Abstract
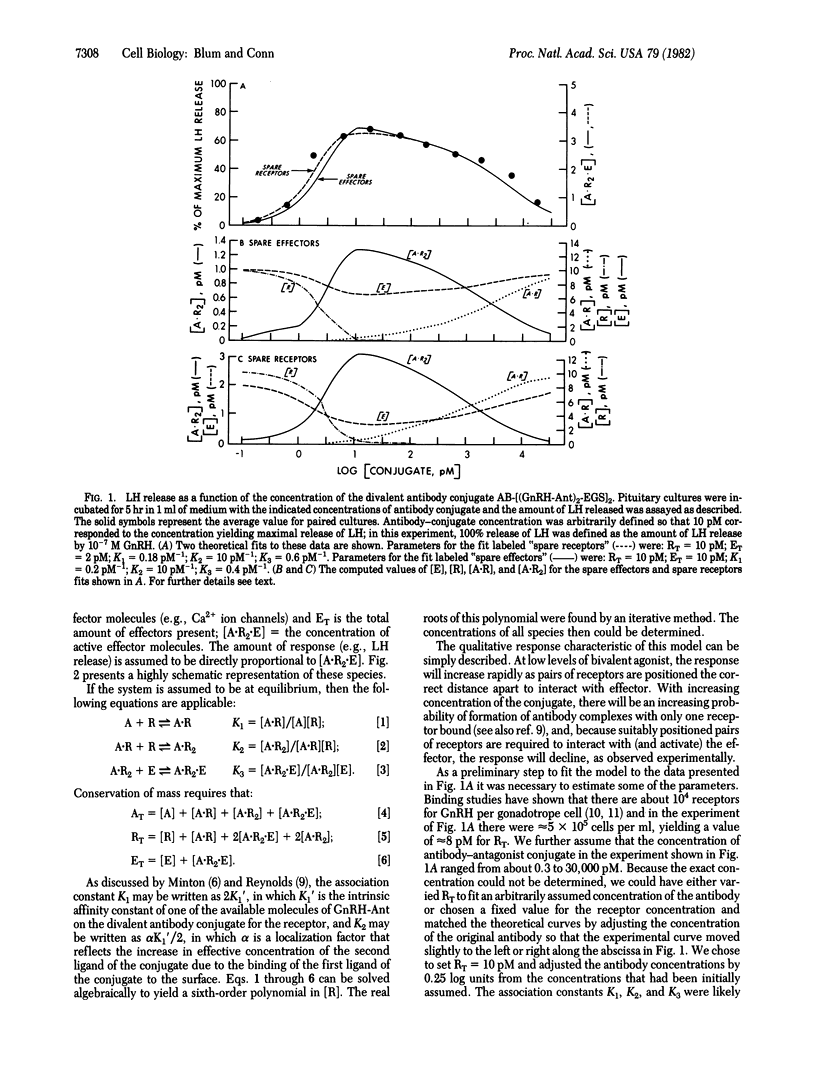
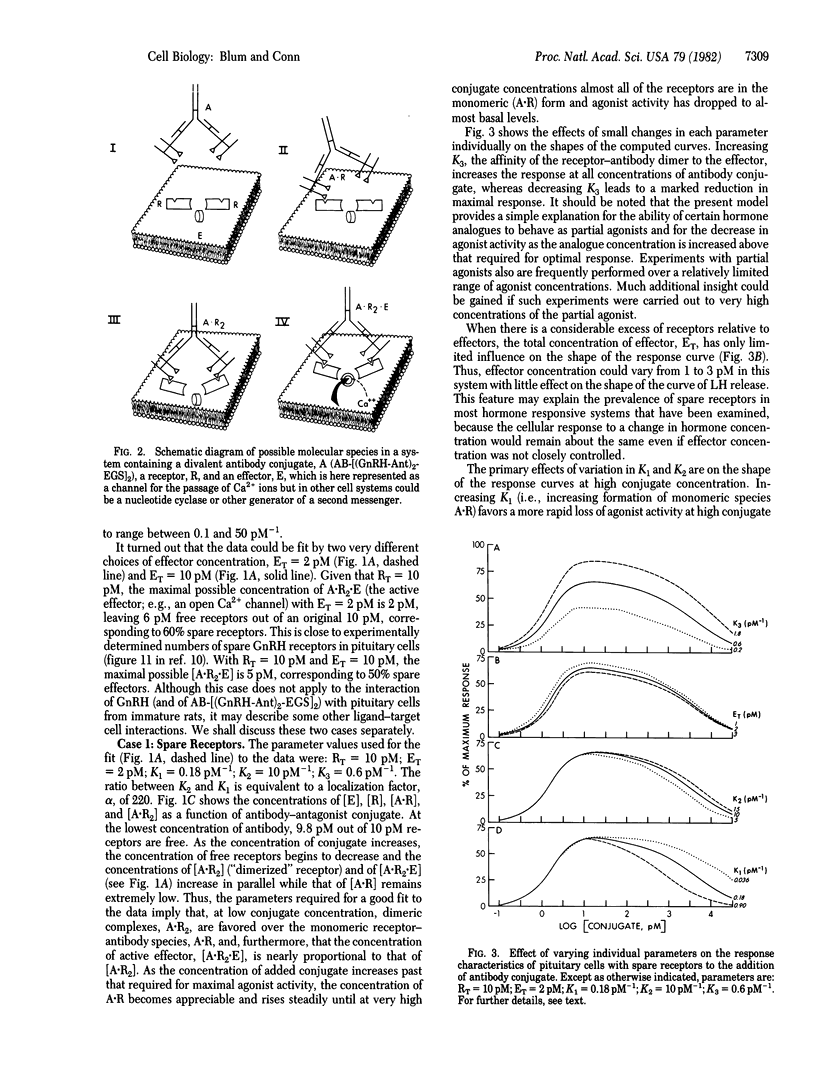
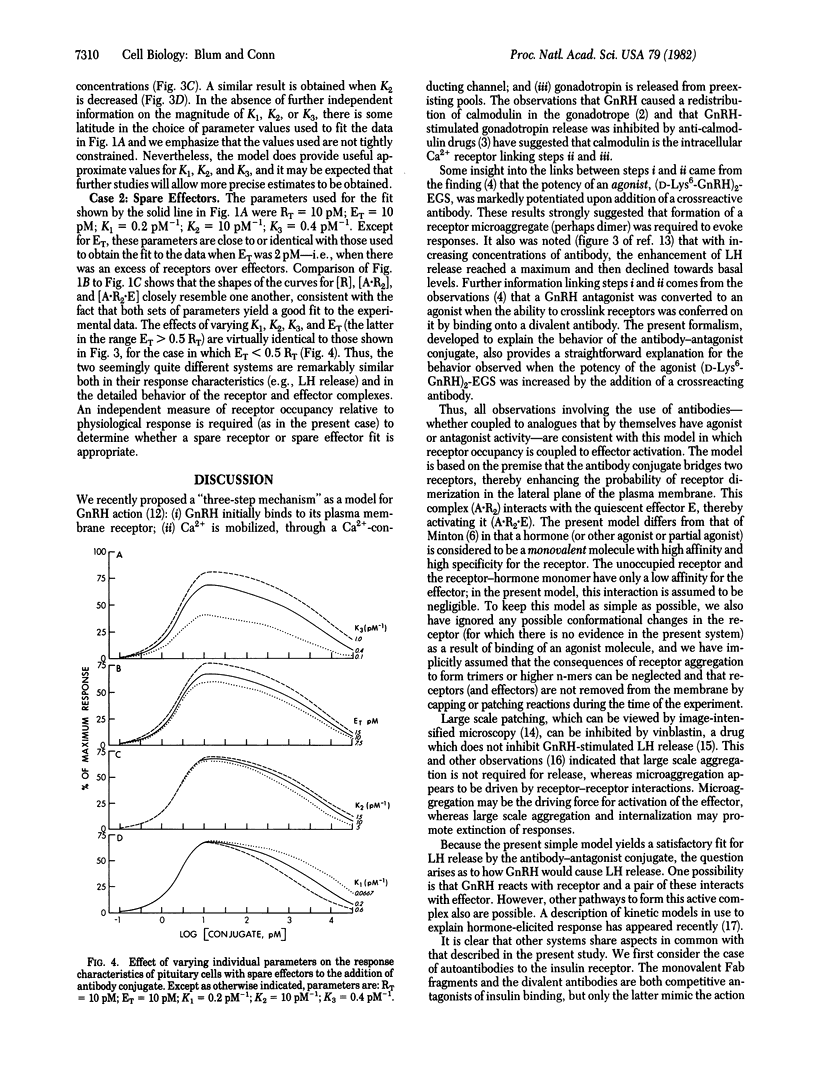
A divalent antibody conjugate of a pure antagonist of gonadotropin-releasing hormone (GnRH) behaved as an agonist--i.e., released luteinizing hormone (LH) from pituitary cultures. Release was measured over a wide range of conjugate concentrations; it rose to a maximum of 66% of the LH released by the optimal concentration of GnRH and declined to basal levels at very high concentrations. This behavior was modeled on the assumption that the antibody conjugate, A, can react with a receptor, R, to form a complex, A . R2. This dimer then can react with a quiescent effector, E (e.g., a closed Ca2+ ion channel), to form A . R2 . E, which contains activated effector and leads to cellular responses. The equilibrium equations governing the behavior of this model were derived, solved, and found to yield a good fit to the experimental data. Consideration of our data in this model system, and of other available data describing the behavior of ligands in other cells, suggests that the present model may be of wide applicability.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin D., Jr, Terris S., Steiner D. F. Characterization of insulin-like actions of anti-insulin receptor antibodies. Effects on insulin binding, insulin degradation, and glycogen synthesis in isolated rat hepatocytes. J Biol Chem. 1980 May 10;255(9):4028–4034. [PubMed] [Google Scholar]
- Conn P. M., Chafouleas J. G., Rogers D., Means A. R. Gonadotropin releasing hormone stimulates calmodulin redistribution in rat pituitary. Nature. 1981 Jul 16;292(5820):264–265. doi: 10.1038/292264a0. [DOI] [PubMed] [Google Scholar]
- Conn P. M., Hazum E. Luteinizing hormone release and gonadotropin-releasing hormone (GnRH) receptor internalization: independent actions of GnRH. Endocrinology. 1981 Dec;109(6):2040–2045. doi: 10.1210/endo-109-6-2040. [DOI] [PubMed] [Google Scholar]
- Conn P. M., Marian J., McMillian M., Stern J., Rogers D., Hamby M., Penna A., Grant E. Gonadotropin-releasing hormone action in the pituitary: a three step mechanism. Endocr Rev. 1981 Spring;2(2):174–185. doi: 10.1210/edrv-2-2-174. [DOI] [PubMed] [Google Scholar]
- Conn P. M., Rogers D. C., McNeil R. Potency enhancement of a GnRH agonist: GnRH-receptor microaggregation stimulates gonadotropin release. Endocrinology. 1982 Jul;111(1):335–337. doi: 10.1210/endo-111-1-335. [DOI] [PubMed] [Google Scholar]
- Conn P. M., Rogers D. C., Sheffield T. Inhibition of gonadotropin-releasing hormone-stimulated luteinizing hormone release by pimozide: evidence for a site of action after calcium mobilization. Endocrinology. 1981 Oct;109(4):1122–1126. doi: 10.1210/endo-109-4-1122. [DOI] [PubMed] [Google Scholar]
- Conn P. M., Rogers D. C., Stewart J. M., Niedel J., Sheffield T. Conversion of a gonadotropin-releasing hormone antagonist to an agonist. Nature. 1982 Apr 15;296(5858):653–655. doi: 10.1038/296653a0. [DOI] [PubMed] [Google Scholar]
- Conn P. M., Smith R. G., Rogers D. C. Stimulation of pituitary gonadotropin release does not require internalization of gonadotropin-releasing hormone. J Biol Chem. 1981 Feb 10;256(3):1098–1100. [PubMed] [Google Scholar]
- DeLisi C., Siraganian R. P. Receptor cross-linking and histamine release. I. The quantitative dependence of basophil degranulation on the number of receptor doublets. J Immunol. 1979 Jun;122(6):2286–2292. [PubMed] [Google Scholar]
- Dembo M., Goldstein B. Theory of equilibrium binding of symmetric bivalent haptens to cell surface antibody: application to histamine release from basophils. J Immunol. 1978 Jul;121(1):345–353. [PubMed] [Google Scholar]
- Endo K., Amir S. M., Ingbar S. H. Development and evaluation of a method for the partial purification of immunoglobulins specific for Graves' disease. J Clin Endocrinol Metab. 1981 Jun;52(6):1113–1123. doi: 10.1210/jcem-52-6-1113. [DOI] [PubMed] [Google Scholar]
- Hazum E., Cuatrecasas P., Marian J., Conn P. M. Receptor-mediated internalization of fluorescent gonadotropin-releasing hormone by pituitary gonadotropes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6692–6695. doi: 10.1073/pnas.77.11.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Chang K. J., Cuatrecasas P. Antibodies to purified insulin receptor have insulin-like activity. Science. 1978 Jun 16;200(4347):1283–1284. doi: 10.1126/science.663609. [DOI] [PubMed] [Google Scholar]
- Kagey-Sobotka A., MacGlashan D. W., Lichtenstein L. M. Role of receptor aggregation in triggering IgE-mediated reactions. Fed Proc. 1982 Jan;41(1):12–16. [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Jarrett D. B., Flier J. S. Direct demonstration that receptor crosslinking or aggregation is important in insulin action. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4209–4213. doi: 10.1073/pnas.75.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. C., Cuatrecasas P. Peptide hormone-induced receptor mobility, aggregation, and internalization. N Engl J Med. 1981 Jul 9;305(2):77–88. doi: 10.1056/NEJM198107093050206. [DOI] [PubMed] [Google Scholar]
- Kurlander R. J., Batker J. The binding of human immunoglobulin G1 monomer and small, covalently cross-linked polymers of immunoglobulin G1 to human peripheral blood monocytes and polymorphonuclear leukocytes. J Clin Invest. 1982 Jan;69(1):1–8. doi: 10.1172/JCI110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marian J., Conn P. M. Gonadotropin releasing hormone stimulation of cultured pituitary cells requires calcium. Mol Pharmacol. 1979 Jul;16(1):196–201. [PubMed] [Google Scholar]
- Maturo J. M., 3rd, Hollenberg M. D. Insulin receptor: interaction with nonreceptor glycoprotein from liver cell membranes. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3070–3074. doi: 10.1073/pnas.75.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdi S. Q., Kriss J. P. Preparation of radiolabeled thyroid-stimulating immunoglobulins (TSI) by recombining TSI heavy chains with 125I-labeled light chains: direct evidence that the product binds to the membrane thyrotropin receptor and stimulates adenylate cyclase. Endocrinology. 1978 Jul;103(1):296–301. doi: 10.1210/endo-103-1-296. [DOI] [PubMed] [Google Scholar]
- Meidan R., Koch Y. Binding of luteinizing hormone-releasing hormone analogues to dispersed rat pituitary cells. Life Sci. 1981 Apr 27;28(17):1961–1967. doi: 10.1016/0024-3205(81)90305-2. [DOI] [PubMed] [Google Scholar]
- Minton A. P. The bivalent ligand hypothesis. A quantitative model for hormone action. Mol Pharmacol. 1981 Jan;19(1):1–14. [PubMed] [Google Scholar]
- Naor Z., Clayton R. N., Catt K. J. Characterization of gonadotropin-releasing hormone receptors in cultured rat pituitary cells. Endocrinology. 1980 Oct;107(4):1144–1152. doi: 10.1210/endo-107-4-1144. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A. Interaction of divalent antibody with cell surface antigens. Biochemistry. 1979 Jan 23;18(2):264–269. doi: 10.1021/bi00569a004. [DOI] [PubMed] [Google Scholar]
- Smith M. A., Vale W. W. Desensitization to gonadotropin-releasing hormone observed in superfused pituitary cells on Cytodex beads. Endocrinology. 1981 Mar;108(3):752–759. doi: 10.1210/endo-108-3-752. [DOI] [PubMed] [Google Scholar]



