Abstract
Store-operated Ca2+ entry (SOCE) is a widespread mechanism in cells to raise cytosolic Ca2+ and to refill Ca2+ stores. T cells critically rely on SOCE mediated by stromal interaction molecules (STIM) and Orai molecules for their activation and regulation of gene transcription; cells such as muscle cells, neurons or melanocytes probably utilize SOCE for the transmission of inducible receptor-mediated function as well as for generalized Ca2+ homeostasis mechanisms. Exposure to environmental or cell-intrinisic reactive oxygen species (ROS) can affect several components involved in Ca2+ homeostasis and thus alter multiple pathways. While all cells have a capacity to produce intracellular ROS, exposure of immune and skin cells to extracellular oxidative stress is particularly high during inflammation and/or with UV exposure. This review briefly summarizes cell-intrinsic sources of ROS and focuses on current findings and controversies regarding the regulation of STIM and Orai by oxidative modifications. We also introduce melanocytes as a new model system to study the function of STIM and Orai isoforms under physiological conditions that include exposure to UV light as an activating stimulus.

Tatiana Kilch (left), Ivan Bogeski (not pictured) and Barbara A. Niemeyer (right) are based in the Department of Biophysics of the Saarland University Medical School in Homburg, Germany. After completing her PhD with Charles Zuker at UCSD patch-clamping Drosophila photoreceptors, Barbara Niemeyer worked on SNARE proteins with Tom Schwarz at Stanford, with mammalian TRP channels in the Department of Pharmacology in Homburg, and currently focuses on the functional properties and regulation of Orai proteins. Ivan Bogeski, whose medical training culminated with a doctorate in Physiology in Homburg, is also an adjunct professor in Štip, Macedonia. Ivan's research interests include understanding how ion transport mechanisms and redox processes regulate cellular physiology and pathophysiology. Tatiana Kilch is finishing her PhD in the Department, applying a wide range of electrophysiological, biochemical and molecular biological techniques to decipher structure and function of STIM1 SAM domain mutants.
Reactive oxygen species (ROS): sources and effects on SOCE
Although many studies concerning the regulation of SOCE by ROS are conducted using the application of exogenous ROS, physiological effects will be mediated by endogenous sources inside cells or by ROS produced by neighbouring cells. The lifetime and microdomain of each species will therefore be important factors in determining their physiological function. Endogenous ROS sources include mitochondrial ROS production via the escape of electrons from the respiratory electron transfer chain, membrane resident activated NADPH oxidases (NOX and DUOX; reviewed in Bedard & Krause, 2007; Bogeski et al. 2011), but also endoplasmic reticulum (ER)-associated flavoproteins such as Ero1p during oxidative protein folding as well as membrane-resident 5-lipoxygenases (Tu & Weissman, 2004; Sevier et al. 2007). When electrons are transferred to molecular oxygen, superoxide radicals are generated which can further dismutate to hydrogen peroxide (H2O2), or in the presence of nitric oxide (NO), to peroxynitrite (ONOO−). External sources of ROS include cells producing high amounts of ROS such as phagocytes, but also environmental factors such as heat, radiation and chemical substances. While high concentrations of ROS are generally deleterious for cell survival, the importance of reactive thiols as activity switches in signalling proteins responding to physiological ROS concentrations is becoming increasingly recognized. In general, reactive cysteines are the major targets of oxidation by ROS. Here, the thiol group can undergo reversible oxidation into sulfenic acid or irreversible oxidation into sulfinic- or sulfonic acid; the thiol group can also be S-glutathionylated or, if reactive nitrogen species are present, nitrosylated. Proteins undergoing oxidative folding within the ER gain disulfides by dithiol-disulfide exchange with the oxidized form of the thioredoxin-like protein Pdi1p (Frand & Kaiser, 1999; Tu & Weissman, 2004). A shift to hyperoxidizing conditions within the ER, however, will inactivate Ero1p which acts as a catalyst for Pdi1p, and thus can lead to an increased presence of reduced thiols in protein domains (such as the EF-SAM domain of STIM) located in the ER lumen (Sevier et al. 2007). Depending on the concentration of reduced glutathione within the ER these reduced thiols may also become S-glutathionylated. As seen below, this regulation may become relevant in the modulation of ER-resident STIM. Here, we summarize recent aspects of SOCE regulation by ROS. Already in the early 90s, regulation of Ca2+ influx and Ca2+ currents by thiol modifying agents had been described. Several of these studies showed that oxidation induced activation of the inositol 1,4,5-trisphosphate (IP3) and ryanodine receptors, leading to depletion of ER stores (Missiaen et al. 1991; Hilly et al. 1993; Poitras et al. 1993; Parekh & Penner, 1995), which may facilitate SOCE activation. However, other studies reported suppression of SOCE by <1 h incubation with the oxidizing agent tBHP with prolonged Ca2+ signals after >2 h incubation (Elliott et al. 1989). Inhibition of SOCE was also found following treatment with thimersoral, tBHP, H2O2 and other oxidants (Tornquist et al. 1999, 2000). Several other groups also report dual concentration or reactive species-dependent effects (Redondo et al. 2004; Suzuki et al. 2009). With the discovery of the molecular correlates of the Ca2+ release-activated Ca2+ current (ICRAC), namely the STIM and Orai protein family members, it has now become possible to investigate redox modifications on these proteins directly.
STIM1
Both murine and human STIM1 proteins contain four cysteine residues in the mature, signal peptide-cleaved protein. C49 and C56 are highly conserved cysteines located in the luminal N-terminal domain upstream of the first Ca2+-bound EF-hand where D76 is the first acidic side chain involved in Ca2+-binding (Stathopulos et al. 2008). C227 is located close to the cytoplasmic exit of the single transmembrane spanning region, and C437 is located within the Orai1 interacting cytosolic CRAC Activation Domain (CAD) STIM1 Orai1-activating region (SOAR) domain. No overall consensus as to the reactivity of these cysteines has been reached. Hawkins et al. (2010 show that the luminal C49 and C56 are not susceptible to oxidation/reduction-induced shifts in molecular weight when the EF-Sterile Alpha Motif domain containing these cysteines is expressed as a fusion protein in bacteria, which is not surprising as they would be unlikely to form disulfide bridges in the bacterial expression system. Prolonged treatment (24 h with Buthionine sulfoximine (BSO) or 30 min with H2O2) of COS-7 cells induced significant S-glutathionylation, clustering of full length STIM1 and activation of SOCE; H2O2-triggered, STIM1-dependent activation of ICRAC was also reported by Grupe et al. (2010), although these results could not be reproduced in our hands. Hawkins et al. went on to show that S-glutathionylation of STIM1 is abolished by mutation of C56. If clustering is due to S-glutathionylation of C56, STIM1 C49/56A mutants, unable to become S-glutathionylated, should not cluster. Unexpectedly, these mutants lead to constitutive Ca2+ entry in STIM1−/− DT40 cells and in HEK cells upon coexpression with Orai1, possibly due to allosteric effects on the Ca2+ binding affinity of the EF-hand (Hawkins et al. 2010). In contrast, Prins et al. recently found that under non-reducing conditions STIM1 protein from STIM1−/− mouse embryonic fibroblasts (MEF) cells transfected with wild-type yellow fluorescent protein (YFP)–STIM1, but not from cells transfected with YFP–STIM1–C49/56A, migrated more slowly in non-reducing compared to reducing conditions, supporting the presence of an intramolecular disulfide bond between C49 and C56. They found that the STIM1 intraluminal domain interacts with the ER resident oxidoreductase ERp57, an interaction which is reduced in the absence of these cysteines. ERp57 deficiency increases SOCE and results in partially pre-clustered STIM1, although disulfide bond formation of STIM1 is not altered in ERp57−/− cells. In their study, rescue of STIM1−/− cells with YFP–STIM1–C49/56A led to substantially reduced STIM1 function with no constitutive activity (Prins et al. 2011). The reason for the discrepancy with the findings of Hawkins et al. is unclear, but may be due to the different cellular systems which may result in different concentrations of endogenous antioxidant proteins, yielding different expression levels of transfected plasmids, or possibly due to different amounts of STIM2 or STIML (see below). In addition, it would be useful to show that, indeed, the C49/C56 STIM1 mutant does not contain other reactive cysteines; preliminary data from our group indicates that the double mutant still contains reactive cysteines (authors and D. Al-Ansary unpublished observations).
Several studies show that STIM1 clusters upon exposure to acute hypoxic conditions, probably due to a slow release of Ca2+ from the stores using mainly sodium dithionite (Na-Dit) to indirectly trigger hypoxia (Mancarella et al. 2011) or as a consequence of hypoxic induction of mitochondrial ROS production (Guzy et al. 2005; Gusarova et al. 2011; Mungai et al. 2011). Inhibition of mitochondrial ROS production prevents the increase of intracellular Ca2+ (Gusarova et al. 2011). Whether treatment with Na-Dit is able to induce mitochondrial ROS production is unclear. A decrease of molecular oxygen should, in the long term, lead to a decrease in ROS. Mancarella et al. show that clustered STIM1 in primary smooth muscle cells and HEK cells exposed to hypoxia does not activate Orai1 due to a concomitant acidification which prevents the interaction between the acidic cluster of the Orai1 C-terminal domain with a basic cluster in the STIM1 CAD region (Mancarella et al. 2011). An earlier study using phaeochromocytoma cells also found that STIM1-dependent SOCE is inhibited by acidosis and hypothermia and increased with alkalosis and hyperthermia (Thompson et al. 2009; Scrimgeour et al. 2012). Scrimgeour et al. recently demonstrated that E106 within the pore of Orai1 is critical for the pH sensitivity of its Ca2+ binding site, which adds another target of pH-dependent regulation of Orai1 (Scrimgeour et al. 2012). While Mungai et al. and Gusarova et al. did not measure pH, they observed STIM1-mediated Ca2+ entry with pharmacological properties related to Calcium Release Activated Calcium (CRAC) in 143B osteosarcoma cells and alveolar type II cells after hypoxic stimuli (Gusarova et al. 2011; Mungai et al. 2011). However, no data concerning the biophysical properties of the induced currents are presented. These recent studies concerning the role of hypoxia and ROS on STIM1 function also raise the question of whether mitochondria-produced ROS might have different effects from externally added ROS (see dual effects of ROS on SOCE above). A further complication in the analyses of hypoxia and ROS on SOCE in primary cells lies in the recent identification of a novel STIM1 splice variant, STIM1L, which is preclustered and bound to Orai1 in resting cells (Darbellay et al. 2011). The STIM1L protein arises by alternative splicing of exon 11 and is characterized by a longer exon 11 with an insertion of 106 additional amino acids also containing an extra cysteine downstream of the Orai interacting domain (CAD, SOAR). In contrast to STIM1 which is ubiquitously expressed in mouse tissues, STIM1L (115 kDa) is found in skeletal muscle, brain, cerebellum, brain stem, spleen and lungs, but not in kidney nor in T-lymphocytes. STIM1 and STIM1L transcripts are also present in neonatal cardiomyocytes but diminish with maturation. An upregulation of STIM1 and STIM1L mRNA and protein was shown in cardiomyocytes exposed to pathological stress, e.g. in cardiomyocytes treated with hypertrophic agonists such as phenylephrine (Luo et al. 2012). A functional difference between STIM1 and STIM1L has been found in myotubes exposed to repetitive Ca2+ release with a high potassium solution. STIM1L-deficient myotubes were not able to sustain continuous Ca2+ peaks and refilling of sarcoplasmic reticulum (SR) Ca2+ stores. Furthermore, Ca2+ influx was delayed in these myotubes. In resting conditions, STIM1L was found to be preclustered in SR regions adjacent to the plasma membrane independently of the filling state of the SR. STIM1L interacts with actin to form these permanent clusters and also preclusters Orai1 channels. However, STIML only activates Orai1 channels upon store depletion. The important role of the STIM1 protein in muscle contraction is highlighted by mice deficient for STIM1, which suffer from muscular fatigue upon tetanic stimulations (Stiber et al. 2008). This phenotype may be due to the concomitant absence of the STIM1L protein, which mediates immediate refilling of the SR stores (Darbellay et al. 2011). Its preclustering with the Orai1 channel may thus be important for maintaining fast, repetitive and persistent contractions in muscle fibres to compensate the Ca2+ efflux during normal contraction. The identification of STIM1L as a permanently clustered STIM1 homologue, which also contains an additional cytosolic cysteine, may help to clarify the divergent results regarding the effects of hyp-oxia and/or ROS on SOCE and activation/inhibition of Orai1-mediated influx. Because preclustered Orai1 is not inhibited by extracellular ROS (see below), cells expressing STIML may not be susceptible to ROS, in contrast to cells that do not express STIML. Knock-down of STIM1 expression by transgenic approaches or siRNA is likely to affect STIM1L expression as well, possibly leading to divergent results depending on the level of STIML expression. It will be important to identify whether both splice variants are expressed in the investigated cell type and to unravel the contribution of STIM1L versus STIM1 in cells where both are co-expressed.
STIM2
Unlike STIM1, the physiological role of STIM2 and its different splice variants is much less clear. Probably due to its low apparent Ca2+ binding affinity of its EF-hand (Zheng et al. 2008), STIM2 has been proposed to regulate basal cytosol and ER store Ca2+ concentrations and can function both in a store-dependent and a store-independent mode (Brandman et al. 2007; Parvez et al. 2008) as well as contribute to SOCE in immune cells (Oh-hora et al. 2008). In neurons, however, STIM2 as well as Orai2 are the predominant isoforms. SOCE mediated by STIM2 but not by STIM1 is essential for ischaemia-induced cytosolic Ca2+ accumulation. Neurons from STIM2−/− mice show significantly increased survival under hypoxic conditions (Berna-Erro et al. 2009). How STIM2 senses hypoxia is unclear. In addition, the expression and function of different STIM2 splice variants (see NCBI database and authors unpublished data), all of which contain additional cysteines, is unknown. Furthermore, a recent study found that the much longer STIM2 signal peptide is responsible for the post-translational production of three distinct STIM2-derived proteins, resulting in an escape of proteins from ER targeting. These cytosolic proteins can directly activate Orai1 in a store-independent manner. A third cleavage product can regulate gene transcription in a Ca2+-independent manner (Graham et al. 2011). The presence and function of different splice variants and cleavage products of STIM2 await further investigation.
Orai
Orai1 and Orai2 proteins contain three cysteines which are localized within transmembrane region (TM) 2 (C126, C143) and at the exit of TM3 (C195). Orai3 lacks C195 but contains two additional cysteines within the extracellular loop between TM3 and TM4. Biochemical analyses indicated that C195 is a reactive cysteine in Orai1 and may serve as a detection system primarily for changes in the extracellular oxidative environment. While acute application of H2O2 to active STIM1/Orai1 complexes does not block permeation, preincubation with H2O2 of Orai1/STIM1 expressing cells (HEK; T cells) inhibited activation of Orai1, but not of Orai3 (Bogeski et al. 2010). Upregulation of Orai3 provides a mechanism to protect SOCE from peroxide mediated inhibition. An Orai1 triple cysteine mutant (C126S/C143S/C195S) does not show H2O2 mediated inhibition, suggesting that redox regulation of STIM1 in these conditions does not play a major role. Reinsertion of a cysteine in the C195 homologous position within Orai3 renders these channels redox sensitive (Bogeski et al. 2010). Because different cells express different ratios of Orai1 and Orai3, redox sensitivity of SOCE may thus depend either on the relative amount of Orai1:Orai3 homotetramers, or on the appearance of Orai1–Orai3 heterotetramers (Fig. 1). The finding that STIM1L is precoupled to Orai1 in cardiomyocytes would lead to our prediction that these complexes would not be redox sensitive. Our findings also indicate that the degree of pre-activation of Orai1- and STIM1-expressing cells would affect the degree of redox sensitivity and may be one reason for divergent results.
Figure 1. Redox sensitivity of SOCE depends on the Orai1:Orai3 ratio.
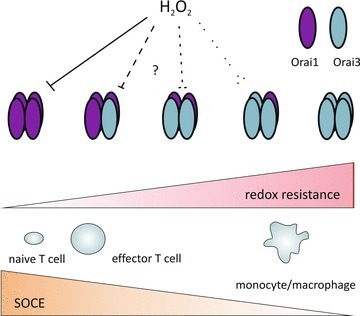
The model explains how the degree of SOCE and its redox sensitivity depend on the relative concentrations of Orai1 and Orai3.
The identification of a new splice variant (STIM1L) of STIM1 with an additional cysteine which is relatively broadly expressed and leads to preclustered STIML–Orai1 complexes, the possible presence of different splice variants and post-translational modifications of STIM2, altered ratios between STIM1 and STIM2, as well as different combinations of Orai1, 2 and 3, necessitate a careful re-evaluation of the diverse effects and the interplay between ROS, hypoxia and STIM mediated activation of SOCE. In addition, STIM1/Orai1 interactions postulated with other proteins such as TRPC channels (see Worley et al. 2007, amongst others) or voltage-gated calcium channels (Park et al. 2010; Wang et al. 2010) may contribute to discrepant results.
In the final section, we would like to introduce a new cellular system that should be well suited to study the function of ROS on SOCE in a physiological context. Skin melanocytes will be exposed to natural ROS sources (i.e. UV light) and respond to a large variety of peptide hormone signals which are able to trigger IP3 production, although a role for Orai proteins had not yet been described.
A physiological role of Orai and STIM proteins in UV-triggered ET-1-induced melanin production
Melanocytes are skin cells of neural origin in the basal epidermal layer where they are exposed to UV light, a known inductor of melanin production (adaptive tanning) but also of oxidative stress. Ca2+ is essential for the melanocyte import of l-phenylalanine as well as for its turnover to l-tyrosine, a precursor of melanin in its bio-synthetic pathway. In contrast to its role in melanin synthesis and activation of transcription factors such as the microphthalmia-associated factor (MITF, see Fig. 2), little is known about the regulation of Ca2+ homeostasis in melanocytes and particularly about the molecular identity of the channels involved in transmembrane Ca2+ influx.
Figure 2. UV radiation-induced Ca2+ homeostasis in primary human melanocytes.
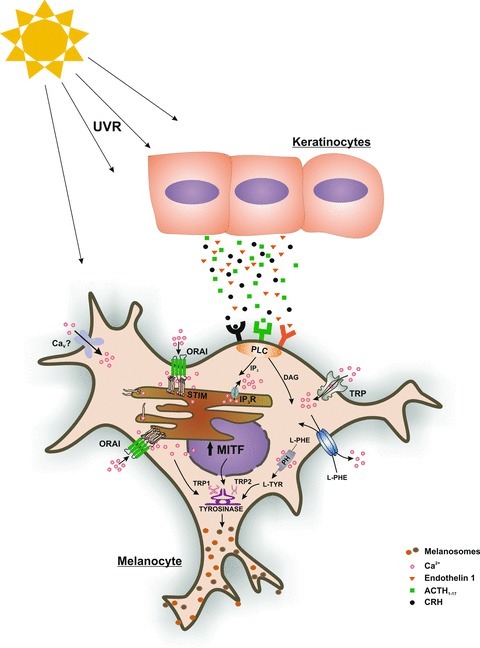
Exposure of human skin to UV radiation induces keratinocytes to secrete peptides and hormones. Many of these activate G-protein coupled receptors of neighbouring melanocytes. This in turn leads to production of IP3, depletion of ER Ca2+ stores, aggregation of STIM proteins and subsequent activation of Orai1 Ca2+ channels. Elevations in [Ca2+]i can lead to a rapid increase in melanin distribution/synthesis, while longer lasting elevations in [Ca2+]i lead to the activation of transcription factors necessary for melanocyte proliferation and melanin synthesis. UVR: ultraviolet radiation; MITF: microphthalmia-associated transcription factor; PLC: phospholipase C; DAG: diacylglycerol; L-PHE: l-phenylalanine; L-TYR: l-tyrosine; CaV: voltage gated Ca2+ channels; TRP: transient receptor potential; PH: phenylalanine hydroxylase; SERCA: sarco-endoplasmic reticulum Ca2+ ATPase; STIM: stromal interaction molecule; TRP1: tyrosinase related protein 1; TRP2: tyrosinase related protein 2; IP3: inositol trisphosphate; IP3R: inositol trisphosphate receptor.
Physiologically important elevations in melanocyte [Ca2+]i are usually triggered via G-protein coupled receptor activation (see Fig. 2 and Slominski et al. 2004). UV radiation triggers keratinocytes and melanocytes to secrete corticotropin-releasing hormone (CRH) and subsequently proopiomelanocortin-derived (POMC) peptides such as ET-1, ACTH, endorphin, amongst others (Slominski et al. 2000; Zbytek et al. 2006). Expression of several Ca2+ channel genes has been reported in primary human melanocytes. The presence of voltage-gated Ca2+ channels (CaV) using pharmacological tools was demonstrated by Wiesner et al. (2003). Using RT-PCR, Das et al. (2012) identified CaV 1.3 and 2.1 isoforms in melanocytes. While no depolarization-induced change in [Ca2+]i could be detected in healthy melanocytes, different melanoma cell lines showed significant depolarization-induced CaV activity. A non-selective cation channel of the transient receptor potential (TRP) family, namely melastatin 1 (TRPM1) was originally identified because of its reduced expression in highly metastatic melanomas in comparison with non-malignant nevi (Duncan et al. 1998); differential expression as well as mutations in TRPM1 have been linked to congenital stationary night blindness and coat spotting patterns in the Appaloosa horse (Bellone et al. 2008). In human melanocytes, Oancea et al. and Devi et al. showed that TRPM1 down-regulation reduces cell growth, differentiation and melanogenesis (Devi et al. 2009; Oancea et al. 2009). In addition to TRPM1, using RT-PCR analyses, Devi et al. identified transcripts of TRPM3, 4, 6 and 7. Earlier, TRPM7 was also identified in human melanocytes and was also shown to regulate melanophore cell death in zebrafish (McNeill et al. 2007). TRP channels of other subfamilies such as TRPA and TRPV have also been identified in human melanocytes (Atoyan et al. 2009; Choi et al. 2009). However, many activating signalling pathways that lead to the activation of a given TRP channel in melanocytes and their functional importance are not completely understood.
While Wicks et al. (2011) showed that UV radiation can also cause ER Ca2+ store depletion and consequent increase in [Ca2+]i, thereby inducing rapid melanin synthesis, the authors do not report which plasma membrane-based Ca2+ channels are involved in this signalling cascade. Recently, we asked if Orai Ca2+ channels control UV-induced specific melanocyte functions. We found that activation of endothelin-1 (ET-1) receptors leads to Ca2+ influx mediated mainly by Orai1 channels, increasing melanocyte proliferation as well as melanin production (Stanisz et al. 2012; see Fig. 2). The Orai1-mediated melanocyte response towards ET-1 as well as to other UV-induced stimulants, therefore, is likely to be involved in the process of ‘adaptive tanning’ rather than in the regulation of basal skin pigmentation. A defective Orai1-dependent Ca2+ entry in melanocytes may thus lead to impaired tanning ability. Besides the finding that Orai1 is highly expressed in primary melanocytes, we also showed expression of Orai3 as well as a STIM2 expression which is stronger than STIM1. Whether Orai3 expression is able to alter melanocyte SOCE sensitivity towards UV-induced ROS production, whether increased STIM2 expression alters specific melanocyte functions and which processes are triggered by too much UV exposure (sunburn) are open questions. The skin will thus be an interesting system to study immune cell function as well as melanocyte function in a physiological setting.
Acknowledgments
We thank members of the laboratory for the critical reading of this article. This work is supported by the AvH Foundation via the joint German-Macedonian project (DEU/1128670 to I.B.) and by the Deutsche Forschungsgemeinschaft to I.B. (BO 3643/2-1) and to B.A.N. (SFB894, NI671/3-1).
References
- Atoyan R, Shander D, Botchkareva NV. Non-neuronal expression of transient receptor potential type A1 (TRPA1) in human skin. J Invest Dermatol. 2009;129:2312–2315. doi: 10.1038/jid.2009.58. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bellone RR, Brooks SA, Sandmeyer L, Murphy BA, Forsyth G, Archer S, Bailey E, Grahn B. Differential gene expression of TRPM1, the potential cause of congenital stationary night blindness and coat spotting patterns (LP) in the Appaloosa horse (Equus caballus. Genetics. 2008;179:1861–1870. doi: 10.1534/genetics.108.088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berna-Erro A, Braun A, Kraft R, Kleinschnitz C, Schuhmann MK, Stegner D, Wultsch T, Eilers J, Meuth SG, Stoll G, Nieswandt B. STIM2 regulates capacitive Ca2+ entry in neurons and plays a key role in hypoxic neuronal cell death. Sci Signal. 2009;2:ra67. doi: 10.1126/scisignal.2000522. [DOI] [PubMed] [Google Scholar]
- Bogeski I, Kappl R, Kummerow C, Gulaboski R, Hoth M, Niemeyer BA. Redox regulation of calcium ion channels: chemical and physiological aspects. Cell Calcium. 2011;50:407–423. doi: 10.1016/j.ceca.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Bogeski I, Kummerow C, Al-Ansary D, Schwarz EC, Koehler R, Kozai D, Takahashi N, Peinelt C, Griesemer D, Bozem M, Mori Y, Hoth M, Niemeyer BA. Differential redox regulation of ORAI ion channels: a mechanism to tune cellular calciumsignaling. Sci Signal. 2010;3:ra24. doi: 10.1126/scisignal.2000672. [DOI] [PubMed] [Google Scholar]
- Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi TY, Park SY, Jo JY, Kang G, Park JB, Kim JG, Hong SG, Kim CD, Lee JH, Yoon TJ. Endogenous expression of TRPV1 channel in cultured human melanocytes. J Dermatol Sci. 2009;56:128–130. doi: 10.1016/j.jdermsci.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Darbellay B, Arnaudeau S, Bader CR, Konig S, Bernheim L. STIM1L is a new actin-binding splice variant involved in fast repetitive Ca2+ release. J Cell Biol. 2011;194:335–346. doi: 10.1083/jcb.201012157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Pushparaj C, Bahi N, Sorolla A, Herreros J, Pamplona R, Vilella R, Matias-Guiu X, Marti RM, Canti C. Functional expression of voltage-gated calcium channels in human melanoma. Pigment Cell Melanoma Res. 2012;25:200–212. doi: 10.1111/j.1755-148X.2012.00978.x. [DOI] [PubMed] [Google Scholar]
- Devi S, Kedlaya R, Maddodi N, Bhat KM, Weber CS, Valdivia H, Setaluri V. Calcium homeostasis in human melanocytes: role of transient receptor potential melastatin 1 (TRPM1) and its regulation by ultraviolet light. Am J Physiol Cell Physiol. 2009;297:C679–C687. doi: 10.1152/ajpcell.00092.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LM, Deeds J, Hunter J, Shao J, Holmgren LM, Woolf EA, Tepper RI, Shyjan AW. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res. 1998;58:1515–1520. [PubMed] [Google Scholar]
- Elliott SJ, Eskin SG, Schilling WP. Effect of t-butyl-hydroperoxide on bradykinin-stimulated changes in cytosolic calcium in vascular endothelial cells. J Biol Chem. 1989;264:3806–3810. [PubMed] [Google Scholar]
- Frand AR, Kaiser CA. Ero1p oxidizes protein disulfide isomerase in a pathway for disulfide bond formation in the endoplasmic reticulum. Mol Cell. 1999;4:469–477. doi: 10.1016/s1097-2765(00)80198-7. [DOI] [PubMed] [Google Scholar]
- Graham SJ, Dziadek MA, Johnstone LS. A cytosolic STIM2 preprotein created by signal peptide inefficiency activates ORAI1 in a store-independent manner. J Biol Chem. 2011;286:16174–16185. doi: 10.1074/jbc.M110.206946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe M, Myers G, Penner R, Fleig A. Activation of store-operated ICRAC by hydrogen peroxide. Cell Calcium. 2010;48:1–9. doi: 10.1016/j.ceca.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusarova GA, Trejo HE, Dada LA, Briva A, Welch LC, Hamanaka RB, Mutlu GM, Chandel NS, Prakriya M, Sznajder JI. Hypoxia leads to Na,K-ATPase downregulation via Ca2+ release-activated Ca2+ channels and AMPK activation. Mol Cell Biol. 2011;31:3546–3556. doi: 10.1128/MCB.05114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Hawkins BJ, Irrinki KM, Mallilankaraman K, Lien YC, Wang Y, Bhanumathy CD, Subbiah R, Ritchie MF, Soboloff J, Baba Y, Kurosaki T, Joseph SK, Gill DL, Madesh M. S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J Cell Biol. 2010;190:391–405. doi: 10.1083/jcb.201004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilly M, Pietri-Rouxel F, Coquil JF, Guy M, Mauger JP. Thiol reagents increase the affinity of the inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1993;268:16488–16494. [PubMed] [Google Scholar]
- Luo X, Hojayev B, Jiang N, Wang ZV, Tandan S, Rakalin A, Rothermel BA, Gillette TG, Hill JA. STIM1-dependent store-operated Ca2+ entry is required for pathological cardiac hypertrophy. J Mol Cell Cardiol. 2012;52:136–147. doi: 10.1016/j.yjmcc.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill MS, Paulsen J, Bonde G, Burnight E, Hsu MY, Cornell RA. Cell death of melanophores in zebrafish trpm7 mutant embryos depends on melanin synthesis. J Invest Dermatol. 2007;127:2020–2030. doi: 10.1038/sj.jid.5700710. [DOI] [PubMed] [Google Scholar]
- Mancarella S, Wang Y, Deng X, Landesberg G, Scalia R, Panettieri RA, Mallilankaraman K, Tang XD, Madesh M, Gill DL. Hypoxia-induced acidosis uncouples the STIM-Orai calcium signaling complex. J Biol Chem. 2011;286:44788–44798. doi: 10.1074/jbc.M111.303081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaen L, Taylor CW, Berridge MJ. Spontaneous calcium release from inositol trisphosphate-sensitive calcium stores. Nature. 1991;352:241–244. doi: 10.1038/352241a0. [DOI] [PubMed] [Google Scholar]
- Mungai PT, Waypa GB, Jairaman A, Prakriya M, Dokic D, Ball MK, Schumacker PT. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol. 2011;31:3531–3545. doi: 10.1128/MCB.05124-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oancea E, Vriens J, Brauchi S, Jun J, Splawski I, Clapham DE. TRPM1 forms ion channels associated with melanin content in melanocytes. Sci Signal. 2009;2:ra21. doi: 10.1126/scisignal.2000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh-hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB, Penner R. Activation of store-operated calcium influx at resting InsP3 levels by sensitization of the InsP3 receptor in rat basophilic leukaemia cells. J Physiol. 1995;489:377–382. doi: 10.1113/jphysiol.1995.sp021058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, Shcheglovitov A, Dolmetsch R. The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science. 2010;330:101–105. doi: 10.1126/science.1191027. [DOI] [PubMed] [Google Scholar]
- Parvez S, Beck A, Peinelt C, Soboloff J, Lis A, Monteilh-Zoller M, Gill DL, Fleig A, Penner R. STIM2 protein mediates distinct store-dependent and store-independent modes of CRAC channel activation. FASEB J. 2008;22:752–761. doi: 10.1096/fj.07-9449com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitras M, Bernier S, Servant M, Richard DE, Boulay G, Guillemette G. The high affinity state of inositol 1,4,5-trisphosphate receptor is a functional state. J Biol Chem. 1993;268:24078–24082. [PubMed] [Google Scholar]
- Prins D, Groenendyk J, Touret N, Michalak M. Modulation of STIM1 and capacitative Ca2+ entry by the endoplasmic reticulum luminal oxidoreductase ERp57. EMBO Rep. 2011;12:1182–1188. doi: 10.1038/embor.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo PC, Salido GM, Pariente JA, Rosado JA. Dual effect of hydrogen peroxide on store-mediated calcium entry in human platelets. Biochem Pharmacol. 2004;67:1065–1076. doi: 10.1016/j.bcp.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Scrimgeour NR, Wilson DP, Rychkov GY. Glu106 in the Orai1 pore contributes to fast Ca2+-dependent inactivation and pH dependence of Ca2+ release-activated Ca2+ (CRAC) current. Biochem J. 2012;441:743–753. doi: 10.1042/BJ20110558. [DOI] [PubMed] [Google Scholar]
- Sevier CS, Qu H, Heldman N, Gross E, Fass D, Kaiser CA. Modulation of cellular disulfide-bond formation and the ER redox environment by feedback regulation of Ero1. Cell. 2007;129:333–344. doi: 10.1016/j.cell.2007.02.039. [DOI] [PubMed] [Google Scholar]
- Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- Stanisz H, Stark A, Kilch T, Schwarz EC, Muller CS, Peinelt C, Hoth M, Niemeyer BA, Vogt T, Bogeski I. ORAI1 Ca2+ channels control endothelin-1-induced mitogenesis and melanogenesis in primary human melanocytes. J Invest Dermatol. 2012;132:1443–1451. doi: 10.1038/jid.2011.478. [DOI] [PubMed] [Google Scholar]
- Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–122. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Stiber J, Hawkins A, Zhang ZS, Wang S, Burch J, Graham V, Ward CC, Seth M, Finch E, Malouf N, Williams RS, Eu JP, Rosenberg P. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat Cell Biol. 2008;10:688–697. doi: 10.1038/ncb1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Yoshimaru T, Inoue T, Ra C. Discrete generations of intracellular hydrogen peroxide and superoxide in antigen-stimulated mast cells: reciprocal regulation of store-operated Ca2+ channel activity. Mol Immunol. 2009;46:2200–2209. doi: 10.1016/j.molimm.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Thompson MA, Pabelick CM, Prakash YS. Role of STIM1 in regulation of store-operated Ca2+ influx in pheochromocytoma cells. Cell Mol Neurobiol. 2009;29:193–202. doi: 10.1007/s10571-008-9311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornquist K, Vainio PJ, Bjorklund S, Titievsky A, Dugue B, Tuominen RK. Hydrogen peroxide attenuates store-operated calcium entry and enhances calcium extrusion in thyroid FRTL-5 cells. Biochem J. 2000;351:47–56. doi: 10.1042/0264-6021:3510047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornquist K, Vainio P, Titievsky A, Dugue B, Tuominen R. Redox modulation of intracellular free calcium concentration in thyroid FRTL-5 cells: evidence for an enhanced extrusion of calcium. Biochem J. 1999;339:621–628. [PMC free article] [PubMed] [Google Scholar]
- Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol. 2004;164:341–346. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Deng X, Mancarella S, Hendron E, Eguchi S, Soboloff J, Tang XD, Gill DL. The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science. 2010;330:105–109. doi: 10.1126/science.1191086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks NL, Chan JW, Najera JA, Ciriello JM, Oancea E. UVA phototransduction drives early melanin synthesis in human melanocytes. Curr Biol. 2011;21:1906–1911. doi: 10.1016/j.cub.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner B, Roloff B, Fechner K, Slominski A. Intracellular calcium measurements of single human skin cells after stimulation with corticotropin-releasing factor and urocortin using confocal laser scanning microscopy. J Cell Sci. 2003;116:1261–1268. doi: 10.1242/jcs.00301. [DOI] [PubMed] [Google Scholar]
- Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium. 2007;42:205–211. doi: 10.1016/j.ceca.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbytek B, Pfeffer LM, Slominski AT. CRH inhibits NF-κB signaling in human melanocytes. Peptides. 2006;27:3276–3283. doi: 10.1016/j.peptides.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Stathopulos PB, Li GY, Ikura M. Biophysical characterization of the EF-hand and SAM domain containing Ca2+ sensory region of STIM1 and STIM2. Biochem Biophys Res Commun. 2008;369:240–246. doi: 10.1016/j.bbrc.2007.12.129. [DOI] [PubMed] [Google Scholar]


