Abstract
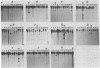
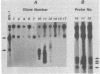
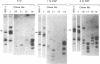
Complementary DNA probes prepared from total polysomal poly(A)+RNA populations were used to identify clones of mouse DNA containing sequences whose expression is specifically enhanced after epidermal growth factor (EGF) stimulation of quiescent mouse embryo cells in culture. Three such clones were isolated and used to study changes in the levels of clone-specific poly(A)+RNA in the polysomes of cells after mitogenic stimulation by EGF. RNA complementary to sequences present in these clones increased approximately equal to 10-fold as a fraction of the total poly(A)+RNA by 6 hr after stimulation. All three clones were found by hybridization criteria to contain sequences related to the class of mouse retrovirus or transposon-like elements termed VL30. These VL30-related sequences were further found to be complementary to EGF-inducible poly(A)+RNAs and enhanced expression was detectable as early as 1 hr after EGF stimulation. In contrast, nine additional clones, including an AKR-type murine leukemia provirus DNA clone, contained no detectable VL30 sequence elements and were complementary to poly(A)+RNA species whose relative concentration was essentially constant in quiescent and EGF-stimulated cells. Therefore, VL30 sequence elements appear distinct in that they encompass members whose expression is specifically regulated in response to a defined peptide growth factor.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Benz E. W., Jr, Getz M. J., Wells D. J., Moses H. L. Nuclear RNA polymerase activities and poly(A)-containing mRNA accumulation in cultured AKR mouse embryo cells stimulated to proliferate. Exp Cell Res. 1977 Aug;108(1):157–165. [PubMed] [Google Scholar]
- Besmer P., Olshevsky U., Baltimore D., Dolberg D., Fan H. Virus-like 30S RNA in mouse cells. J Virol. 1979 Mar;29(3):1168–1176. doi: 10.1128/jvi.29.3.1168-1176.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron J. R., Loh E. Y., Davis R. W. Evidence for transposition of dispersed repetitive DNA families in yeast. Cell. 1979 Apr;16(4):739–751. doi: 10.1016/0092-8674(79)90090-4. [DOI] [PubMed] [Google Scholar]
- Courtney M. G., Elder P. K., Steffen D. L., Getz M. J. Evidence for an early evolutionary origin and locus polymorphism of mouse VL30 DNA sequences. J Virol. 1982 Aug;43(2):511–518. doi: 10.1128/jvi.43.2.511-518.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney M. G., Schmidt L. J., Getz M. J. Organization and expression of endogenous virus-like (VL30) DNA sequences in nontransformed and chemically transformed mouse embryo cells in culture. Cancer Res. 1982 Feb;42(2):569–576. [PubMed] [Google Scholar]
- Finnegan D. J., Rubin G. M., Young M. W., Hogness D. S. Repeated gene families in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1053–1063. doi: 10.1101/sqb.1978.042.01.106. [DOI] [PubMed] [Google Scholar]
- Gates B. J., Friedkin M. Mid-G1 marker protein(s) in 3T3 mouse fibroblast cells. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4959–4961. doi: 10.1073/pnas.75.10.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz M. J., Elder P. K., Benz E. W., Jr, Stephens R. E., Moses H. L. Effect of cell proliferation on levels and diversity of poly(A)-containing mRNA. Cell. 1976 Feb;7(2):255–65. doi: 10.1016/0092-8674(76)90025-8. [DOI] [PubMed] [Google Scholar]
- Getz M. J., Reiman H. M., Jr, Siegal G. P., Quinlan T. J., Proper J., Elder P. K., Moses H. L. Gene expression in chemically transformed mouse embryo cells: selective enhancement of the expression of C type RNA tumor virus genes. Cell. 1977 Aug;11(4):909–921. doi: 10.1016/0092-8674(77)90302-6. [DOI] [PubMed] [Google Scholar]
- Hershko A., Mamont P., Shields R., Tomkins G. M. "Pleiotypic response". Nat New Biol. 1971 Aug;232(33):206–211. [PubMed] [Google Scholar]
- Highfield D. P., Dewey W. C. Inhibition of DNA synthesis in synchronized Chinese hamster cells treated in G1 or early S phase with cycloheximide or puromycin. Exp Cell Res. 1972 Dec;75(2):314–320. doi: 10.1016/0014-4827(72)90435-1. [DOI] [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. "Contact inhibition" of cell division in 3T3 cells. Proc Natl Acad Sci U S A. 1968 May;60(1):300–304. doi: 10.1073/pnas.60.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howk R. S., Troxler D. H., Lowy D., Duesberg P. H., Scolnick E. M. Identification of a 30S RNA with properties of a defective type C virus in murine cells. J Virol. 1978 Jan;25(1):115–123. doi: 10.1128/jvi.25.1.115-123.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. L., Ostrowski M. C., Berard D., Hager G. L. Glucocorticoid regulation of the Ha-MuSV p21 gene conferred by sequences from mouse mammary tumor virus. Cell. 1981 Dec;27(2 Pt 1):245–255. doi: 10.1016/0092-8674(81)90408-6. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., Itin A. Patterns of genomic distribution and sequence heterogeneity of a murine "retrovirus-like" multigene family. J Virol. 1982 Jul;43(1):50–58. doi: 10.1128/jvi.43.1.50-58.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., Shaul Y., Kaminchik J., Aviv H. Heterogeneity of "virus-like" genes encoding retrovirus-associated 30S RNA and their organization within the mouse genome. Cell. 1980 Jun;20(2):431–439. doi: 10.1016/0092-8674(80)90629-7. [DOI] [PubMed] [Google Scholar]
- Keshet E., Shaul Y. Terminal direct repeats in a retrovirus-like repeated mouse gene family. Nature. 1981 Jan 1;289(5793):83–85. doi: 10.1038/289083a0. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN I., ABRAMS R., OVE P. Changes in the metabolism of ribonucleic acid preceding the synthesis of deoxyribonucleic acid in mammalian cells cultured from the animal. J Biol Chem. 1963 Jun;238:2141–2149. [PubMed] [Google Scholar]
- Levinson B., Khoury G., Vande Woude G., Gruss P. Activation of SV40 genome by 72-base pair tandem repeats of Moloney sarcoma virus. Nature. 1982 Feb 18;295(5850):568–572. doi: 10.1038/295568a0. [DOI] [PubMed] [Google Scholar]
- Maizels N. Dictyostelium 17S, 25S, and 5S rDNAs lie within a 38,000 base pair repeated unit. Cell. 1976 Nov;9(3):431–438. doi: 10.1016/0092-8674(76)90088-x. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Moses H. L., Branum E. L., Proper J. A., Robinson R. A. Transforming growth factor production by chemically transformed cells. Cancer Res. 1981 Jul;41(7):2842–2848. [PubMed] [Google Scholar]
- Moses H. L., Proper J. A., Volkenant M. E., Wells D. J., Getz M. J. Mechanism of growth arrest of chemically transformed cells in culture. Cancer Res. 1978 Sep;38(9):2807–2812. [PubMed] [Google Scholar]
- Pledger W. J., Hart C. A., Locatell K. L., Scher C. D. Platelet-derived growth factor-modulated proteins: constitutive synthesis by a transformed cell line. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4358–4362. doi: 10.1073/pnas.78.7.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ringold G. M. Glucocorticoid regulation of mouse mammary tumor virus gene expression. Biochim Biophys Acta. 1979 Dec 19;560(4):487–508. doi: 10.1016/0304-419x(79)90014-3. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Lamb L. C., Newton D. L., Sporn M. B., De Larco J. E., Todaro G. J. Transforming growth factors: isolation of polypeptides from virally and chemically transformed cells by acid/ethanol extraction. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3494–3498. doi: 10.1073/pnas.77.6.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossow P. W., Riddle V. G., Pardee A. B. Synthesis of labile, serum-dependent protein in early G1 controls animal cell growth. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4446–4450. doi: 10.1073/pnas.76.9.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin S. A., Rapp U. R., Benveniste R. E., Sen A., Todaro G. J. Rescue of endogenous 30S retroviral sequences from mouse cells by baboon type C virus. J Virol. 1978 May;26(2):257–264. doi: 10.1128/jvi.26.2.257-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John T. P., Davis R. W. Isolation of galactose-inducible DNA sequences from Saccharomyces cerevisiae by differential plaque filter hybridization. Cell. 1979 Feb;16(2):443–452. doi: 10.1016/0092-8674(79)90020-5. [DOI] [PubMed] [Google Scholar]
- Thomas G., Thomas G., Luther H. Transcriptional and translational control of cytoplasmic proteins after serum stimulation of quiescent Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5712–5716. doi: 10.1073/pnas.78.9.5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Fryling C., De Larco J. E. Transforming growth factors produced by certain human tumor cells: polypeptides that interact with epidermal growth factor receptors. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5258–5262. doi: 10.1073/pnas.77.9.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells D. J., Stoddard L. S., Getz M. J., Moses H. L. alpha-Amanitin and 5-fluorouridine inhibition of serum-stimulated DNA synthesis in quiescent AKR-2B mouse embryo cells. J Cell Physiol. 1979 Aug;100(2):199–214. doi: 10.1002/jcp.1041000202. [DOI] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]
- Williams J. G., Penman S. The messenger RNA sequences in growing and resting mouse fibroblasts. Cell. 1975 Oct;6(2):197–206. doi: 10.1016/0092-8674(75)90010-0. [DOI] [PubMed] [Google Scholar]