Abstract
Low-load resistance training with blood flow restriction has been shown to elicit substantial increases in muscle mass and muscle strength; however, the effect on myogenic stem cells (MSCs) and myonuclei number remains unexplored. Ten male subjects (22.8 ± 2.3 years) performed four sets of knee extensor exercise (20% 1RM) to concentric failure during blood flow restriction (BFR) of the proximal thigh (100 mmHg), while eight work-matched controls (21.9 ± 3.0 years) trained without BFR (control, CON). Twenty-three training sessions were performed within 19 days. Maximal isometric knee extensor strength (MVC) was examined pre- and post-training, while muscle biopsies were obtained at baseline (Pre), after 8 days intervention (Mid8) and 3 (Post3) and 10 days (Post10) post training to examine changes in myofibre area (MFA), MSC and myonuclei number. MVC increased by 7.1% (Post5) and 10.6% (Post12) (P < 0.001) with BFR training, while type I and II MFA increased by 38% (Mid8), 35–37% (Post3) and 31–32% (Post10) (P < 0.001). MSCs per myofibre increased with BFR training from 0.10 ± 0.01 (Pre) to 0.38 ± 0.02 (Mid8), 0.36 ± 0.04 (Post3) and 0.25 ± 0.02 (Post10) (P < 0.001). Likewise, myonuclei per myofibre increased from 2.49 ± 0.07 (Pre) to 3.30 ± 0.22 (Mid8), 3.20 ± 0.16 (Post3) and 3.11 ± 0.11 (Post10), (P < 0.01). Although MFA increased in CON at Mid8, it returned to baseline at Post3. No changes in MSC or myonuclei number were observed in CON. This study is the first to show that short-term low-load resistance exercise performed with partial blood flow restriction leads to marked proliferation of myogenic stem cells and resulting myonuclei addition in human skeletal muscle, which is accompanied by substantial myofibre hypertrophy.
Key points
In the last decade muscle training performed using a combination of low external loads and partial restriction of blood flow to the exercising limb has gained increasing interest, since it leads to significant gains in muscle strength and muscle mass.
The cellular mechanisms responsible for the muscular adaptations induced by this training paradigm are not fully understood.
This study shows that 3 weeks of high-frequency, low-intensity muscle exercise with partial blood flow restriction induces increases in maximal muscle strength accompanied by highly marked gains in muscle fibre size.
Furthermore, the results indicate that these muscular adaptations rely on a considerable upregulation in myogenic satellite cells number, resulting in nuclear addition to the exercised myofibres.
The results contribute to a better understanding of the physiological mechanisms underlying the gain in muscle strength and muscle mass observed with blood flow restricted low-intensity resistance exercise.
Introduction
Repetitive muscle loading performed using a combination of low external load (20–50% 1RM) and blood flow restriction (BFR) has recently gained interest, as it appears to increase human skeletal muscle mass and maximal muscle strength to a similar or greater extent (Takarada et al. 2002) as seen with heavy-load resistance training (Aagaard et al. 2001). In addition, BFR training appears to show superior results on these parameters compared to low-load resistance training without BFR (Abe et al. 2006; Holm et al. 2008), although recent results have suggested a hypertrophic role of low-intensity resistance training as well (Mitchell et al. 2012). However, the underlying mechanisms responsible for the adaptive changes in muscle morphology in response to BFR training remain largely unknown. Recent studies show increased protein synthesis following acute bouts of BFR training, accompanied by post-translation regulation in the AKT/mTOR pathway (Fujita et al. 2007; Fry et al. 2010). Moreover, a reduced expression of the proteolysis-related genes FOXO3a, Atrogin and Murf-1, as well as the negative regulator of muscle mass, myostatin, recently were observed 8 and 48 h after acute BFR exercise (Manini et al. 2011; Laurentino et al. 2012). In contrast, mRNA expression of other myogenic- and proteolysis-related targets did not change or differ between BFR and non-occluded exercise conditions (Drummond et al. 2008; Manini et al. 2011). The activation and proliferation of MSCs have been implied to be involved in accelerated hypertrophy signalling in human skeletal muscle, where the amount of myonuclei in the myofibre has been proposed to impose a ceiling effect on myofibre hypertrophy (Kadi et al. 2004; Petrella et al. 2008).
Myogenic stem cells (MSCs) are quiescent cells positioned between the sarcolemma and the basal lamina of myofibres (Mauro, 1961) that provide the only source of myogenic-derived nuclei with the ability of mitogenesis. It is well-documented that MSCs activate and proliferate in response to prolonged heavy-resistance training in human skeletal muscle (Kadi & Thornell, 2000; Kadi et al. 2004; Olsen et al. 2006; Petrella et al. 2008; Mackey et al. 2010). The newly formed daughter cells differentiate to become fusion-capable myoblasts that either return to quiescence or irreversibly withdraw from the cell cycle to fuse with pre-existing myofibres with the purpose of assisting in myofibre regeneration or providing myonuclei addition. In human intervention studies, addition of myonuclei only seems to take place concurrently with marked myofibre hypertrophy (Kadi & Thornell, 2000; Kadi et al. 2004; Olsen et al. 2006; Petrella et al. 2008; Mackey et al. 2010), which suggests that these factors are interlinked in human skeletal muscle. Hence, MSCs most likely play an essential role in conditions of amplified muscle protein synthesis by providing additional DNA content for mRNA transcription. However, the MSC response including the aspect of myonuclei addition has not yet been examined during and after BFR training.
The aim of the present study was to investigate whether the hypertrophy response observed with BFR training involves MSC proliferation and myonuclei addition. To our knowledge the present data are the first to demonstrate that substantial MSC proliferation and myonuclei addition can be evoked by short-term (3 weeks) BFR training in human skeletal muscle, which is accompanied by significant gains in myofibre size and contractile muscle function.
Methods
Subjects
Twenty healthy male subjects were included in the study, twelve performing blood flow restricted training (BFR) (body mass 82.3 ± 13.7 kg; height 181.2 ± 6.4 cm; age 22.8 ± 2.1 years) (mean ± SD), while eight served as controls performing non-occluded work-matched bouts of exercise (CON) (body mass 80.2 ± 11.4 kg; height 182.9 ± 8.8 cm; age 21.9 ± 3.0 years) (mean ± SD). None of the subjects had participated in systematic strength training within a year prior to the study and they did not participate in any structured training regimes in addition to the present intervention. The study was approved by the local Ethics Committee (S-200900070) in accordance with the Declaration of Helsinki, and written informed consent was obtained from subjects prior to inclusion.
Protocol overview
Muscle mechanical function was tested using isokinetic dynamometry before (Pre) and 5 and 12 days after cessation of training (Post5 and Post12). Muscle biopsies were obtained from m. vastus lateralis (VL) 3 days before training (Pre), at day 8 during training (Mid8) as well as 3 and 10 days post-training (Post3 and Post10) in BFR subjects, and at Pre, Mid8 and Post3 in control subjects. One week prior to the intervention period, subjects underwent a familiarization session for the strength test procedures and had their 1RM determined. All measurements were obtained in the experimental leg, which was chosen by paired within group randomization between the dominant and non-dominant leg. Subjects were carefully instructed not to deviate from their normal pattern of food intake, not to engage in any supplementary training and to refrain from any alcohol intake during the intervention period. All strength measurements and biopsy samplings were conducted at the same time point of the day to control for diurnal variations.
Training intervention
The subjects participated in a 3 week supervised training programme consisting of 23 training sessions. Training was conducted once per day (Mon–Wed) and twice per day (Thu–Fri) during the first week, and twice per day (Mon–Fri) during week 2 and 3. Exceptions were Mon–Tue in week 2 as well as Friday in week 3 when only a single training session was conducted. Successive training sessions were separated by at least 4 h (Abe et al. 2006). Before each training session, a pneumatic cuff (15.0 cm width) (9-7350-003, Delfi Medical, Vancouver BC, Canada) was placed around the proximal portion of the thigh. The cuff was connected to a computerized tourniquet system (Zimmer A.T.S. 750, Warsaw, IN, USA) that ensured automatic regulation of cuff pressure. Following a brief warm up (15–20 repetitions without load) and inflation of the cuff to 100 mmHg, BFR subjects performed four sets of unilateral dynamic knee extensions (Cybex VR4850, Medway, MA, USA) at 20% 1RM to concentric failure. Successive sets were separated by 30 s rest periods. Restriction of muscular blood flow was maintained for the entire training session (7.91 ± 1.06 min, including rest periods), and was released immediately upon completion of the fourth set. Control subjects performed a work-matched exercise protocol without BFR.
Assessment of maximal isometric muscle strength
Knee extensor MVC were performed at 70 deg knee joint angle (0 deg = full extension) using an isokinetic dynamometer (Kinetic Communicator 500H, Chattecx Corp., Hixson, TN, USA) (Aagaard et al. 2001). Subjects were placed with the lateral epicondyle of the knee aligned with the rotational axis of the dynamometer. The hip and thigh were carefully fastened to the dynamometer, while the lower leg was attached to the dynamometer lever arm 2 cm above the medial malleolus. Subjects performed a standardized 5 min warm-up on a stationary bike, followed by ∼10 submaximal dynamic contractions in the dynamometer. The test consisted of five 3 s maximal isometric contractions (45 s pause), during which subjects were instructed to contract as hard as possible. Strong verbal encouragement and online visual feedback of the exerted force was provided. All trials with visible countermovement contractions were disregarded and repeated. Force signals were sampled at 1000 Hz and corrected for gravity of the lower leg (Aagaard et al. 2001). The trial with the highest MVC was selected for further analysis.
Muscle biopsy sampling
Muscle biopsies (∼150 μg) were obtained from VL muscle using a 5 mm Bergström biopsy needle under sterile conditions and local anaesthesia (1% lidocaine, Amgros 742122, Copenhagen, Denmark), as described in detail previously (Aagaard et al. 2001). Biopsies were obtained from the same region and depth of the VL muscle and placed approximately 2–3 cm apart. The sequence of the four biopsies was randomized. Muscle samples were aligned and mounted in Tissue-Tec (4583, Sakura Finetek, Alphen aan den Rijn, The Netherlands) and subsequently frozen in isopentane pre-cooled with liquid nitrogen and stored at –80°C for later analysis.
Immunofluorescence microscopy
Transverse serial sections (8 μm) of the embedded muscle biopsy specimen were cut at −22°C using a cryostat (HM560; Microm, Walldorf, Germany) and were mounted on glass slides.
Immunohistochemical stainings were fixed for 10 min at room temperature in a 4% formaldehyde fixation buffer containing 0.05% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA). After fixation the staining procedure consisted of three steps that were identical. Each step was initiated with a 1:10 wash buffer (Dako, S3006, Glostrup, Denmark) and subsequently blocked (Dako, X0909) for 10 min. Next primary and secondary antibodies were applied for 60 min incubation separated by a wash sequence. MSCs were visualized with an antibody against Pax7 (Pax7, Hybridoma Bank, Iowa City, IA, USA 1:100), while laminin (Dako, Z0097, 1:100) and MHC-I (M8421, 1:2000) were added in that order for distinction of the myofibre border and myofibre type slow, respectively. Specific secondary antibodies (order listed: Alexa-555 goat anti-mouse (Invitrogen, A21424, Life Technologies Denmark, Naerum, Denmark, 1:1000), Alexa-488 goat anti-rabbit (Invitrogen, A11034, 1:1000) and Alexa-350 goat anti-mouse (Invitrogen, A11045, 1:500)) were applied after each primary antibody. Finally, sections were mounted with a fluorescent anti-fade medium containing DAPI (which stains nuclei) (Invitrogen, P36935), and subsequently slides and coverglass were pasted together and stored protected from light at 5°C.
Biopsy stainings were visualized on a computer screen using a light microscope (Carl Zeiss Axio Imager M1, Germany) and a high-resolution AxioCam (Carl Zeiss), and all morphometric analysis were performed using a digital analysis program (Carl Zeiss, AxioVision 4.6). Type I (stained) and type II (unstained) myofibres were differentiated, and MFA was determined. On average 371 ± 103 and 618 ± 158 myofibres (mean ± SD) were analysed per biopsy for the assessment of MFA and fibre type distribution, respectively. Furthermore, nuclei were identified either as MSC or myonuclei using the following criteria: MSC-derived nuclei had to stain positive for Pax7 and be placed within the basal lamina; nuclei with a sublaminar placement were considered myonuclei (Fig. 1). The number of Pax7+ nuclei was expressed relative to the number of type I and II myofibres, myofibre area and the proportion of myonuclei, while the number of myonuclei was expressed relative to the number of type I and II myofibres as well as MFA (Mackey et al. 2010). A total of 75 myofibres for each fibre type were analysed per biopsy in accordance with previous reliability analysis (Mackey et al. 2009) to ensure a reliable estimate of MSC and myonuclei number. Myofibres were analysed in three or more separate areas of the cross section. All analyses were carried out manually by the same investigator, who was blinded with respect to subject-ID and time point. Nine data points are reported at Mid8 in the BFR group as one subjects failed to turn up to this biopsy sampling, while a dataset was omitted from CON due to inadequate biopsy quality.
Figure 1. Representative immunohistochemical staining (BFR, Mid8) containing Pax7 (red), laminin (green) and MHC I/Dapi (blue).
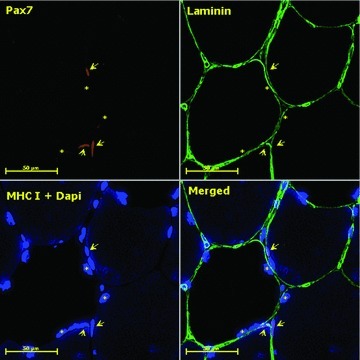
Arrows denote Dapi+/Pax7+ myogenic stem cells and stars mark myonuclei (scale bar = 50 μm).
Statistical analysis
Statistical analysis was performed using a linear mixed-model. Variables were analysed with subject-ID as a random effect and time and group as fixed effects. Myofibre types were analysed separately. Variables with skewed distributions were appropriately transformed. Furthermore, training data were analysed with a one-way ANOVA, while associations between relevant parameters were evaluated with linear regression and Pearson's product–moment correlation. All statistical analyses were performed with STATA 10.1 (StataCorp, College Station, TX, USA). Values are presented as means ± SEM, unless otherwise stated. The level of statistical significance was set at P≤ 0.05.
Results
Subjects
Two subjects from the BFR group left the project prematurely for reasons not related to the intervention. The remaining subjects (BFR: n = 10; CON: n = 8) completed 22.8 ± 0.4 (mean ± SD) of 23 possible training sessions. No changes in body weight were observed during the intervention.
Training progression
Averaged over the intervention period subjects performed 40.5 ± 5.2, 12.1 ± 3.9, 8.0 ± 3.1 and 6.6 ± 3.5 repetitions in the first, second, third and fourth set, respectively; this summed to a total of 66.2 ± 11.3 repetitions per session (mean ± SD). The mean training load (20% 1RM) was 19.5 ± 2.6 and 20.9 ± 4.0 kg (mean ± SD) during BFR intervention and in controls, respectively. Total work increased proportionally with the total repetitions performed, as training-load and range of motion remained unchanged during the intervention. To evaluate changes within the training period, the 23 training sessions were divided into three phases (A: session 1–7, B: session 8–16, C: session 17–23). Total repetitions per training session increased from A (51.5 ± 6.7) to B (66.3 ± 3.7) and C (75.9 ± 2.2) (P < 0.01) (mean ± SD), while also increasing between B and C (P < 0.01). Repetitions per set increased from A to B and C in all sets (P < 0.05) and between B and C in set 1 and 3 (P < 0.05). Training data are presented for the BFR group only, as all training in CON was work-matched.
Maximal isometric quadriceps strength
Knee extensor MVC increased with BFR training from 271.6 ± 47.5 N m (Pre) to 290.7 ± 54.1 (Post5) and 300.4 ± 60.9 N m (Post12) (mean ± SD) (Fig. 2), corresponding to relative increases of 7.0 and 10.6%, respectively (P < 0.001). No changes were observed in CON (Fig. 2).
Figure 2. Maximal isometric knee extensor muscle strength at baseline (Pre), and 5 and 12 days after cessation of training (Post5 and Post12).
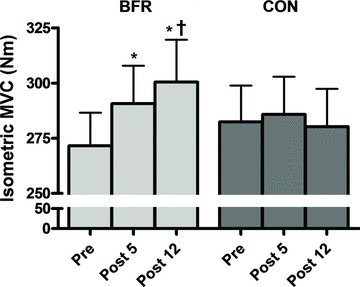
Pre to Post differences: *P < 0.001. Mid5 to Post12 difference: †P < 0.05. Values are means ± SEM; BFR: n = 10; C: n = 8.
Myofibre cross sectional area and fibre type distribution
Type II MFA was larger than type I MFA at Pre in both intervention groups (P < 0.01). Type I MFA increased with BFR training from 3781 ± 191 μm2 (Pre) to 5201 ± 321 (Mid8), 5103 ± 256 (Post3) and 4960 ± 226 μm2 (Post10), corresponding to relative increases of 37.6, 35.0 and 31.2% (P < 0.001) (Fig. 3A). Likewise type II MFA increased from 4170 ± 192 μm2 (Pre) to 5772 ± 390 (Mid8), 5718 ± 300 (Post3) and 5512 ± 253 μm2 (Post10), corresponding to relative increases of 38.4, 37.1 and 32.2% (P < 0.001) (Fig. 3B). In CON, MFA increased from 4041 ± 250 and 4975 ± 350 to 5058 ± 582 and 6396 ± 620 μm2 at Mid8 for type I and type II myofibres, corresponding to increases of 25.2 and 28.6% (P < 0.05), but returned to baseline level values at Post3 (Fig. 3A and B). In both groups myofibre type distribution remained unchanged during the intervention period.
Figure 3. Myofibre cross sectional area at baseline (Pre), 8 days into the training intervention (Mid8), and 3 and 10 days after cessation of training (Post3 and Post10).
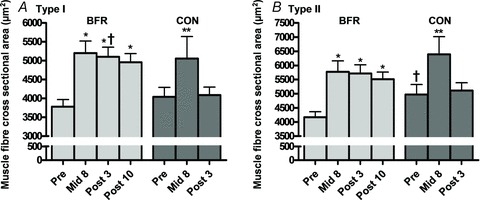
A, BFR and CON response in type slow myofibres; B, BFR and CON response in type fast myofibres. Pre to Mid8/Post differences: *P < 0.001. Pre to Mid8 difference: **P < 0.01. Between group difference: †P < 0.05. Values are means ± SEM; BFR: n = 10 at Mid8 n = 9; C: n = 7.
Myogenic stem cell content
Pax7+ cells per type I myofibre increased with BFR training from 0.11 ± 0.01 (Pre) to 0.41 ± 0.04 (Mid8), 0.36 ± 0.05 (Post3) and 0.26 ± 0.03 (Post10), corresponding to increases of 292.4, 242.6 and 143.5% (P < 0.001) (Fig. 4A and C). Pax7+ cells per type II myofibre increased from 0.10 ± 0.01 (Pre) to 0.37 ± 0.03 (Mid8), 0.35 ± 0.03 (Post3) and 0.23 ± 0.02 (Post10), corresponding to relative gains of 276.2, 264.7 and 143.9% (P < 0.001) (Fig. 4B and D). No changes were observed in CON (Fig. 4A–D).
Figure 4. Pax7 positive cells at baseline (Pre), 8 days into the training intervention (Mid8), and 3 and 10 days after cessation of training (Post3 and Post10).
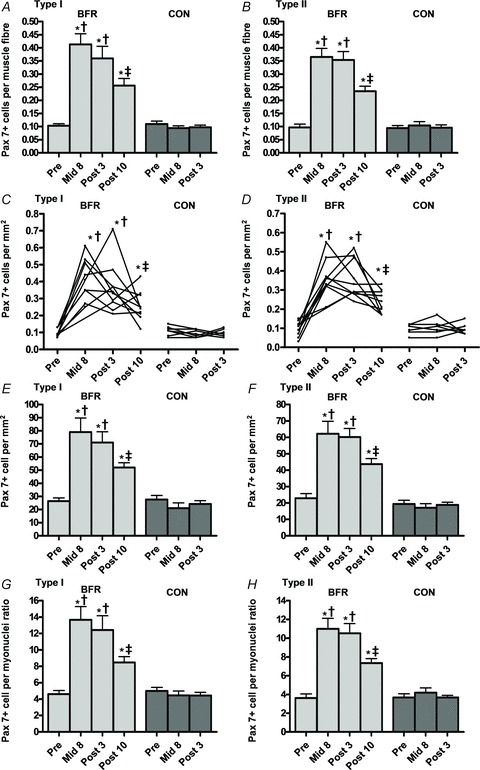
A and B, Pax7+ myogenic stem cells per myofibre, type I and II myofibres (group response). C and D, Pax7+ myogenic stem cells per myofibre, type I and II myofibres (individual response). E and F, Pax7+ myogenic stem cells per myofibre cross sectional area (mm2) (group response). G and H, Pax7+ myogenic stem cells to myonuclei ratio (group response). Pre to Mid8/Post differences: *P < 0.001. Mid8/Post3 to Post12 differences: ‡P < 0.01. Between group difference: †P < 0.05. Values are means ± SEM; BFR: n = 10 at Mid8 n = 9; C: n = 7.
In BFR-subjects Pax7+ cells expressed relative to type I MFA (mm2) increased from 26.5 ± 2.4 cells mm-2 (Pre) to 79.0 ± 10.7 (Mid8), 71.2 ± 8.1 (Post3) and 52.0 ± 3.6 cells mm-2 (Post10) corresponding to gains of 198.5, 168.8 and 96.5% (P < 0.001) (Fig. 4E). Likewise, Pax7+ cells increased in type II myofibres from 22.9 ± 2.8 cells mm-2 (Pre) to 62.2 ± 7.5 (Mid8), 60.2 ± 5.1 (Post3) and 43.7 ± 3.4 cells mm-2 (Post10), corresponding to relative gains of 171.7, 163.1 and 91.0% (P < 0.001) (Fig. 4F). No changes were observed in CON for this parameter (Fig. 4E and F).
The ratio of Pax7+ cells to myonuclei number increased with BFR training in type I myofibres from 4.6 ± 0.4 (Pre) to 13.7 ± 1.6 (Mid8), 12.4 ± 1.7 (Post3) and 8.5 ± 0.7 (Post10), corresponding to relative gains of 196.9, 170.0 and 84.2% (P < 0.001) (Fig. 4G). In type II myofibres the ratio of Pax7+ cells to myonuclei number increased from 3.6 ± 0.4 (Pre) to 11.0 ± 1.1 (Mid8), 10.5 ± 1.0 (Post3) and 7.3 ± 0.5 (Post10), corresponding to increases of 203.1, 189.8 and 102.3% (P < 0.001) (Fig. 4H). No changes were observed in CON for this parameter (Fig. 4G and H).
Myonuclei content
BFR training lead to an increased number of myonuclei per type I myofibre from 2.34 ± 0.08 (Pre) to 3.11 ± 0.21 (Mid8), 2.98 ± 0.18 (Post3) and 2.99 ± 0.10 (Post10), corresponding to relative gains of 33.2, 27.4 and 28.0% (P < 0.001) (Fig. 5A). Likewise, in type II myofibres the number of myonuclei per myofibre increased from 2.65 ± 0.11 (Pre) to 3.43 ± 0.25 (Mid8), 3.43 ± 0.16 (Post3) and 3.23 ± 0.13 (Post10), corresponding to relative increases by 29.5, 29.6 and 22.0% (P < 0.001) (Fig. 5B). No changes were observed in CON for this parameter (Fig. 5A and B).
Figure 5. Myonuclei number at baseline (Pre), 8 days into the training intervention (Mid8), and 3 and 10 days after cessation of training (Post3 and Post10).
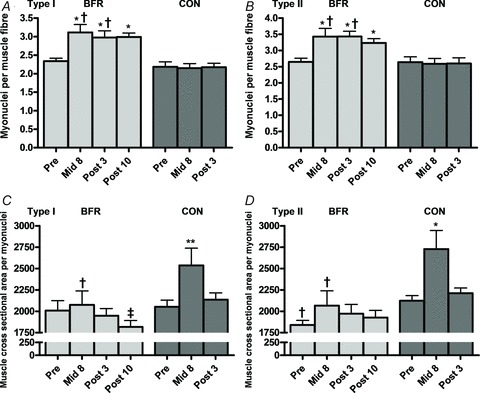
A and B, myonuclei number per myofibre, type I and II myofibres. C and D, cross sectional area (μm2) per myonuclei, type I and II myofibres. Pre to Mid8/Post differences: *P < 0.001, **P < 0.01. Mid8 to Post10 difference: ‡P < 0.05. Between group difference: †P < 0.05. Values are means ± SEM; BFR: n = 10 at Mid8 n = 9; C: n = 7.
MFA per myonuclei decreased for type I myofibres during BFR training from 2075 ± 165 to 1816 ± 76 μm2 between Post3 and Post10, corresponding to a 12.5% decrease (P < 0.05) (Fig. 5C). In CON an increase in myonuclei domain from 2052 ± 77 to 2536 ± 202 μm2 and from 2123 ± 61 to 2728 ± 217 μm2 was observed between Pre and Mid8 in type I and II myofibres, respectively, corresponding to increases of 23.6 and 28.5% (P < 0.01) (Fig. 5C and D). However, myonuclei domain returned to baseline levels in CON at Post3 for both myofibre types (Fig. 5C and D).
Correlations
Following BFR training a positive relationship emerged between the relative change in myonuclei number per fibre and MFA (r = 0.51; P < 0.01), and a similar relationship was observed between the relative change in MSC per fibre and MFA (r = 0.58; P < 0.01).
Discussion
The present study is the first to examine the effect of low-load resistance exercise with blood flow restriction on MSC and myonuclei number in human skeletal muscle. Several notable findings emerged. Firstly, it was demonstrated that the expression of Pax7+ cells was markedly increased (∼3- to 4-fold up-regulated) relative to baseline values in response to 19 days of BFR resistance training and continued to remain elevated in the following 10 days of detraining. Secondly, BFR training led to an increased number of myonuclei per myofibre, strongly indicating the presence of MSC/myoblast fusion with existing myofibres. Thirdly, type I and II myofibre area increased markedly already in the very initial phase of training (5 days of training; Mid8) irrespectively of intervention modality; however, MFA remained elevated throughout the study period with BFR training only, thus returning to baseline levels in controls at the end of the training period (19 days of training, Post3). Thus, the present data demonstrate that low-load BFR training can elicit marked MSC proliferation as well as myonuclei addition in human skeletal muscle, which is accompanied by substantial gains in MFA.
The increase in MFA (∼30–40%) presently observed in the BFR group is highly unique taking into consideration the low intensity of loading (≤20% 1RM) and short duration of training (19 days). Previous studies utilising BFR-exercise have reported substantial increases in anatomical quadriceps CSA using MR imaging (Takarada et al. 2002; Abe et al. 2006), while training using low-load training without BFR combined with normal or high training frequency showed only minor (3%) or no change in quadriceps CSA (Abe et al. 2006; Holm et al. 2008), although more substantial gains in anatomical quadriceps volume (∼7%) recently have been reported as well (Mitchell et al. 2012). It is well-documented that heavy resistance training can lead to significant gains in MFA. Thus, 15–20% increases in mean MFA were reported after 12–16 weeks of heavy resistance training in young untrained men (Aagaard et al. 2001; Kadi et al. 2004; Olsen et al. 2006), while Petrella and co-workers reported an MFA increase of ∼37% in individuals characterized as hypertrophy responders after 16 weeks of heavy-load resistance training (Petrella et al. 2008). In contrast, 12 weeks of high-volume low-load resistance training (15.5% 1RM) without BFR did not lead to any change in MFA (Mackey et al. 2010). However, increased mean MFA (∼18%) recently was reported following 10 weeks (30 sessions) of low-load (30% 1RM) knee extensor training performed until fatigue onset (Mitchell et al. 2012).
Interestingly, a large increase in the MFA of both myofibre types in the BFR group was observed after only 8 days of the intervention (Mid8), and remained elevated at 3 and 10 days after cessation of the training intervention lasting 19 days. Control subjects matched the increase at Mid8, but MFA returned to baseline level at Post3. The transitory change in MFA observed in the control subjects indicates that the cell volume was influenced by factors related to the early habituation to exercise other than protein accretion. Such factors could include cell swelling and/or changes in glycogen/mitochondria content. However, the latter factors seem unlikely to affect MFA in the present range, as an increase in glycogen content and mitochondria volume is thought to increase MFA by less than 5% (Nygren et al. 2000). Lasting cellular swelling (∼3 days) could be explained by hypoxia-induced modification of homeostasis regulating membrane channels (Korthuis et al. 1985), stretch-induced opening of membrane channels (Singh & Dhalla, 2010) or microfocal damage to the plasma membrane (Grembowicz et al. 1999).
It is likely that the initial increase in MFA observed following 5 days of intervention (Mid8) irrespectively of training modality was influenced by exercise-induced cell-swelling, and as the observed MFA increase during such a short time frame is unlikely to be accounted for by a positive protein turnover alone. This notion is further supported by the finding that MFA returned to reach baseline values during the latter period of training in the control subjects. Conversely, it is plausible that the late-phase gain in MFA observed with BFR training occurred mainly due to accumulation of myofibrillar proteins, as supported by the finding that MFA remained elevated 3–10 days post-training along with a 7–11% persistent increase in MVC. It cannot be excluded that this gain in MVC could arise at least in part from elevated neuromuscular activity, but this parameter has been reported to remain unchanged after low-load BFR training and to only increase with heavy resistance training (Kubo et al. 2006).
Furthermore, indirect evidence for longitudinal myofibrillar protein accretion following low-load BFR training is provided in several acute studies where an augmented protein turnover was observed. Thus, mixed muscle protein synthesis appears to increase with acute BFR exercise in parallel with increased signalling in anabolic mitogen-activated protein kinase pathways related directly to protein synthesis along with a down-regulated proteolysis and reduced myostatin signalling (Fujita et al. 2007; Drummond et al. 2008; Fry et al. 2010; Manini et al. 2011; Laurentino et al. 2012). In contrast, mixed protein synthesis and related signalling pathways were largely unaffected after acute low-intensity exercise without BFR (Fujita et al. 2007; Drummond et al. 2008; Fry et al. 2010; Manini et al. 2011; Laurentino et al. 2012), although increases in myofibrillar synthesis and elevated anabolic signalling have also been reported with acute bouts of low-intensity (30% 1RM) resistance training performed to fatigue (Burd et al. 2010).
The observation of a consistent elevation in MFA 3–10 days following cessation of the BFR training protocol along with no detectable pre to post change in MFA in our control subjects indicates that cellular swelling was unlikely to be the primary cause of the observed gains in MFA during the later phase of training. Consequently, elevated myofibrillar protein content seems to mainly explain the gain in MFA observed following the relatively short period (∼3 weeks) of BFR training.
In the present study individuals exposed to 19 days of BFR resistance training demonstrated a marked increase in Pax7+ cells illustrated by relative gains of ∼280% (Mid), ∼250% (Post3) and ∼140% (Post10), in both type I and type II myofibres, while no changes were observed in the controls performing a work-matched training protocol without BFR. These changes in MSC number evoked by BFR training were paralleled by increases in the number of myonuclei per myofibre and MFA, respectively, resulting in an unchanged MFA per myonuclei (e.g. unaltered myonuclei domain). Previous studies have examined the expression of selected MSC markers (NCAM/CD56, Pax7) in human skeletal muscle within a number of different contexts. Hitherto, the largest up-regulation in MSCs per myofibre (157%) and in the MSC to myonuclei ratio (112–192%) were reported between 24 and 96 h after acute bouts of high-volume eccentric muscle contractions in untrained young subjects (Crameri et al. 2004; Dreyer et al. 2006). Following varying periods of heavy-load resistance training in young men and woman, more modest changes of 30–50% in MSCs per myofibre are typically observed (Kadi & Thornell, 2000; Kadi et al. 2004; Olsen et al. 2006). Interestingly, 12 weeks of low-load (15.5% 1RM) resistance training without BFR showed a minor, yet significant change in MSCs per fibre of 18% (Mackey et al. 2010). Notably, Mackey et al. did not find any difference between low-load and high-load training which in combination with the present data suggests that both active muscle contractions and vascular occlusion are required to obtain highly amplified elevations in MSC proliferation and differentiation.
As the main and novel finding in the present study, short-term high-frequency BFR training using low external loading appears effective for inducing a marked proliferation of MSCs compared to other training modalities. Previous human studies have demonstrated significant proliferation and differentiation of MSCs during muscle regeneration or when myonuclei are needed during myofibre hypertrophy (Dreyer et al. 2006; Petrella et al. 2008). The unusually large increase in Pax7+ cells observed in the present study could reflect both myofibre regeneration and/or a need for myonuclei addition. The latter notion was supported by the present finding of a very large increase in MFA. Thus, it has been hypothesized that the number of myonuclei per fibre sets the ceiling for myofibre hypertrophy, when the volume of cytoplasm per myonuclei exceeds the myonuclei's ability to transcribe mRNA. Most human studies support this notion, since myonuclei addition has been observed concomitantly with an increase in MFA greater than 25% and/or a myonuclei domain of or greater than 2000 μm2 (Kadi et al. 2004; Petrella et al. 2008). Interestingly, the initial increase in MFA observed in the control subjects was not paralleled by signs of increased MSC number or myonuclei addition, which implies that MSC proliferation and differentiation are not per se sensitive to changes in myofibre area/myonuclei volume, but may need more stable (i.e. non-transient) changes to emerge.
In the present study myonuclei domain remained unchanged or a little reduced throughout the experimental period (Fig. 5C and D) and a positive relationship (r = 0.51; P < 0.01) between the relative change in myonuclei per fibre and MFA was found in the BFR subjects. These findings indicate that MSC proliferation and myonuclei addition are at least in part responsible for an amplified transcription rate and myofibrillar protein synthesis with BFR training.
Furthermore, the increase in myonuclei addition might represent an important mechanism for the maintenance of muscle mass following cessation of training. Significant myofibre type II atrophy was reported already 10 days after cessation of a 90 day heavy-resistance training intervention (Jespersen et al. 2011), while no myofibre atrophy was observed 3 or 10 days post-training in the present study. Interestingly, a lack of myonuclei addition was noted in the study by Jespersen and colleagues (Kadi et al. 2004), which potentially could explain the disparate time courses of myofibre atrophy despite a similar period of detraining (10 days). This suggestion of an atrophy protective effect achieved by training-induced myonuclei addition is supported by recent animal data, where overload-induced myonuclei addition in mice partially protected the affected muscle from subsequent atrophy (Bruusgaard et al. 2010).
Although differentiation of MSCs was evident following BFR training as reflected by an increase in the myonuclei-to-fibre ratio, the present data suggest an unusually high proliferation rate of MSCs. Increases in cyclin-dependent kinase inhibitor-1 and myoblast determination protein-1 mRNA have been observed 3 h after an acute bout of low intensity BFR exercise (Drummond et al. 2008), which indirectly supports the presence of MSC activation and proliferation with this type of exercise. Corresponding changes were observed in control subjects performing similar exercise without BFR; however, it cannot be excluded that differential MSC responses might have been observed if evaluated at later time points (>3 h). Interestingly, a reduced myostatin expression in the exercised muscle tissue recently was reported 48 h after acute bouts of BFR exercise (Laurentino et al. 2012), which indicates a potential regulating role for myostatin, since it is known to regulate both MSC proliferation and differentiation negatively.
Previously, the largest reported changes in MSC content have been observed after unaccustomed high-volume eccentric exercise, where signs of microfocal damage and cellular regeneration may be evident (Crameri et al. 2004; Dreyer et al. 2006). With BFR resistance exercise it is possible that the muscle cell membrane undergoes microfocal damage followed by subsequent regeneration (Grembowicz et al. 1999) due to external loading, hypoxia or a combination of both. However, in terms of potential microfocal damage, we did not find any visible signs of damage to the basal lamina as indicated in our laminin stainings.
Another possible explanation for the high degree of MSC proliferation with BFR training could be the role of hypoxia in combination with stretch and/or contraction on different MSC mediators. Thus, activation and proliferation of MSCs may be stimulated acutely by BFR-induced stretch-, hypoxia- and/or contraction-induced nitric oxide (NO) secretion (Blitzer et al. 1996; Pattwell et al. 2004; Tatsumi et al. 2006). In support of this notion, NO has been demonstrated to release hepatocyte growth-factor, which has been identified to activate MSCs directly in vivo (Tatsumi et al. 2006). Furthermore, inhibition of NO secretion has been shown to inhibit MSC proliferation (Tatsumi et al. 2006), which underlines a potential role of NO in MSC activation that should be investigated in future studies.
In summary, the present study reports a rapid and pronounced increase in myofibre area concomitantly with marked increases in the proliferation and differentiation of MSCs during and following 3 weeks of BFR muscle exercise. The increase in MFA was accompanied by corresponding increases in contractile function (elevated MVC). These data indicate that the unusually large increase in MFA observed with BFR training, at least in part, may rely on an increase in MSC proliferation and differentiation that results in the donation of additional myonuclei to the myofibres. In turn, this incorporation of MSC-derived myonuclei provides an improved capacity for myofibrillar gene transcription, which is likely to contribute to an enhanced activity of cellular protein synthesis.
The present findings may have useful clinical implications. Thus, an amplified proliferation, differentiation and self-renewal of MSCs by means of BFR training potentially could benefit clinical patients with loss in skeletal muscle mass and elderly sarcopaenic individuals. Future studies should address these important aspects of skeletal muscle homeostasis in selected patient populations.
Acknowledgments
We wish to express our gratitude to all the subjects who volunteered to participate in the study. Also, we thank the Danish Rheumatism Association (Gigtforeningen) and the Foundation of A.P. Møller and Wife Chastine Mc-Kinney Møller for their support. None of the authors declare any conflict of interest.
Glossary
- BFR
blood flow restricted
- CSA
cross sectional area
- MFA
myofibre area
- MSC
myogenic stem cell
- MVC
maximal isometric voluntary contraction
- RM
repetition maximum
- VL
m. vastus lateralis
Author contributions
The project was performed at the Institute of Sports Science and Clinical Biomechanics, University of Southern Denmark, Denmark. The contributions of the authors were as follows: conception and design of the study: J.N., P.A., M.B. and U.F.; collection, analysis and interpretation of data: J.N., P.A., R.B., T.B., L.G., C.S. and U.F.; drafting the article or revising it critically for important intellectual content: J.N., P.A., R.B., T.B., M.B., L.G., C.S. and U.F. All authors have approved the final version of the manuscript.
References
- Aagaard P, Andersen JL, Dyhre-Poulsen P, Leffers AM, Wagner A, Magnusson SP, Halkjaer-Kristensen J, Simonsen EB. A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. J Physiol. 2001;534:613–623. doi: 10.1111/j.1469-7793.2001.t01-1-00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe T, Kearns CF, Sato Y. Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J Appl Physiol. 2006;100:1460–1466. doi: 10.1152/japplphysiol.01267.2005. [DOI] [PubMed] [Google Scholar]
- Blitzer ML, Loh E, Roddy MA, Stamler JS, Creager MA. Endothelium-derived nitric oxide regulates systemic and pulmonary vascular resistance during acute hypoxia in humans. J Am Coll Cardiol. 1996;28:591–596. doi: 10.1016/0735-1097(96)00218-5. [DOI] [PubMed] [Google Scholar]
- Bruusgaard JC, Johansen IB, Egner IM, Rana ZA, Gundersen K. Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining. Proc Natl Acad Sci U S A. 2010;107:15111–15116. doi: 10.1073/pnas.0913935107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd NA, West DW, Staples AW, Atherton PJ, Baker JM, Moore DR, Holwerda AM, Parise G, Rennie MJ, Baker SK, Phillips SM. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS One. 2010;5:e12033. doi: 10.1371/journal.pone.0012033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crameri RM, Langberg H, Magnusson P, Jensen CH, Schroder HD, Olesen JL, Suetta C, Teisner B, Kjaer M. Changes in satellite cells in human skeletal muscle after a single bout of high intensity exercise. J Physiol. 2004;558:333–340. doi: 10.1113/jphysiol.2004.061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer HC, Blanco CE, Sattler FR, Schroeder ET, Wiswell RA. Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve. 2006;33:242–253. doi: 10.1002/mus.20461. [DOI] [PubMed] [Google Scholar]
- Drummond MJ, Fujita S, Abe T, Dreyer HC, Volpi E, Rasmussen BB. Human muscle gene expression following resistance exercise and blood flow restriction. Med Sci Sports Exerc. 2008;40:691–698. doi: 10.1249/MSS.0b013e318160ff84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CS, Glynn EL, Drummond MJ, Timmerman KL, Fujita S, Abe T, Dhanani S, Volpi E, Rasmussen BB. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol. 2010;108:1199–1209. doi: 10.1152/japplphysiol.01266.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S, Abe T, Drummond MJ, Cadenas JG, Dreyer HC, Sato Y, Volpi E, Rasmussen BB. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol. 2007;103:903–910. doi: 10.1152/japplphysiol.00195.2007. [DOI] [PubMed] [Google Scholar]
- Grembowicz KP, Sprague D, McNeil PL. Temporary disruption of the plasma membrane is required for c-fos expression in response to mechanical stress. Mol Biol Cell. 1999;10:1247–1257. doi: 10.1091/mbc.10.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm L, Reitelseder S, Pedersen TG, Doessing S, Petersen SG, Flyvbjerg A, Andersen JL, Aagaard P, Kjaer M. Changes in muscle size and MHC composition in response to resistance exercise with heavy and light loading intensity. J Appl Physiol. 2008;105:1454–1461. doi: 10.1152/japplphysiol.90538.2008. [DOI] [PubMed] [Google Scholar]
- Jespersen JG, Nedergaard A, Andersen LL, Schjerling P, Andersen JL. Myostatin expression during human muscle hypertrophy and subsequent atrophy: increased myostatin with detraining. Scand J Med Sci Sports. 2011;21:215–223. doi: 10.1111/j.1600-0838.2009.01044.x. [DOI] [PubMed] [Google Scholar]
- Kadi F, Schjerling P, Andersen LL, Charifi N, Madsen JL, Christensen LR, Andersen JL. The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol. 2004;558:1005–1012. doi: 10.1113/jphysiol.2004.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadi F, Thornell LE. Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochem Cell Biol. 2000;113:99–103. doi: 10.1007/s004180050012. [DOI] [PubMed] [Google Scholar]
- Korthuis RJ, Granger DN, Townsley MI, Taylor AE. The role of oxygen-derived free radicals in ischemia-induced increases in canine skeletal muscle vascular permeability. Circ Res. 1985;57:599–609. doi: 10.1161/01.res.57.4.599. [DOI] [PubMed] [Google Scholar]
- Kubo K, Komuro T, Ishiguro N, Tsunoda N, Sato Y, Ishii N, Kanehisa H, Fukunaga T. Effects of low-load resistance training with vascular occlusion on the mechanical properties of muscle and tendon. J Appl Biomech. 2006;22:112–119. doi: 10.1123/jab.22.2.112. [DOI] [PubMed] [Google Scholar]
- Laurentino GC, Ugrinowitsch C, Roschel H, Aoki MS, Soares AG, Neves M, Jr, Aihara AY, Fernandes Ada R, Tricoli V. Strength training with blood flow restriction diminishes myostatin gene expression. Med Sci Sports Exerc. 2012;44:406–412. doi: 10.1249/MSS.0b013e318233b4bc. [DOI] [PubMed] [Google Scholar]
- Mackey AL, Holm L, Reitelseder S, Pedersen TG, Doessing S, Kadi F, Kjaer M. Myogenic response of human skeletal muscle to 12 weeks of resistance training at light loading intensity. Scand J Med Sci Sports. 2010;21:773–782. doi: 10.1111/j.1600-0838.2010.01178.x. [DOI] [PubMed] [Google Scholar]
- Mackey AL, Kjaer M, Charifi N, Henriksson J, Bojsen-Moller J, Holm L, Kadi F. Assessment of satellite cell number and activity status in human skeletal muscle biopsies. Muscle Nerve. 2009;40:455–465. doi: 10.1002/mus.21369. [DOI] [PubMed] [Google Scholar]
- Manini TM, Vincent KR, Leeuwenburgh CL, Lees HA, Kavazis AN, Borst SE, Clark BC. Myogenic and proteolytic mRNA expression following blood flow restricted exercise. Acta Physiol (Oxf) 2011;201:255–263. doi: 10.1111/j.1748-1716.2010.02172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CJ, Churchward-Venne TA, West DW, Burd NA, Breen L, Baker SK, Phillips SM. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J Appl Physiol. 2012;113:71–77. doi: 10.1152/japplphysiol.00307.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygren AT, Sundberg CJ, Goransson H, Esbjornsson-Liljedahl M, Jansson E, Kaijser L. Effects of dynamic ischaemic training on human skeletal muscle dimensions. Eur J Appl Physiol. 2000;82:137–141. doi: 10.1007/s004210050663. [DOI] [PubMed] [Google Scholar]
- Olsen S, Aagaard P, Kadi F, Tufekovic G, Verney J, Olesen JL, Suetta C, Kjaer M. Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J Physiol. 2006;573:525–534. doi: 10.1113/jphysiol.2006.107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell DM, McArdle A, Morgan JE, Patridge TA, Jackson MJ. Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Radic Biol Med. 2004;37:1064–1072. doi: 10.1016/j.freeradbiomed.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol. 2008;104:1736–1742. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- Singh RB, Dhalla NS. Ischemia-reperfusion-induced changes in sarcolemmal Na+/K+-ATPase are due to the activation of calpain in the heart. Can J Physiol Pharmacol. 2010;88:388–397. doi: 10.1139/Y10-012. [DOI] [PubMed] [Google Scholar]
- Takarada Y, Sato Y, Ishii N. Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur J Appl Physiol. 2002;86:308–314. doi: 10.1007/s00421-001-0561-5. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Liu X, Pulido A, Morales M, Sakata T, Dial S, Hattori A, Ikeuchi Y, Allen RE. Satellite cell activation in stretched skeletal muscle and the role of nitric oxide and hepatocyte growth factor. Am J Physiol Cell Physiol. 2006;290:C1487–1494. doi: 10.1152/ajpcell.00513.2005. [DOI] [PubMed] [Google Scholar]


