Abstract
Following the start of low-intensity exercise in healthy humans, it has been established that the kinetics of skeletal muscle O2 delivery is faster than, and does not limit, the kinetics of muscle O2 uptake (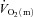 ). Direct data are lacking, however, on the question of whether O2 delivery might limit
). Direct data are lacking, however, on the question of whether O2 delivery might limit 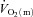 kinetics during high-intensity exercise. Using multiple exercise transitions to enhance confidence in parameter estimation, we therefore investigated the kinetics of, and inter-relationships between, muscle blood flow (
kinetics during high-intensity exercise. Using multiple exercise transitions to enhance confidence in parameter estimation, we therefore investigated the kinetics of, and inter-relationships between, muscle blood flow (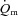 ), a–
), a–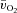 difference and
difference and 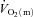 following the onset of low-intensity (LI) and high-intensity (HI) exercise. Seven healthy males completed four 6 min bouts of LI and four 6 min bouts of HI single-legged knee-extension exercise. Blood was frequently drawn from the femoral artery and vein during exercise and
following the onset of low-intensity (LI) and high-intensity (HI) exercise. Seven healthy males completed four 6 min bouts of LI and four 6 min bouts of HI single-legged knee-extension exercise. Blood was frequently drawn from the femoral artery and vein during exercise and 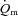 , a–
, a–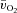 difference and
difference and 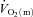 were calculated and subsequently modelled using non-linear regression techniques. For LI, the fundamental component mean response time (MRTp) for
were calculated and subsequently modelled using non-linear regression techniques. For LI, the fundamental component mean response time (MRTp) for 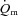 kinetics was significantly shorter than
kinetics was significantly shorter than 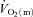 kinetics (mean ± SEM, 18 ± 4 vs. 30 ± 4 s; P < 0.05), whereas for HI, the MRTp for
kinetics (mean ± SEM, 18 ± 4 vs. 30 ± 4 s; P < 0.05), whereas for HI, the MRTp for 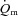 and
and 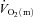 was not significantly different (27 ± 5 vs. 29 ± 4 s, respectively). There was no difference in the MRTp for either
was not significantly different (27 ± 5 vs. 29 ± 4 s, respectively). There was no difference in the MRTp for either 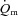 or
or 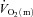 between the two exercise intensities; however, the MRTp for a–
between the two exercise intensities; however, the MRTp for a–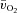 difference was significantly shorter for HI compared with LI (17 ± 3 vs. 28 ± 4 s; P < 0.05). Excess O2, i.e. oxygen not taken up (
difference was significantly shorter for HI compared with LI (17 ± 3 vs. 28 ± 4 s; P < 0.05). Excess O2, i.e. oxygen not taken up (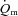 ×
× ), was significantly elevated within the first 5 s of exercise and remained unaltered thereafter, with no differences between LI and HI. These results indicate that bulk O2 delivery does not limit
), was significantly elevated within the first 5 s of exercise and remained unaltered thereafter, with no differences between LI and HI. These results indicate that bulk O2 delivery does not limit 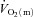 kinetics following the onset of LI or HI knee-extension exercise.
kinetics following the onset of LI or HI knee-extension exercise.
Key points
Following the start of low-intensity exercise in healthy humans, it has been established that the kinetics of muscle O2 delivery is faster than, and does not limit, the kinetics of muscle O2 uptake.
Direct data are lacking, however, on the question of whether O2 delivery might limit O2 uptake kinetics during high-intensity exercise.
In this study, we made frequent measurements of muscle blood flow, arterial-to-venous O2 difference (a–
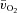 difference) and O2 uptake following the onset of multiple transitions of both low-intensity and high-intensity knee-extension exercise in the same subjects.
difference) and O2 uptake following the onset of multiple transitions of both low-intensity and high-intensity knee-extension exercise in the same subjects.We show that although blood flow kinetics is slower for high-intensity compared with low-intensity exercise, this does not result in slower O2 uptake kinetics.
These results indicate that muscle O2 delivery does not limit O2 uptake during knee-extension exercise in healthy humans.
Introduction
Following the onset of exercise, the rate of ATP resynthesis in the active myocytes increases immediately to prevent a rapid fall in ATP concentration ([ATP]) and to sustain contractions. It has been known since the early work of Krogh & Lindhard (1920), however, that the rate of pulmonary O2 uptake (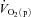 ) rises more slowly, only reaching a steady state several minutes following the start of exercise. This obligates an increased rate of ATP supply from non-oxidative metabolic pathways in this transient phase, resulting in a reduction of muscle [PCr] and an accumulation of muscle lactate, the magnitude of which will depend on the work rate.
) rises more slowly, only reaching a steady state several minutes following the start of exercise. This obligates an increased rate of ATP supply from non-oxidative metabolic pathways in this transient phase, resulting in a reduction of muscle [PCr] and an accumulation of muscle lactate, the magnitude of which will depend on the work rate.
The limitation(s) to the dynamic adaptation of skeletal muscle O2 utilization (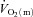 ) following the onset of contractions remains unclear. In particular, controversy continues to surround the extent to which the systemic (bulk) delivery of O2 to muscle might constrain
) following the onset of contractions remains unclear. In particular, controversy continues to surround the extent to which the systemic (bulk) delivery of O2 to muscle might constrain 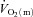 kinetics as opposed to some intrinsic cellular limitation to O2 utilization (Hughson et al. 2001; Grassi, 2006; Poole et al. 2008). It has been suggested that muscle blood flow (
kinetics as opposed to some intrinsic cellular limitation to O2 utilization (Hughson et al. 2001; Grassi, 2006; Poole et al. 2008). It has been suggested that muscle blood flow (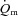 ) may limit
) may limit 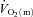 kinetics in the transition from rest to exercise, at least in certain circumstances. For example, Hughson et al. (1996) reported that forearm
kinetics in the transition from rest to exercise, at least in certain circumstances. For example, Hughson et al. (1996) reported that forearm 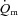 and
and 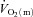 increased more rapidly at the onset of intermittent static handgrip exercise when the arm was positioned below compared with above the level of the heart. It has also been reported that reducing O2 availability to the working muscles using interventions such as hypoxia (Engelen et al. 1996) and β-blockade (Hughson, 1984), and during exercise in the supine compared with the upright position (Koga et al. 1999), can slow
increased more rapidly at the onset of intermittent static handgrip exercise when the arm was positioned below compared with above the level of the heart. It has also been reported that reducing O2 availability to the working muscles using interventions such as hypoxia (Engelen et al. 1996) and β-blockade (Hughson, 1984), and during exercise in the supine compared with the upright position (Koga et al. 1999), can slow 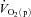 kinetics. On the other hand, there is evidence to suggest that a limited extraction of O2 by the contracting muscle cells causes the delay in
kinetics. On the other hand, there is evidence to suggest that a limited extraction of O2 by the contracting muscle cells causes the delay in 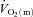 in the initial phase of exercise, at least during low-intensity exercise. Using an isolated canine gastrocnemius model, Grassi et al. (1998) reported that
in the initial phase of exercise, at least during low-intensity exercise. Using an isolated canine gastrocnemius model, Grassi et al. (1998) reported that 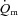 kinetics was significantly faster than
kinetics was significantly faster than 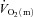 kinetics following the onset of contractions requiring ∼60%
kinetics following the onset of contractions requiring ∼60%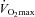 but that
but that 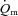 and
and 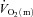 kinetics were similar following the onset of contractions requiring ∼100%
kinetics were similar following the onset of contractions requiring ∼100%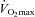 (Grassi et al. 2000).
(Grassi et al. 2000).
Few studies have addressed the question of whether 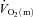 kinetics is limited by
kinetics is limited by 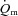 kinetics in humans. Grassi et al. (1996) made direct measurements of
kinetics in humans. Grassi et al. (1996) made direct measurements of 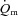 and arterio-venous O2 content difference across the leg during the transition from unloaded pedalling to moderate-intensity (i.e. below the lactate threshold, LT) cycle exercise. The results of this study indicated that
and arterio-venous O2 content difference across the leg during the transition from unloaded pedalling to moderate-intensity (i.e. below the lactate threshold, LT) cycle exercise. The results of this study indicated that 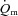 (and O2 delivery) increased considerably faster than
(and O2 delivery) increased considerably faster than 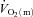 over the first 10–15 s of exercise, after which
over the first 10–15 s of exercise, after which 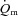 and
and 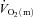 increased with a similar time course to their respective steady-state values. Bangsbo et al. (2000) reported that the difference between muscle O2 delivery and
increased with a similar time course to their respective steady-state values. Bangsbo et al. (2000) reported that the difference between muscle O2 delivery and 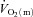 was greatest at the onset of intense exercise (becoming reduced to a constant level after ∼15 s), indicating that O2 supply exceeds demand in the initial phase of dynamic exercise and that O2 delivery is not limiting
was greatest at the onset of intense exercise (becoming reduced to a constant level after ∼15 s), indicating that O2 supply exceeds demand in the initial phase of dynamic exercise and that O2 delivery is not limiting 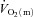 kinetics. The possibility cannot be excluded, however, that a non-maximal muscle O2 extraction in the initial phase of exercise is due to an inefficient flow distribution, i.e. heterogeneity of local blood flow relative to metabolic rate. Using non-invasive Doppler ultrasound techniques, a number of other studies have indicated that
kinetics. The possibility cannot be excluded, however, that a non-maximal muscle O2 extraction in the initial phase of exercise is due to an inefficient flow distribution, i.e. heterogeneity of local blood flow relative to metabolic rate. Using non-invasive Doppler ultrasound techniques, a number of other studies have indicated that 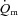 adapts more rapidly than
adapts more rapidly than 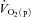 such that bulk muscle O2 delivery cannot be considered to limit the rate at which
such that bulk muscle O2 delivery cannot be considered to limit the rate at which 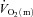 increases, at least following the onset of low-intensity exercise (MacDonald et al. 1998; van Beekvelt et al. 2001; Fukuba et al. 2004; Koga et al. 2005). However, the ‘excess’ of O2 delivery relative to O2 utilisation appears to be reduced during high-intensity compared with low-intensity exercise such that O2 availability might play a role in limiting
increases, at least following the onset of low-intensity exercise (MacDonald et al. 1998; van Beekvelt et al. 2001; Fukuba et al. 2004; Koga et al. 2005). However, the ‘excess’ of O2 delivery relative to O2 utilisation appears to be reduced during high-intensity compared with low-intensity exercise such that O2 availability might play a role in limiting 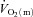 kinetics during more intense exercise (Grassi et al. 2000; Koga et al. 2005; Poole et al. 2008). If so, this would be expected to be manifest in a slowing of the initial
kinetics during more intense exercise (Grassi et al. 2000; Koga et al. 2005; Poole et al. 2008). If so, this would be expected to be manifest in a slowing of the initial 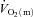 kinetics for high-intensity compared with low-intensity exercise. The possibility that
kinetics for high-intensity compared with low-intensity exercise. The possibility that 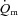 might limit
might limit 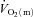 kinetics in an intensity-dependent fashion has never been directly investigated.
kinetics in an intensity-dependent fashion has never been directly investigated.
To address this issue, we aimed to extend the work of Grassi et al. (1996) by investigating the relationship between the kinetics of 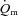 (and hence muscle O2 delivery), a–
(and hence muscle O2 delivery), a–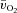 difference and
difference and 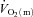 following the onset of low-intensity and high-intensity knee-extensor exercise. We reasoned that any slowing of
following the onset of low-intensity and high-intensity knee-extensor exercise. We reasoned that any slowing of 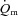 kinetics during high-intensity (HI) compared with low-intensity (LI) exercise would not appreciably impact on
kinetics during high-intensity (HI) compared with low-intensity (LI) exercise would not appreciably impact on 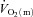 kinetics given the apparent surplus of O2 delivery relative to O2 utilization for this mode of exercise (Bangsbo et al. 2000; Nyberg et al. 2010). We hypothesized that: (1)
kinetics given the apparent surplus of O2 delivery relative to O2 utilization for this mode of exercise (Bangsbo et al. 2000; Nyberg et al. 2010). We hypothesized that: (1) 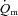 kinetics would be faster than
kinetics would be faster than 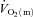 kinetics during LI but not HI exercise; (2) a–
kinetics during LI but not HI exercise; (2) a–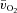 difference kinetics would be faster in HI compared with LI exercise; and (3)
difference kinetics would be faster in HI compared with LI exercise; and (3) 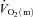 kinetics would not be significantly different between LI and HI exercise.
kinetics would not be significantly different between LI and HI exercise.
Methods
Subjects
Seven healthy male subjects participated in the experiment. The subjects had a mean ± SD age, height, mass and body fat percentage of 23 ± 2 years, 1.82 ±0.03 m, 78.4 ± 6.9 kg, and 18.2 ± 5.1%, respectively. The subjects were untrained or recreationally active with a peak O2 uptake (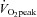 ) during cycle ergometry of 3.84 ± 0.50 l min−1 or 49.0 ± 5.1 ml min−1 kg−1. The subjects were fully informed of the risks and discomforts associated with the experimental procedures, and all provided written consent. The study was carried out in accordance with the guidelines contained in the Declaration of Helsinki and was approved by the Ethics Committee of Copenhagen and Frederiksberg communities (H-B-2007-098).
) during cycle ergometry of 3.84 ± 0.50 l min−1 or 49.0 ± 5.1 ml min−1 kg−1. The subjects were fully informed of the risks and discomforts associated with the experimental procedures, and all provided written consent. The study was carried out in accordance with the guidelines contained in the Declaration of Helsinki and was approved by the Ethics Committee of Copenhagen and Frederiksberg communities (H-B-2007-098).
Exercise model and pre-experimental procedures
On the first day a progressive cycle ergometer test was performed for determination of whole-body 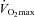 . The subject cycled for 4 min at 100 W after which the load was increased by 20 W each 30 s until volitional exhaustion. Pulmonary gas exchange and ventilation were measured (CPX/D MedGraphics, St Paul, MN, US) throughout the incremental test.
. The subject cycled for 4 min at 100 W after which the load was increased by 20 W each 30 s until volitional exhaustion. Pulmonary gas exchange and ventilation were measured (CPX/D MedGraphics, St Paul, MN, US) throughout the incremental test. 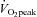 was defined as the highest 30 s mean value recorded before the subject's volitional termination of the test. On a separate day, a resting needle muscle biopsy sample was obtained under local anaesthesia (1 ml of 20 mg ml−1 lidocaine) from m. vastus lateralis of the experimental (right) leg (Bergstøm et al. 1962).
was defined as the highest 30 s mean value recorded before the subject's volitional termination of the test. On a separate day, a resting needle muscle biopsy sample was obtained under local anaesthesia (1 ml of 20 mg ml−1 lidocaine) from m. vastus lateralis of the experimental (right) leg (Bergstøm et al. 1962).
Subjects performed dynamic single-legged knee-extension exercise in a semi-supine position on an ergometer that permitted the exercise to be confined to the quadriceps muscle (Andersen et al. 1985; Bangsbo et al. 1990). The subjects had several visits to the laboratory in order to become familiarized with the exercise model. After at least three familiarization sessions, the subjects performed a single-legged incremental knee-extension test in order to determine the maximal power output, which was 66 ± 4 (54–82) W. Force tracings and kicking frequency were continuously monitored and pulmonary gas exchange and ventilation were measured throughout the incremental test. 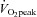 was defined as the highest 30 s mean value recorded before the subject's volitional termination of the test. The gas exchange threshold (GET) was determined from a cluster of measures including: (1) the first disproportionate increase in carbon dioxide output (
was defined as the highest 30 s mean value recorded before the subject's volitional termination of the test. The gas exchange threshold (GET) was determined from a cluster of measures including: (1) the first disproportionate increase in carbon dioxide output (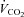 ) from visual inspection of individual plots of
) from visual inspection of individual plots of 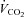 vs.
vs.
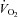 ; (2) an increase in
; (2) an increase in 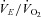 (
(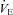 , expiratory ventilation) with no increase in
, expiratory ventilation) with no increase in 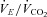 ; (3) an increase in end-tidal O2 tension with no fall in end-tidal CO2 tension. The power outputs that would require 60% of the GET (i.e. low-intensity exercise, LI: 18 ± 1 (14–22) W) and 50% of the difference (Δ) between the GET and
; (3) an increase in end-tidal O2 tension with no fall in end-tidal CO2 tension. The power outputs that would require 60% of the GET (i.e. low-intensity exercise, LI: 18 ± 1 (14–22) W) and 50% of the difference (Δ) between the GET and 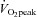 (i.e. high-intensity exercise, HI: 47 ± 3 (38–58) W) were calculated and used in the main experiment. These power outputs were selected in order that the subjects could complete several like-transitions at each intensity (to increase confidence in the model fits) without fatigue.
(i.e. high-intensity exercise, HI: 47 ± 3 (38–58) W) were calculated and used in the main experiment. These power outputs were selected in order that the subjects could complete several like-transitions at each intensity (to increase confidence in the model fits) without fatigue.
Main experiment
Subject preparation
The subjects arrived at the laboratory at 8.00 a.m. after consuming a standard breakfast including fruit juice and cereal. Subjects were placed in the supine position and one arterial and two venous catheters were placed under local anaesthesia. The arterial catheter, used for collection of blood samples and for green dye measurement, was inserted antegrade into the femoral artery of the right leg (experimental leg) with the tip positioned ∼2 cm proximal to the inguinal ligament. The second catheter, used for collection of venous blood samples and for green dye measurements, was placed retrograde in the femoral vein of the left leg with the tip positioned 6 cm distal to the inguinal ligament. The third catheter, used for measurements of thigh blood flow, was placed antegrade into the femoral vein with the tip positioned 2 cm distal to the inguinal ligament. A thermistor (Edslab, T.D. Probe, 94-030-2.5F, Baxter A/S, Allerod, Denmark) for measurement of blood temperature was advanced ∼8 cm beyond the tip of the venous catheter.
Experimental protocol
The exercise protocol (Fig. 1) consisted of four 6 min LI bouts (EX 1–4) followed by four 6 min HI bouts (EX 5–8). The LI bouts and HI bouts were interspersed with 30 min and 45 min rest periods, respectively. These recovery durations were selected to ensure that muscle blood flow, blood gases and metabolites, and  had returned to baseline before the commencement of the next exercise bout. An occlusion cuff placed below the knee was inflated (240 mmHg) 30 s prior to exercise and remained inflated throughout exercise in order to avoid contribution of blood from the lower leg. Before and during EX1–2 and EX5–6, blood flow was measured and femoral arterial and venous blood samples were obtained. During EX3 and EX6, venous blood samples were obtained at rest, during passive exercise and frequently during exercise. For 180 s prior to the onset of the single-legged knee-extension exercise, the leg was passively moved in order to accelerate the ergometer flywheel and ensure a constant power output from the onset of exercise. Blood was drawn from the femoral artery and vein during passive exercise and frequently during the initial phase of exercise, i.e. five arterial and six venous samples were obtained between −3 and 20 s of exercise using racks of stop-cocks (Bangsbo et al. 2000; Krustrup, 2004). Further arterial and venous samples were collected at 30, 45, 60 and 75 s and 1.5, 2, 2.5, 3, 4, 5 and 6 min of exercise. Blood flow was measured at rest, during passive exercise, and as frequently as possible during the first 60 s of exercise (8–10 measurements in EX1–2 and EX5–6) as well as from 70–85, 100–115, 140–155, 175–190, 240–255, 295–310 and 330–345 s of exercise (see Fig. 1). The multiple transitions used in the present study allowed us to obtain a high time resolution for the measurement of blood flow and arterio-venous difference across the exercise transient, i.e. the high measurement frequency was obtained by using a composite of measurements made at different times during the repeat exercise transitions.
had returned to baseline before the commencement of the next exercise bout. An occlusion cuff placed below the knee was inflated (240 mmHg) 30 s prior to exercise and remained inflated throughout exercise in order to avoid contribution of blood from the lower leg. Before and during EX1–2 and EX5–6, blood flow was measured and femoral arterial and venous blood samples were obtained. During EX3 and EX6, venous blood samples were obtained at rest, during passive exercise and frequently during exercise. For 180 s prior to the onset of the single-legged knee-extension exercise, the leg was passively moved in order to accelerate the ergometer flywheel and ensure a constant power output from the onset of exercise. Blood was drawn from the femoral artery and vein during passive exercise and frequently during the initial phase of exercise, i.e. five arterial and six venous samples were obtained between −3 and 20 s of exercise using racks of stop-cocks (Bangsbo et al. 2000; Krustrup, 2004). Further arterial and venous samples were collected at 30, 45, 60 and 75 s and 1.5, 2, 2.5, 3, 4, 5 and 6 min of exercise. Blood flow was measured at rest, during passive exercise, and as frequently as possible during the first 60 s of exercise (8–10 measurements in EX1–2 and EX5–6) as well as from 70–85, 100–115, 140–155, 175–190, 240–255, 295–310 and 330–345 s of exercise (see Fig. 1). The multiple transitions used in the present study allowed us to obtain a high time resolution for the measurement of blood flow and arterio-venous difference across the exercise transient, i.e. the high measurement frequency was obtained by using a composite of measurements made at different times during the repeat exercise transitions.
Figure 1. Schematic representation of the experimental protocol.
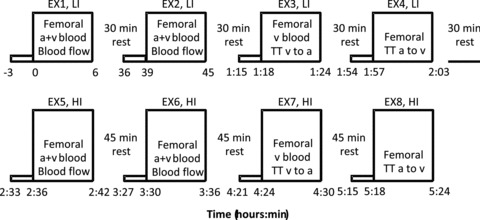
Four consecutive 6 min low-intensity single-legged knee-extension exercise bouts (LI, EX1–4) were performed interspersed with 30 min rest periods, followed by four 6 min high-intensity single-legged knee-extension exercise bouts (HI, EX5–8) interspersed with 45 min rest periods. During EX1–2 and EX5–6, femoral arterial blood samples (BSa) and venous blood samples (BSv) were collected and thigh blood flow was measured. During EX3 and EX7, the vein-to-artery transit time (TTv–a) was determined and venous blood samples were collected. During EX4 and EX8, the artery-to-vein transit time (TTa–v) was determined.
Between 0.5 to 1.5 min of exercise the arterial blood samples were taken approximately 6 s before the venous samples and for the remainder of the exercise 5 s before, in order to account for the transit time of blood from the artery through the muscle capillary bed and to the collection point at the vein (Bangsbo et al. 2000). Afterwards, all measurements were time-corrected to represent the time in the muscle capillaries based on the individual artery-to-vein transit times determined in EX4 and EX8. During EX4 and EX8, artery-to-vein mean transit time was measured at rest, during passive exercise and as frequently as possible during exercise, i.e. after 6, 35, 60, 90, 120, 180 and 270 s of exercise (Fig. 1).
Measurements and analyses
Thigh blood flow measurements
Femoral venous blood flow (i.e. thigh blood flow) was measured by the constant infusion thermodilution technique (Andersen & Saltin, 1985). Briefly, venous and infusate temperatures were measured continuously before and during ice-cold saline infusion (10–15 s) at a rate of 120 ml min−1 to achieve a drop in venous blood temperature of ∼0.6–2°C. Resting blood flow measurements were made with an infusion rate of ∼30 ml min−1 for 30–45 s. Venous temperature was measured with the thermistor positioned through the venous catheter. Infusate temperature (0–4°C) was measured at the site of entry to the catheter (Edslab flow-through thermister). Venous blood temperature and saline infusate temperatures were recorded at 400 Hz analog-to-digital sampling rate (Powerlab 16 s data acquisition system, Chart v4.13 software, ADInstruments, Sydney, Australia) onto the hard drive of a computer.
Artery-to-vein transit times
To determine the femoral artery-to-vein mean transit time (MTTa–v), 3 mg of indocyanine green (ICG, Becton Dickenson) at a concentration of 5 mg ml−1 was rapidly injected into the femoral artery, immediately followed by a flush of 5 ml isotonic saline. Blood was withdrawn from the femoral vein at a rate of 30 ml min−1 for measurements of ICG concentration with a linear densitometer. The densitometer output was sampled at a frequency of 100 Hz. The time from injection to the time of appearance and the time when the curve peaked was calculated as described by Bangsbo et al. (2000) and corrected by the transit time of the catheter (i.e. the dead space of the catheter divided by the pump flow). The mean transit time from femoral artery to the muscle capillaries and from the muscle capillaries to the femoral vein was estimated to be 1/3 and 2/3, respectively, of the total femoral artery-to-vein transit time (Bangsbo et al. 2000).
Blood analyses
Arterial and venous blood samples were immediately analysed for 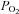 , O2 saturation and haemoglobin (ABL510, Radiometer, Copenhagen, Denmark) from which O2 content was calculated. For the determination of blood lactate and glucose (YSI 2300, Yellow Spring Instruments (YSI), Yellow Springs, OH, USA), 200 μl of whole blood was haemolysed within 10 s of sampling by adding to 200 μl of buffer (YSI; 0.5% Triton X-100).
, O2 saturation and haemoglobin (ABL510, Radiometer, Copenhagen, Denmark) from which O2 content was calculated. For the determination of blood lactate and glucose (YSI 2300, Yellow Spring Instruments (YSI), Yellow Springs, OH, USA), 200 μl of whole blood was haemolysed within 10 s of sampling by adding to 200 μl of buffer (YSI; 0.5% Triton X-100).
Muscle fibre type and capillary determination
Muscle biopsies were mounted in an embedding medium (OCT Compound Tissue-Tek, Sakura Finetek, Zoeterwoude, The Netherlands) and frozen in isopentane that was cooled to the freezing point in liquid nitrogen. These samples were stored at –80°C prior to histochemical analysis for fibre type distribution and fibre type specific capillarisation. Five serial 10-μm-thick sections were cut at –20°C and incubated for myofibrillar ATPase reactions at pH 9.4 after preincubation at pH 4.3, 4.6 and 10.3. Based on the myofibrillar ATP staining, individual fibres were classified under light microscopy as slow twitch (ST), fast twitch (FT)a or FTx.
Quadriceps muscle mass determination
The mass of the quadriceps femoris muscle of the experimental leg was estimated anthropometrically by measurements of the thigh length, and thigh circumference and skin fold thickness at three sites and corrected based on a comparison between MR-scan and anthropometric determinations (Krustrup et al. 2004c).
Calculations and mathematical modelling
Thigh 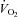 was calculated by multiplying
was calculated by multiplying 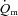 with the a–
with the a–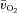 difference, and thigh lactate release was calculated by multiplying
difference, and thigh lactate release was calculated by multiplying 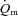 with the venous–arterial lactate difference. A continuous blood flow curve was constructed for each subject by linear interpolation of the measured blood flow data points to obtain time-matched values of
with the venous–arterial lactate difference. A continuous blood flow curve was constructed for each subject by linear interpolation of the measured blood flow data points to obtain time-matched values of 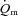 with the blood variables.
with the blood variables.
For 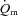 and
and 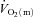 , the mean value for each of the sampling periods throughout exercise was calculated and used in subsequent curve fitting. The data were modelled from the onset of exercise using eqn (1) for LI and eqn (2) for HI.
, the mean value for each of the sampling periods throughout exercise was calculated and used in subsequent curve fitting. The data were modelled from the onset of exercise using eqn (1) for LI and eqn (2) for HI.
| (1) |
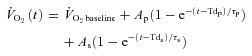 |
(2) |
where: 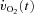 represents the absolute
represents the absolute 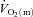 at a given time t;
at a given time t; 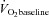 represents the mean
represents the mean 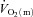 in the final 30 s of the baseline period; Ap, Tdp and τp represent the amplitude, time delay and time constant, respectively, describing the fundamental increase in
in the final 30 s of the baseline period; Ap, Tdp and τp represent the amplitude, time delay and time constant, respectively, describing the fundamental increase in 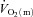 above baseline; and As, Tds and τs represent the amplitude, time delay before the onset of, and time constant describing the development of, the
above baseline; and As, Tds and τs represent the amplitude, time delay before the onset of, and time constant describing the development of, the 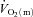 slow component, respectively. The
slow component, respectively. The 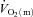 slow component was also described as the difference in
slow component was also described as the difference in 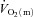 between 6 min and 2 min of exercise (6–2 min). An iterative process was used to minimize the sum of the squared errors between the fitted function and the observed values.
between 6 min and 2 min of exercise (6–2 min). An iterative process was used to minimize the sum of the squared errors between the fitted function and the observed values. 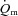 and a–
and a–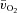 difference data were modelled in the same way.
difference data were modelled in the same way.
For 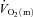 , the mean response time (MRTp) for the fundamental phase of the response in both LI and HI was calculated by summing the Tdp and τp since this parameter best describes the overall
, the mean response time (MRTp) for the fundamental phase of the response in both LI and HI was calculated by summing the Tdp and τp since this parameter best describes the overall 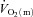 kinetics following the onset of contractions (Koga et al. 2005; Whipp & Rossiter, 2005). In addition, the mean response time for the entire
kinetics following the onset of contractions (Koga et al. 2005; Whipp & Rossiter, 2005). In addition, the mean response time for the entire 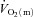 response (MRTt) was calculated by fitting a single exponential curve through the data from the onset of exercise.
response (MRTt) was calculated by fitting a single exponential curve through the data from the onset of exercise.
Statistics
Data were analysed using a two-factor (condition × time) repeated measure analysis of variance (ANOVA), with significance set at P < 0.05. Significant interactions and main effects were subsequently analysed using a Newman–Keuls post hoc test. Differences in 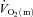 ,
, 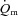 and a–
and a–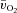 difference kinetics were tested using the Student's paired t test and relationships were explored using Pearson product moment correlation coefficients. Data are presented as means ± SEM, unless otherwise stated.
difference kinetics were tested using the Student's paired t test and relationships were explored using Pearson product moment correlation coefficients. Data are presented as means ± SEM, unless otherwise stated.
Results
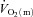 ,
, 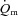 and a–
and a–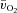 difference kinetics for LI and HI
difference kinetics for LI and HI
The parameters derived from modelling the 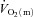 ,
, 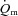 and a–
and a–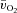 difference data for LI and HI are presented in Table 1 and the responses are illustrated in Fig. 2 and Fig. 3. Equation (1) provided an adequate fit to the
difference data for LI and HI are presented in Table 1 and the responses are illustrated in Fig. 2 and Fig. 3. Equation (1) provided an adequate fit to the 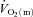 ,
, 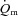 and a–
and a–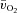 difference data for LI. However, for HI, eqn (2) provided a better fit to the
difference data for LI. However, for HI, eqn (2) provided a better fit to the 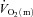 data in four subjects and a better fit to the
data in four subjects and a better fit to the 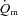 data in two subjects.
data in two subjects.
Table 1.
Muscle blood flow (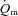 ), arterio-venous O2 difference (a–v O2 diff) and oxygen uptake (
), arterio-venous O2 difference (a–v O2 diff) and oxygen uptake (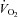 ) kinetics variables for 6 min bouts of low-intensity (LI) and high-intensity (HI) knee-extensor exercise
) kinetics variables for 6 min bouts of low-intensity (LI) and high-intensity (HI) knee-extensor exercise
| Low intensity | High intensity | |||||
|---|---|---|---|---|---|---|
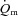 (l min−1) (l min−1) |
a–v O2 diff (ml l−1) |
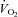 (ml min−1) (ml min−1) |
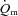 (l min−1) (l min−1) |
a–v O2 diff (ml l−1) |
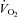 (ml min−1) (ml min−1) |
|
| Baseline values | 1.08 ± 0.08 | 47 ± 8 | 48 ± 6 | 1.52 ± 0.11 | 43 ± 7 | 46 ± 8 |
| Td (s) | −1 ± 2 | 8 ± 1 | 4 ± 1 | 1 ± 1 | 5 ± 0 | 4 ± 1 |
| Tau (s) | 19 ± 4 | 21 ± 3 | 26 ± 3 | 26 ± 5 | 12 ± 3$*# | 25 ± 4 |
| Amplitude | 2.06 ± 0.29 | 70 ± 6 | 327 ± 32 | 4.32 ± 0.47# | 89 ± 5 | 687 ± 55# |
| MRTp (s) | 18 ± 4* | 28 ± 4 | 30 ± 4 | 27 ± 5 | 17 ± 3$*# | 29 ± 4 |
| 6–2 min | 0.07 ± 0.09 | 5 ± 2 | 32 ± 8 | 0.47 ± 0.19# | 11 ± 3 | 112 ± 21# |
| End exercise | 3.20 ± 0.3 | 117 ± 5 | 375 ± 37 | 6.05 ± 0.59# | 132 ± 6# | 790 ± 61# |
| MRTt (s) | 16 ± 4* | 35 ± 5 | 34 ± 4 | 31 ± 7 | 21 ± 3 | 37 ± 5 |
Data are presented as means ± SEM. Td, time delay; MRTp, mean response time for the fundamental phase of the response; 6–2 min, the difference in 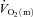 between 6 min and 2 min of exercise; MRTt, mean response time for the entire
between 6 min and 2 min of exercise; MRTt, mean response time for the entire 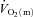 response; End exercise, end-exercise value for
response; End exercise, end-exercise value for 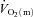 . *Significantly different from
. *Significantly different from 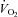 . $Significantly different from
. $Significantly different from 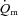 . #Significantly different from LI.
. #Significantly different from LI.
Figure 2. Thigh blood flow (A), arterial and venous O2 content (B) and muscle O2 extraction (C) before and during 6 min of low-intensity (LI, filled symbols) and high-intensity (HI, open symbols) single-legged knee-extension exercise.
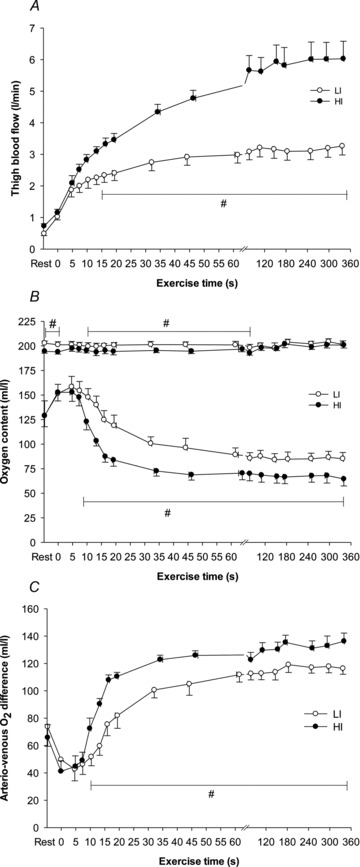
Values are mean ± SEM. #LI significantly different from HI.
Figure 3. Muscle oxygen uptake before and during 6 min of low-intensity (LI, filled symbols) and high-intensity (HI, open symbols) single-legged knee-extension exercise.
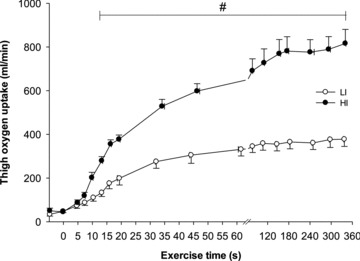
Values are mean ± SEM. #LI significantly different from HI.
For LI, following a short time delay of ∼4 s, 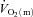 rose exponentially with a τp of 26 ± 3 s.
rose exponentially with a τp of 26 ± 3 s. 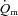 increased with no discernible delay at the onset of exercise with a τp of 19 ± 4 s. The MRTp for
increased with no discernible delay at the onset of exercise with a τp of 19 ± 4 s. The MRTp for 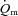 kinetics was smaller (P < 0.05) than the MRTp for
kinetics was smaller (P < 0.05) than the MRTp for 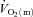 kinetics, i.e. the kinetic adaptation was faster. Similarly, the MRTt for
kinetics, i.e. the kinetic adaptation was faster. Similarly, the MRTt for 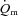 was smaller (P < 0.05) than the MRTt for
was smaller (P < 0.05) than the MRTt for 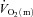 . The a–
. The a–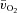 difference was unchanged for ∼8 s after which it increased with a τp of 21 ± 3 s. The MRTp for a–
difference was unchanged for ∼8 s after which it increased with a τp of 21 ± 3 s. The MRTp for a–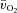 difference was not different from the MRTp for
difference was not different from the MRTp for 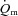 or
or 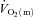 (Table 1).
(Table 1).
For HI, following a modelled time delay of ∼4 s, 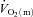 rose with a τp of 25 ± 4 s.
rose with a τp of 25 ± 4 s. 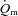 increased with no delay at the onset of exercise with a τp of 26 ± 5 s. The MRTp for
increased with no delay at the onset of exercise with a τp of 26 ± 5 s. The MRTp for 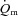 kinetics and the MRTp for
kinetics and the MRTp for 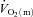 kinetics were not different. The a–
kinetics were not different. The a–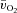 difference was unchanged for ∼5 s after which it increased with a τp of 12 ± 3 s. The MRTp for a–
difference was unchanged for ∼5 s after which it increased with a τp of 12 ± 3 s. The MRTp for a–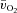 difference was shorter than the MRTp for both
difference was shorter than the MRTp for both 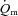 and
and 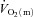 (P < 0.05; Table 1).
(P < 0.05; Table 1).
Differences in 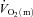 ,
, 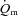 and a–
and a–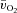 difference between LI and HI
difference between LI and HI
There was no difference in the baseline values of 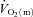 ,
, 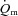 and a–
and a–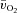 difference between LI and HI. However,
difference between LI and HI. However, 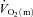 ,
, 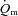 and a–
and a–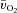 difference for HI were higher (P < 0.05) than for LI from 13, 10 and 16 s, respectively, and throughout exercise (Figs 2 and 3) with approximately 2-fold higher (P < 0.01) end-exercise values for
difference for HI were higher (P < 0.05) than for LI from 13, 10 and 16 s, respectively, and throughout exercise (Figs 2 and 3) with approximately 2-fold higher (P < 0.01) end-exercise values for 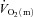 (790 ± 61 vs. 375 ± 37 ml min−1) and
(790 ± 61 vs. 375 ± 37 ml min−1) and 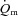 (6.05 ± 0.59 vs. 3.20 ± 0.30 l min−1) and 17% higher (P < 0.05) end-exercise a–
(6.05 ± 0.59 vs. 3.20 ± 0.30 l min−1) and 17% higher (P < 0.05) end-exercise a–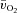 difference (132 ± 6 vs. 117 ± 5 ml l−1). The amplitude for
difference (132 ± 6 vs. 117 ± 5 ml l−1). The amplitude for 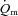 and
and 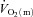 were greater for HI compared with LI (P < 0.01), but the a–
were greater for HI compared with LI (P < 0.01), but the a–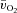 difference amplitude was not different between LI and HI. The ratio of the amplitude of
difference amplitude was not different between LI and HI. The ratio of the amplitude of 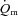 to the amplitude of
to the amplitude of 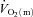 , i.e.
, i.e. 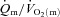 , was ∼6.3 for both LI and HI.
, was ∼6.3 for both LI and HI.
No ‘slow component’ for 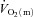 ,
, 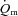 and a–
and a–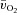 difference data was evident for LI whereas a
difference data was evident for LI whereas a 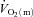 slow component could be discerned in four subjects and a
slow component could be discerned in four subjects and a 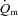 slow component was measured in two subjects during HI. The change in variables between 2 and 6 min of exercise was greater (P < 0.05) in HI compared with LI for
slow component was measured in two subjects during HI. The change in variables between 2 and 6 min of exercise was greater (P < 0.05) in HI compared with LI for 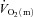 and
and 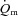 but not for a–
but not for a–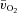 difference (Table 1).
difference (Table 1).
The Td, τp and MRTp values were not significantly different between LI and HI for either 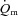 or
or 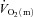 . The Td for a–
. The Td for a–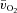 difference was not different between LI and HI; however, both the τp and MRTp for a–
difference was not different between LI and HI; however, both the τp and MRTp for a–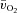 difference was significantly smaller (i.e. the kinetics were faster) for HI compared with LI (P < 0.05; Table 1). Excess O2, i.e. O2 not taken up (
difference was significantly smaller (i.e. the kinetics were faster) for HI compared with LI (P < 0.05; Table 1). Excess O2, i.e. O2 not taken up (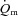 ×
×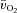 ), was ∼160 ml min−1 during passive exercise, was elevated 2-fold within the first 5 s of exercise in LI and HI, and remained unaltered during exercise, with no significant differences (P = 0.08) between LI and HI (Fig. 4).
), was ∼160 ml min−1 during passive exercise, was elevated 2-fold within the first 5 s of exercise in LI and HI, and remained unaltered during exercise, with no significant differences (P = 0.08) between LI and HI (Fig. 4).
Figure 4. Excess muscle oxygen before and during 6 min of low-intensity (LI, filled symbols) and high-intensity (HI, open symbols) single-legged knee-extension exercise.
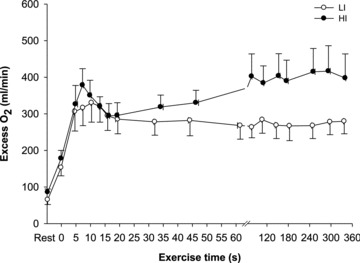
Values are mean ± SEM.
Figure 5 shows the changes in 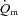 ,
, 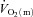 and a–
and a–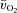 difference for the initial phase of LI (panel A) and HI (panel B) exercise when the responses are normalized to 100% of the amplitude attained after 2 min of exercise. The relatively faster kinetics of
difference for the initial phase of LI (panel A) and HI (panel B) exercise when the responses are normalized to 100% of the amplitude attained after 2 min of exercise. The relatively faster kinetics of 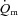 relative to
relative to 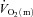 is evident, especially for LI exercise, while the a–
is evident, especially for LI exercise, while the a–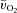 difference falls more rapidly in HI compared with LI.
difference falls more rapidly in HI compared with LI.
Figure 5. Schematic illustration, based on the group mean model fits, of the relative changes in muscle blood flow, O2 uptake and arterio-venous O2 difference for the initial phase of low-intensity (A) and high-intensity (B) exercise.
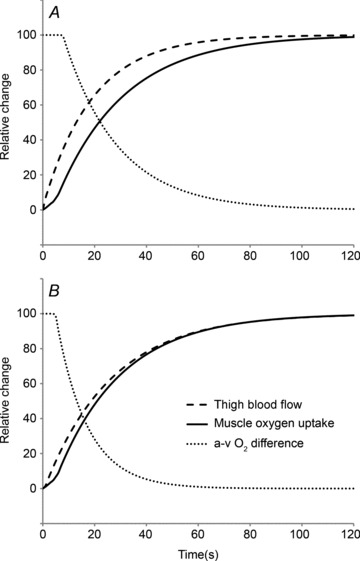
Notice that muscle blood flow kinetics are faster than muscle O2 uptake kinetics for low-intensity exercise but that the kinetics of muscle blood flow and muscle O2 uptake are similar for high-intensity exercise. Notice also that the arterio-venous O2 difference falls more rapidly following the onset of HI compared with LI exercise.
Blood lactate, pH, 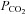 and HCO3
−
and HCO3
−
Blood variables for LI and HI are summarized in Table 2. No difference was observed in baseline values for blood lactate between LI and HI. However, after 30 s and 6 min of exercise, venous blood lactate was 2- and 7-fold higher (P < 0.05) for HI compared with LI (6 min: 5.3 ± 0.9 vs. 0.8 ± 0.1 mmol l−1), with corresponding end-exercise venous pH values of 7.19 ± 0.01 and 7.30 ± 0.01 (Table 2). No differences were observed between LI and HI in blood pH, 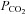 and HCO3− during the first 30 s of exercise (Table 2).
and HCO3− during the first 30 s of exercise (Table 2).
Table 2.
Blood variables at rest and during 6 min of low- (LI) and high-intensity (HI) knee-extensor exercise
| Low intensity | High intensity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rest | 30 s | 60 s | 3 min | 6 min | Rest | 30 s | 60 s | 3 min | 6 min | |
| Blood lactate (mmol−1) | ||||||||||
| Artery | 0.7 ± 0.0 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.9 ±0.1 | 0.9 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 1.1 ± 0.1 | 2.4 ± 0.4# | 3.3 ± 0.6# |
| Vein | 0.7 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 | 1.6 ± 0.1# | 2.5 ± 0.3# | 4.2 ± 0.7# | 5.3 ± 0.9# |
| Blood pH (−log H+) | ||||||||||
| Artery | 7.40 ± 0.01 | 7.38 ± 0.01 | 7.38 ± 0.01 | 7.37 ± 0.01 | 7.38 ± 0.01 | 7.38 ± 0.01# | 7.38 ± 0.01 | 7.36 ± 0.00# | 7.35 ± 0.01# | 7.35 ± 0.01# |
| Vein | 7.34 ± 0.01 | 7.36 ± 0.01 | 7.33 ± 0.01 | 7.31 ± 0.01 | 7.30 ± 0.01 | 7.35 ± 0.01 | 7.34 ± 0.01 | 7.27 ± 0.01# | 7.21 ± 0.01# | 7.19 ± 0.01# |
Blood 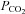 (mmHg) (mmHg) | ||||||||||
| Artery | 41.6 ± 0.7 | 43.8 ± 0.9 | 43.0 ± 0.5 | 43.6 ± 0.8 | 43.3 ± 1.3 | 42.4 ± 0.7 | 41.4 ± 0.6 | 42.9 ± 0.3 | 42.0 ± 0.6 | 38.6 ± 0.3# |
| Vein | 57.2 ± 2.5 | 52.3 ± 1.4 | 57.2 ± 1.7 | 60.8 ± 2.1 | 63.8 ± 1.9 | 51.1 ± 1.3# | 50.3 ± 0.7 | 60.6 ± 1.5 | 73.6 ± 2.8# | 74.5 ± 3.1# |
| Blood HCO3− (mmol l−1) | ||||||||||
| Artery | 24.5 ± 0.3 | 25.0 ± 0.4 | 24.4 ± 0.3 | 24.5 ± 0.4 | 24.4 ± 0.7 | 24.0 ± 0.2 | 23.6 ± 0.2 | 23.7 ± 0.2 | 22.3 ± 0.1# | 20.6 ± 0.2# |
| Vein | 28.6 ± 0.9 | 27.7 ± 0.4 | 28.7 ± 0.6 | 29.0 ± 0.6 | 29.7 ± 0.7 | 26.5 ± 0.4 | 28.7 ± 1.5 | 27.1 ± 0.2# | 27.1 ± 0.3# | 26.1 ± 0.3# |
| Plasma K+ (mmol l−1) | ||||||||||
| Artery | 4.0 ± 0.0 | 4.1 ± 0.1 | 4.2 ± 0.0 | 4.3 ± 0.1 | 4.2 ± 0.0 | 4.2 ± 0.1 | 4.3 ± 0.1 | 4.6 ± 0.0# | 4.9 ± 0.1# | 5.0 ± 0.1# |
| Vein | 4.1 ± 0.0 | 4.7 ± 0.1 | 4.8 ± 0.1 | 4.8 ± 0.1 | 4.7 ± 0.0 | 4.3 ± 0.1 | 5.3 ± 0.1# | 5.4 ± 0.1# | 5.7 ± 0.1# | 5.6 ± 0.1# |
| Hb (g dl−1) | ||||||||||
| Artery | 9.2 ± 0.1 | 9.0 ± 0.2 | 9.1 ± 0.1 | 9.1 ± 0.2 | 9.1 ± 0.2 | 8.8 ± 0.1 | 8.9 ± 0.1 | 8.9 ± 0.1 | 9.0 ± 0.1 | 9.1 ± 0.2 |
| Vein | 9.1 ± 0.2 | 9.2 ± 0.2 | 9.2 ± 0.2 | 9.2 ± 0.1 | 9.2 ± 0.1 | 8.8 ± 0.1 | 8.9 ± 0.1 | 9.0 ± 0.1 | 9.1 ± 0.1 | 9.2 ± 0.2 |
Data are presented as means ± SEM.
Denotes significant difference from LI.
Muscle mass, fibre types and capillaries
The quadriceps muscle mass of the experimental leg was 2.34 ± 0.11 (2.01–2.69) kg. The distribution of ST, FTa and FTx fibres in m. vastus lateralis was 50 ± 5 (34–68)%, 32 ± 5 (17–49)% and 18 ± 1 (15–23)%, respectively. The number of capillaries per fibre was 4.0 ± 0.5 (2.6–6.7) and the number of capillaries per mm2 was 645 ± 113 (315–1278). No correlations were observed between either the fraction of ST fibres or muscle capillarisation and the parameters describing the 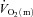 ,
, 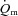 and a–
and a–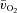 difference kinetics. The fraction of FTx fibres was correlated (P < 0.05) with
difference kinetics. The fraction of FTx fibres was correlated (P < 0.05) with 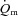 MRTp for LI (r = 0.85) and
MRTp for LI (r = 0.85) and 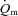 6–2 min for HI (r = 0.77). The correlation between FTx fibres and the end-exercise
6–2 min for HI (r = 0.77). The correlation between FTx fibres and the end-exercise 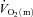 ‘gain’ (i.e. Δ
‘gain’ (i.e. Δ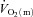 /ΔWR) for HI approached significance (r = 0.71; P = 0.07).
/ΔWR) for HI approached significance (r = 0.71; P = 0.07).
Discussion
The purpose of the present study was to investigate the interactions of 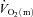 ,
, 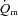 and O2 extraction during LI and HI knee-extension exercise in humans. The results confirm our experimental hypotheses and indicate that O2 delivery does not limit
and O2 extraction during LI and HI knee-extension exercise in humans. The results confirm our experimental hypotheses and indicate that O2 delivery does not limit 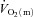 kinetics during LI or HI exercise. Despite a tendency for
kinetics during LI or HI exercise. Despite a tendency for 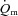 kinetics to become slower at the higher exercise intensity, such that
kinetics to become slower at the higher exercise intensity, such that 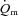 kinetics was faster than
kinetics was faster than 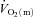 kinetics for LI but not HI, the time constant for
kinetics for LI but not HI, the time constant for 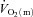 kinetics was not significantly different between LI and HI (τ = 26 and 25 s, respectively). The somewhat slower
kinetics was not significantly different between LI and HI (τ = 26 and 25 s, respectively). The somewhat slower 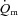 kinetics in HI compared with LI was apparently compensated by a more rapid muscle O2 extraction with both the time delay and the time constant for a–
kinetics in HI compared with LI was apparently compensated by a more rapid muscle O2 extraction with both the time delay and the time constant for a–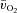 difference being significantly reduced in HI compared with LI (Fig. 5).
difference being significantly reduced in HI compared with LI (Fig. 5).
While our study confirms the results of Grassi et al. (1996) for moderate-intensity cycle exercise, this is the first study, to our knowledge, to directly investigate the inter-relationships between 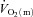 ,
, 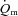 and O2 extraction kinetics during both LI and HI exercise. This enabled us to address the controversial question of whether
and O2 extraction kinetics during both LI and HI exercise. This enabled us to address the controversial question of whether 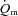 (and thus muscle O2 delivery) might limit
(and thus muscle O2 delivery) might limit 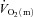 kinetics following the onset of exercise and, in particular, whether any such limitation might be exercise intensity dependent. Previous studies addressing the influence of exercise intensity on the control of
kinetics following the onset of exercise and, in particular, whether any such limitation might be exercise intensity dependent. Previous studies addressing the influence of exercise intensity on the control of 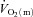 kinetics in humans have typically estimated
kinetics in humans have typically estimated 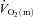 from
from 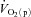 , requiring multiple transitions due to the noise inherent in breath-to-breath data, and/or estimated
, requiring multiple transitions due to the noise inherent in breath-to-breath data, and/or estimated 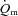 using non-invasive Doppler ultrasound techniques (van Beekvelt et al. 2001; Koga et al. 2005). The few studies that have directly measured
using non-invasive Doppler ultrasound techniques (van Beekvelt et al. 2001; Koga et al. 2005). The few studies that have directly measured 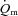 and
and 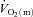 kinetics at more than one intensity in the same study did not use multiple exercise transitions and did not describe the kinetic features of the response (Krustrup et al. 2003, 2004a). Key advances of the present study include that we: (1) made direct and high-frequency measurements of
kinetics at more than one intensity in the same study did not use multiple exercise transitions and did not describe the kinetic features of the response (Krustrup et al. 2003, 2004a). Key advances of the present study include that we: (1) made direct and high-frequency measurements of 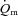 and a–
and a–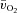 difference across the rest-to-exercise transient and subsequently calculated
difference across the rest-to-exercise transient and subsequently calculated 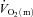 from the Fick equation; (2) used the artery-to-vein mean transit time to correct our measurements for the transit time of blood from the artery through the muscle capillary bed and to the collection point at the vein; and (3) asked the subjects to complete four repeat exercise transitions at both LI and HI, providing increased confidence in the kinetic parameters derived from the curve-fitting procedures (Lamarra et al. 1987).
from the Fick equation; (2) used the artery-to-vein mean transit time to correct our measurements for the transit time of blood from the artery through the muscle capillary bed and to the collection point at the vein; and (3) asked the subjects to complete four repeat exercise transitions at both LI and HI, providing increased confidence in the kinetic parameters derived from the curve-fitting procedures (Lamarra et al. 1987).
Our results indicate that 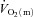 kinetics is not limited by
kinetics is not limited by 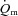 during LI or HI knee-extension exercise. This interpretation is based on the following evidence: (1) the τp for
during LI or HI knee-extension exercise. This interpretation is based on the following evidence: (1) the τp for 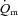 was significantly shorter than the τp for
was significantly shorter than the τp for 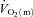 during LI, and there was no significant difference between the τp for
during LI, and there was no significant difference between the τp for 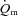 and
and 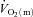 during HI; (2) the τp for
during HI; (2) the τp for 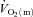 was not significantly different between LI and HI, with group mean values of 26 s and 25 s, respectively; and (3) there was an excess of O2 delivery relative to O2 utilization during both LI and HI with a larger excess in the initial phase of exercise (Fig. 4). Nevertheless, although the difference in
was not significantly different between LI and HI, with group mean values of 26 s and 25 s, respectively; and (3) there was an excess of O2 delivery relative to O2 utilization during both LI and HI with a larger excess in the initial phase of exercise (Fig. 4). Nevertheless, although the difference in 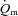 kinetics between LI (∼19 s) and HI (∼25 s) was not statistically significant, it is evident that O2 extraction increased more rapidly in HI than LI. This suggests that the slight slowing of
kinetics between LI (∼19 s) and HI (∼25 s) was not statistically significant, it is evident that O2 extraction increased more rapidly in HI than LI. This suggests that the slight slowing of 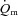 in HI was compensated by faster O2 extraction kinetics to prevent a slowing of
in HI was compensated by faster O2 extraction kinetics to prevent a slowing of 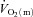 kinetics. However, although these data imply that the system was closer to the so-called ‘tipping point’ beyond which muscle O2 delivery might begin to limit
kinetics. However, although these data imply that the system was closer to the so-called ‘tipping point’ beyond which muscle O2 delivery might begin to limit 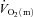 kinetics in HI (Poole et al. 2008), the similarity of τp for
kinetics in HI (Poole et al. 2008), the similarity of τp for 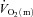 for HI and LI indicates that the tipping point was not crossed and that
for HI and LI indicates that the tipping point was not crossed and that 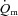 cannot be considered to be ‘limiting’
cannot be considered to be ‘limiting’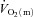 even in HI (Poole et al. 2008).
even in HI (Poole et al. 2008).
Our interpretation regarding the role of O2 in regulating 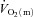 kinetics is consistent with several previous studies. Grassi et al. (1996) reported that the half-time for
kinetics is consistent with several previous studies. Grassi et al. (1996) reported that the half-time for 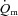 (∼27 s) and
(∼27 s) and 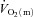 (∼28 s) following the onset of LI cycling were not significantly different. However,
(∼28 s) following the onset of LI cycling were not significantly different. However, 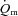 increased rapidly in the first 10–15 s of exercise whereas
increased rapidly in the first 10–15 s of exercise whereas 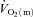 rose only slowly, leading to a surplus of O2 supply relative to O2 requirement in the early phase. Also, Bangsbo et al. (2000) reported that the difference between thigh O2 delivery and thigh O2 uptake increased in the early phase of intense knee-extension exercise and was never smaller than the value measured at baseline, suggesting that O2 availability is not limiting
rose only slowly, leading to a surplus of O2 supply relative to O2 requirement in the early phase. Also, Bangsbo et al. (2000) reported that the difference between thigh O2 delivery and thigh O2 uptake increased in the early phase of intense knee-extension exercise and was never smaller than the value measured at baseline, suggesting that O2 availability is not limiting 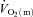 kinetics. Koga et al. (2005) used Doppler ultrasonography and measurements of femoral venous O2 content to estimate
kinetics. Koga et al. (2005) used Doppler ultrasonography and measurements of femoral venous O2 content to estimate 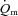 and
and 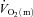 during knee-extension exercise. These authors reported that
during knee-extension exercise. These authors reported that 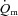 kinetics was significantly faster than
kinetics was significantly faster than 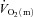 kinetics. The majority of the other investigations that have addressed the question of an exercise intensity-dependent modulation of
kinetics. The majority of the other investigations that have addressed the question of an exercise intensity-dependent modulation of 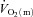 kinetics by muscle O2 delivery have used Doppler techniques to estimate
kinetics by muscle O2 delivery have used Doppler techniques to estimate 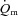 kinetics and
kinetics and 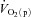 kinetics as a surrogate for
kinetics as a surrogate for 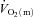 kinetics (Grassi et al. 1996; Krustrup et al. 2009). The consensus from these studies is that
kinetics (Grassi et al. 1996; Krustrup et al. 2009). The consensus from these studies is that 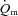 kinetics is faster than
kinetics is faster than 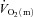 kinetics and that the latter is not limited by the former (MacDonald et al. 1998, 2000; Fukuba et al. 2004; Paterson et al. 2005). Our results are also consistent with previous animal studies. Based on measurements of capillary red blood cell flux and microvascular
kinetics and that the latter is not limited by the former (MacDonald et al. 1998, 2000; Fukuba et al. 2004; Paterson et al. 2005). Our results are also consistent with previous animal studies. Based on measurements of capillary red blood cell flux and microvascular 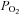 in rats, Behnke et al. (2002) also conclude that, in healthy muscle,
in rats, Behnke et al. (2002) also conclude that, in healthy muscle, 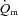 kinetics are faster than, and do not limit,
kinetics are faster than, and do not limit, 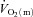 kinetics. Using an isolated canine gastrocnemius model, Grassi et al. reported that
kinetics. Using an isolated canine gastrocnemius model, Grassi et al. reported that 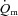 kinetics was significantly faster than
kinetics was significantly faster than 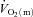 kinetics following the onset of contractions requiring ∼60%
kinetics following the onset of contractions requiring ∼60%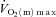 (Grassi et al. 1998) but that
(Grassi et al. 1998) but that 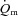 and
and 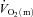 kinetics was similar following the onset of contractions requiring ∼100%
kinetics was similar following the onset of contractions requiring ∼100%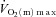 (Grassi et al. 2000). Interestingly, in those studies, fixing
(Grassi et al. 2000). Interestingly, in those studies, fixing 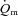 to the steady-state value from the onset of contractions did not alter
to the steady-state value from the onset of contractions did not alter 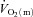 kinetics at ∼60%
kinetics at ∼60%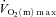 (Grassi et al. 1998) but resulted in a significant speeding of
(Grassi et al. 1998) but resulted in a significant speeding of 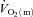 kinetics at ∼100%
kinetics at ∼100%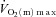 (Grassi et al. 2000). In contrast to the present study, these results suggest an intensity-related O2 dependency of
(Grassi et al. 2000). In contrast to the present study, these results suggest an intensity-related O2 dependency of 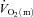 kinetics in this preparation.
kinetics in this preparation.
It should be noted that the extent to which 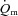 might limit
might limit 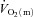 kinetics will be related to factors such as the muscle mass recruited, muscle contraction regimen, exercise modality, body position and inspired O2 fraction (Jones & Poole, 2005). It has been shown, for example that, for the same exercise intensity,
kinetics will be related to factors such as the muscle mass recruited, muscle contraction regimen, exercise modality, body position and inspired O2 fraction (Jones & Poole, 2005). It has been shown, for example that, for the same exercise intensity, 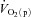 kinetics is slower in hypoxia relative to normoxia (Linnarsson et al. 1974; Engelen et al. 1996) and in the supine compared with the upright body position (MacDonald et al. 1998; Koga et al. 1999).
kinetics is slower in hypoxia relative to normoxia (Linnarsson et al. 1974; Engelen et al. 1996) and in the supine compared with the upright body position (MacDonald et al. 1998; Koga et al. 1999). 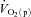 kinetics is also slowed relative to the control condition following ß-blockade (Hughson, 1984). These results indicate that interventions which reduce muscle O2 delivery have the potential to slow
kinetics is also slowed relative to the control condition following ß-blockade (Hughson, 1984). These results indicate that interventions which reduce muscle O2 delivery have the potential to slow 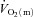 kinetics. In contrast, interventions which may enhance muscle O2 delivery such as priming exercise (MacDonald et al. 1997; Burnley et al. 2000) and hyperoxia (Linnarsson et al. 1974; Wilkerson et al. 2006) do not consistently speed
kinetics. In contrast, interventions which may enhance muscle O2 delivery such as priming exercise (MacDonald et al. 1997; Burnley et al. 2000) and hyperoxia (Linnarsson et al. 1974; Wilkerson et al. 2006) do not consistently speed 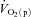 kinetics. Moreover, some studies which have reduced
kinetics. Moreover, some studies which have reduced 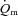 using lower body negative pressure (Williamson et al. 1996) or double blockade to inhibit the synthesis of nitric oxide and prostanoids (Nyberg et al. 2010) have not resulted in altered
using lower body negative pressure (Williamson et al. 1996) or double blockade to inhibit the synthesis of nitric oxide and prostanoids (Nyberg et al. 2010) have not resulted in altered 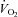 kinetics. We would point out that, although our results suggest that
kinetics. We would point out that, although our results suggest that 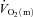 kinetics is not limited by
kinetics is not limited by 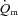 during either LI or HI, our experiments were confined to single-leg knee-extension exercise performed in the semi-supine position. It is known that the quadriceps muscle is well-perfused in this type of exercise (Andersen & Saltin, 1985) and we cannot exclude the possibility that
during either LI or HI, our experiments were confined to single-leg knee-extension exercise performed in the semi-supine position. It is known that the quadriceps muscle is well-perfused in this type of exercise (Andersen & Saltin, 1985) and we cannot exclude the possibility that 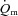 might limit
might limit 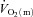 kinetics during HI exercise which engages a larger muscle mass.
kinetics during HI exercise which engages a larger muscle mass.
Our observation of a short time delay (of ∼4 s) before 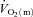 increased appreciably following the onset of exercise is worthy of comment. Such a delay has been noted previously (Grassi et al. 1998; Bangsbo et al. 2000) but is inconsistent with prevailing theories of metabolic control which relate the regulation of mitochondrial respiration to changes in the concentrations of high-energy phosphates in the cytosol (see Poole et al. 2007, 2008 for review). Oxidative phosphorylation begins to increase with the first muscle contraction in isolated single myocytes (Kindig et al. 2003) and rat skeletal muscle with intact blood supply (Behnke et al. 2002). It is likely therefore that
increased appreciably following the onset of exercise is worthy of comment. Such a delay has been noted previously (Grassi et al. 1998; Bangsbo et al. 2000) but is inconsistent with prevailing theories of metabolic control which relate the regulation of mitochondrial respiration to changes in the concentrations of high-energy phosphates in the cytosol (see Poole et al. 2007, 2008 for review). Oxidative phosphorylation begins to increase with the first muscle contraction in isolated single myocytes (Kindig et al. 2003) and rat skeletal muscle with intact blood supply (Behnke et al. 2002). It is likely therefore that 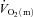 increases with essentially no delay. In this regard, it is important to note that the ‘time delay’ derived from the modelling procedure is simply a parameter that permits the best possible fit of the model to the data. In fact,
increases with essentially no delay. In this regard, it is important to note that the ‘time delay’ derived from the modelling procedure is simply a parameter that permits the best possible fit of the model to the data. In fact, 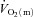 increased within the first few seconds of exercise, albeit at a slower rate than was observed later in the transition.
increased within the first few seconds of exercise, albeit at a slower rate than was observed later in the transition.
Few studies have investigated the influence of exercise intensity on 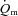 kinetics following the onset of exercise. In the present study, we observed that
kinetics following the onset of exercise. In the present study, we observed that 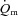 increased immediately at the onset of loaded contractions and then rose with a τp of ∼19 s for LI and ∼26 s for HI. When the entire
increased immediately at the onset of loaded contractions and then rose with a τp of ∼19 s for LI and ∼26 s for HI. When the entire 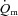 response was considered, the MRTt was ∼16 s for LI and ∼31 s for HI. Although these differences were not statistically significant, they suggest that
response was considered, the MRTt was ∼16 s for LI and ∼31 s for HI. Although these differences were not statistically significant, they suggest that 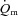 may be somewhat slower at higher relative work rates. In the study of Koga et al. (2005) in which
may be somewhat slower at higher relative work rates. In the study of Koga et al. (2005) in which 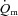 was estimated using Doppler techniques during two-legged upright knee-extension exercise, the MRTt was ∼35 s for LI and ∼46 s for HI, but this ∼30% slowing of
was estimated using Doppler techniques during two-legged upright knee-extension exercise, the MRTt was ∼35 s for LI and ∼46 s for HI, but this ∼30% slowing of 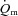 at the higher exercise intensity was not statistically significant. Other studies which measured
at the higher exercise intensity was not statistically significant. Other studies which measured 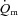 at more than one intensity appear to show a slower
at more than one intensity appear to show a slower 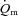 response at higher work rates; however, the
response at higher work rates; however, the 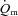 kinetics was not formally characterized in these studies (van Beekvelt et al. 2001; Krustrup et al. 2004a). The control of vascular conductance and hence
kinetics was not formally characterized in these studies (van Beekvelt et al. 2001; Krustrup et al. 2004a). The control of vascular conductance and hence 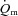 is complex and incompletely understood, involving both ‘instantaneous’ processes such as the muscle pump and immediate vasodilatation of unknown origin, as well as subsequent feedback control linked to shear-induced nitric oxide (NO) release, ATP (and NO) released from erythrocytes, prostaglandins, conducted vasodilatation, sympatholysis and metabolite accumulation (Clifford & Hellsten, 2004; Delp & O’Leary, 2004; Tschakovsky & Joyner, 2008). These feedback processes may be inherently slower at higher work rates. Additionally, further recruitment of type II fibres with time (Krustrup et al. 2004b), along with the development of a
is complex and incompletely understood, involving both ‘instantaneous’ processes such as the muscle pump and immediate vasodilatation of unknown origin, as well as subsequent feedback control linked to shear-induced nitric oxide (NO) release, ATP (and NO) released from erythrocytes, prostaglandins, conducted vasodilatation, sympatholysis and metabolite accumulation (Clifford & Hellsten, 2004; Delp & O’Leary, 2004; Tschakovsky & Joyner, 2008). These feedback processes may be inherently slower at higher work rates. Additionally, further recruitment of type II fibres with time (Krustrup et al. 2004b), along with the development of a 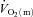 slow component in HI, would probably act to slow the overall
slow component in HI, would probably act to slow the overall 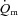 dynamics during HI compared with LI.
dynamics during HI compared with LI.
Following the onset of LI exercise, there was a delay of approximately 8 s before the a–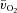 difference began to increase (τp of ∼21 s), whereas for HI exercise, both the time delay (∼5 s) and τp (∼12 s) were significantly shorter. An initial time delay of 6–15 s before a widening of the a–
difference began to increase (τp of ∼21 s), whereas for HI exercise, both the time delay (∼5 s) and τp (∼12 s) were significantly shorter. An initial time delay of 6–15 s before a widening of the a–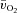 difference has been reported previously (Bangsbo et al. 2000; Grassi et al. 2000). This time delay arises as a consequence of a constant (for HI) or slightly higher (for LI) venous O2 content over the first 5–10 s of exercise which, given a constant arterial O2 content, leads to a similar (for HI) or slightly reduced (for LI) a–
difference has been reported previously (Bangsbo et al. 2000; Grassi et al. 2000). This time delay arises as a consequence of a constant (for HI) or slightly higher (for LI) venous O2 content over the first 5–10 s of exercise which, given a constant arterial O2 content, leads to a similar (for HI) or slightly reduced (for LI) a–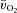 difference (Fig. 2). It has been proposed that this initial time delay for a–
difference (Fig. 2). It has been proposed that this initial time delay for a–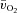 difference represents a period of time where
difference represents a period of time where 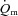 is at least adequate to support
is at least adequate to support 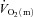 (Grassi et al. 2003). As noted previously (Grassi et al. 2005), it is striking that the directly measured values for Td and τ describing the change in a–
(Grassi et al. 2003). As noted previously (Grassi et al. 2005), it is striking that the directly measured values for Td and τ describing the change in a–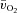 difference following the onset of exercise are very similar to the Td and τ values that are often reported for the changes in deoxyhaemoglobin concentration measured with near-infra-red spectroscopy and used to estimate changes in muscle fractional O2 extraction (DeLorey et al. 2003; Grassi et al. 2003; Jones et al. 2009). On the one hand, a more rapid increase of the a–
difference following the onset of exercise are very similar to the Td and τ values that are often reported for the changes in deoxyhaemoglobin concentration measured with near-infra-red spectroscopy and used to estimate changes in muscle fractional O2 extraction (DeLorey et al. 2003; Grassi et al. 2003; Jones et al. 2009). On the one hand, a more rapid increase of the a–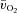 difference for HI compared with LI might indicate that O2 delivery has become somewhat less sufficient to support oxidative processes in the myocytes. On the other hand, the fact that τp for
difference for HI compared with LI might indicate that O2 delivery has become somewhat less sufficient to support oxidative processes in the myocytes. On the other hand, the fact that τp for 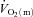 kinetics was not different between LI and HI indicates that O2 delivery remains sufficient (an interpretation supported by the excess O2 data) and that
kinetics was not different between LI and HI indicates that O2 delivery remains sufficient (an interpretation supported by the excess O2 data) and that 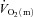 kinetics is limited by an inability to increase the a–
kinetics is limited by an inability to increase the a–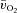 difference more rapidly following the onset of exercise. This may be a consequence of intrinsic inertia of muscle oxidative metabolism, perhaps related to feedback control through changes in high-energy phosphate concentrations and/or to slow activation of rate-limiting oxidative metabolic enzymes (Grassi, 2006; Korzeniewski & Zoladz, 2006; Poole et al. 2008). Alternatively, it is possible that, whereas bulk O2 delivery to muscle following the onset of exercise is rapid, the regional distribution of O2 within the active musculature is heterogeneous and not well matched to local metabolic rate (Kalliokoski et al. 2003; Koga et al. 2007; Poole et al. 2008).
difference more rapidly following the onset of exercise. This may be a consequence of intrinsic inertia of muscle oxidative metabolism, perhaps related to feedback control through changes in high-energy phosphate concentrations and/or to slow activation of rate-limiting oxidative metabolic enzymes (Grassi, 2006; Korzeniewski & Zoladz, 2006; Poole et al. 2008). Alternatively, it is possible that, whereas bulk O2 delivery to muscle following the onset of exercise is rapid, the regional distribution of O2 within the active musculature is heterogeneous and not well matched to local metabolic rate (Kalliokoski et al. 2003; Koga et al. 2007; Poole et al. 2008).
Several studies have indicated that muscle fibre type and fibre recruitment during exercise can influence 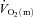 kinetics (Barstow et al. 1996; Pringle et al. 2003; Krustrup et al. 2004b, 2008). In the present study, the fraction of FTx fibres in the vastus lateralis tended to be correlated (r = 0.71; P = 0.07) with the end-exercise
kinetics (Barstow et al. 1996; Pringle et al. 2003; Krustrup et al. 2004b, 2008). In the present study, the fraction of FTx fibres in the vastus lateralis tended to be correlated (r = 0.71; P = 0.07) with the end-exercise 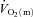 gain for HI. Given the relatively small sample size in the present study, this may support the notion that a greater fraction of less oxidative, more fatigable fibres may be associated with reduced efficiency (Pringle et al. 2003). The fraction of FTx fibres was also correlated with several indices of
gain for HI. Given the relatively small sample size in the present study, this may support the notion that a greater fraction of less oxidative, more fatigable fibres may be associated with reduced efficiency (Pringle et al. 2003). The fraction of FTx fibres was also correlated with several indices of 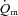 kinetics including the MRT for LI and the
kinetics including the MRT for LI and the 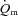 6–2 min for HI. These results tentatively suggest that the fraction of FTx fibres might be linked to slower
6–2 min for HI. These results tentatively suggest that the fraction of FTx fibres might be linked to slower 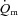 kinetics perhaps as a consequence of differences in the local control of blood flow (McAllister, 2003; Ferreira et al. 2006; Hellsten et al. 2009). Indeed, in the rat, there is evidence that O2 delivery is lower and fractional O2 extraction is higher in muscles that are considered to be FT (e.g. gastrocnemius and peroneal) compared with muscles that are considered to be ST such as the soleus (Behnke et al. 2003; McDonough et al. 2005). The greater sustained microvascular O2 pressure head following the onset of con-tractions in ST muscles might enable a greater oxidative contribution to ATP turnover by facilitating an enhanced blood myocyte O2 flux (McDonough et al. 2005). Applying these results to the present study, it is possible that the recruitment of additional FT fibres in HI compared with LI altered vascular control (and the local relationship between
kinetics perhaps as a consequence of differences in the local control of blood flow (McAllister, 2003; Ferreira et al. 2006; Hellsten et al. 2009). Indeed, in the rat, there is evidence that O2 delivery is lower and fractional O2 extraction is higher in muscles that are considered to be FT (e.g. gastrocnemius and peroneal) compared with muscles that are considered to be ST such as the soleus (Behnke et al. 2003; McDonough et al. 2005). The greater sustained microvascular O2 pressure head following the onset of con-tractions in ST muscles might enable a greater oxidative contribution to ATP turnover by facilitating an enhanced blood myocyte O2 flux (McDonough et al. 2005). Applying these results to the present study, it is possible that the recruitment of additional FT fibres in HI compared with LI altered vascular control (and the local relationship between 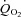 and
and 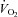 ) and mandated a faster and greater change in fractional O2 extraction following the transition from passive to loaded exercise.
) and mandated a faster and greater change in fractional O2 extraction following the transition from passive to loaded exercise.
In conclusion, we have, for the first time, investigated the dynamic interactions of 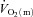 ,
, 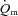 and O2 extraction kinetics following the onset of exercise as a function of exercise intensity (HI vs. LI) in humans. Our results show that
and O2 extraction kinetics following the onset of exercise as a function of exercise intensity (HI vs. LI) in humans. Our results show that 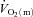 kinetics is not limited by bulk O2 delivery during knee-extension exercise, at least at intensities up to approximately 75% of the peak power output elicited during incremental exercise. During LI exercise,
kinetics is not limited by bulk O2 delivery during knee-extension exercise, at least at intensities up to approximately 75% of the peak power output elicited during incremental exercise. During LI exercise, 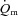 kinetics was significantly faster than
kinetics was significantly faster than 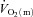 kinetics, whereas during HI exercise there was no significant difference between
kinetics, whereas during HI exercise there was no significant difference between 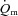 kinetics and
kinetics and 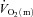 kinetics. The a–
kinetics. The a–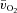 difference increased more rapidly in HI resulting in there being no slowing of
difference increased more rapidly in HI resulting in there being no slowing of 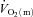 kinetics in HI compared with LI. At both intensities, the excess O2 increased between passive exercise and loaded exercise, suggesting a surplus of O2 delivery relative to O2 utilization. These results indicate that, at least for this exercise modality and at these exercise intensities,
kinetics in HI compared with LI. At both intensities, the excess O2 increased between passive exercise and loaded exercise, suggesting a surplus of O2 delivery relative to O2 utilization. These results indicate that, at least for this exercise modality and at these exercise intensities, 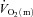 kinetics is not limited by bulk muscle O2 delivery.
kinetics is not limited by bulk muscle O2 delivery.
Acknowledgments
Some of the 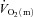 data in this study were previously presented in Krustrup et al. (2009) which focused on the agreement between muscular and pulmonary
data in this study were previously presented in Krustrup et al. (2009) which focused on the agreement between muscular and pulmonary 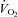 kinetics. We thank the subjects for their committed participation. The excellent technical assistance of Jens Jung Nielsen, Winnie Taagerup and Helgi W Olsen is greatly appreciated. The study was supported by the Danish National Research Foundation (504-14) and by the Danish Ministry of Culture (Kulturministeriets Udvalg for Idrætsforskning).
kinetics. We thank the subjects for their committed participation. The excellent technical assistance of Jens Jung Nielsen, Winnie Taagerup and Helgi W Olsen is greatly appreciated. The study was supported by the Danish National Research Foundation (504-14) and by the Danish Ministry of Culture (Kulturministeriets Udvalg for Idrætsforskning).
Glossary
- GET
gas exchange threshold
- HI/LI
high/low intensity
- MRT
mean response time
- Q̇m
muscle blood flow
- V̇O2
muscle O2 uptake
Author contributions
A.M.J. contributed to the design of the experiment, analysis and interpretation of the data, and writing of this article. P.K. and J.B. contributed to the design of the experiment, collection, analysis and interpretation of the data, and critical revision of this article. D.P.W., N.J.B. and J.A.C. contributed to the collection and analysis of the data, and critical revision of this article. All authors approved the final version of the manuscript.
References
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Adams RP, Sjøgaard G, Thorboe A, Saltin B. Dynamic knee extension as a model for the study of an isolated exercising muscle in man. J Appl Physiol. 1985;59:1647–1653. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Gollnick PD, Graham TE, Juel C, Kiens B, Mizuno M, Saltin B. Anaerobic energy production and O2 deficit–debt relationship during exhaustive exercise in humans. J Physiol. 1990;422:539–559. doi: 10.1113/jphysiol.1990.sp018000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J, Krustrup P, González-Alonso J, Boushel R, Saltin B. Muscle oxygen uptake kinetics at onset of intense dynamic exercise. Am J Physiol Regul Integr Comp Physiol. 2000;279:R899–R906. doi: 10.1152/ajpregu.2000.279.3.R899. [DOI] [PubMed] [Google Scholar]
- Barstow TJ, Jones AM, Nguyen PH, Casaburi R. Influence of muscle fiber type and pedal frequency on oxygen uptake kinetics of heavy exercise. J Appl Physiol. 1996;81:1624–1650. doi: 10.1152/jappl.1996.81.4.1642. [DOI] [PubMed] [Google Scholar]
- Behnke BJ, Barstow TJ, Kindig CA, McDonough P, Musch TI, Poole DC. Dynamics of oxygen uptake following exercise onset in rat skeletal muscle. Respir Physiol Neurobiol. 2002;133:229–239. doi: 10.1016/s1569-9048(02)00183-0. [DOI] [PubMed] [Google Scholar]
- Behnke BJ, McDonough P, Padilla DJ, Musch TI, Poole DC. Oxygen exchange profile in rat muscles of contrasting fibre types. J Physiol. 2003;549:597–605. doi: 10.1113/jphysiol.2002.035915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstøm J. Muscle electrolytes in man. Scand J Clin Lab Invest. 1962;68:1–101. [Google Scholar]
- Burnley M, Jones AM, Carter H, Doust JH. Effects of prior heavy exercise on phase II pulmonary oxygen uptake kinetics during heavy exercise. J Appl Physiol. 2000;89:1387–1396. doi: 10.1152/jappl.2000.89.4.1387. [DOI] [PubMed] [Google Scholar]
- Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;97:393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- DeLorey DS, Kowalchuk JM, Paterson DH. Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. J Appl Physiol. 2003;95:113–120. doi: 10.1152/japplphysiol.00956.2002. [DOI] [PubMed] [Google Scholar]
- Delp MD, O’Leary DS. Integrative control of the skeletal muscle microcirculation in the maintenance of arterial pressure during exercise. J Appl Physiol. 2004;97:1112–1118. doi: 10.1152/japplphysiol.00147.2003. [DOI] [PubMed] [Google Scholar]
- Engelen M, Porszasz J, Riley M, Wasserman K, Maehara K, Barstow TJ. Effects of hypoxic hypoxia on O2 uptake and heart rate kinetics during heavy exercise. J Appl Physiol. 1996;81:2500–2508. doi: 10.1152/jappl.1996.81.6.2500. [DOI] [PubMed] [Google Scholar]
- Ferreira LF, McDonough P, Behnke BJ, Musch TI, Poole DC. Blood flow and O2 extraction as a function of O2 uptake in muscles composed of different fibre types. Respir Physiol Neurobiol. 2006;153:237–249. doi: 10.1016/j.resp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Fukuba Y, Ohe Y, Miura A, Kitano A, Endo M, Sato H, Miyachi M, Koga S, Fukuda O. Dissociation between the time courses of femoral artery blood flow and pulmonary during repeated bouts of heavy knee extension exercise in humans. Exp Physiol. 2004;89:243–253. doi: 10.1113/expphysiol.2003.026609. [DOI] [PubMed] [Google Scholar]
- Grassi B. Limitation of skeletal muscle kinetics by inertia of cellular respiration. In: Jones AM, Poole DC, editors. Oxygen Uptake Kinetics in Sport, Exercise and Medicine. London: Routledge; 2005. pp. 212–229. [Google Scholar]
- Grassi B. Oxygen uptake kinetics: Why are they so slow? And what do they tell us? J Physiol Pharmacol. 2006;57:53–65. [PubMed] [Google Scholar]
- Grassi B, Gladden LB, Samaja M, Stary CM, Hogan MC. Faster adjustment of O2 delivery does not affect on-kinetics in isolated in situ canine muscle. J Appl Physiol. 1998;85:1394–1403. doi: 10.1152/jappl.1998.85.4.1394. [DOI] [PubMed] [Google Scholar]
- Grassi B, Hogan MC, Kelley KM, Aschenbach WG, Hamann JJ, Evans RK, Patillo RE, Gladden LB. Role of convective O2 delivery in determining on-kinetics in canine muscle contracting at peak. J Appl Physiol. 2000;89:1293–1301. doi: 10.1152/jappl.2000.89.4.1293. [DOI] [PubMed] [Google Scholar]
- Grassi B, Pogliaghi S, Rampichini S, Quaresima V, Ferrari M, Marconi C, Cerretelli P. Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transitions in humans. J Appl Physiol. 2003;95:149–158. doi: 10.1152/japplphysiol.00695.2002. [DOI] [PubMed] [Google Scholar]
- Grassi B, Poole DC, Richardson RS, Knight DR, Erickson BK, Wagner PD. Muscle O2 uptake kinetics in humans: implications for metabolic control. J Appl Physiol. 1996;80:988–998. doi: 10.1152/jappl.1996.80.3.988. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Krustrup P, Iaia FM, Secher NH, Bangsbo J. Partial neuromuscular blockade in humans enhances muscle blood flow during exercise independently of muscle oxygen uptake and acetylcholine receptor blockade. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1106–R1112. doi: 10.1152/ajpregu.90477.2008. [DOI] [PubMed] [Google Scholar]
- Hughson RL. Alterations in the oxygen deficit-oxygen debt relationships with β-adrenergic receptor blockade in man. J Physiol. 1984;349:375–387. doi: 10.1113/jphysiol.1984.sp015161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughson RL, Shoemaker JK, Tshakovsky ME, Kowalchuk JM. Dependence of muscle on blood flow dynamics at onset of forearm exercise. J Appl Physiol. 1996;81:1619–1626. doi: 10.1152/jappl.1996.81.4.1619. [DOI] [PubMed] [Google Scholar]
- Hughson RL, Tschakovsky ME, Houston ME. Regulation of oxygen consumption at the onset of exercise. Exerc Sport Sci Rev. 2001;29:129–133. doi: 10.1097/00003677-200107000-00008. [DOI] [PubMed] [Google Scholar]
- Jones AM, Davies RC, Ferreira LF, Barstow TJ, Koga S, Poole DC. Evaluation of the dynamics of muscle oxygenation by near-infrared-based tissue oximeters. Reply to Quaresima and Ferrari. J Appl Physiol. 2009;107:372–373. doi: 10.1152/japplphysiol.00215.2009. [DOI] [PubMed] [Google Scholar]
- Jones AM, Poole DC. Oxygen uptake dynamics: from muscle to mouth – an introduction to the symposium. Med Sci Sports Exerc. 2005;37:1542–1550. doi: 10.1249/01.mss.0000177466.01232.7e. [DOI] [PubMed] [Google Scholar]
- Kalliokoski KK, Laaksonen MS, Takala TO, Knuuti J, Nuutila P. Muscle oxygen extraction and perfusion heterogeneity during continuous and intermittent static exercise. J Appl Physiol. 2003;94:953–958. doi: 10.1152/japplphysiol.00731.2002. [DOI] [PubMed] [Google Scholar]
- Kindig CA, Kelley KM, Howlett RA, Stary CM, Hogan MC. Assessment of O2 uptake dynamics in isolated single skeletal myocytes. J Appl Physiol. 2003;94:353–357. doi: 10.1152/japplphysiol.00559.2002. [DOI] [PubMed] [Google Scholar]
- Koga S, Poole DC, Ferreira LF, Whipp BJ, Kondo N, Saitoh T, Ohmae E, Barstow TJ. Spatial heterogeneity of quadriceps muscle deoxygenation kinetics during cycle exercise. J Appl Physiol. 2007;103:2049–2056. doi: 10.1152/japplphysiol.00627.2007. [DOI] [PubMed] [Google Scholar]
- Koga S, Poole DC, Shiojiri T, Kondo N, Fukuba Y, Miura A, Barstow TJ. Comparison of oxygen uptake kinetics during knee extension and cycle exercise. Am J Physiol Regul Integr Comp Physiol. 2005;288:R212–R220. doi: 10.1152/ajpregu.00147.2004. [DOI] [PubMed] [Google Scholar]
- Koga S, Shiojiri T, Shibasaki M, Kondo N, Fukuba Y, Barstow TJ. Kinetics of oxygen uptake during supine and upright heavy exercise. J Appl Physiol. 1999;87:253–260. doi: 10.1152/jappl.1999.87.1.253. [DOI] [PubMed] [Google Scholar]
- Korzeniewski B, Zoladz JA. Biochemical background of the on-kinetics in skeletal muscles. J Physiol Sci. 2006;56:1–12. doi: 10.2170/physiolsci.m93. [DOI] [PubMed] [Google Scholar]
- Krogh A, Lindhard J. The changes in respiration at the transition from work to rest. J Physiol. 1920;53:431–437. doi: 10.1113/jphysiol.1920.sp001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P. Muscle oxygen uptake and energy turnover during dynamic exercise in humans. Denmark: University of Copenhagen; 2004. PhD thesis, ISBN: 87-89361-87-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P, Ferguson RA, Kjær M, Bangsbo J. ATP and heat production in human skeletal muscle during dynamic exercise: higher efficiency of anaerobic than aerobic ATP resynthesis. J Physiol. 2003;549:255–269. doi: 10.1113/jphysiol.2002.035089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P, Hellsten Y, Bangsbo J. Intense interval training enhances human skeletal muscle oxygen uptake in the initial phase of dynamic exercise at high but not at low intensities. J Physiol. 2004a;559:335–345. doi: 10.1113/jphysiol.2004.062232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P, Jones AM, Wilkerson DP, Calbet JA, Bangsbo J. Muscular and pulmonary O2 uptake kinetics during moderate- and high-intensity sub-maximal knee-extensor exercise in humans. J Physiol. 2009;587:1843–1856. doi: 10.1113/jphysiol.2008.166397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P, Secher NH, Relu MU, Hellsten Y, Söderlund K, Bangsbo J. Neuromuscular blockade of slow twitch muscle fibres elevates muscle oxygen uptake and energy turnover during submaximal exercise in humans. J Physiol. 2008;586:6037–6048. doi: 10.1113/jphysiol.2008.158162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P, Söderlund K, Mohr M, Bangsbo J. The slow component of oxygen uptake during intense sub-maximal exercise in man is associated with additional fibre recruitment. Pflügers Arch. 2004b;447:855–866. doi: 10.1007/s00424-003-1203-z. [DOI] [PubMed] [Google Scholar]
- Krustrup P, Söderlund K, Mohr M, Gonzalez-Alonso J, Bangsbo J. Recruitment of fibre types and quadriceps muscle portions during repeated intense knee-extensor exercise in humans. Pflügers Archiv. 2004c;449:56–65. doi: 10.1007/s00424-004-1304-3. [DOI] [PubMed] [Google Scholar]
- Lamarra N, Whipp BJ, Ward SA, Wasserman K. Effect of interbreath fluctuations on characterizing exercise gas exchange kinetics. J Appl Physiol. 1987;62:2003–2012. doi: 10.1152/jappl.1987.62.5.2003. [DOI] [PubMed] [Google Scholar]
- Linnarsson D, Karlsson J, Fagraeus L, Saltin B. Muscle metabolites and oxygen deficit with exercise in hypoxia and hyperoxia. J Appl Physiol. 1974;36:399–402. doi: 10.1152/jappl.1974.36.4.399. [DOI] [PubMed] [Google Scholar]
- McAllister RM. Endothelium-dependent vasodilation in different rat hindlimb skeletal muscles. J Appl Physiol. 2003;94:1777–1784. doi: 10.1152/japplphysiol.00901.2002. [DOI] [PubMed] [Google Scholar]
- MacDonald M, Pedersen PK, Hughson RL. Acceleration of kinetics in heavy submaximal exercise by hyperoxia and prior high-intensity exercise. J Appl Physiol. 1997;83:1318–1325. doi: 10.1152/jappl.1997.83.4.1318. [DOI] [PubMed] [Google Scholar]
- MacDonald MJ, Shoemaker K, Tshakovsky ME, Hughson RL. Alveolar oxygen uptake and femoral artery blood flow dynamics in upright and supine leg exercise in humans. J Appl Physiol. 1998;85:1622–1628. doi: 10.1152/jappl.1998.85.5.1622. [DOI] [PubMed] [Google Scholar]
- MacDonald MJ, Tarnopolsky MA, Hughson RL. Effect of hyperoxia and hypoxia on leg blood flow and pulmonary and leg oxygen uptake at the onset of kicking exercise. Can J Physiol Pharmacol. 2000;78:67–74. doi: 10.1139/cjpp-78-1-67. [DOI] [PubMed] [Google Scholar]
- McDonough P, Behnke BJ, Padilla DJ, Musch TI, Poole DC. Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J Physiol. 2005;563:903–913. doi: 10.1113/jphysiol.2004.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg M, Mortensen SP, Saltin B, Hellsten Y, Bangsbo J. Low blood flow at onset of moderate-intensity exercise does not limit muscle oxygen uptake. Am J Physiol Regul Integr Comp Physiol. 2010;298:R843–R848. doi: 10.1152/ajpregu.00730.2009. [DOI] [PubMed] [Google Scholar]
- Paterson ND, Kowalchuk JM, Paterson DH. Kinetics of and femoral artery blood flow during heavy-intensity, knee-extension exercise. J Appl Physiol. 2005;99:683–690. doi: 10.1152/japplphysiol.00707.2004. [DOI] [PubMed] [Google Scholar]
- Poole DC, Barstow TJ, McDonough P, Jones AM. Control of oxygen uptake during exercise. Med Sci Sports Exerc. 2008;40:462–474. doi: 10.1249/MSS.0b013e31815ef29b. [DOI] [PubMed] [Google Scholar]
- Poole DC, Ferreira LF, Behnke BJ, Barstow TJ, Jones AM. The final frontier: oxygen flux into muscle at exercise onset. Exerc Sport Sci Rev. 2007;35:166–173. doi: 10.1097/jes.0b013e318156e4ac. [DOI] [PubMed] [Google Scholar]
- Pringle JS, Doust JH, Carter H, Tolfrey K, Campbell IT, Sakkas GK, Jones AM. Oxygen uptake kinetics during moderate, heavy and severe intensity “submaximal” exercise in humans: the influence of muscle fibre type and capillarisation. Eur J Appl Physiol. 2003;89:289–300. doi: 10.1007/s00421-003-0799-1. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Joyner MJ. Nitric oxide and muscle blood flow in exercise. Appl Physiol Nutr Metab. 2008;33:151–161. doi: 10.1139/H07-148. [DOI] [PubMed] [Google Scholar]
- Van Beekvelt MC, Shoemaker JK, Tschakovsky ME, Hopman MT, Hughson RL. Blood flow and muscle oxygen uptake at the onset and end of moderate and heavy dynamic forearm exercise. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1741–R1747. doi: 10.1152/ajpregu.2001.280.6.R1741. [DOI] [PubMed] [Google Scholar]
- Whipp BJ, Rossiter HB. The kinetics of oxygen uptake: physiological inferences from the parameters. In: Jones AM, Poole DC, editors. Oxygen Uptake Kinetics in Sport, Exercise and Medicine. London: Routledge; 2005. pp. 62–94. [Google Scholar]
- Wilkerson DP, Berger NJ, Jones AM. Influence of hyperoxia on pulmonary O2 uptake kinetics following the onset of exercise in humans. Respir Physiol Neurobiol. 2006;153:92–106. doi: 10.1016/j.resp.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Williamson JW, Raven PB, Whipp BJ. Unaltered oxygen uptake kinetics at exercise onset with lower-body positive pressure in humans. Exp Physiol. 1996;81:695–705. doi: 10.1113/expphysiol.1996.sp003970. [DOI] [PubMed] [Google Scholar]


