kaufman MP. Role of muscle afferents in the inhibition of motoneurons during fatigue. Adv Exp Med Biol. 1995;384:271–278. doi: 10.1007/978-1-4899-1016-5_21. [DOI] [PubMed] [Google Scholar]
- Green AL, Wang S, Purvis S, Owen SL, Bain PG, Stein JF, Guz A, Aziz TZ, Paterson DJ. Identifying cardiorespiratory neurocircuitry involved in central command during exercise in humans. J Physiol. 2007;578:605–612. doi: 10.1113/jphysiol.2006.122549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haouzi P, Chenuel B, Huszczuk A. Sensing vascular distension in skeletal muscle by slow conducting afferent fibers: neurophysiological basis and implication for respiratory control. J Appl Physiol. 2004;96:407–418. doi: 10.1152/japplphysiol.00597.2003. [DOI] [PubMed] [Google Scholar]
- Haouzi P, Hill JM, Lewis BK, Kaufman MP. Responses of group III and IV muscle afferents to distension of the peripheral vascular bed. J Appl Physiol. 1999;87:545–553. doi: 10.1152/jappl.1999.87.2.545. [DOI] [PubMed] [Google Scholar]
- Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol. 1997;82:1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Hanson P, Dempsey JA. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol. 1998;85:609–618. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- Harms CA, Wetter TJ, St Croix CM, Pegelow DF, Dempsey JA. Effects of respiratory muscle work on exercise performance. J Appl Physiol. 2000;89:131–138. doi: 10.1152/jappl.2000.89.1.131. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, Kaufman MP. Cyclooxygenase blockade attenuates responses of group III and IV muscle afferents to dynamic exercise in cats. Am J Physiol Heart Circ Physiol. 2006;290:H2239–H2246. doi: 10.1152/ajpheart.01274.2005. [DOI] [PubMed] [Google Scholar]
- Hill JM. Discharge of group IV phrenic afferent fibers increases during diaphragmatic fatigue. Brain Res. 2000;856:240–244. doi: 10.1016/s0006-8993(99)02366-5. [DOI] [PubMed] [Google Scholar]
- Hill JM, Kaufman MP. Attenuation of reflex pressor and ventilatory responses to static muscular contraction by intrathecal opioids. J Appl Physiol. 1990;68:2466–2472. doi: 10.1152/jappl.1990.68.6.2466. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Richardson RS, Haseler LJ. Human muscle performance and PCr hydrolysis with varied inspired oxygen fractions: a 31P-MRS study. J Appl Physiol. 1999;86:1367–1373. doi: 10.1152/jappl.1999.86.4.1367. [DOI] [PubMed] [Google Scholar]
- Hornbein TF, Sorensen SC, Parks CR. Role of muscle spindles in lower extremities in breathing during bicycle exercise. J Appl Physiol. 1969;27:476–479. doi: 10.1152/jappl.1969.27.4.476. [DOI] [PubMed] [Google Scholar]
- Hussain SN, Chatillon A, Comtois A, Roussos C, Magder S. Chemical activation of thin-fibre phrenic afferents. 2. Cardiovascular responses. J Appl Physiol. 1991;70:77–86. doi: 10.1152/jappl.1991.70.1.77. [DOI] [PubMed] [Google Scholar]
- Jammes Y, Balzamo E. Changes in afferent and efferent phrenic activities with electrically induced diaphragmatic fatigue. J Appl Physiol. 1992;73:894–902. doi: 10.1152/jappl.1992.73.3.894. [DOI] [PubMed] [Google Scholar]
- Kaufman MP. Control of breathing during dynamic exercise by thin fibre muscle afferents. J Appl Physiol. 2010;109:947–948. doi: 10.1152/japplphysiol.00892.2010. [DOI] [PubMed] [Google Scholar]
- Kaufman MP. The exercise pressor reflex in animals. Exp Physiol. 2012;97:51–58. doi: 10.1113/expphysiol.2011.057539. [DOI] [PubMed] [Google Scholar]
- Kjaer M, Hanel B, Worm L, Perko G, Lewis SF, Sahlin K, Galbo H, Secher NH. Cardiovascular and neuroendocrine responses to exercise in hypoxia during impaired neural feedback from muscle. Am J Physiol Regul Integr Comp Physiol. 1999;277:R76–R85. doi: 10.1152/ajpregu.1999.277.1.R76. [DOI] [PubMed] [Google Scholar]
- Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol. 2008;100:1184–1201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell AK, Lomax M. The influence of inspiratory muscle work history and specific inspiratory muscle training upon human limb muscle fatigue. J Physiol. 2006;577:445–457. doi: 10.1113/jphysiol.2006.117614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltais F, LeBlanc P, Whittom F, Simard C, Marquis K, Belanger M, Breton MJ, Jobin J. Oxidative enzyme activities of the vastus lateralis muscle and the functional status in patients with COPD. Thorax. 2000;55:848–853. doi: 10.1136/thorax.55.10.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar M. Blood flow to the respiratory and limb muscles and to abdominal organs during maximal exertion in ponies. J Physiol. 1986;377:25–35. doi: 10.1113/jphysiol.1986.sp016174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcora S. Counterpoint: Afferent feedback from fatigued locomotor muscles is not an important determinant of endurance exercise performance. J Appl Physiol. 2010;108:454–456. doi: 10.1152/japplphysiol.00976.2009a. [DOI] [PubMed] [Google Scholar]
- Meintjes AF, Nobrega AC, Fuchs IE, Ally A, Wilson LB. Attenuation of the exercise pressor reflex. Effect of opioid agonist on substance P release in L-7 dorsal horn of cats. Circ Res. 1995;77:326–334. doi: 10.1161/01.res.77.2.326. [DOI] [PubMed] [Google Scholar]
- Miller JD, Smith CA, Hemauer SJ, Dempsey JA. The effects of inspiratory intrathoracic pressure production on the cardiovascular response to submaximal exercise in health and chronic heart failure. Am J Physiol Heart Circ Physiol. 2007;292:H580–H592. doi: 10.1152/ajpheart.00211.2006. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Reeves DR, Jr, Rogers HB, Secher NH. Epidural anaesthesia and cardiovascular responses to static exercise in man. J Physiol. 1989;417:13–24. doi: 10.1113/jphysiol.1989.sp017787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin D, Viala D. Coordinations of locomotor and respiratory rhythms in vitro are critically dependent on hindlimb sensory inputs. J Neurosci. 2002;22:4756–4765. doi: 10.1523/JNEUROSCI.22-11-04756.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch TI. Elevated diaphragmatic blood flow during submaximal exercise in rats with chronic heart failure. Am J Physiol Heart Circ Physiol. 1993;265:H1721–H1726. doi: 10.1152/ajpheart.1993.265.5.H1721. [DOI] [PubMed] [Google Scholar]
- Nielsen AM, Bisgard GE, Vidruk EH. Carotid chemoreceptor activity during acute and sustained hypoxia in goats. J Appl Physiol. 1988;65:1796–1802. doi: 10.1152/jappl.1988.65.4.1796. [DOI] [PubMed] [Google Scholar]
- Olson TP, Joyner MJ, Dietz NM, Eisenach JH, Curry TB, Johnson BD. Effects of respiratory muscle work on blood flow distribution during exercise in heart failure. J Physiol. 2010;588:2487–2501. doi: 10.1113/jphysiol.2009.186056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickar JG, Hill JM, Kaufman MP. Dynamic exercise stimulates group III muscle afferents. J Neurophysiol. 1994;71:753–760. doi: 10.1152/jn.1994.71.2.753. [DOI] [PubMed] [Google Scholar]
- Plowey ED, Kramer JM, Beatty JA, Waldrop TG. In vivo electrophysiological responses of pedunculopontine neurons to static muscle contraction. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1008–R1019. doi: 10.1152/ajpregu.00075.2002. [DOI] [PubMed] [Google Scholar]
- Pomeroy G, Ardell JL, Wurster RD. Spinal opiate modulation of cardiovascular reflexes in the exercising dog. Brain Res. 1986;381:385–389. doi: 10.1016/0006-8993(86)90095-8. [DOI] [PubMed] [Google Scholar]
- Road JD. Phrenic afferents and ventilatory control. Lung. 1990;168:137–149. doi: 10.1007/BF02719685. [DOI] [PubMed] [Google Scholar]
- Rodman JR, Henderson KS, Smith CA, Dempsey JA. Cardiovascular effects of the respiratory muscle metaboreflexes in dogs: rest and exercise. J Appl Physiol. 2003;95:1159–1169. doi: 10.1152/japplphysiol.00258.2003. [DOI] [PubMed] [Google Scholar]
- Romer LM, Lovering AT, Haverkamp HC, Pegelow DF, Dempsey JA. Effect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humans. J Physiol. 2006;571:425–439. doi: 10.1113/jphysiol.2005.099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer LM, McConnell AK, Jones DA. Effects of inspiratory muscle training on time-trial performance in trained cyclists. J Sports Sci. 2002;20:547–562. doi: 10.1080/026404102760000053. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Ideas about control of skeletal and cardiac muscle blood flow (1876–2003): cycles of revision and new vision. J Appl Physiol. 2004;97:384–392. doi: 10.1152/japplphysiol.01220.2003. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Hermansen L, Blackmon JR. Human cardiovascular and respiratory responses to graded muscle ischemia. J Appl Physiol. 1976;41:693–701. doi: 10.1152/jappl.1976.41.5.693. [DOI] [PubMed] [Google Scholar]
- Rowell LB, O’Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol. 1990;69:407–418. doi: 10.1152/jappl.1990.69.2.407. [DOI] [PubMed] [Google Scholar]
- St Croix CM, Morgan BJ, Wetter TJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J Physiol. 2000;529:493–504. doi: 10.1111/j.1469-7793.2000.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz HD. Angiotensin and carotid body chemoreception in heart failure. Curr Opin Pharmacol. 2011;11:144–149. doi: 10.1016/j.coph.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Johnson DG, Fregosi RF. Hypoxia potentiates exercise-induced sympathetic neural activation in humans. J Appl Physiol. 1991;71:1032–1040. doi: 10.1152/jappl.1991.71.3.1032. [DOI] [PubMed] [Google Scholar]
- Sheel AW, Derchak PA, Morgan BJ, Pegelow DF, Jacques AJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J Physiol. 2001;537:277–289. doi: 10.1111/j.1469-7793.2001.0277k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheel AW, Derchak PA, Pegelow DF, Dempsey JA. Threshold effects of respiratory muscle work on limb vascular resistance. Am J Physiol Heart Circ Physiol. 2002;282:H1732–H1738. doi: 10.1152/ajpheart.00798.2001. [DOI] [PubMed] [Google Scholar]
- Smith SA, Querry RG, Fadel PJ, Gallagher KM, Stromstad M, Ide K, Raven PB, Secher NH. Partial blockade of skeletal muscle somatosensory afferents attenuates baroreflex resetting during exercise in humans. J Physiol. 2003;551:1013–1021. doi: 10.1113/jphysiol.2003.044925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling EH. On the circulatory changes associated with exercise. J Royal Army Med Corps. 1920;34:258–272. [Google Scholar]
- Stickland MK, Fuhr DP, Haykowsky MJ, Jones KE, Paterson DI, Ezekowitz JA, McMurtry MS. Carotid chemoreceptor modulation of blood flow during exercise in healthy humans. J Physiol. 2011;589:6219–6230. doi: 10.1113/jphysiol.2011.218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickland MK, Miller JD, Smith CA, Dempsey JA. Carotid chemoreceptor modulation of regional blood flow distribution during exercise in health and chronic heart failure. Circ Res. 2007;100:1371–1378. doi: 10.1161/01.RES.0000266974.84590.d2. [DOI] [PubMed] [Google Scholar]
- Stickland MK, Morgan BJ, Dempsey JA. Carotid chemoreceptor modulation of sympathetic vasoconstrictor outflow during exercise in healthy humans. J Physiol. 2008;586:1743–1754. doi: 10.1113/jphysiol.2007.147421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange S, Secher NH, Pawelczyk JA, Karpakka J, Christensen NJ, Mitchell JH, Saltin B. Neural control of cardiovascular responses and of ventilation during dynamic exercise in man. J Physiol. 1993;470:693–704. doi: 10.1113/jphysiol.1993.sp019883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced activity of carotid body chemoreceptors in rabbits with heart failure: role of nitric oxide. J Appl Physiol. 1999;86:1273–1282. doi: 10.1152/jappl.1999.86.4.1273. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Gandevia SC. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J Appl Physiol. 2008;104:542–550. doi: 10.1152/japplphysiol.01053.2007. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Dahan A. The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol Rev. 2010;90:675–754. doi: 10.1152/physrev.00012.2009. [DOI] [PubMed] [Google Scholar]
- Thornton JM, Guz A, Murphy K, Griffith AR, Pedersen DL, Kardos A, Leff A, Adams L, Casadei B, Paterson DJ. Identification of higher brain centres that may encode the cardiorespiratory response to exercise in humans. J Physiol. 2001;533:823–836. doi: 10.1111/j.1469-7793.2001.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibes U. Reflex inputs to the cardiovascular and respiratory centers from dynamically working canine muscles. Some evidence for involvement of group III or IV nerve fibers. Circ Res. 1977;41:332–341. doi: 10.1161/01.res.41.3.332. [DOI] [PubMed] [Google Scholar]
- Verges S, Lenherr O, Haner AC, Schulz C, Spengler CM. Increased fatigue resistance of respiratory muscles during exercise after respiratory muscle endurance training. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1246–R1253. doi: 10.1152/ajpregu.00409.2006. [DOI] [PubMed] [Google Scholar]
- Vissing J, MacLean DA, Vissing SF, Sander M, Saltin B, Haller RG. The exercise metaboreflex is maintained in the absence of muscle acidosis: insights from muscle microdialysis in humans with McArdle's disease. J Physiol. 2001;537:641–649. doi: 10.1111/j.1469-7793.2001.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogiatzis I, Athanasopoulos D, Boushel R, Guenette JA, Koskolou M, Vasilopoulou M, Wagner H, Roussos C, Wagner PD, Zakynthinos S. Contribution of respiratory muscle blood flow to exercise-induced diaphragmatic fatigue in trained cyclists. J Physiol. 2008;586:5575–5587. doi: 10.1113/jphysiol.2008.162768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogiatzis I, Athanasopoulos D, Habazettl H, Kuebler WM, Wagner H, Roussos C, Wagner PD, Zakynthinos S. Intercostal muscle blood flow limitation in athletes during maximal exercise. J Physiol. 2009;587:3665–3677. doi: 10.1113/jphysiol.2009.171694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrop TG, Iwamoto GA. Point: Supraspinal locomotor centers do contribute significantly to the hyperpnea of dynamic exercise. J Appl Physiol. 2006;100:1077–1079. doi: 10.1152/japplphysiol.01528.2005. [DOI] [PubMed] [Google Scholar]
- Waldrop TG, Mullins DC, Millhorn DE. Control of respiration by the hypothalamus and by feedback from contracting muscles in cats. Respir Physiol. 1986;64:317–328. doi: 10.1016/0034-5687(86)90125-8. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Olson EB, Jr, Bjorling DE, Mitchell GS, Bisgard GE. Sustained hypoxia-induced proliferation of carotid body type I cells in rats. J Appl Physiol. 2008;104:803–808. doi: 10.1152/japplphysiol.00393.2007. [DOI] [PubMed] [Google Scholar]
- Weil JV, Byrne-Quinn E, Sodal IE, Kline JS, McCullough RE, Filley GF. Augmentation of chemosensitivity during mild exercise in normal man. J Appl Physiol. 1972;33:813–819. doi: 10.1152/jappl.1972.33.6.813. [DOI] [PubMed] [Google Scholar]
-
Wetter TJ, Harms CA, Nelson WB, Pegelow DF, Dempsey JA. Influence of respiratory muscle work on
 and leg blood flow during submaximal exercise. J Appl Physiol. 1999;87:643–651. doi: 10.1152/jappl.1999.87.2.643. [DOI] [PubMed] [Google Scholar]
and leg blood flow during submaximal exercise. J Appl Physiol. 1999;87:643–651. doi: 10.1152/jappl.1999.87.2.643. [DOI] [PubMed] [Google Scholar] - Witt JD, Guenette JA, Rupert JL, McKenzie DC, Sheel AW. Inspiratory muscle training attenuates the human respiratory muscle metaboreflex. J Physiol. 2007;584:1019–1028. doi: 10.1113/jphysiol.2007.140855. [DOI] [PMC free article] [PubMed] [Google Scholar]


