Abstract
The nucellus is a complex maternal grain tissue that embeds and feeds the developing cereal endosperm and embryo. Differential screening of a barley (Hordeum vulgare) cDNA library from 5-d-old ovaries resulted in the isolation of two cDNA clones encoding nucellus-specific homologs of the vacuolar-processing enzyme of castor bean (Ricinus communis). Based on the sequence of these barley clones, which are called nucellains, a homolog from developing corn (Zea mays) grains was also identified. In dicots the vacuolar-processing enzyme is believed to be involved in the processing of vacuolar storage proteins. RNA-blot and in situ-hybridization analyses detected nucellain transcripts in autolysing nucellus parenchyma cells, in the nucellar projection, and in the nucellar epidermis. No nucellain transcripts were detected in the highly vacuolate endosperm or in the other maternal tissues of developing grains such as the testa or the pericarp. Using an antibody raised against castor bean vacuolar-processing protease, a single polypeptide was recognized in protein extracts from barley grains. Immunogold-labeling experiments with this antibody localized the nucellain epitope not in the vacuoles, but in the cell walls of all nucellar cell types. We propose that nucellain plays a role in processing and/or turnover of cell wall proteins in developing cereal grains.
The grass caryopsis, or grain, is a one-seeded fruit containing a well-developed embryo within a copious endosperm in which the seed coat or testa is adnate to the surrounding pericarp. Major events in the developmental pathway from ovule to grain are well documented: embryo-sac formation (Bouman, 1984), fertilization (Cass and Jensen, 1970), embryogenesis (Engell, 1989), and endosperm development (Bosnes et al., 1992; Lopes and Larkins, 1993; Brown et al., 1994; Olsen et al., 1995).
The nucellus, a maternal tissue immediately surrounding the central cell, is often neglected in studies of angiosperm reproduction, with most investigators concentrating instead on the more dynamic aspects of embryo and endosperm development. In addition to supplying nutrients, intercellular contacts between the ovule and megagametophyte may be important for embryo-sac differentiation (Willemse and van Went, 1984). Support for the importance of the nucellus in seed development has recently been confirmed by the isolation of several female-sterile mutants of Arabidopsis, making possible a preliminary genetic dissection of the pathways that regulate ovule and embryosac development (Robinson-Beers et al., 1992; Reiser and Fischer, 1993).
In the cereals the nucellus consists of three main cell types: the nucellus parenchyma cells, the nucellus epidermis, and the nucellar projection. Starting before fertilization and lasting until approximately 5 DAP, the nucellus parenchyma cells of barley (Hordeum vulgare) undergo complete autolysis (Norstog, 1974). After this stage the nucellar epidermis differentiates and persists throughout most of seed development, finally autolysing and forming the hyaline layer (Duffus and Cochrane, 1992). Concomitant with the development of the nucellar epidermis, the nucellus cells in the ventral crease of the barley grain differentiate into the nucellar projection. The nucellar projection is the terminal maternal tissue in a route along which nutrients are transported from the vascular tissue of the pericarp to the developing endosperm and embryo (Cochrane and Duffus, 1979, 1980). In wheat the differentiation into transfer cells occurs as a continuum from the base of the nucellar projection to the endosperm cavity (Wang et al., 1994). Similar studies of the nucellar projection have thus far been lacking for barley.
Molecular studies of the ovule, including the nucellus, are few. Factors contributing to this situation include the rapidity of ovule development, the position of the nucellus within the ovary (which makes isolation difficult and time consuming), and the small amount of tissue that can be isolated at any given stage. The isolation of corn (Zea mays) embryo sacs was reported for the first time within the last decade (Wagner et al., 1989; Mol et al., 1993). To our knowledge, differential screening experiments based on isolated ovules have so far been reported only for petunia (Decroocq-Ferrant et al., 1995) and the orchid Phalaenopsis (Nadeau et al., 1996), in which the prolonged synchronous development of large numbers of ovules made such studies feasible.
This report describes cDNA clones from barley and corn that encode nucellain, the monocot homolog of a dicot VPE (Hara-Nishimura et al., 1993b). The first dicot VPE isolated from developing castor bean (Ricinus communis) and soybean seeds showed significant similarity to hemoglobinase from the blood fluke Schistosoma mansoni (Klinkert et al., 1989; Hara-Nishimura et al., 1993b; Shimada et al., 1994). The term VPE was coined by Hara-Nishimura and co-workers (Hara-Nishimura et al., 1993b; Hiraiwa et al., 1993) after the demonstration by transmission electron microscopic immunogold labeling that the VPE antigen is present in the protein storage vacuoles of castor bean. Localization to this subcellular compartment is compatible with the belief that storage-protein processing occurs in protein storage vacuoles.
Many seed-storage proteins are characteristically processed at Asn residues (Hara-Nishimura et al., 1993a; Shimada et al., 1994) and, based on the observation that VPEs have a specificity for Asn in the P1 position of the cleavage site, it is believed that these proteins play a role in seed- storage-protein processing and in the mobilization of nitrogen reserves during seed germination (Hara-Nishimura and Nishimura, 1987; Hara-Nishimura et al., 1991, 1993b; Shimada et al., 1994). Subsequent to the discovery of the castor bean VPE, the term has been adopted to designate other enzymes with high sequence identity to the castor bean enzyme, although data regarding subcellular localization is lacking. Recently, this group of proteins has been numbered EC 3.4.22.34 and forms the C13 family of Cys proteinases, the legumains. Although problematic (see below), we use the term VPE for nucellain throughout this paper.
cDNA clones for VPEs have been reported from a variety of dicot nonseed tissues, including hypocotyls, roots, leaves, stems, buds, and flowers (Hiraiwa et al., 1993; Kinoshita et al., 1995a). The specificity of proteases from nonseed tissues is unclear. Recently, homologs of VPEs have also been characterized from yeast (Benghezal et al., 1996) and human (Chen et al., 1997) sources. The yeast homolog is not a VPE, but is anchored to the ER membrane by a membrane-spanning C-terminal domain, where it appears to be involved in the transaminidation of glycosylphosphatidylinositol-anchored membrane protein precursors to the glycosylphosphatidylinositol glycolipid. Recently, Chen and Foolad (1997) reported the isolation of cDNAs and the corresponding gene encoding a putative aspartic protease homolog, termed nucellin, which is differentially expressed in degrading nucellar tissues in a pattern similar to that of nucellain.
To our knowledge, the nucellains reported here represent the first monocot grain homologs of the dicot VPEs. Unlike the castor bean enzyme, nucellain is localized in maternal nucellar tissues, excluding a role in endosperm or embryo storage-protein processing. Furthermore, using immunogold-labeling experiments with an antibody recognizing the castor bean VPE, an epitope was recognized not in vacuoles, but in cell walls. No labeling was detectable in the abundant nucellar vacuoles or in vacuoles or cell walls of other maternal seed tissues.
MATERIALS AND METHODS
Barley (Hordeum vulgare L. cv Bomi) was grown under controlled environmental conditions, with 16-h light periods at 15°C and 8-h dark periods at 10°C, as described previously (Kalla et al., 1994). Hand-pollinated grains were harvested at the appropriate developmental stages, rapidly frozen in liquid nitrogen, and stored at −80°C. Individual 5-DAP ovaries were thawed for manual separation of the pericarp (negative probe) and the embryo sac with adhering nucellus cell layers (positive probe). After dissection, both tissue fractions were rapidly refrozen and stored at −80°C. Material for northern-blot analysis was harvested at the appropriate stages, hand dissected, refrozen in liquid nitrogen, and stored at −80°C.
Isolation of cDNA Clones
Two barley nucellain cDNA clones, HvNP1 and HvNP2, were isolated by differential screening of a cDNA library of 5-DAP intact ovaries, as described by Doan et al. (1996). Positive and negative probes for the differential screening experiment were made from total RNA extracted from embryo sacs with adhering testa and nucellus and pericarp, respectively. Differential screening was performed by sequential hybridization of duplicate filters with pericarp- and ovule-specific probes. Individual plaques that hybridized exclusively with the positive probe were chosen and rescreened using the same probes. Confirmed positive-hybridizing phages were excised and converted into pBluescript (Stratagene) recombinants using R408 helper phage according to the manufacturer's protocol. For further experimental details, see Doan et al. (1996).
The putative corn (Zea mays cv Pioneer) nucellain cDNA homolog ZmNP1, representing a full-length sequence of 1920 bp from a cDNA library of 5-DAP whole grains, was identified in the corn EST database based on sequence homology to barley HvNP1.
During the preparation of this paper, a 414-bp EST similar to ZmNP1 was published (Smart et al., 1995). The accession number of this sequence is A43551.
In Situ Hybridization
Localization of nucellain mRNA corresponding to the cDNA clone HvNP1 was demonstrated by in situ hybridization. Tissues younger than 10 DAP were fixed in 3.7% formaldehyde, 5% acetic acid, and 50% ethanol. For older tissues the fixative was 1% glutaraldehyde and 100 mm sodium phosphate buffer, pH 7.0. Dehydration of fixed tissue was through an ethanol and tert-butyl alcohol series, and embedding was in Histowax (Leica). Sections were cut 15 to 18 μm thick and mounted on glass slides coated with poly-l-Lys hydrobromide (Sigma). Mounted sections were deparaffinized with xylene and rehydrated through an ethanol series. Sections were incubated sequentially at room temperature in 200 mm sodium phosphate buffer, pH 7.0, for 5 min, in predigested pronase (0.25 mg/mL predigested pronase in 50 mm Tris-HCl, pH 7.5, and 5 mm EDTA) for 10 min, and were then postfixed in 1% glutaraldehyde and 100 mm sodium phosphate buffer, pH 7.0, for 20 min. The sections were then dehydrated using increasing ethanol concentrations.
RNA probes were made using the MAXIscript kit (Ambion, Austin, TX) and SmaI (antisense)- or XhoI (sense)-digested HvNP1 DNA in the presence of [33P]UTP (BT1002, Amersham). Nonincorporated ribonucleotides were removed by filtration through a Sephadex G-50 (fine) column and probes were subjected to carbonate hydrolysis to reduce probe length to approximately 100 nucleotides.
For 12-h in situ hybridizations at 50°C, 200 ng of RNA probe was used per milliliter of hybridization mixture containing 50% deionized formamide, 10% dextran sulfate, 0.3 m NaCl, 10 mm Tris, 1 mm EDTA, 1× Denhardt's solution, 1 mg/mL tRNA, and 0.5 mg/mL poly(A+) RNA. For removal of excess probe and nonspecifically bound RNA, the slides were washed in the following solutions: 1× SSC and 50% formamide three times for 1 h each at 50°C; 1× SSC for 5 min at room temperature (approximately 20°C); 20 mg of RnaseA per milliliter of 0.5 m NaCl, 10 mm Tris, pH 8.0, 1 mm EDTA for 30 min at 37°C; 1× SSC and 50% formamide three times for 1 h each at 50°C; and 1× SSC twice for 20 min each at room temperature. The slides were dehydrated using increasing ethanol concentrations. For microautoradiography, slides were dipped in nuclear track emulsion (NTB 2, Kodak) diluted 1:1 in 0.6 m ammonium acetate, pH 7.0. The slides were developed after 6 to 7 weeks of exposure. Images were recorded using a microscope (Axioplan, Zeiss) with a camera (model MC100, Zeiss) and Kodak EPY64T film. Sense-probe control experiments were negative at all stages investigated.
Northern-Blot Analysis
Poly(A+)-rich RNA from various grain and vegetative tissues was isolated using magnetic oligo(dT) beads (Dynal A/S, Oslo, Norway) (Jakobsen et al., 1990). Approximately 100 ng of poly(A+)-rich RNA from each sample was separated by 1.4% agarose gel electrophoresis and transferred onto nylon membrane filters (Amersham) (Sambrook et al., 1989). Generation of single-stranded, antisense, 32P-labeled cDNA probes was according to the method of Espelund et al. (1990). Filters were hybridized at 42°C in the presence of 50% formamide, 1 m NaCl, 0.1% sodium pyrophosphate, and 0.05 m Tris-HCl, pH 7.5. Washing conditions were 2× SSC twice for 10 min each at 25°C; 2× SSC and 1% (w/v) SDS twice for 30 min each at 68°C; and 0.2× SSC twice for 30 min each at 25°C. Filters were exposed to film (Hyperfilm, Amersham) for 1 to 3 d. The probe used was the same as for the in situ-hybridization experiments.
Western-Blot Analysis
Protein from developing barley and castor bean (Ricinus communis) seeds was extracted in SDS sample buffer containing 5% SDS and Tris, pH 8.0. Samples of 10 μg of protein were subjected to SDS-PAGE in precast 10% to 20% gels (Bio-Rad) in Laemmli buffer and blotted onto a PVDF membrane (Immobilon P, Millipore) using cleaved-amplified polymorphic sequence buffer (Matsudaira, 1987) in a semidry blotter apparatus (Hoefer Scientific Instruments/Pharmacia). Primary antibody was the castor bean anti-VPE antibody RDaPE (1:1000 dilution), which was kindly provided by Dr. I. Hara-Nishimura.
Transmission Electron Microscopy
Grains grown under the conditions described above were collected at regular intervals after hand pollination, sliced approximately in one-half along the proximal distal axis, fixed in 4% glutaraldehyde in 0.1 m phosphate buffer, pH 6.9, postfixed in osmium ferricyanide (Hepler, 1981), dehydrated in a graded acetone series, and infiltrated with Spurr's resin (all at room temperature). Thin sections (approximately 0.5 μm) were stained with methylene-blue borax for study with transmitted light microscopy (Postek and Tucker, 1976). Ultrathin sections were stained in 7.5% aqueous uranyl magnesium acetate followed by lead citrate and studied with a transmission electron microscope (model H-600, Hitachi, Tokyo, Japan).
Immunogold Labeling
For immunogold labeling, 3- to 5-DAP grains were fixed in 4% glutaraldehyde in 0.1 m phosphate buffer, pH 6.9. Samples were dehydrated in graded series of ethanol and embedded in London White resin (London Resin Co. Ltd., London, UK). Thin sections (approximately 90 nm) were colleced on coated nickel grids, blocked with 5% goat serum in PBS, and incubated with VPE antibody from castor bean (Hara-Nishimura et al., 1993b) at a 1:45 dilution for 1 h at room temperature. After a wash in PBS, sections were incubated with goat anti-rabbit IgG conjugated to 10 nm gold diluted 1:45 for 1 h at room temperature. After washing in buffer and distilled water, sections were either poststained in uranyl acetate or viewed unstained. Controls in which the primary antibody was either omitted or replaced with an inappropriate primary antibody resulted in no specific staining.
RESULTS
Isolation of Barley cDNAs for Nucellain, a Homolog of Dicot VPEs
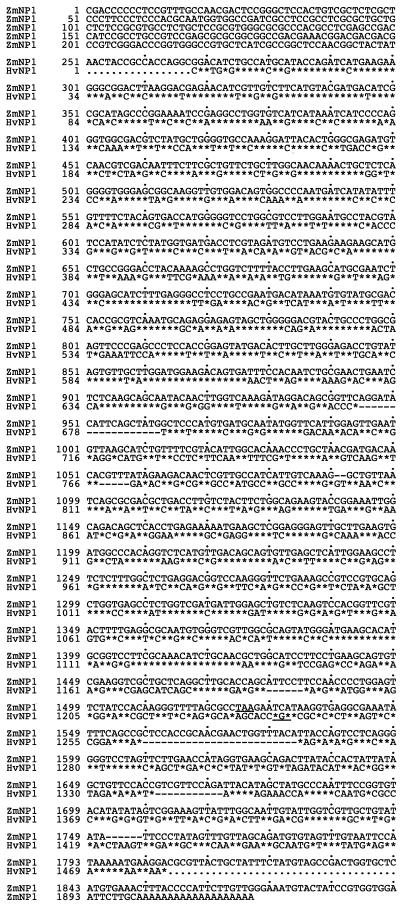
In the differential screening experiment of a 5-DAP intact barley grain cDNA library carried out by Doan et al. (1996), the cDNA clones HvNP1 and HvNP2 hybridized exclusively to the positive probe, indicating that they were differentially expressed in the embryo sac, the nucellar tissues, or both. (For anatomical details of the tissue used in the differential screening experiment, see Doan et al., 1996.) Alignment of the sequences to databases in the public domain identified HvNP1 (Fig. 1) and HvNP2 (sequence not shown) as homologs of dicot VPEs, the derived HvNP1 peptide (Fig. 2a) being 61% identical to the castor bean enzyme. The nucleotide sequences of HvNP1 and HvNP2 were very similar: 96% identical in the predicted open reading frame and 86% identical in the 3′-untranslated region (data not shown). Because of the localization of the HvNP transcripts strictly in nucellar tissues (see below), we designated the predicted proteins as nucellains.
Figure 1.
Nucleotide sequences of the barley nucellain HvNP1 cDNA clone and its homolog from corn, ZmNP1. Identical nucleotides are indicated by asterisks, spaces are indicated by dashes, and stop codons are underlined. Both sequences represent polyadenylated transcripts.
Figure 2.
Alignment and structure of monocot nucellain and other class C13 Cys proteases. a, Alignment of the derived amino acid sequences of barley (hvnp1; accession no. AF082346; this paper) and corn nucellain (zmnp1; accession no. AF082347; this paper); VPEs from the dicots Citrus sinensis (cscysprns; accession no. Z47793; Alonso and Granell, 1995) and castor bean (vpe ricco; accession no. D17401; Hara-Nishimura et al., 1993b); and proteases from blood fluke hemoglobinase (hglb schja; accession no. X70967; Merckelbach et al., 1994), human (hslegumain; accession no. Y09862; Chen et al., 1997), and Saccharomyces cerevisiae (scgpi8; accession no. U32517; Benghezal et al., 1996). Putative sites for proteolytic cleavage of propeptides are indicated by arrowheads (Hara-Nishimura et al., 1993b; Shimada et al., 1994). b, Common domain structure of nucellain and dicot VPEs. Note that three proteolytic events function to remove the N-terminal signal sequence and the N- and C-terminal propeptides. c, Cladogram of monocot nucellain, dicot VPEs, and homologs from highly divergent species based on the alignment shown in part a using software from the Genetics Computer Group (Madison, WI).
Developing Corn Grains Express a Putative Homolog of the Barley Nucellain
To investigate the presence of nucellains in other cereals, the corn EST database was searched using the HvNP1 sequence, which identified the ZmNP1 cDNA. This full-length sequence was isolated from a cDNA library of 5-DAP whole grains and was 70% identical to HvNP1 (Fig. 1). In addition to ZmNP1, several incomplete cDNAs representing the same transcript were also present in cDNA libraries of 5- and 9-DAP grains. RNA-blot analysis from dissected grain tissues using the 3′-untranslated region of the ZmNP1 cDNA as a probe showed that the transcript was present in the maternal tissues and absent from the endosperm and embryo, supporting the conclusion that this sequence encodes a putative corn homolog of barley nucellain. As shown in Figure 2b, the domain structure of the predicted corn nucellain was similar to that of the dicot VPEs, with an identifiable signal peptide in the N terminus and a probable signal peptide cleavage site.
Monocot Nucellains and Dicot VPEs Do Not Represent Evolutionarily Diverged Subgroups
Members of the hemoglobinase protein superfamily were subjected to phylogenetic analysis to elucidate sequence heterogeneity. As shown in Figure 2c, the plant sequences branched off from the blood fluke sequences at an early point in time, forming a separate branch in the cladogram. The cereal nucellains do not form a subgroup separate from the dicot VPEs, confirming that all plant sequences of this protein family are highly conserved.
Nucellain Transcripts Are Preferentially Expressed in All Nucellar Cell Types of Barley Grains
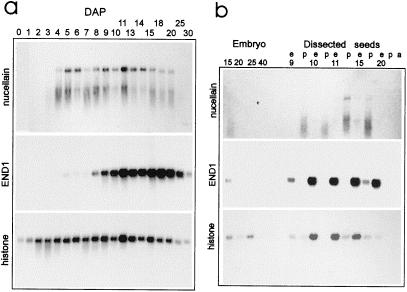
RNA-blot experiments using poly(A+) RNA of intact barley ovaries 0 to 30 DAP showed that the nucellain transcripts were first detectable in the developing grain at 4 DAP, increased until 6 DAP, and persisted at a relatively high level in the seed until 20 DAP (Fig. 3). Five days later, HvNP mRNA was undetectable. As is apparent from both nucellain northern blots in Figure 3, HvNP transcripts were highly prone to degradation, an effect not seen with the control probes END1 and histone H3 in the same blot. Using poly(A+)-rich RNA in northern-blot analysis from hand-dissected material, we demonstrated that the nucellain transcripts were present in the pericarp fraction of 10-, 11-, and 15-DAP grains and absent from extruded endosperm (Fig. 3b). A weak signal was detectable in the endosperm fraction at 15 DAP, most likely the result of contamination with nucellar tissue, an interpretation supported by the presence of the endosperm-specific END1 transcript in the 15-DAP pericarp lane (Fig. 3b).
Figure 3.
RNA-blot analysis of barley HvNP transcripts in intact grains and dissected grain tissues. a, Hybridization of HvNP1 probe (upper panel) to poly(A+) (100 ng per lane) of intact grains harvested between 0 and 30 DAP detects nucellain transcripts of approximately 1900 nucleotides. As controls, the same blot was probed with END1 cDNA, hybridizing to an endosperm-specific transcript of 920 nucleotides (Doan et al., 1996), and histone H3 cDNA, hybridizing to a constitutively expressed transcript of around 900 nucleotides. b, Hybridization of single-stranded nucellain HvNP1 probe to poly(A+) RNA (100 ng per lane) of isolated embryos, extruded endosperm (e), pericarp with adhering nucellar tissues (p), and 20-DAP aleurone layers (a). Control probes are the same as for blot a; for size of hybridizing bands, see legend for a.
We inferred that the HvNP signal in 20-DAP embryos was the result of cross-contamination from the nucellar epidermis adjacent to the embryo (see below). The presence of the nearly constitutively expressed histone H3 transcript in the endosperm fraction was caused by cell division being more frequent in this tissue than in the pericarp after 10 DAP. As shown by Kalla et al. (1994), manual extrusion of the endosperm in the interval between 10 and 15 DAP leaves the aleurone layer and the nucellar tissues attached to the pericarp. This observation, combined with the fact that the HvNP cDNAs hybridized exclusively to the positive probe in the differential screening experiment, led us to conclude that nucellain transcripts are highly enriched in the nucellar tissues. No HvNP transcript was detectable using poly(A+)-rich RNA from leaf, stem, root, stigma, and germinating grains (data not shown).
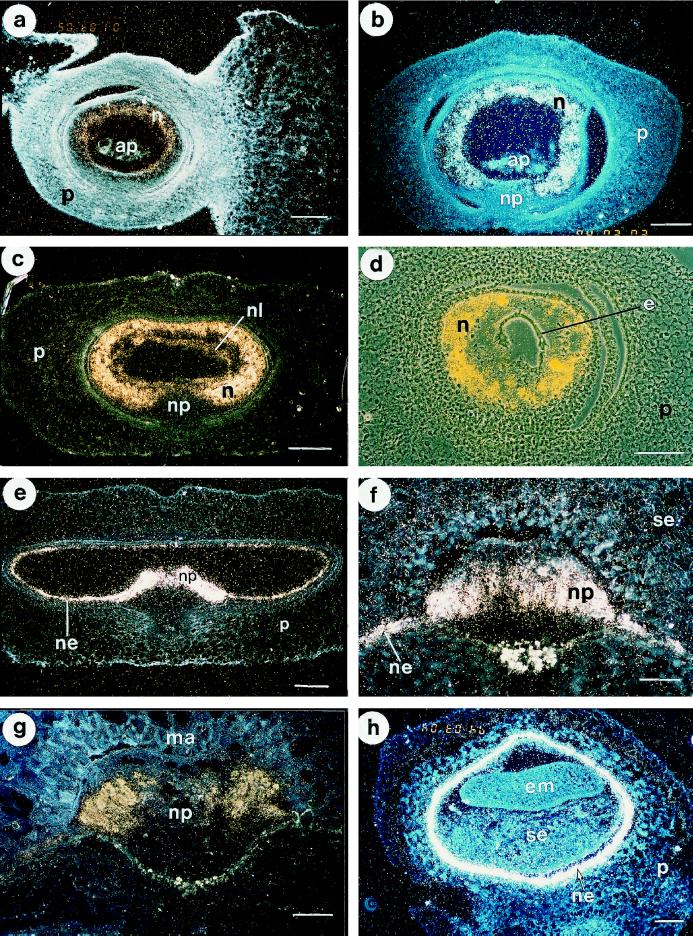
To obtain information on HvNP expression at the cellular level, in situ-hybridization experiments were carried out on transverse sections of barley grains from 0 to 30 DAP (Fig. 4). Anatomical studies of thin plastic sections were carried out to facilitate identification of individual cell types (Fig. 5). In situ-hybridization analysis showed that HvNP transcripts were first detectable in the nucellus of unfertilized ovules (Fig. 4a). At this stage the level of expression seemed to be higher toward the inner layers of the nucellus, where autolysis is initiated. No accumulation of silver grains over background level was detectable in any other tissue. At 2 DAP the nucellus retained a considerable degree of cellular integrity, consisting of 5 to 10 cell layers (Fig. 5a). Two days after fertilization, HvNP transcripts were present in the entire bulk of nucellar parenchyma cells, whereas no nucellain transcript was detectable in the antipodal cells or in the nucellar projection (Fig. 4b).
Figure 4.
In situ-hybridization analysis of HvNP1 expression in barley grain at different developmental stages. For anatomical details, see Figure 5. a, Longitudinal section of unfertilized barley ovary. HvNP transcripts are differentially expressed in the autolysing nucellus parenchyma cells. b, Cross-section of 2-DAP barley grain showing expression of HvNP in nucellar parenchyma cells. No signal above background was detectable in the nucellar projection, testa, cross-cells, pericarp, or antipodal cells. c, Cross-section of 4-DAP barley grain showing the presence of HvNP mRNA in nucellus parenchyma cells. Hybridization signals are also detected in the nucellar lysate immediately surrounding the endosperm coenocyte. d, Cross-section toward the distal end of the 4-DAP grain in c, showing that the endosperm coenocyte is void of HvNP transcript. This micrograph is a double exposure of a phase-contrast photograph and a dark-field micrograph using a yellow filter. e, Cross-section of 6-DAP grain showing localization of HvNP transcripts in the remaining autolysing nucellus parenchyma cells and the nucellar epidermis. f, Nucellar projection of barley grain at 10 DAP showing the presence of HvNP mRNA in the nucellar epidermis and periphery through the mid-part of the nucellar projection. g, HvNP expression detected in the lateral lobes of the nucellar projection of a 15-DAP grain. h, Transverse section of the proximal region of an 18-DAP grain demonstrating the presence of HvNP transcripts in the nucellar epidermis. Bars in a to d = 200 μm; bars in f to h = 100 μm. n, Nucellus parenchyma cells; np, nucellar projection; ne, nucellar epidermis; nl, nucellar lysate; p, pericarp; e, endosperm coenocyte; ap, antipodal cells; se, starchy endosperm; em, embryo; ma, modified aleurone cells.
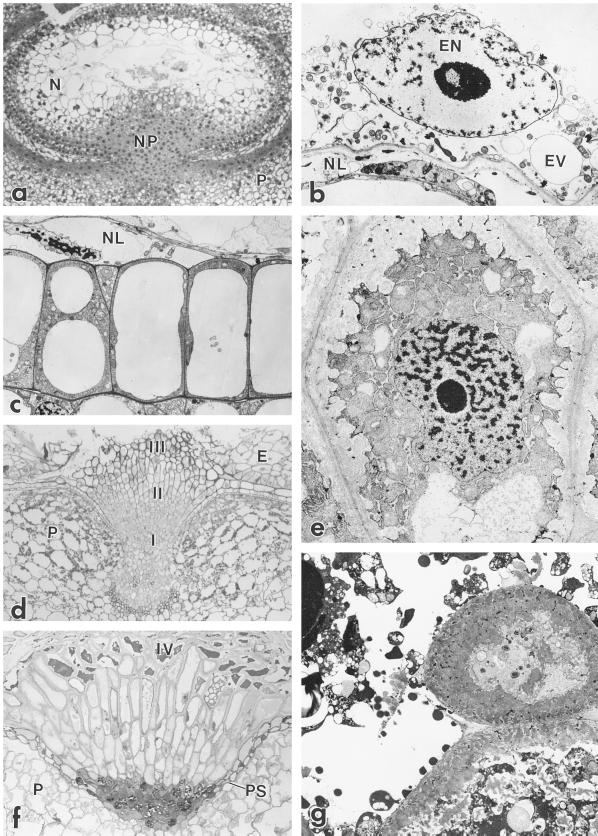
Figure 5.
Ultrastructure of the developing nucellus. a, Light micrograph showing cross-section through a 2-DAP barley grain. Several layers of nucellar parenchyma cells (N) remain intact at this stage. Degradation of the nucellar parenchyma cells starts next to the coenocytic endosperm. At 2 DAP the nucellar projection (NP) largely consists of undifferentiated meristematic cells above the ventral part of the pericarp (P). Magnification, ×55. b, Details of the autolysing nucellar parenchyma cells (NL) beneath the endosperm coenocyte at 4 DAP. EN, Endosperm nucleus; EV, endosperm vacuole. Magnification, ×2410. c, Transmission electron micrograph showing details of the highly vacuolate nucellar epidermis at 6 DAP. Magnification, ×2528. d, Light micrograph showing differentiation of types I, II, and III nucellar projection cells of 9-DAP grain with cellularized endosperm (E). Magnification, ×55. e, Type III nucellar projection cell showing extensive labyrinthine walls with thin strands of cytoplasm trapped in the wall matrix. Magnification, ×3160. f, Light micrograph of 15-DAP nucellar projection. All cell types are elongate. Type IV cells are autolysed with dense amorphous contents. A pigment strand (PS) separates the nucellar projection from the pericarp. Magnification, ×55. g, Type IV cells showing extensive labyrinthine walls and autolysing contents. Magnification, ×2528.
Two days later, at around 4 DAP, the endosperm expanded rapidly while the nucellus continued to break down. At this time, the nucellus contained a high level of HvNP transcripts and no signal was detectable in the endosperm (Fig. 4, c and d). As the walls gradually disappeared, a heterogeneous nucellar lysate was conspicuous immediately outside of the endosperm (Fig. 5b). HvNP expression was detectable 4 d earlier by in situ-hybridization analysis than by northern-blot analysis. In our interpretation, the lack of nucellain mRNA between 0 and 3 DAP was caused by the low relative proportion of the HvNP transcripts in the young grains, as is apparent from Figure 4a.
After the degradation of most of the peripheral nucellar parenchyma cells at 5 DAP, HvNP expression was detected in the nucellar epidermis and the persistent wings of nucellar parenchyma on either side of the nucellar projection (Fig. 4e). Shortly before this stage, the cells of the nucellar epidermis became highly vacuolated (Fig. 5c), a condition that lasted until grain maturation. High levels of HvNP expression persisted in the epidermis nearly 2 weeks later (Fig. 4h), when these cells appeared almost empty, and HvNP mRNA could no longer be detected in mature grains. Remnants of the nucellar epidermis appear as an unpigmented layer of wall material referred to as the hyaline layer (Duffus and Cochrane, 1992).
By 9 DAP, cells in the nucellar projection had become differentiated, and the nucellar projection was composed of about 18 to 20 rows of cells that exhibited a zonation along the radial axis (Fig. 5d). Densely cytoplasmic and isodiametric cells (type I) were found near the base of the nucellar projection, adjacent to the vascular tissue. Cells in the middle zone of the nucellar projection were radially elongate (type II), and cells in the zone adjacent to the endosperm cavity were more cuboidal, with conspicuously thickened cell walls (type III). These cells developed pronounced, flange-like wall ingrowths, with microvillus-like projections of cytoplasm extending far into the labyrinthine wall, giving the cytoplasm a spiked appearance (Fig. 5e). At this stage HvNP expression was detected in the nucellar projection and in the nucellar epidermis (Fig. 4f). The pattern of expression was uniform throughout the nucellar projection except for the zone of undifferentiated cells adjacent to the vascular tissue.
Between 9 and 15 DAP the nucellar projection cells continued to elongate (Fig. 5f). The level of HvNP expression during the interval from 10 to 15 DAP appeared to be higher in the lateral lobes of the nucellar projection than in the central portion (Fig. 4g). At 15 DAP a pigment strand delimited the base of the nucellar projection. At this stage the type IV cell was observable, representing the autolysing cells at the extreme margin of the nucellar projection (Fig. 5g). These cells exhibited massive wall ingrowths and an osmiophilic, disorganized cytoplasm.
Immunogold Labeling Detects Nucellain in Nucellar Cell Walls
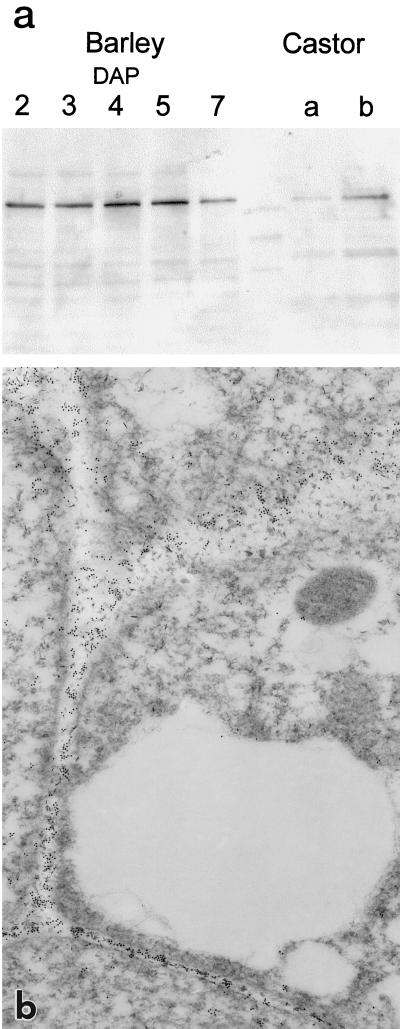
Using the antibody that localized the castor bean VPE to vacuoles (Hara-Nishimura et al., 1993b), a single band was recognized in western-blot analysis of extracts from developing barley grains (Fig. 6a). The molecular mass of this protein was similar to that of the major protein species recognized by the same antibody in extracts from castor bean seeds (37 kD). In an independent experiment the HvNP1 cDNA was expressed in Escherichia coli as a fusion protein with thioredoxin. Western-blot analysis using this protein demonstrated that it is recognized by the castor bean antibody, strongly supporting the conclusion that the protein band recognized in extracts from developing barley grains was nucellain (data not shown).
Figure 6.
The castor bean VPE antibody detects an epitope in nucellar cell walls of developing barley grains. a, Immunoblot using the castor bean VPE antibody with protein extracts from castor bean and intact barley grains harvested at different developmental stages. Lane a from castor bean contains a lower amount of protein than lane b. The polypeptide recognized in barley seeds has a molecular mass similar to that of the VPE from castor bean (37 kD). b, Immunogold electron micrograph showing that the epitope recognized by the castor bean VPE antibody is concentrated throughout the walls of the nucellar projection cells but is absent from the vacuole. Magnification, ×22,910.
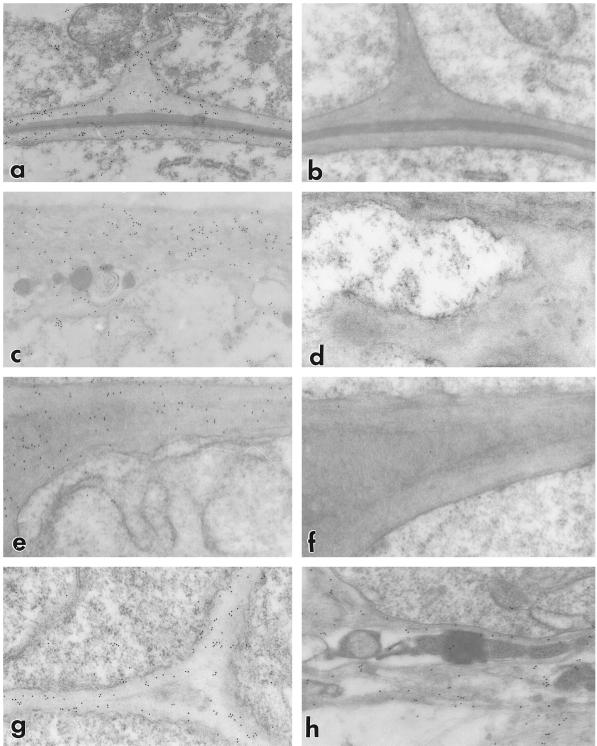
The castor bean antibody was therefore used in immunogold-labeling experiments in the transmission electron microscope with sections from barley ovaries (Figs. 6b and 7). In these experiments the VPE antibody localized the epitope to cell walls, not to vacuoles (Fig. 6b). In the nucellar epidermis (Fig. 7, a and b) the antibody recognized all cell walls, whereas in the nucellar projection the VPE antibody only detected the epitope in type II and III cells (Fig. 7, c–g). No significant variation was seen in the amount of label in the various wall types, including the transfer or labyrinthine walls of the nucellar projection (Fig. 7, c and d) and the lysate resulting from cell degradation (Fig. 7h). No specific localization of the protein was detectable in the cytoplasm or in cell walls of adjacent tissues.
Figure 7.
The walls of all barley nucellar cell types contain an epitope that is recognized by the antibody that was raised against the castor bean VPE. Magnification, ×28,500 for all micrographs. a, Walls of the persistent nucellar epidermis are labeled. This micrograph shows portions of the outer periclinal and radial wall. b, Controls in which an inappropriate primary antibody was substituted were not labeled. c, Immunogold distribution follows transfer wall ingrowths. d, Control preparation of transfer-type walls. e, Thickened wall of nucellar projection cell in zone II showing wall-specific labeling. f, Control showing the absence of label. g, Thin wall of type I nucellar projection cells in 9-DAP grains exhibit specific labeling. h, The fibrillar component (thought to be derived from degrading walls) of the nucellar lysate below the modified aleurone is labeled by the anti-VPE antibody.
DISCUSSION
Sequence alignment between the predicted nucellain from barley and corn reveals high similarity to the VPE from castor bean (Hara-Nishimura et al., 1993b) (Fig. 2a). Interestingly, nucellain represents the second protease isolated recently from the barley nucellus, suggesting that a vacuolar processing type of activity as well as a putative aspartic protease are active during the process of nucellus autolysis. Subsequent to the discovery of castor bean VPE, members of the dicot VPE family were shown to fall into two main groups, depending on whether they were localized in seeds or in vegetative tissues (Kinoshita et al., 1995a, 1995b). Although very few of these enzymes have been characterized biochemically, their high degree of similarity to the blood fluke hemoglobinase suggests that they represent functional proteases. Typically, the similarity between the different members of this protein family in plants, including dicot VPE and the nucellains, is in the range of 50% to 60%. As shown in Figure 2b, both the dicot VPE and the putative corn nucellain homolog display a complex structure consisting of a transit peptide, N- and C-terminal propeptides, and the domain representing the active enzyme.
Overall, the highest degree of conservation is in the segment from the N terminus through the mid-part of the protein, including Cys and His residues known to be required for proteolytic activity in several Cys proteinases (Hussain and Lowe, 1970). These residues, which are also conserved in the predicted barley and corn sequences, may be part of the active site(s) of these enzymes. Based on the lines of evidence presented here, we conclude that HvNP1, HvNP2, and ZmNP1 represent the first monocot members of the plant protease family from grains with similarity to the hemoglobinase from the blood fluke S. mansoni. Furthermore, as shown in the cladogram in Figure 2c, the monocot and dicot family members are highly conserved, falling into the same phylogenetic class. This class diverged from the proteases of blood flukes and humans.
In the young caryopsis HvNP expression is first detectable in autolysing nucellus cells. The data shown here support the earlier description by Norstog (1974), which suggested that autolysis starts in the cell layer nearest to the endosperm and that the last cells to disappear are those close to the nucellar projection (Fig. 4e). The mechanism driving nucellus autolysis is unknown, but the process bears striking similarities to programmed cell death or apoptosis in animal cells (Cory, 1994; Martin and Green, 1995). Because proteases have been shown to play a major role in this process in animals and plants (Pennel and Lamb, 1997), the presence of proteolytic enzymes in the nucellus parenchyma cells is not surprising. Whether nucellains play a role in autolysis remains to be determined.
Barley nucellar projection cells lying between the vascular tissue and the endosperm develop transfer walls during the period of grain filling. In these cells the pattern of HvNP expression seems to correlate with the cell-maturation process, being detectable from 6 to 20 DAP. In the in situ-hybridization analyses, HvNP expression appears to be located predominantly in nucellar cell types II and III (Fig. 4, f and g), corresponding to mature transfer cells close to the endosperm cavity and the underlying, differentiating cells of the nucellar projection.
One problem that remains unresolved is the individual pattern of expression of HvNP1 and HvNP2. In the present analysis the probe used in the northern-blot and in situ analyses recognizes both types of mRNA because the differences between the two at the nucleotide level are very small. It is hoped that experiments using cDNA-specific probes will resolve whether the transcripts overlap or are uniquely distributed within the nucellar cell types.
Using the antibody raised against the castor bean VPE in barley grains, an epitope was shown to be present in the walls of all nucellar cell types in which HvNP transcripts are detectable by RNA-blot and in situ-hybridization analysis (Figs. 6 and 7). The only possible exception is the nucellar projection, in which the HvNP transcripts are present mainly in cell types II and III (Fig. 4, f and g). The apparent discrepancy between the two methods may be explained by a lower steady-state level of the transcript in type I cells that escapes detection by in situ-hybridization analysis.
The localization of nucellain to the nucellus reported here excludes a role for storage-protein processing in the seed like that proposed to occur in castor bean. Moreover, localization of nucellain in cell walls is surprising not only because of the targeting of the castor bean enzyme to vacuoles, but also based on the fact that many plant proteases are targeted to this subcellular compartment (Boller and Kende, 1979). However, targeting of the dicot VPE to vacuoles has been directly demonstrated only for the proteins from castor bean and jack bean. Whether the situation is the same for the other dicot VPEs in nonseed storage tissues such as leaves, flowers, stems, and roots remains to be determined. The subcellular localization of nucellain from other cereal species such as corn awaits further experiments. As pointed out in the introduction, the yeast homolog is anchored to the ER membrane by a membrane-spanning C-terminal domain.
The biochemical basis for the complex structural changes in the nucellus during grain development is unknown. To our knowledge, the isolation of nucellin (Chen and Foolad, 1997), nucellain, and 28 other groups of barley cDNAs in the same experiment represents the first systematic approach to elucidating the molecular biology of the grass nucellus. In addition to nucellain, two other clones representing nucellar transcripts have been characterized, NUC1 (Doan et al., 1996) and a novel extensin (Sturaro et al., 1998). Both of these probes hybridize to transcripts with a similar pattern of expression as nucellain, indicating a highly coordinated expression of several abundant transcripts in this tissue. It is interesting to note that a common feature of all three nucellus cell types is the dynamic nature of their cell walls. This may be reflected by the high level of expression of the novel extensin.
At this stage speculations about a role for nucellain in nucellus development may be premature. However, as pointed out by Varner and Lin (1989), cell wall morphology in differentiating cells often involves reorganization of cell wall structural components, the enzymes for which may be located in the cell walls themselves. One example of this is peroxidases, which are believed to cause reduction of cell wall extensibility by forming bridges between phenolic residues on neighboring cell wall proteins or polysaccharides (Kim et al., 1989). Similarly, proteinases have also been suggested to modify cell wall polypeptides during growth (Van Der Wilden et al., 1983; Varner and Lin, 1989). Therefore, we propose that nucellain plays a role in processing and/or turnover of cell wall proteins. The corn nucellain gene maps to or near the Etched-1 gene (Stadler, 1940). Reverse genetic investigations using a collection of corn plants containing a high frequency of Mutator insertions developed at Pioneer Hi-Bred International (Bensen et al., 1995) to study nucellain function in corn are currently under way.
ACKNOWLEDGMENTS
Elisabeth Bakker, Virginia Dress, Linda LeMont, Berit Morken, Hege Munck, and Peter Sekkelsten are acknowledged for their excellent technical support. The castor bean VPE antibody was a kind gift from Dr. I. Hara-Nishimura.
Abbreviations:
- DAP
days after pollination
- EST
expressed sequence tag
- VPE
vacuolar-processing enzyme
Footnotes
This work was funded in part by the Biotechnology Program of the Norwegian Research Council.
LITERATURE CITED
- Alonso JM, Granell A. A putative vacuolar processing protease is regulated by ethylene and also during fruit ripening in citrus fruit. Plant Physiol. 1995;109:541–547. doi: 10.1104/pp.109.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benghezal M, Benachour A, Rusconi S, Aebi M, Conzelmann A. Yeast Gpi8p is essential for GPI anchor attachment onto proteins. EMBO J. 1996;15:6575–6583. [PMC free article] [PubMed] [Google Scholar]
- Bensen RJ, Johal GS, Crane VC, Tossberg JT, Schnable PS, Meeley RB, Briggs SP. Cloning and characterization of the maize An1 gene. Plant Cell. 1995;7:75–84. doi: 10.1105/tpc.7.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T, Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979;63:1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnes M, Weideman F, Olsen O-A. Endosperm differentiation in barley wild-type and sex mutants. Plant J. 1992;2:661–674. [Google Scholar]
- Bouman F. The ovule. In: Johri BM, editor. Embryology of Angiosperms. Berlin: Springer-Verlag; 1984. pp. 123–158. [Google Scholar]
- Brown RC, Lemmon BE, Olsen O-A. Endosperm development in barley: microtubule involvement in the morphogenetic pathway. Plant Cell. 1994;6:1241–1252. doi: 10.1105/tpc.6.9.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass DD, Jensen WA. Fertilization in barley. Am J Bot. 1970;57:62–70. [Google Scholar]
- Chen F, Foolad MR. Molecular organization of a gene in barley which encodes a protein similar to aspartic protease and its specific expression in nucellar cells during degeneration. Plant Mol Biol. 1997;35:821–831. doi: 10.1023/a:1005833207707. [DOI] [PubMed] [Google Scholar]
- Chen JM, Dando PM, Rawlings ND, Brown MA, Young NE, Stevens RA, Hewitt E, Watts C, Barrett AJ. Cloning, isolation, and characterization of mammalian legumain, an asparaginyl endopeptidase. J Biol Chem. 1997;227:8090–8098. doi: 10.1074/jbc.272.12.8090. [DOI] [PubMed] [Google Scholar]
- Cochrane MP, Duffus CM. Morphology and ultrastructure of immature cereal grains in relation to transport. Ann Bot. 1979;44:67–72. [Google Scholar]
- Cochrane MP, Duffus CM. The nucellar projection and modified aleurone in the crease region of developing caryopses of barley (Hordeum vulgare L. var. Distichum) Protoplasma. 1980;103:361–375. [Google Scholar]
- Cory S. Apoptosis: fascinating death factor. Nature. 1994;367:317–318. doi: 10.1038/367317a0. [DOI] [PubMed] [Google Scholar]
- Decroocq-Ferrant V, Decroocq S, van Went J, Schmidt E, Kreis M. A homologue of the MAP/ERK family of protein kinase genes is expressed in vegetative and in female reproductive organs of Petunia hybrida. Plant Mol Biol. 1995;27:339–350. doi: 10.1007/BF00020188. [DOI] [PubMed] [Google Scholar]
- Doan DNP, Linnestad C, Olsen O-A. Isolation of molecular markers from the barley endosperm coenocyte and the surrounding nucellus cell layers. Plant Mol Biol. 1996;31:877–886. doi: 10.1007/BF00019474. [DOI] [PubMed] [Google Scholar]
- Duffus CM, Cochrane MP (1992) Grain structure and composition. In PR Shewry, ed, Barley: Genetics, Biochemistry, Molecular Biology and Biotechnology. CAB International, Wallingford, CT, pp 291–317
- Engell K. Embryology of barley: time course and analysis of controlled fertilization and early embryo formation after fertilization of the egg cell. Nord J Bot. 1989;9:265–280. [Google Scholar]
- Espelund M, Stacy RAP, Jakobsen KS. A simple method for generating single-stranded DNA probes labeled to high activities. Nucleic Acids Res. 1990;18:6157–6158. doi: 10.1093/nar/18.20.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Nishimura I, Inoue K, Nishimura M. A unique vacuolar processing enzyme responsible for conversion of several proprotein precursors into the mature forms. FEBS Lett. 1991;294:89–93. doi: 10.1016/0014-5793(91)81349-d. [DOI] [PubMed] [Google Scholar]
- Hara-Nishimura I, Nishimura M. Proglobulin processing enzyme in vacuoles isolated from developing pumpkin cotyledons. Plant Physiol. 1987;85:440–445. doi: 10.1104/pp.85.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Nishimura I, Takeuchi Y, Inoue K, Nishimura M. Vesicle transport and processing of the precursor to 2S albumin in pumpkin. Plant J. 1993a;4:793–800. doi: 10.1046/j.1365-313x.1993.04050793.x. [DOI] [PubMed] [Google Scholar]
- Hara-Nishimura I, Takeuchi Y, Nishimura M. Molecular characterization of a vacuolar processing enzyme related to a putative cysteine proteinase of Schistosoma mansoni. Plant Cell. 1993b;5:1651–1659. doi: 10.1105/tpc.5.11.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler PK. The structure of the endosplasmic reticulum revealed by osmium tetroxide-potassium ferricyanide staining. Eur J Cell Biol. 1981;26:102–111. [PubMed] [Google Scholar]
- Hiraiwa N, Takeuchi Y, Nishimura M, Hara-Nishimura I. A vacuolar processing enzyme in maturing and germinating seeds: its distribution and associated changes during development. Plant Cell Physiol. 1993;34:1197–1204. [Google Scholar]
- Hussain SS, Lowe GT. The amino acid sequence around the active-site cysteine and histidine residues of stem bromelain. Biochem J. 1970;117:341–346. doi: 10.1042/bj1170341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen KS, Breivold E, Hornes E. Purification of mRNA directly from crude plant tissue in 15 minutes using magnetic oligo dT microsphere. Nucleic Acids Res. 1990;18:3669. doi: 10.1093/nar/18.12.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalla R, Shimamoto K, Potter R, Nielsen PS, Linnestad C, Olsen O-A. The promoter of the barley aleurone specific gene encoding a putative 7 kDa lipid transfer protein confers aleurone specific gene expression in transgenic rice. Plant J. 1994;4:849–860. doi: 10.1046/j.1365-313x.1994.6060849.x. [DOI] [PubMed] [Google Scholar]
- Kim SH, Shinkle JR, Roux SJ. Phytochrome induces changes in the immunodetectable level of wall peroxidase that precede growth changes in maize seedlings. Proc Natl Acad Sci USA. 1989;86:9866–9870. doi: 10.1073/pnas.86.24.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Nishimura M, Hara-Nishimura I. Homologues of a vacuolar processing enzyme that are expressed in different organs in Arabidopsis thaliana. Plant Mol Biol. 1995a;29:81–89. doi: 10.1007/BF00019120. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Nishimura M, Hara-Nishimura I. The sequence and expression of the gamma-VPE gene, one member of a family of three genes for vacuolar processing enzymes in Arabidopsis thaliana. Plant Cell Physiol. 1995b;36:1555–1562. [PubMed] [Google Scholar]
- Klinkert M-Q, Felleisen R, Link G, Ruppel A, Beck E. Primary structure of Sm31/32 diagnostic proteins of Schistosoma mansoni and their identification as proteases. Mol Biochem Parasitol. 1989;33:113–122. doi: 10.1016/0166-6851(89)90025-x. [DOI] [PubMed] [Google Scholar]
- Lopes MA, Larkins B. Endosperm origin, development and function. Plant Cell. 1993;5:1383–1399. doi: 10.1105/tpc.5.10.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Green DR. Protease activation during apoptosis: death by a thousand cuts? Cell. 1995;82:349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidine difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- Merckelbach A, Hasse S, Dell R, Eschlbeck A, Ruppel A. cDNA sequences of Schistosoma japonicum coding for two cathepsin B-like proteins and Sj32. Trop Med Parasitol. 1994;45:193–198. [PubMed] [Google Scholar]
- Mol R, Matthys-Rochon E, Dumas C. In vitro culture of fertilized embryo sacs of maize: zygotes and two-celled proembryos can develop into plants. Planta. 1993;189:213–217. [Google Scholar]
- Nadeau JA, Zhang XS, Li J, O'Neill SD. Ovule development: identification of stage-specific and tissue-specific cDNAs. Plant Cell. 1996;8:213–239. doi: 10.1105/tpc.8.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norstog K. Nucellus during early embryogeny in barley: fine structure. Bot Gaz. 1974;135:97–103. [Google Scholar]
- Olsen O-A, Brown R, Lemmon BE. Pattern and process of wall formation in developing endosperm. BioEssays. 1995;17:803–812. [Google Scholar]
- Pennel RI, Lamb C. Programmed cell death in plants. Plant Cell. 1997;9:1157–1168. doi: 10.1105/tpc.9.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postek MP, Tucker SC. A new short chemical dehydration method for light microscopy preparations of plant material. Can J Bot. 1976;59:1738–1748. [Google Scholar]
- Reiser L, Fischer RL. The ovule and the embryo sac. Plant Cell. 1993;5:1291–1301. doi: 10.1105/tpc.5.10.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Beers K, Pruitt RE, Gasser CS. Ovule development in wild-type Arabidopsis and two female-sterile mutants. Plant Cell. 1992;4:1237–1249. doi: 10.1105/tpc.4.10.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Frisch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Shimada T, Hiraiwa N, Nishimura M, Hara-Nishimura I. Vacuolar processing enzyme of soybean that converts proproteins to the corresponding mature forms. Plant Cell Physiol. 1994;35:713–718. doi: 10.1093/oxfordjournals.pcp.a078648. [DOI] [PubMed] [Google Scholar]
- Smart CM, Thomas H, Hosken SE, Schuch WW, Drake CR, Grierson D, Farrell A, John I (1995) Regulation of senescence. Patent WO 9507993. Zeneca Ltd, UK
- Stadler LJ. Gene list and linkage map of corn (maize) Maize Genet Coop Newsl. 1940;14:26–27. [Google Scholar]
- Sturaro M, Linnestad C, Kleinhofs A, Olsen O-A, Doan DNP (1998) Characterization of a cDNA encoding a putative extensin from developing barley grains (Hordeum vulgare L.). J Exp Bot (in press)
- Van Der Wilden W, Seegers JHL, Chrispeels MJ. Cell walls of Phaseolus vulgaris leaves contain the azocoll digesting protease. Plant Physiol. 1983;73:576–578. doi: 10.1104/pp.73.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner J, Lin LS. Plant cell wall architecture. Cell. 1989;56:231–239. doi: 10.1016/0092-8674(89)90896-9. [DOI] [PubMed] [Google Scholar]
- Wagner VT, Song YC, Matthys-Rochon E, Dumas C. Observations on the isolated embryo sac of Zea mays L. Plant Sci. 1989;59:127–132. [Google Scholar]
- Wang HL, Offler CE, Patrick JW. Nucellar projection transfer cells in the developing wheat grain. Protoplasma. 1994;182:39–52. [Google Scholar]
- Willemse MTM, van Went JL. The female gametophyte. In: Johri BM, editor. Embryology of Angiosperms. Berlin: Springer-Verlag; 1984. pp. 159–196. [Google Scholar]