Abstract
Passive leg movement is associated with a ∼3-fold increase in blood flow to the leg but the underlying mechanisms remain unknown. The objective of the present study was to examine the role of nitric oxide (NO) for the hyperaemia observed during passive leg movement. Leg haemodynamics and metabolites of NO production (nitrite and nitrate; NOx) were measured in plasma and muscle interstitial fluid at rest and during passive leg movement with and without inhibition of NO formation in healthy young males. The hyperaemic response to passive leg movement and to ACh was also assessed in elderly subjects and patients with peripheral artery disease. Passive leg movement (60 r.p.m.) increased leg blood flow from 0.3 ± 0.1 to 0.9 ± 0.1 litre min−1 at 20 s and 0.5 ± 0.1 litre min−1 at 3 min (P < 0.05). Mean arterial pressure remained unchanged during the trial. When passive leg movement was performed during inhibition of NO formation (NG-mono-methyl-l-arginine; 29–52 mg min−1), leg blood flow and vascular conductance were increased after 20 s (P < 0.05) and then returned to baseline levels, despite an increase in arterial pressure (P < 0.05). Passive leg movement increased the femoral venous NOx levels from 35 ± 5 at baseline to 62 ± 11 μmol l−1 during passive leg movement (P < 0.05), whereas muscle interstitial NOx levels remained unchanged. The hyperaemic response to passive leg movement were correlated with the vasodilatation induced by ACh (r2 = 0.704, P < 0.001) and with age (r2 = 0.612, P < 0.001). Leg blood flow did not increase during passive leg movement in individuals with peripheral arterial disease. These results suggest that the hypaeremia induced by passive leg movement is NO dependent and that the source of NO is likely to be the endothelium. Passive leg movement could therefore be used as a non-invasive tool to evaluate NO dependent endothelial function of the lower limb.
Key points
Passive leg movement is associated with a ∼3-fold increase in blood flow to the leg, but the underlying mechanisms remain unknown.
Passive leg movement increased venous levels of metabolites of nitric oxide (NO) in young subjects, whereas they remained unaltered in the muscle interstitial space. Inhibition of NO synthesis lowered the vasodilatory response to passive leg movement by ∼90%.
The increase in leg blood flow was lower in elderly subjects compared to young subjects and leg blood flow did not increase when passive leg movement was performed by elderly with peripheral artery disease.
The results suggest that the hyperaemia induced by passive leg movement is NO dependent. The hyperaemic response to passive leg movement and to ACh was also assessed in elderly subjects and patients with peripheral artery disease.
Introduction
Endothelial dysfunction is associated with a reduced formation of endothelium-dependent vasodilators including nitric oxide (NO) (Widlansky et al. 2003) and plays a key role in the pathogenesis of micro- and macrovascular complications observed in pathological conditions such as diabetes, atherosclerosis, hypertension and peripheral artery disease (Tooke, 1995; Taddei et al. 1997; Ross, 1999; Tendera et al. 2011). Evaluation of endothelial function and especially NO function is an important clinical tool, but the currently available methods for quantification of endothelial function are invasive or induce vasodilatation not only by NO-dependent pathways (Widlansky et al. 2003; Tschakovsky & Pyke, 2005).
Passive leg movement increases limb blood flow (Rådegran & Saltin, 1998; Krustrup et al. 2004; Wray et al. 2005), with no increase in muscle activity (Hellsten et al. 2008) and little (González-Alonso et al. 2008; Høier et al. 2010) or no (Krustrup et al. 2004; Hellsten et al. 2008) increase in metabolism. Furthermore, the increase in leg blood flow occurs independently of the arousal invoked by passive movement or the thought of passive leg movement (Venturelli et al. 2011). Mechanical factors are therefore likely to be involved in the increase in blood flow during passive leg movement, but to what extent locally formed vasodilating compounds mediate the increase in flow remains unknown. One study has suggested NOS inhibition has no effect on blood flow during passive movement, but in this study only five to seven passive leg movements were performed to accelerate the leg to 60 r.p.m. (Rådegran & Saltin, 1999). In vitro studies have demonstrated that shear stress increases the formation of NO (Pohl et al. 1986) and in vivo studies have shown that flow-mediated dilatation is partly mediated by NO (Joannides et al. 1995; Kooijman et al. 2008) and that eNOS expression is upregulated after a period of passive leg movement training (Hellsten et al. 2008; Høier et al. 2010). Both increased shear stress and stretch are inherent to passive leg movement (Høier et al. 2010) and NO is therefore a likely candidate for the mediation of passive flow. Peripheral arterial disease is characterized by a reduced endothelial NO function (Böger et al. 1997) and these patients are therefore likely to have a reduced hyperaemic response, if passive leg movement increases NO formation.
The purpose of the present study was to examine the role of NO in the hyperaemic response to passive limb movement and to determine if blood flow is lower during passive leg movement in the elderly and patients with peripheral artery disease. To accomplish this, we measured leg haemodynamics during passive leg movement with and without inhibition of NO formation and determined metabolites of NO synthesis in venous plasma and muscle interstitial fluid at rest and during passive leg movement and compared the hyperaemic response to elderly and patients with peripheral artery disease. To avoid possible confounding factors during blood sampling, we used an intravascular microdialysis probe to separate NO metabolites from blood in vivo. We hypothesized that passive leg movement results in vasodilatation via NO formation such that inhibition of NO formation would lower the hyperaemic response to passive leg movement.
Methods
Subjects
A total of 38 subjects participated in four studies (Table 1). All of the healthy young and elderly subjects were recreationally active and were not receiving any medication. Subjects with peripheral artery disease had limiting intermittent claudication and a resting ankle to brachial blood pressure index <0.9 in the affected limbs. The subjects were informed of any risks and discomforts associated with the experiments before giving their informed oral and written consent to participate. The study was approved by the Ethical committee of the Capitol Region of Denmark and the Human Research Ethics Committee of the University of the Sunshine Coast and all of the procedures followed were in accordance with institutional guidelines. The study conformed to the Declaration of Helsinki.
Table 1.
Subject characteristics
| Young | Middle aged | Elderly | PAD | |
|---|---|---|---|---|
| Subjects (n) | 15 | 4 | 11 | 8 |
| Men/women | 15/0 | 3/1 | 11/0 | 6/2 |
| Age (years) | 24 ± 5 | 46 ± 4 | 65 ± 1 | 68 ± 2 |
| Weight (kg) | 79 ± 8 | 75 ± 7 | 82 ± 2 | 83 ± 2 |
| Height (cm) | 182 ± 5 | 173 ± 4 | 176 ± 2 | 177 ± 2 |
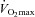 (ml min−1 kg−1) (ml min−1 kg−1) |
48 ± 7 | 33 ± 4 | 34 ± 2 | − |
PAD: peripheral artery disease.
Experimental protocols
Study 1: Effect of NOS inhibition on the hyperaemic response to passive leg movement in young subjects
One week prior to the experimental day, the subjects visited the laboratory to become accustomed to the one-leg knee-extensor model. The subjects refrained from caffeine, alcohol and exercise for 24 h before the study. On the day of the experiment, the subjects were instructed to ingest a light breakfast 1 h before reporting to the laboratory at 08.00 h.
Catheters were placed into the femoral artery of the non-experimental leg (blood sampling and blood pressure measurement) and into the femoral artery (drug infusion) and vein (blood sampling) of the experimental leg under local anaesthesia (lidocaine). Following 30 min of supine rest, the subjects (n = 7) completed 3 min of passive leg movement under the following conditions: (1) control and (2) NG-mono-methyl-l-arginine (l-NMMA; nitric oxide synthase (NOS) inhibitor). During passive leg movement, the subjects were in an upright seated position and the lower leg was strapped to the ergometer and was moved passively at a frequency of 60 r.p.m. Saline (control) or l-NMMA (4.0 mg min−1 (kg leg mass)−1; Clinalfa, Bachem, Weil am Rhein, Germany) was infused into the femoral artery of the experimental leg for 4 min prior to passive leg movement and during the 3 min of passive leg movement. A similar dose of l-NMMA has been found to lower ACh induced increase in leg blood flow by ∼65% (Mortensen et al. 2009b). Blood samples (1–5 ml) were drawn simultaneously from the femoral artery and vein at rest and during passive leg movement (30, 60, 90, 120, 150 and 180 s). The two trials were separated by a 30 min rest period. Due to the potential long lasting effects of l-NMMA, the control trial was always performed first. To test the effect of time and reproducibility of the hyperaemic response, four subjects performed three trials of passive exercise (separated by one hour) on a separate day. No differences in the blood flow response to passive leg movement were observed.
Study 2: Effect of passive leg movement on plasma and muscle interstitial metabolites in young subjects
A catheter was placed in the femoral vein, 4–5 cm below the inguinal ligament and advanced 10 cm in the proximal direction. A microdialysis probe (CMA 70 bolt, CMA Microdialysis, Solna, Sweden) with a 10 mm membrane (20 kDa cut-off) was inserted into this catheter such that the membrane was placed in the vein. Three microdialysis probes (CMA63) were inserted into the vastus lateralis muscle of the experimental leg under local anethesia (lidocaine). Thirty minutes after insertion of the probes, the subjects performed 10 min of light (10 W, i.e. <20% maximal workload, WLmax) knee-extensor exercise with the purpose of minimizing the tissue response to insertion trauma. To re-establish resting conditions, the subjects rested for another 30 min. The subjects (n = 6) then completed 10 min of passive leg movement (60 r.p.m.). Microdialysate was collected for 10 min before and during the passive leg movement.
Study 3: Hyperaemic response to passive leg movement and arterial ACh infusion
Twelve healthy subjects were catheterized as in study 1. After 30 min of rest, femoral arterial blood flow and arterial blood pressure were measured before and during 3 min of seated passive leg movement and 3 min of supine femoral arterial ACh infusion (25 μg min−1 (kg leg mass)−1). The two trials were separated by 60 min of supine rest.
Study 4: Hyperaemic response to passive leg movement in healthy subjects and patients with peripheral artery disease
Femoral arterial blood flow was measured at rest and during 3 min of passive leg movement in four middle-aged individuals, eight elderly men and eight individuals diagnosed with peripheral artery disease.
Measurements
Femoral arterial blood flow (study 1, 3 and 4)
Femoral arterial blood flow was measured with an ultrasound machine equipped with a linear probe operating an imaging frequency of 7–9 MHz and Doppler frequency of 4.2–5.0 MHz (Logic E9, GE Healthcare in the healthy subjects and Mindray M5 in patients with peripheral artery disease). The site of blood velocity measurements in the common femoral artery was distal to the inguinal ligament but above the bifurcation into the superficial and profound femoral branch to avoid turbulence from the bifurcation. All recordings were obtained at the lowest possible insonation angle and always below 60 deg. The sample volume was maximized according to the width of the vessel, and kept clear of the vessel walls. A low-velocity filter (velocities <1.8 m s−1) rejected noises caused by turbulence at the vascular wall. Doppler tracings and B-mode images were recorded continuously and Doppler tracings were averaged over eight heart cycles (10 s values) whereas the area under the curve (AUC) was calculated from the continuous Doppler tracings. Vessel diameter was determined during each Doppler recording. Arterial diameter was calculated as 1/3 of the diameter during the systole and 2/3 of the diameter during the diastole.
Microdialysis procedure (study 2)
The microdialysis probes were continuously perfused with a Ringer solution (Fresenius Kabi AB, Sweden) with a high-precision syringe pump (CMA 102, CMA microdialysis, Solna, Sweden) at a rate of 5 μl min−1. The intravascular probe was also perfused with Dalteparin (25 IE ml−1; Fragmin, Pfizer Inc., USA) to avoid blood clotting in the membrane. A small amount (2.7 nm) of [2-3H]ATP (<0.1 μCi ml−1) was added to the perfusate for the calculation of probe recovery. The purpose of determining probe recovery was to correct for differences in recovery occurring in the transition from rest to passive leg movement. After collection of samples, the microdialysate was weighed, and the actual flow rate was calculated to estimate any loss of fluid or abnormal decrease in perfusion rate and the samples were then stored at −80°C for later analysis. The relative loss for each probe was determined according to the internal reference method (Scheller & Kolb, 1991; Jansson et al. 1994). The molecular probe recovery (PR) was calculated as [PR = (dpminfusate× dpmdialysate)/dpminfusate], where dpm denotes disintegrations per minute. The 3H activity (in dpm) was measured on a liquid scintillation counter (Tri-Carb 2000; Copenhagen; Denmark) after addition of the infusate and dialysate (5 μl each) to 3.0 ml of Ultima Gold scintillation liquid (Packard Instruments, Gronningen, The Netherlands).
The concentration of stable metabolites of NO, nitrate and nitrite (NOx), was measured using fluorometric assay kit (Cayman Chemical Co., Ann Arbor, MI, USA) according to the manufacturer's instructions.
Data acquisition and analysis
Heart rate was obtained from an electrocardiogram, while arterial pressures were monitored with transducers positioned at the level of the heart (Pressure Monitoring Kit, Baxter, Deerfield, IL, USA). Blood gases and haemoglobin concentrations were measured using an ABL725 analyzer (Radiometer, Copenhagen, Denmark). Leg mass was calculated from whole-body dual-energy X-ray absorptiometry scanning (Prodigy, GE Healthcare, Chalfont St Giles, UK). Pulmonary oxygen uptake was measured online (Quark CPET, Cosmed, Italy). Maximal oxygen uptake (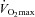 ) in the young and elderly subjects was determined during an incremental bicycle ergometer exercise test (Excalibur Sport, Lode, The Netherlands) in which the oxygen uptake was determined with a metabolic system (Quark b2 system, Cosmed, Rome, Italy).
) in the young and elderly subjects was determined during an incremental bicycle ergometer exercise test (Excalibur Sport, Lode, The Netherlands) in which the oxygen uptake was determined with a metabolic system (Quark b2 system, Cosmed, Rome, Italy).
Statistical analysis
A two-way repeated measures ANOVA was performed to test significance within and between trials. A two-way ANOVA was used to test significance between the young, elderly and peripheral artery disease subjects within trials. Following a significant F test, pair-wise differences were identified using Tukey's honestly significant difference (HSD) post hoc procedure. The significance level was set at P < 0.05 and data are means ± SEM unless otherwise indicated.
Results
The effect of NOS inhibition on leg haemodynamics at rest and during passive leg movement
Baseline leg blood flow was 0.32 ± 0.03 and increased during passive leg movement to a peak value of 0.92 ± 0.14 litre min−1 (20 s) and remained elevated during the 2 min of passive leg movement (P < 0.05; Fig. 1). The increase in leg blood flow was paralleled by an increase in LVC (P < 0.05) as MAP did not change. The increase in leg O2 delivery was paralleled by a decrease in a-vO2 difference (Table 2; P < 0.05). However, leg 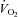 was increased by 5–12 ml min−1 during the first 2 min of passive leg movement (P < 0.05).
was increased by 5–12 ml min−1 during the first 2 min of passive leg movement (P < 0.05).
Figure 1. Leg haemodynamics during baseline conditions and passive leg movement.

Data are means ± SEM for 7 subjects. *Different from baseline conditions, P < 0.05; #different from control, P < 0.05.
Table 2.
Blood gas variables and 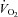 during baseline conditions and passive leg movement with and without inhibition of NO formation
during baseline conditions and passive leg movement with and without inhibition of NO formation
| Control (s) | l-NMMA (s) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 30 | 60 | 90 | 120 | 150 | 180 | Baseline | 30 | 60 | 90 | 120 | 150 | 180 | |
| Haemoglobin (g l−1) | ||||||||||||||
| a | 146 ± 2 | 146 ± 2 | 147 ± 2 | 147 ± 2 | 147 ± 2 | 146 ± 3 | 148 ± 1 | 149 ± 3 | 148 ± 3 | 148 ± 3 | 148 ± 3 | 150 ± 3 | 151 ± 3 | 163 ± 3 |
| v | 146 ± 2 | 147 ± 2 | 148 ± 2 | 147 ± 2 | 147 ± 3 | 148 ± 3 | 150 ± 2 | 148 ± 3 | 147 ± 3 | 147 ± 3 | 147 ± 2 | 150 ± 3 | 151 ± 4 | 152 ± 4 |
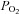 (mmHg) (mmHg) |
||||||||||||||
| a | 100 ± 2 | 96 ± 2 | 96 ± 2 | 98 ± 2 | 98 ± 3 | 98 ± 3 | 96 ± 2 | 105 ± 3 | 98 ± 1 | 98 ± 1 | 97 ± 3 | 97 ± 3 | 96 ± 3 | 97 ± 1 |
| v | 33 ± 1 | 38 ± 3 | 40 ± 2 | 40 ± 2 | 38.8 ± 3 | 37 ± 2 | 39 ± 1 | 26 ± 1 | 26 ± 1 | 28 ± 1 | 29 ± 2 | 28 ± 2 | 27 ± 2 | 29 ± 2 |
| O2 saturation (%) | ||||||||||||||
| a | 97.9 ± 0.2 | 97.6 ± 0.2 | 97.6 ± 0.2 | 97.5 ± 0.3 | 97.5 ± 0.3 | 97.7 ± 0.2 | 97.6 ± 0.1 | 98.1 ± 0.2 | 97.8 ± 0.1 | 97.8 ± 0.1 | 97.5 ± 0.3 | 97.4 ± 0.3 | 97.6 ± 0.3 | 97.6 ± 0.2 |
| v | 58.1 ± 3.3 | 70.6 ± 4.1* | 70.5 ± 3.9* | 70.2 ± 4.5* | 67.0 ± 5.2* | 65.3 ± 5.0* | 69.1 ± 2.9* | 40.9 ± 3.8# | 42.0 ± 3.0# | 46.5 ± 4.2# | 46.7 ± 4.1*# | 46.1 ± 4.0# | 43.6 ± 3.7# | 47.3 ± 3.7# |
| O2 content (ml l−1) | ||||||||||||||
| a | 195 ± 3 | 194 ± 2 | 195 ± 3 | 195 ± 3 | 196 ± 3 | 195 ± 4 | 198 ± 2 | 199 ± 4 | 197 ± 3 | 197 ± 3 | 197 ± 3 | 199 ± 4 | 200 ± 4 | 203 ± 4 |
| v | 115 ± 7 | 120 ± 9* | 142 ± 9* | 141 ± 11* | 134 ± 12* | 132 ± 12* | 140 ± 7* | 82 ± 7# | 84 ± 6# | 92 ± 7# | 93 ± 8# | 94 ± 8*# | 90 ± 8# | 97 ± 6# |
| Leg a-vO2 difference (ml l−1) | ||||||||||||||
| 80 ± 7 | 54 ± 8* | 54 ± 8* | 54 ± 8* | 60 ± 10* | 63 ± 11* | 58 ± 6* | 117 ± 8# | 113 ± 5# | 104 ± 9*# | 103 ± 8*3 | 105 ± 5# | 110 ± 7# | 106 ± 9# | |
Leg  (ml min−1) (ml min−1) |
||||||||||||||
| 24 ± 3 | 43 ± 3* | 31 ± 3* | 29 ± 4* | 33 ± 5* | 32 ± 5 | 29 ± 1 | 22 ± 2 | 23 ± 3# | 24 ± 3 | 22 ± 2 | 22 ± 3 | 23 ± 3 | 25 ± 2 | |
 (mmHg) (mmHg) |
||||||||||||||
| a | 41 ± 1 | 42 ± 0 | 42 ± 0 | 42 ± 1 | 42 ± 1 | 42 ± 1 | 42 ± 1 | 41 ± 1 | 42 ± 1 | 42 ± 1 | 41 ± 1 | 41 ± 1 | 40 ± 0 | 40 ± 1 |
| v | 47 ± 2 | 46 ± 2 | 49 ± 1 | 49 ± 2 | 49 ± 2 | 50 ± 1 | 49 ± 2 | 49 ± 2 | 49 ± 2 | 51 ± 1 | 51 ± 1 | 51 ± 1 | 51 ± 1 | 51 ± 2 |
| pH | ||||||||||||||
| a | 7.40 ± 0.01 | 7.40 ± 0.00 | 7.40 ± 0.00 | 7.40 ± 0.01 | 7.40 ± 0.01 | 7.40 ± 0.01 | 7.40 ± 0.01 | 7.40 ± 0.01 | 7.40 ± 0.01 | 7.40 ± 0.01 | 7.40 ± 0.01 | 7.40 ± 0.01 | 7.41 ± 0.01 | 7.41 ± 0.01 |
| v | 7.38 ± 0.01 | 7.37 ± 0.01 | 7.37 ± 0.01 | 7.37 ± 0.01 | 7.37 ± 0.01 | 7.36 ± 0.01 | 7.37 ± 0.01 | 7.37 ± 0.01 | 7.38 ± 0.01 | 7.37 ± 0.01 | 7.37 ± 0.01 | 7.36 ± 0.01 | 7.36 ± 0.01 | 7.37 ± 0.01 |
| Lactate (mmol l−1) | ||||||||||||||
| a | 1.9 ± 0.3 | 1.8 ± 0.2 | 1.8 ± 0.2 | 1.9 ± 0.2 | 1.9 ± 0.2 | 1.7 ± 0.2 | 1.7 ± 0.2 | 1.6 ± 0.2 | 1.7 ± 0.2 | 1.7 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.5 ± 0.1 | 1.5 ± 0.2 |
| v | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.1 | 1.6 ± 0.2 | 1.6 ± 0.3 | 1.6 ± 0.2 | 1.8 ± 0.2 | 1.4 ± 0.1 | 1.3 ± 0.2 | 1.5 ± 0.2 | 1.5 ± 0.1 | 1.7 ± 0.2 | 1.6 ± 0.1 | 1.8 ± 0.2 |
| Glucose (mmol l−1) | ||||||||||||||
| a | 5.5 ± 0.2 | 5.6 ± 0.3 | 5.6 ± 0.3 | 5.6 ± 0.3 | 5.6 ± 0.3 | 5.7 ± 0.3 | 5.7 ± 0.3 | 5.6 ± 0.3 | 5.6 ± 0.2 | 5.6 ± 0.2 | 5.6 ± 0.2 | 5.6 ± 0.2 | 5.4 ± 0.1 | 5.3 ± 0.0 |
| v | 5.1 ± 0.3 | 4.7 ± 0.3 | 5.1 ± 0.3 | 5.1 ± 0.2 | 5.1 ± 0.3 | 5.0 ± 0.2 | 5.0 ± 0.3 | 4.7 ± 0.2 | 4.8 ± 0.3 | 4.9 ± 0.2 | 4.9 ± 0.2 | 4.8 ± 0.2 | 4.8 ± 0.2 | 4.8 ± 0.2 |
Data are mean ± SEM for 7 subjects. *different from baseline conditions, P < 0.05; #different from control, P < 0.05.
During NOS inhibition, baseline leg blood flow, vascular conductance and O2 delivery were lower compared to control conditions (P < 0.05), whereas the a-vO2 difference was increased (P < 0.05) and MAP and leg  were similar. During passive leg movement, leg blood flow and vascular conductance increased at 20 s, but then returned to baseline values, whereas leg a-vO2 difference and leg
were similar. During passive leg movement, leg blood flow and vascular conductance increased at 20 s, but then returned to baseline values, whereas leg a-vO2 difference and leg 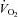 did not increase compared to baseline. MAP increased at 60, 120, 150 and 210 s (P < 0.05). Accounting for the change in baseline leg blood flow with NOS inhibition, leg blood flow and vascular conductance were lower during passive leg movement in the presence of NOS inhibition compared to control (P < 0.05). Passive exercise did not alter heart rate in either the control or the NOS inhibition trials and there was no difference between the two trials.
did not increase compared to baseline. MAP increased at 60, 120, 150 and 210 s (P < 0.05). Accounting for the change in baseline leg blood flow with NOS inhibition, leg blood flow and vascular conductance were lower during passive leg movement in the presence of NOS inhibition compared to control (P < 0.05). Passive exercise did not alter heart rate in either the control or the NOS inhibition trials and there was no difference between the two trials.
Venous plasma and muscle interstitial NOx levels during passive leg movement
Passive leg movement increased venous NOx levels from 35 ± 5 to 62 ± 11 μmol l−1 (P < 0.05; Fig. 2), whereas NOx levels remained unchanged in the muscle interstitium during passive leg movement.
Figure 2. Femoral venous plasma and muscle interstitial NOx concentrations during baseline conditions and passive leg movement.
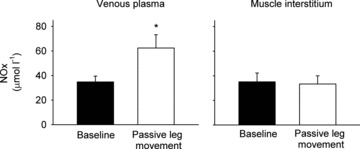
Data are means ± SEM for 6 subjects. *Significantly different from baseline, P < 0.05.
Hyperaemic response to passive leg movement and arterial ACh infusion
Leg blood flow and vascular conductance (AUC) during passive leg movement was correlated to leg blood flow (r2 = 0.570, P = 0.005) and vascular conductance (r2 = 0.704, P < 0.001) during arterial ACh infusion (Fig. 3).
Figure 3. Leg blood flow (upper panel) and vascular conductance (lower panel) during passive leg movement in 20- to 70-year-old individuals plotted against the same variables during ACh infusion.

Data are means ± SEM for 12 subjects.
Hyperaemic response to passive leg movement in elderly and patients with peripheral artery disease
Baseline leg blood flow was lower in the elderly and peripheral artery disease subjects (0.22 ± 0.05 and 0.13 ± 0.03 litre min−1, respectively), compared to the young subjects (P < 0.05). Passive leg movement increased leg blood flow in the elderly subjects at 20, 30 and 40 s (P < 0.05) where after leg blood flow returned to baseline levels. Leg blood flow was lower throughout passive leg movement in the elderly compared to the young subjects (P < 0.05; Fig. 4). Passive leg movement did not increase leg blood flow in the patients with peripheral artery disease (Fig. 5). Compared to the young subjects, leg blood flow (AUC) was lower before and during passive leg movement in both groups (P < 0.05).
Figure 4. Leg blood flow during baseline conditions and passive leg movement in young and elderly subjects.

Data are means ± SEM for 14 subjects. *Different from baseline conditions, P < 0.05; #different from young, P < 0.05.
Figure 5. Leg hyperaemia induced by passive leg movement in healthy individuals and individuals with peripheral arterial disease (PAD).
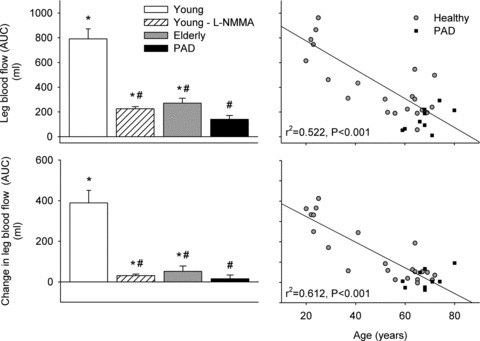
Leg blood flow and the change in leg blood flow from baseline to passive leg movement in young and elderly healthy subjects and patients with peripheral artery disease (left). Individual leg blood flow response and the changes in leg blood flow expressed in relation to age (right). Area under the curve (AUC) was calculated from continuous Doppler tracings during 90 s of passive leg movement. Displayed correlations are for healthy subjects. *Significantly different from baseline, P < 0.05; #different from young subjects, P < 0.05.
Discussion
The main findings of this study were that: (1) inhibition of NO synthesis lowered the vasodilatory response to passive leg movement by ∼90%, (2) passive leg movement increased venous plasma NOx levels, whereas interstitial NOx levels remained unchanged, (3) the hyperaemic response to passive leg movement was correlated to the vasodilatory response to ACh, (4) leg blood flow increased in the healthy elderly subjects, but the increase was lower compared to the young subjects, and (5) leg blood flow did not increase with passive exercise in subjects with peripheral artery disease. These results suggest that the hyperaemic response to passive leg movement is dependent on NO and that the source is likely to be the endothelium. Passive leg movement can therefore be used to test endothelial NO function in the leg.
The present study demonstrates that inhibition of NO formation reduces the hyperaemia observed during passive leg movement in young, healthy individuals by ∼90%. NO formation can be stimulated both chemically and mechanically and given that passive leg movement involves no alteration in muscle activation (Høier et al. 2010) and only a small increase in metabolism, it may be assumed that the enhanced NO formation with passive movement mainly occurs via mechanical stimuli. l-NMMA is likely to inhibit both nNOS and eNOS, but the increase in venous NOx and not muscle interstitial fluid during passive movement suggest that the source of NO is likely to be eNOS in the endothelium and not nNOS in the muscle tissue. Passive movement leads to alterations in both shear stress and passive stretch of the tissue (Cheng et al. 2009), both of which have been found to stimulate NO formation in vitro (Pohl et al. 1986; Joannides et al. 1995; Kooijman et al. 2008). In contrast, experimental compression of endothelial cells in vitro does not appear to have an effect on NO release (Dai et al. 2002). Collectively, these observations suggest that the enhanced NO formation during passive movement is likely to be induced by either shear stress or passive stretch or potentially both factors combined. It is noteworthy that NOS blockade almost completely abolished the flow response to passive movement. Leg blood flow was only increased after 20 s of passive leg movement during NOS inhibition, which is consistent with a slower endothelial response to shear stress (Pyke et al. 2004; Shipley et al. 2005). Despite this initial NO-independent increase in leg blood flow, the overall hyperaemic response was 90% lower during the first 90 s of passive leg movement, suggesting that NO plays an important role in the hyperaemic response to passive leg movement. This was further supported by the correlation between the hyperaemia during passive leg movement and infusion of the endothelium dependent vasodilator ACh (Furchgott & Zawadzki, 1980). The 90% lower hyperaemic response when NO formation was inhibited also refute the hypothesis that a central response to passive leg movement is an important component of the hyperaemic response (Trinity et al. 2010).
To gain further insight into the role of the endothelium in the hyperaemic response to passive leg movement, we compared the young individuals to two populations known to have reduced endothelial function. When passive leg movement was performed in the elderly subjects, the hyperaemic response was attenuated (McDaniel et al. 2010), which is in agreement with a reduced endothelial function with advancing ageing (Taddei et al. 1995). We did not measure or test NO function or test endothelium-independent vasodilatation in these individuals and therefore it cannot be excluded that the lower hyperaemic response in the elderly subjects was associated with other mechanisms. However, ageing appears not to affect endothelium-independent vasodilatation (Celermajer et al. 1994; Kirby et al. 2010), suggesting that the lower hyperaemic response was not caused by a reduced response to NO. Leg blood flow did not increase during passive leg movement in elderly individuals with peripheral artery disease, which is a disease known to be associated with severely reduced NO function (Böger et al. 1997). The observations in healthy elderly and elderly with peripheral artery disease therefore appear to support our observations in healthy young men, suggesting that the hyperaemic response to passive leg movement is NO dependent.
In contrast to the present findings during passive leg movement, NOS inhibition does not alter exercise hyperaemia in the leg (Rådegran & Saltin, 1999; Frandsen et al. 2001) and only up to ∼35% in combination with pharmacological inhibition of other vasodilator systems (Boushel et al. 2002; Mortensen et al. 2007,2009b). This discrepancy is likely to be a reflection of the mechanisms that mediate hyperaemia under the different conditions. During exercise, blood flow appears to be regulated by an interaction between several vasodilator systems, including NO, which ensures adequate O2 delivery even when one system is impaired. In contrast, the hyperaemia in response to passive movement occurs without need for an increased oxygen supply and is simply the result of a mechanical signal. Interestingly, the small (∼20 ml min−1) increase in leg 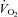 during passive leg movement (González-Alonso et al. 2008; Høier et al. 2010) was abolished during NOS inhibition. A direct effect of NO on mitochondrial respiration does not appear to explain this observation, because leg
during passive leg movement (González-Alonso et al. 2008; Høier et al. 2010) was abolished during NOS inhibition. A direct effect of NO on mitochondrial respiration does not appear to explain this observation, because leg  was not altered during baseline conditions. Instead, mechanical stress and/or the increase tissue O2 delivery may have caused a local increase in metabolism. In support of the latter, we have observed a similar tendency during arterial ATP infusion (Mortensen et al. 2009a).
was not altered during baseline conditions. Instead, mechanical stress and/or the increase tissue O2 delivery may have caused a local increase in metabolism. In support of the latter, we have observed a similar tendency during arterial ATP infusion (Mortensen et al. 2009a).
Implications
Flow mediated dilatation (FMD) in the brachial artery is a widely used method to evaluate endothelial function in patients (Celermajer et al. 1992; Thijssen et al. 2011a), and although FMD is commonly interpreted to reflect eNOS function, other vasodilators contribute to the hyperaemic response following occlusion of the artery (Tschakovsky & Pyke, 2005). Atherosclerosis and peripheral artery disease can specifically affect one leg or be more pronounced in the leg compared to the arm (Fukudome et al. 1997; Tendera et al. 2011) and it is likely that there are differences in endothelium-dependent and endothelium-independent vasodilatation between the arms and legs (Newcomer et al. 2004) due to large differences in hydrostatic pressure (Rowell, 1993). Furthermore, the larger volume of the legs may provide a better estimate of overall cardiovascular function than the forearm (Thijssen et al. 2011b). The findings that passive leg movement is NO dependent and the hyperaemic response is correlated to the response to ACh suggest that passive leg movement can be used to specifically test endothelial NO function of the legs.
In conclusion, the present results demonstrate that the hyperaemic response to passive leg movement is NO dependent and that the source of NO is likely to be the endothelium. Although the precise mechanisms remain unclear, it is suggested that mechanical forces such as shear stress and passive stretch cause endothelial release of NO. Furthermore, the hyperaemic response is attenuated in elderly and abolished in patients with peripheral artery disease. The hyperaemic response is also correlated to the vasodilatation induced by ACh. Passive leg movement could therefore be a simple, non-invasive method to specifically evaluate NO function in humans.
Acknowledgments
This study was supported by a grant from the Lundbeck foundation, the Danish Council for Independent Research – Medical Sciences and from the University of the Sunshine Coast. S.P.M. was supported by a grant from the Danish Council for Independent Research – Medical Sciences. M.N. was supported by a grant from the Lundbeck foundation. C.A. is a member of the National Health and Medical Research Council funded National Centre of Research Excellence for Peripheral Arterial Disease (NCRE-PAD, Australia).
Translational perspective
Endothelial dysfunction plays a key role in the pathogenesis of micro- and macrovascular complications observed in pathological conditions such as diabetes, atherosclerosis, hypertension and peripheral artery disease, and it is a risk factor for cardiovascular events. The findings that the hyperaemia during passive leg movements is mediated by NO and correlated to ACh induced vasodilatation suggest that passive leg movement can be used to test endothelial function. A test for endothelial function in the leg is important because there are differences in vascular function between arms and legs and some disease states only affect the legs. In addition, the >10-fold larger volume of the leg compared to the arm is more likely to reflect the vascular function of the cardiovascular system. In a standardized set-up, passive leg movement can therefore be a simple, non-invasive tool to evaluate endothelial NO function of the lower limb.
Glossary
- l-NMMA
NG-mono-methyl-l-arginine
- LVC
leg vascular conductance
- NO
nitric oxide
- NOx
nitrate/nitrite
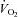
oxygen uptake
Author contributions
The contributions of the authors were as follows. Conception and design of the study: S.P.M, M.N., C.D.A, M.W. and Y.H.; collection, analysis, and interpretation of data: S.P.M, M.N. C.D.A, M.W. and Y.H.; drafting the article or revising it critically for important intellectual content: S.P.M, M.N., C.D.A, M.W. and Y.H. All authors approved the final version.
References
- Böger RH, Bode-Böger SM, Thiele W, Junker W, Alexander K, Frölich JC. Biochemical evidence for impaired nitric oxide synthesis in patients with peripheral arterial occlusive disease. Circulation. 1997;95:2068–2074. doi: 10.1161/01.cir.95.8.2068. [DOI] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Gemmer C, Olesen J, Crameri R, Scheede C, Sander M, Kjaer M. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol. 2002;543:691–698. doi: 10.1113/jphysiol.2002.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Cheng TH, Chen JJW, Shih NL, Lin JW, Liu JC, Chen YL, Chen CH, Chen JJ. Mechanical stretch induces endothelial nitric oxide synthase gene expression in neonatal rat cardiomyocytes. Clin Exp Pharmacol Physiology. 2009;36:559–566. doi: 10.1111/j.1440-1681.2008.05100.x. [DOI] [PubMed] [Google Scholar]
- Dai G, Tsukurov O, Chen M, Gertler JP, Kamm RD. Endothelial nitric oxide production during in vitro simulation of external limb compression. Am J Physiol Heart Circ Physiol. 2002;282:H2066–H2075. doi: 10.1152/ajpheart.00288.2001. [DOI] [PubMed] [Google Scholar]
- Frandsen U, Bangsbo J, Sander M, Hoffner L, Betak A, Saltin B, Hellsten Y. Exercise-induced hyperaemia and leg oxygen uptake are not altered during effective inhibition of nitric oxide synthase with NG-nitro-l-arginine methyl ester in humans. J Physiol. 2001;531:257–264. doi: 10.1111/j.1469-7793.2001.0257j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukudome Y, Fujii K, Abe I, Ohya Y, Fukuhara M, Kaseda S, Onaka U, Tsuchihashi T, Fujishima M. Ultrasonographic assessment of regional differences in atherosclerotic lesions in patients with hypertension, diabetes mellitus, or both. Hypertens Res. 1997;20:175–181. doi: 10.1291/hypres.20.175. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Mortensen SP, Jeppesen TD, Ali L, Barker H, Damsgaard R, Secher NH, Dawson EA, Dufour SP. Haemodynamic responses to exercise, ATP infusion and thigh compression in humans: insight into the role of muscle mechanisms on cardiovascular function. J Physiol. 2008;586:2405–2417. doi: 10.1113/jphysiol.2008.152058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten Y, Rufener N, Nielsen JJ, Hoier B, Krustrup P, Bangsbo J. Passive leg movement enhances interstitial VEGF protein, endothelial cell proliferation, and eNOS mRNA content in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2008;294:R975–R982. doi: 10.1152/ajpregu.00677.2007. [DOI] [PubMed] [Google Scholar]
- Høier B, Rufener N, Bojsen-Møller J, Bangsbo J, Hellsten Y. The effect of passive movement training on angiogenic factors and capillary growth in human skeletal muscle. J Physiol. 2010;588:3833–3845. doi: 10.1113/jphysiol.2010.190439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson PA, Veneman T, Nurjhan N, Gerich J. An improved method to calculate adipose tissue interstitial substrate recovery for microdialysis studies. Life Sci. 1994;54:1621–1624. doi: 10.1016/0024-3205(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Lüscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Vasodilatory responsiveness to adenosine triphosphate in ageing humans. J Physiol. 2010;588:4017–4027. doi: 10.1113/jphysiol.2010.197814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman M, Thijssen DHJ, de Groot PCE, Bleeker MWP, van Kuppevelt HJM, Green DJ, Rongen GA, Smits P, Hopman MTE. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J Physiol. 2008;586:1137–1145. doi: 10.1113/jphysiol.2007.145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P, Hellsten Y, Bangsbo J. Intense interval training enhances human skeletal muscle oxygen uptake in the initial phase of dynamic exercise at high but not at low intensities. J Physiol. 2004;559:335–345. doi: 10.1113/jphysiol.2004.062232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel J, Hayman MA, Ives S, Fjeldstad AS, Trinity JD, Wray DW, Richardson RS. Attenuated exercise induced hyperaemia with age: mechanistic insight from passive limb movement. J Physiol. 2010;588:4507–4517. doi: 10.1113/jphysiol.2010.198770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, González-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol. 2007;581:853–861. doi: 10.1113/jphysiol.2006.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Gonzalez-Alonso J, Bune LT, Saltin B, Pilegaard H, Hellsten Y. ATP-induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins, and adenosine. Am J Physiol Regul Integr Comp Physiol. 2009a;296:R1140–R1148. doi: 10.1152/ajpregu.90822.2008. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Nyberg M, Thaning P, Saltin B, Hellsten Y. Adenosine contributes to blood flow regulation in the exercising human leg by increasing prostaglandin and nitric oxide formation. Hypertension. 2009b;53:993–999. doi: 10.1161/HYPERTENSIONAHA.109.130880. [DOI] [PubMed] [Google Scholar]
- Newcomer SC, Leuenberger UA, Hogeman CS, Handly BD, Proctor DN. Different vasodilator responses of human arms and legs. J Physiol. 2004;556:1001–1011. doi: 10.1113/jphysiol.2003.059717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension. 1986;8:37–44. doi: 10.1161/01.hyp.8.1.37. [DOI] [PubMed] [Google Scholar]
- Pyke KE, Dwyer EM, Tschakovsky ME. Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J Appl Physiol. 2004;97:499–508. doi: 10.1152/japplphysiol.01245.2003. [DOI] [PubMed] [Google Scholar]
- Rådegran G, Saltin B. Muscle blood flow at onset of dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 1998;274:H314–H322. doi: 10.1152/ajpheart.1998.274.1.H314. [DOI] [PubMed] [Google Scholar]
- Rådegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol Heart Circ Physiol. 1999;276:H1951–H1960. doi: 10.1152/ajpheart.1999.276.6.H1951. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Cardiovascular Control. Ann Intern Med. 1993;119:1234–123b. [Google Scholar]
- Scheller D, Kolb J. The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialysate samples. J Neurosci Methods. 1991;40:31–38. doi: 10.1016/0165-0270(91)90114-f. [DOI] [PubMed] [Google Scholar]
- Shipley RD, Kim SJ, Muller-Delp JM. Time course of flow-induced vasodilation in skeletal muscle: contributions of dilator and constrictor mechanisms. Am J Physiol Heart Circ Physiol. 2005;288:H1499–H1507. doi: 10.1152/ajpheart.00489.2004. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Fasolo CB, Sudano I, Salvetti A. Hypertension causes premature aging of endothelial function in humans. Hypertension. 1997;29:736–743. doi: 10.1161/01.hyp.29.3.736. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- Tendera M, Aboyans V, Bartelink ML, Baumgartner I, Clément D, Collet JP, Cremonesi A, De Carlo M, Erbel R, Fowkes FG, Heras M, Kownator S, Minar E, Ostergren J, Poldermans D, Riambau V, Roffi M, Röther J, Sievert H, van Sambeek M, Zeller T. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases. Eur Heart J. 2011;32:2851–2906. doi: 10.1093/eurheartj/ehr211. [DOI] [PubMed] [Google Scholar]
- Thijssen DHJ, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011a;300:H2–H12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen DHJ, Rowley N, Padilla J, Simmons GH, Laughlin MH, Whyte G, Cable NT, Green DJ. Relationship between upper and lower limb conduit artery vasodilator function in humans. J Appl Physiol. 2011b;111:244–250. doi: 10.1152/japplphysiol.00290.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooke JE. Microvascular function in human diabetes. A physiological perspective. Diabetes. 1995;44:721–726. doi: 10.2337/diab.44.7.721. [DOI] [PubMed] [Google Scholar]
- Trinity JD, Amann M, McDaniel J, Fjeldstad AS, Barrett-O’Keefe Z, Runnels S, Morgan DE, Wray DW, Richardson RS. Limb movement-induced hyperemia has a central hemodynamic component: evidence from a neural blockade study. Am J Physiol Heart Circ Physiol. 2010;299:H1693–H1700. doi: 10.1152/ajpheart.00482.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschakovsky ME, Pyke KE. Counterpoint: Flow-mediated dilation does not reflect nitric oxide-mediated endothelial function. J Appl Physiol. 2005;99:1235–1237. doi: 10.1152/japplphysiol.00607.2005. [DOI] [PubMed] [Google Scholar]
- Venturelli M, Amann MK, McDaniel J, Trinity JD, Fjeldstad AS, Richardson RS. Central and peripheral hemodynamic responses to passive-limb movement: the role of arousal. Am J Physiol Heart Circ Physiol. 2011;302:H333–339. doi: 10.1152/ajpheart.00851.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- Wray DW, Donato AJ, Uberoi A, Merlone JP, Richardson RS. Onset exercise hyperaemia in humans: partitioning the contributors. J Physiol. 2005;565:1053–1060. doi: 10.1113/jphysiol.2005.084327. [DOI] [PMC free article] [PubMed] [Google Scholar]


