Abstract
Biphasic effects of cannabinoids have been shown in processes such as feeding behavior, motor activity, motivational processes and anxiety responses. Using two different tests for the characterization of anxiety-related behavior (elevated plus-maze and holeboard), we first identified in wild-type C57BL/6N mice, two doses of the synthetic CB1 cannabinoid receptor agonist CP-55,940 with anxiolytic (1 μg/kg) and anxiogenic properties (50 μg/kg), respectively. To clarify the role of CB1 receptors in this biphasic effect, both doses were applied to two different conditional CB1 receptor knockout (KO) mouse lines, GABA-CB1-KO (CB1 receptor inactivation in forebrain GABAergic neurons) and Glu-CB1-KO (CB1 receptor inactivation in cortical glutamatergic neurons). We found that the anxiolytic-like effects of the low dose of cannabinoids are mediated via the CB1 receptor on cortical glutamatergic terminals, because this anxiolytic-like response was abrogated only in Glu-CB1-KO mice. On the contrary, the CB1 receptor on the GABAergic terminals is required to induce an anxiogenic-like effect under a high-dose treatment because of the fact that this effect was abolished specifically in GABA-CB1-KO mice. These experiments were carried out in both sexes, and no differences occurred with the doses tested in the mutant mice. Interestingly, the positive allosteric modulation of GABAB receptor with GS-39783 was found to largely abrogate the anxiogenic-like effect of the high dose of CP-55,940. Our results shed new light in further understanding the biphasic effects of cannabinoids at the molecular level and, importantly, pave the way for the development of novel anxiolytic cannabinoid drugs, which may have favorable effect profiles targeting the CB1 receptor on glutamatergic terminals.
Keywords: CB1 receptor; CP-55,940; GS-39783; anxiety; GABA; glutamate
INTRODUCTION
Fear is an adaptive component of the acute stress response to stimuli, which potentially threaten the integrity of the individual. However, when disproportional, it constitutes a maladaptive response and may be symptomatic of an anxiety-related disorder. A well-known demonstration of the influence of cannabinoids on emotion processing, such as anxiety and fear, is derived from the wide range of reactions observed after marijuana consumption. However, the relation between consumption and behavioral reaction is not directly proportional to the dose, but biphasic effects were observed (Viveros et al, 2005). Cannabinoid activity governs in a bimodal manner not only the regulation of anxiety responses (Marco et al, 2004) but also various other behaviors, including motivational processing (Maldonado, 2002), feeding behavior (Wiley et al, 2005; Bellocchio et al, 2010), novelty seeking (Lafenêtre et al, 2009), and locomotion and exploration (Genn et al, 2004; Häring et al, 2011).
The broad distribution of CB1 receptors in the central nervous system (Marsicano and Lutz, 1999), together with its modulatory role on several neurotransmitter systems (Kano et al, 2009), complicates the search on the mechanisms underlying the biphasic effect on anxiety. Although it has been clearly demonstrated that cannabinoid signaling is involved in the control of anxiety, it has been difficult to define the exact role of this signaling, because anxiolytic- and anxiogenic-like effects have been reported both with cannabinoid receptor agonists (low and high doses, respectively; Viveros et al, 2005) and with CB1 receptor antagonists (Navarro et al, 1997; Haller et al, 2002). In this context, the endocannabinoid (eCB) anandamide has been shown to exert its effects on anxiety via the transient receptor potential vanilloid type 1 channel, demonstrating the promiscuity of eCBs and adding a new level of complexity to the study of cannabinoid transmission in the regulation of emotions (Moreira et al, 2012). Additionally, specific brain areas, which are usually involved in emotional homeostasis (ie, prefrontal cortex, hippocampus, and amygdala), seem to have opposite but complementary roles in the regulation of anxiety by cannabinoids (Rubino et al, 2008). These discrepant observations might be explained by the distribution of CB1 receptors in the brain. The recent development of cell type-specific genetic modification of CB1 receptor function has provided a powerful tool in understanding the cannabinoid action (Monory et al, 2007; Puighermanal et al, 2009; Bellocchio et al, 2010). Thus, we aimed at combining cannabinoid pharmacology and its effects on anxiety with the use of conditional CB1 receptor mutant mice. We hypothesize that the regulation of the balance between GABAergic and glutamatergic neurotransmission by the eCB system is responsible for the bidirectional properties of cannabinoids. To investigate the differential role of the CB1 receptor on the regulation of anxiety states after cannabinoid treatment, we tested two different conditional knockout (KO) mouse lines (Monory et al, 2006), in which the CB1 receptors were deleted either from forebrain GABAergic neurons (GABA-CB1-KO) or from cortical glutamatergic neurons (Glu-CB1-KO), in two different but complementary paradigms for anxiety (elevated plus-maze (EPM)) and exploratory activity behavior (holeboard (HB)).
Additionally, we propose that the cross-talk between GABAergic and glutamatergic transmission is a key element in the anxiogenic-like effects of cannabinoids, which have been largely reported when these compounds were administered at high doses. Remarkably, the metabotropic GABAB receptor has recently emerged as a promising regulator of anxiety and depression (Cryan and Kaupmann, 2005). Because of the presynaptic presence of the GABAB receptors in both neuronal subpopulations, they are able to reduce GABA and glutamate release by inhibiting Ca2+ influx via voltage-activated Ca2+ channels (Bowery et al, 2002). However, although eg, baclofen as a GABAB receptor agonist was reported to have significant side effects (Cryan and Kaupmann, 2005), the recent development of novel pharmacological tools targeting GABAB receptor has enabled a much more precise study of the role of this receptor in anxiety. In fact, in our study, by using the positive allosteric modulator (PAM) of the GABAB receptor GS-39783 (Urwyler et al, 2003) alone or in combination with the CB1 receptor agonist CP-55,940, we aimed at characterizing the molecular basis of the anxiogenic-like effect of cannabinoids administered at high dose.
MATERIALS AND METHODS
Animals
Experimental procedures were approved by the Committees on Animal Health and Care of local governments. Mice (70–90 days of age) were housed in groups of three to four, maintained at 22±2 °C and exposed to a reversed 12 : 12 h light–dark photoperiod (lights off at 1300 hours). They had free access to food and water. Experiments were performed either on wild-type (WT) C57BL/6N male mice or on the following mutant lines: Glu-CB1-KO (CB1floxed/floxed;Nex-Cre) and GABA-CB1-KO (CB1floxed/floxed;Dlx5/6-Cre) male and female mice with respective phenotypically WT controls (CB1floxed/floxed: named Glu-CB1-WT and GABA-CB1-WT). Mutants were bred and genotyped as previously described (Monory et al, 2006; Massa et al, 2010). CB1floxed/floxed;Cre+ males were bred with CB1floxed/floxed;Cre− females to avoid potential influences of the mother's genotype on the phenotype of the experimental offspring. All lines were in a mixed genetic background (backcrossed at least six times into C57BL/6N). Animals used in the experiments were littermates. Animals were not single-housed prior to testing to exclude any possible influence of the isolation on anxiety levels. Each experimental group contained individuals from at least three different cages to minimize inter-cage variability.
Drugs
2-[(1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl) cyclohexyl]-5-(2-methyloctan-2-yl)phenol (CP-55,940, Sigma Aldrich, Munich, Germany), a high-affinity synthetic CB1/CB2-receptor agonist, was freshly prepared and dissolved in ethanol:cremophor:saline (1 : 1 : 18; cremophor; Sigma Aldrich) immediately prior to testing on each experimental day. Based on previous studies (Genn et al, 2004) and the dose-response curve performed in our laboratory, two doses of CP-55,940 were chosen; a low (1 μg/kg) and a high dose (50 μg/kg) to induce biphasic effects.
N,N′-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine (GS-39783, Tocris, Wiesbaden, Germany), a PAM of GABAB receptor, was freshly prepared and dissolved in 0.5% methyl cellulose (Sigma Aldrich) immediately prior to testing on each experimental day.
Behavioral Tests
The EPM consisted of a cross-shaped plastic apparatus, elevated 100 cm from the floor, with two opposite open arms (OAs) and two opposite enclosed arms. The floor of the arms was made of white plastic, 35 cm long and 6 cm wide and connected by a central platform of 6 × 6 cm2. Walls in black plastic of 20 cm height surrounded the enclosed arms. Animals were placed into the centre of the apparatus, facing the enclosed arms, and were allowed to explore it during 5 min. We measured frequency and duration of arm visits, separately for OA and closed arm. An arm was considered to be visited when the animal entered it with the four limbs. The percentage of entries and time spent in the OAs were calculated in relation to the total values for the OA and enclosed arm through the following formula: % of OA=100 × open/(open+enclosed). Secondary parameters such as risk assessment (exploratory posture in which the head is stuck out of the rim in an OA, looking to the floor) or stretched-attend postures (SAPs; exploratory posture in which the body is stretched forward and then retracted to the original position without any forward locomotion) were measured as well.
The HB apparatus was made of white Plexiglas with an arena (40 × 40 × 30 cm3) divided into 36 squares by black-color strips. In the center, there were four holes (diameter: 1.8 cm; depth: 7.5 cm) where the animal can poke its nose, but their diameter did not allow the exploration with the whole body. The animals were always placed in the same corner of the arena and were allowed to explore it during 5 min. The parameters recorded were external (number of line crossings in the 20 peripheral squares) and internal ambulation (number of line crossing in the 16 central squares), rearing (number of times when the animal stood on its rear limbs) and frequency and duration (seconds) of head dipping. This test provided independent measures of motor activity (internal and external ambulation) and directed exploration (head dipping frequency and duration).
Experimental Design
Experiment 1; CB1 receptor agonist administration
Mice were intraperitoneally (i.p.) injected with either CP-55,940 (1–50 μg/kg) or its vehicle (ethanol:cremophor:saline; 1 : 1 : 18) in a volume of 10 ml/kg 30 min prior to testing. Animals were kept in their home cages during this period and afterwards they were tested on the EPM immediately followed by the HB test. Vaginal smears were done on females to evaluate a possible influence of the estrous cycle on the anxiety responses. Female mice were synchronized and tested during estrus and proestrus phases for >80% of the subjects on every experimental group. All the experiments were done under red light (25–30 lux) between 1400 and 1700 hours in the activity phase of the animals.
Experiment 2; GABAB receptor PAM+CB1 receptor agonist administration
GS-39783 (GABAB receptor PAM) or its vehicle (0.5% methyl cellulose; vehicle 1) were administered orally (p.o.) in a volume of 10 ml/kg 1 h prior to testing. CP-55,940 was administered (i.p.) as described in Experiment 1, and animals were kept in their cages between drug administrations and before the behavioral assays.
Statistical Analysis
For multiple comparisons, data were analyzed using one-, two-, or three-way analysis of variance (ANOVA) followed by post-hoc Bonferroni's test when necessary (GraphPad, San Diego, CA, USA). Differences were considered statistically significant if p<0.05. Data were presented as mean±SEM.
RESULTS
The Role of CB1 Receptor in Different Neuronal Populations in the Biphasic Effect of Cannabinoids on Anxiety
In order to monitor anxiety-like behaviors, two assays (EPM and HB) were applied to C57BL/6N, GABA-CB1-KO, Glu-CB1-KO and corresponding littermate controls after CP-55,940 and their corresponding vehicle treatment. The experiments were performed in both sexes. Three-way ANOVA (sex × genotype × treatment) did not reveal major sex differences, except of three parameters in Glu-CB1-KO (SAP in the EPM and head dipping frequency and time in the HB; see Supplementary Text and Supplementary Tables 1 and 2). Therefore, only the behavioral analyses of males will be presented in the Result section as Figures 1, 2 and 3, but it will be referred to the Supplementary Information for the data on females.
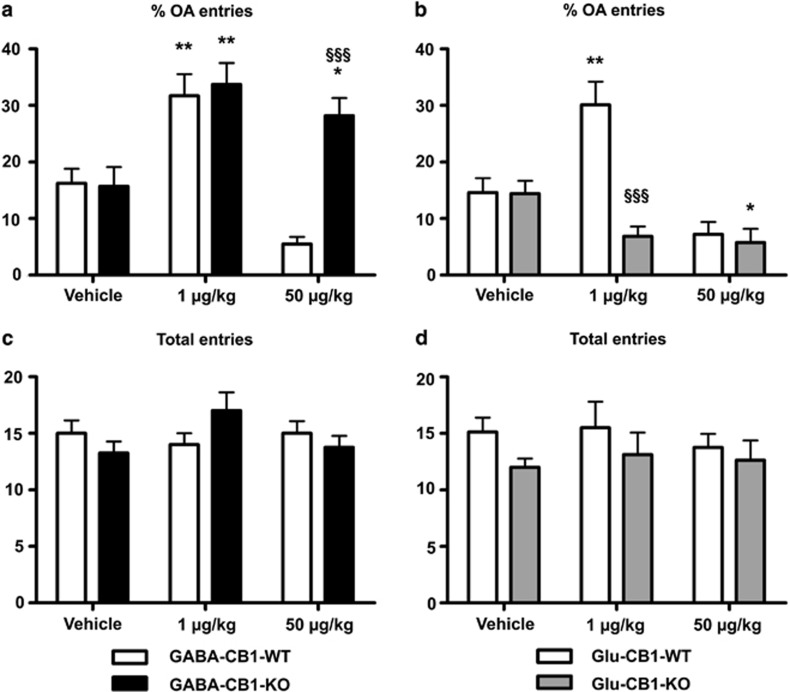
Figure 1.
Behavioral responses of GABA-CB1-KO (left panels) and Glu-CB1-KO (right panels) male mice and their corresponding GABA-CB1-WT and Glu-CB1-WT mice, evaluated in the elevated plus-maze (EPM), treated either with vehicle or CP-55,940 (1 and 50 μg/kg). (a and b) Percentage of open arm (OA) entries (% of OAs=100 × open/(open+enclosed)) instead of absolute values in order to avoid any underlying interaction of treatment or genotype on the locomotion activity. (c and d) Total number of entries in open+enclosed arms. Data are presented as mean±SEM (8–10 animals per experimental group). Significant differences: *p<0.05 and **p<0.01 when compared with vehicles (same genotype) (one-way analysis of variance (ANOVA) followed by Bonferroni's test); §§§p<0.001 when compared with wild-type (WT) littermates (same treatment) (two-way ANOVA followed by Bonferroni's test).
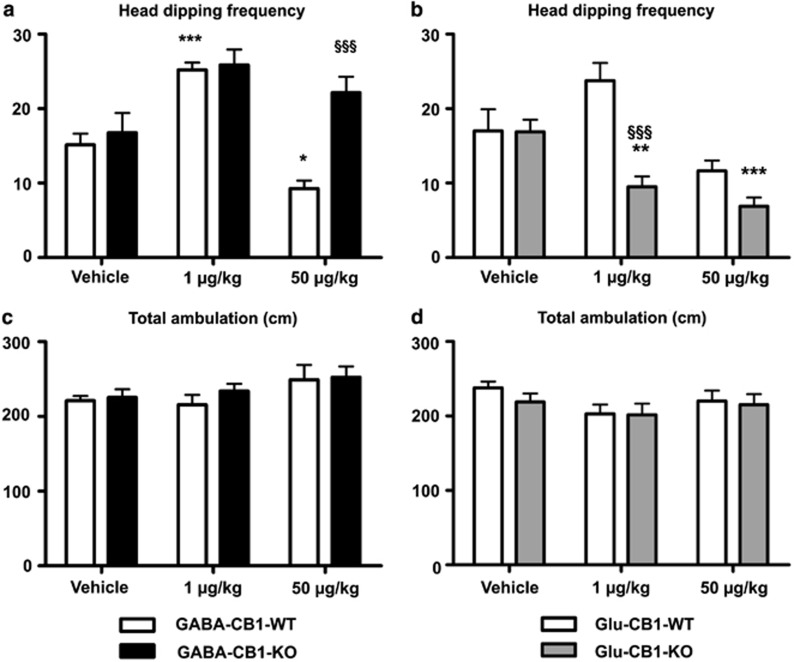
Figure 2.
Behavioral responses of GABA-CB1-KO (left panels) and Glu-CB1-KO (right panels) male mice and their corresponding GABA-CB1-WT and Glu-CB1-WT mice, evaluated in the holeboard (HB) paradigm, treated either with vehicle or CP-55,940 (1 and 50 μg/kg). (a and b) Number of head dippings performed by the animals (only when the mice were facing at least their snouts to the bottom of the holes. (c and d) Number of square lines crossed by the animals. Data are presented as mean±SEM (8–10 animals per experimental group). Significant differences: *p<0.05; **p<0.01, and ***p<0.001 when compared with vehicles (same genotype) (one-way ANOVA followed by Bonferroni's test); §§§p<0.001 when compared with WT littermates (same treatment) (two-way ANOVA followed by Bonferroni's test).
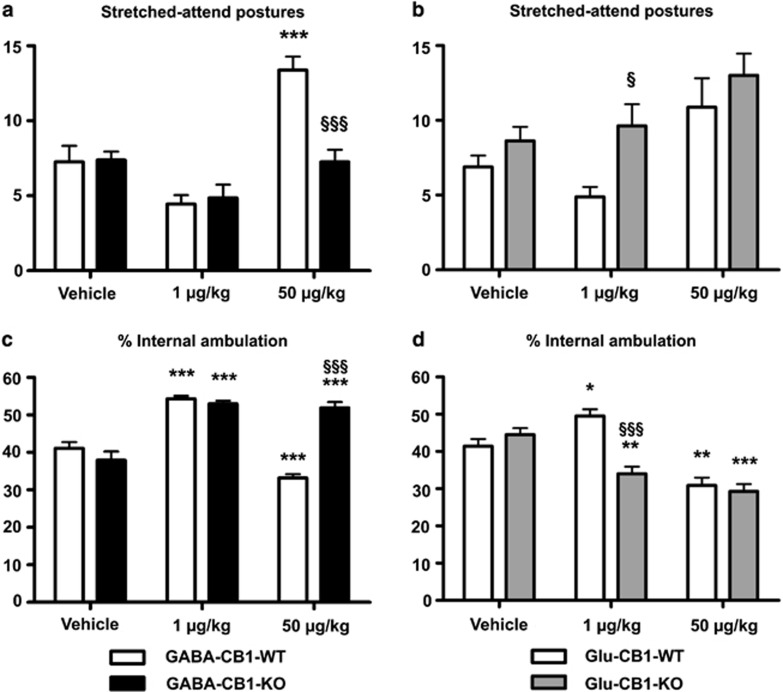
Figure 3.
Secondary behavioral parameters of GABA-CB1-KO (left panels) and Glu-CB1-KO (right panels) male mice and their corresponding GABA-CB1-WT and Glu-CB1-WT mice, evaluated in the EPM and the HB paradigm, treated either with vehicle or CP-55,940 (1 and 50 μg/kg). (a and b) Number of stretched-attend postures (SAPs) performed by the mice (exploratory posture in which the body is stretched forward then retracted to the original position without any forward locomotion). (c and d) Percentage of square lines crossed in the internal area (% of internal area=100 × internal/(internal+external)). Data are presented as mean±SEM (6–10 animals per experimental group). Significant differences: *p<0.05; **p<0.01, and ***p<0.001 when compared with vehicles (same genotype) (one-way ANOVA followed by Bonferroni's test); §p<0.05 and §§§p<0.001 when compared with WT littermates (same treatment) (two-way ANOVA followed by Bonferroni's test).
EPM
Based on the natural aversion of rodents to open spaces, and the anxiety associated to this avoidance, the EPM was used in order to evaluate the biphasic effect of CB1 receptor activation in anxiety processing. In this paradigm, the percentage of OA entries is considered as a reliable parameter of the anxiety state of the subjects, being sensitive to the effects of both anxiolytic and anxiogenic agents (Lister, 1987). As such, an increased percentage of OA entries is usually associated with a reduction of anxiety and vice versa. In our study, the two-way ANOVA comparison of percentage of entries into the OAs in GABA-CB1-KO and GABA-CB1-WT showed a significant interaction between genotype and treatment in both sexes (males: F2,42=8.16, p<0.01; females: F2,40=6.39, p<0.01). Bonferroni's post-hoc test revealed a highly significant difference between GABA-CB1-KO and GABA-CB1-WT mice treated with the high dose of CP-55,940 (50 μg/kg) in both males (Figure 1a; t14=5.091, p<0.001) and females (Supplementary Figure S1a; t13=5.710, p<0.001), when compared with the vehicle-treated group. In all the sexes and genotypes, the percentage of entries into the OAs was increased after the treatment with the low dose (males (Figure 1a) GABA-CB1-WT: (t15=3.807, p<0.01), GABA-CB1-KO: (t13=3.651, p<0.01); females (Supplementary Figure S1a) GABA-CB1-WT: (t16=5.312, p<0.001), GABA-CB1-KO: (t12=4,288, p<0.01)), indicating an anxiolytic-like effect in the GABA-CB1 line treated with the low dose of cannabinoids. However, when treated with the high dose of CP-55,940, neither males (t14=2.618, p>0.05) nor females (t12=2.228, p>0.05) of the KO group showed the decrease of percentage of OA entries displayed by the WT group, but a significant anxiolytic-like effect was observed in both males (Figure 1a) and females (Supplementary Figure S1a) of the KO groups. These results demonstrate that the CB1 receptor in GABAergic neurons is required to mediate the anxiogenic-like effect induced by the treatment with a high dose of CP-55,940. Consistently, similar results were seen in percentage of time spent in the OAs (Supplementary Figures S4a and S6a).
The two-way ANOVA for percentage of entries into the OAs in the Glu-CB1-KO mouse line also revealed a significant interaction of genotype × treatment, both in males (Figure 1b; F2,42=11.90, p<0.001) and females (Supplementary Figure S1b; F2,42=4.03, p<0.05). We failed to observe a significant biphasic effect on percentage OA entries in the WT group of both sexes, although a bimodal tendency was still recognizable. In this case, the percentage of time spent in the OAs was also similar to the percentage of OA entries (Supplementary Figures S4b and S6b). Remarkably, we observed a loss of the anxiolytic-like effect associated with the low dose in the Glu-CB1-KO, indicating that the CB1 receptor in cortical glutamatergic neurons is necessary to promote the anxiolytic-like effect of cannabinoids at low dose.
Despite the fact that the eCB system is clearly involved in the regulation of locomotion (El Manira and Kyriakatos, 2010), no differences were obtained in the total number of arm entries between the groups, independently of sex or mouse line (Figures 1c and d, Supplementary Figures S1c and S1d). Therefore, the anxiety-related effects of the treatments used are not caused by changes in locomotion mediated by CP-55,940 treatment.
HB
The head dipping frequency displayed by rodents during the HB test is considered as an appropriate parameter evaluating exploration (File and Wardill, 1975). Interestingly, CB1 receptor activation was shown to mediate changes in exploratory activity in mice (Marco et al, 2004; Hernández-Tristán et al, 2000). Thus, the CB1 receptor conditional mutant mice were exposed to the HB assay in order to further characterize a possible biphasic effect of CP-55,940 in exploration.
The two-way ANOVA for head dipping frequency in GABA-CB1-KO and GABA-CB1-WT male mice showed a significant interaction between genotype and treatment (F242=7.07, p<0.01) for the high dose of CP-55,940 (50 μg/kg; Figure 2a), and Bonferroni's post-hoc test confirmed this observation (t14=5.043, p<0.001). The head dipping frequency (Figure 2a), as well as the time spent exploring the holes (Supplementary Figure S5a), was increased in GABA-CB1 mice treated with the low dose of CP-55,940, independently of the sex and the genotype (Supplementary Figures S2a, S5a and S7a). The opposite effect was shown in the GABA-CB1-WT animals treated with the high dose (Figure 2a; t14=3.418, p<0.05), presumably caused by an increase in their anxiety state. This anxiogenic-associated property of the high dose of CP-55,940 was completely absent in the GABA-CB1-KO mice (Figure 2a; t14=1.663, p>0.05). In accordance with the results obtained from the EPM, the CB1 receptor in GABAergic neurons has a fundamental role both in the reduction of the exploratory behavior, and the anxiogenic-like response mediated by the high dose of CP-55,940.
In contrast to GABA-CB1-KO mice, Glu-CB1-KO animals showed a different regulation of exploratory behavior in the HB test. A significant bimodal effect of CP-55,940 was not detected in the WT group, however, both directions (low dose, anxiolytic-like effect and high dose, anxiogenic-like effect) are easily identifiable for head dipping frequency (Figure 2b, Supplementary Figure S2b) and time (Supplementary Figures S5b and S7b). Nevertheless, the two-way ANOVA for head dipping frequency in Glu-CB1-KO male mice revealed a significant interaction sex × treatment (Figure 2b; F2,42=7.020, p<0.001), and Bonferroni's post-hoc test showed a significant difference between Glu-CB1-KO and Glu-CB1-WT mice (t14=5.243, p<0.001). Both doses of CP-55,940 reduced the exploratory activity in Glu-CB1-KO males and females (Figure 2b, Supplementary Figure S2b), as compared with the vehicle-injected group, suggesting that the CB1 receptor on cortical glutamatergic neurons mediates the anxiolytic-associated increase of head dipping frequency observed after the treatment with the low dose of CP-55,940. Locomotor activity was also measured in this test, in order to avoid any interference of the basal motor level in explorative behavior. Rearing behavior was scored and considered as a measurement of vertical locomotor activity. No differences were found in neither of the experimental groups (Figures 2c and d, Supplementary Figures S2c, S2d, S5c, S5d, S7c, and S7d), independently of sex, genotype and treatment, confirming that the effects identified were not caused by the underlying properties of the CB1 receptor agonist on locomotion.
Secondary parameters
In addition to the indices, which are typically scored in the EPM to evaluate anxiety (eg, OA avoidance and general locomotion), behavioral scoring is usually extended to include several stereotypic behaviors, which contribute to a better understanding of the performance of the mice. Accordingly, SAPs measured in the EPM are usually considered as an indicator of fear or cautious behavior, and they were shown to be sensitive to both anxiolytic and anxiogenic drugs (Grewal et al, 1997). On the contrary, risk assessment is directly related to the level of OA exploration and consequently, it is normally increased when the general anxiety of the animal is reduced. Moreover, the inherent thigmotaxis of rodents allowed us to interpret the percentage of internal ambulation in the HB paradigm as an indicator of exploration, being increased when the anxiety level is reduced and vice versa.
In agreement with these observations, the high/anxiogenic dose of CP-55,940 induced an increase of the SAP in GABA-CB1-WT mice, but not in GABA-CB1-KO (Figure 3a, Supplementary Figure S3a). Both genotypes of the GABA-CB1 mouse line showed no differences in this stereotypic behavior when treated with the low/anxiolytic dose of CP-55,940 (Figure 3a, Supplementary Figure S3a). In contrast to the GABA-CB1 mice, the Glu-CB1 mice showed a differential regulation of SAP when treated with the low/anxiolytic dose of CP-55,940, but not with the high/anxiogenic dose of the CB1 receptor agonist. In this case, only WT mice showed a tendency to a reduction of SAP, whereas Glu-CB1-KO mice did not display this reduction (Figure 3b, Supplementary Figure S3b).
Accordingly, percentage of internal ambulation in the HB arena was increased in all the groups treated with 1 μg/kg of CP-55,940, except of Glu-CB1-KO mice (Figures 3c and d, Supplementary Figures S3c and S3d), whereas this same parameter was reduced in all the groups injected with 50 μg/kg of CP-55,940 except of GABA-CB1-KO mice (Figures 3c and d, Supplementary Figures S3c and S3d). Finally, the same pattern was found regarding the risk assessment (Supplementary Figures S4c, S4d, S6c, and S6d), demonstrating that the low dose of cannabinoids is anxiolytic as long as the CB1 receptor in glutamatergic terminals is expressed and that the high dose of CP-55,940 is anxiogenic as long as the GABAergic terminals express CB1 receptors.
The Role of GABAB Receptor in the Anxiogenic-Like Effects of Cannabinoids Administered at High Doses
Based on the clearly differentiated processes that the CB1 receptor exerts in GABAergic vs glutamatergic neuronal subpopulations in the regulation of anxiety, it is tempting to predict that a fine-tuned balance of GABAergic vs glutamatergic transmission mediates the regulation of anxiety by cannabinoids. Accordingly, the cross-talk between these neurotransmitter systems has a potential key role where GABAB receptors acquire a remarkable therapeutic interest (Cryan and Kaupmann, 2005; Bowery et al, 2002). In order to study the role of the GABAB receptor in the anxiogenic-like effect of CP-55,940, we performed a treatment with the GABAB receptor PAM GS-39783 in combination with the high/anxiogenic dose of CP-55,940 tested above (50 μg/kg). We aimed at evaluating the capacity of GS-39783 to counteract the anxiogenic-like effect of CP-55,940. However, it was not our purpose to compensate the anxiety promoted by the CB1 receptor agonist with a high/anxiolytic dose of GS-39783, but rather to use a subthreshold dose without apparent anxiolytic-like effects under basal conditions. To identify the highest dose with mild or absent anxiolytic-like effects, we first performed a dose-response curve, where four different doses of this compound (0.5, 2, 10, and 50 mg/kg) were administered (p.o.) alone 1 h prior to behavioral testing. In this assay, both 10 and 50 mg/kg doses were found to promote a classical anxiolytic response as characterized by a significant increase in the OA exploration (Supplementary Figures S8a and S10c), risk assessment (Supplementary Figure S8c) and a decrease of the SAPs (Supplementary Figure S8e). As previously described, none of the doses tested induced a locomotion effect (Supplementary Figures S9c and d). Consequently, 2 mg/kg was the dose chosen for the experiment in combination with CP-55,940 (50 μg/kg).
EPM
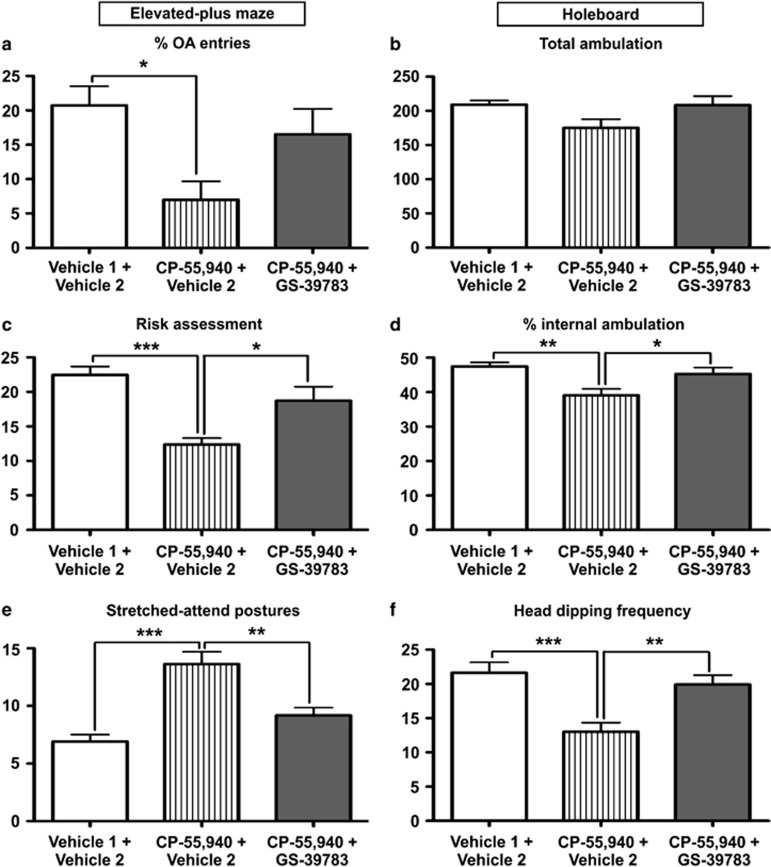
In this experiment, the pretreatment with GS-39783 (subthreshold dose; 2 mg/kg) was found to counteract at least partially the anxiogenic-like effects of CP-55,940 (50 μg/kg) in terms of OA exploration (Figure 4a, Supplementary Figure S10a). Therefore, considering that a subthreshold positive modulation of GABAB receptor was enough to compensate the adverse effects of the high dose of CP-55,940, it can be concluded that the high dose of CP-55,940 mediates its anxiogenic-like effects at least partially through GABAergic inhibition.
Figure 4.
Behavioral responses of C57BL/6N male mice treated first with either GS-39783 (subthreshold dose; 2 mg/kg; orally) or its vehicle (vehicle 1) and 30 min later with either CP-55,940 (anxiogenic-like dose; 50 μg/kg; intraperitoneally) or its vehicle (vehicle 2). Mice were evaluated in the EPM (left panels) and HB test (right panels)1 h after the first treatment. During the EPM, (a) the percentage of OA entries, (c) risk-assessment behavior, and (e) SAPs were scored. In the HB test, (b) total ambulation was used as a measurement of locomotion, and (d) percentage of internal ambulation and (f) head dipping frequency indicated the level of exploration (11 mice per experimental group). Significant differences: *p<0.05, **p<0.01 and ***p<0.001 when compared with vehicles (one-way ANOVA followed by Bonferroni's test).
HB
Regarding the dose curve for GS-39783 described above, the analysis of the behavior in the HB task confirmed on one hand the absence of locomotion effects of any of the doses used (Figure 4b, Supplementary Figures S8b and S9d), and on the other hand, the increase in internal exploration and head dipping (frequency and time) commonly associated with anxiolytic-like behaviors. In our experiments, this effect was clearly identified again in the animals treated with 10 and 50 mg/kg (Supplementary Figures S8d, f and S10d). Interestingly, when GS-39783 (subthreshold dose; 2 mg/kg) was p.o. administered before the injection of CP-55,940, the reduction in exploration was abolished and mice showed a profile similar to the vehicle-treated group (Figures 4d and f and Supplementary Figure S10b). Consequently, the data obtained in the HB task corroborated that the anxiogenic-like response of mice to a high dose of CP-55,940 was mediated by a reduction of the availability of GABA at GABAB receptors.
Secondary parameters
Interestingly, SAP and risk-assessment behavior were differentially affected in mice treated with CP-55,940 in comparison with the vehicle-treated group. In both cases, anxiogenic-like effects of CP-55,940 (reduction in SAP and increase in risk assessment; Figures 4e and c, respectively) were abrogated by pretreatment with GS-39783 (subthreshold dose; 2 mg/kg). These results confirmed the reproducibility of the anxiogenic-like effect of CP-55,940 at high dose and strongly corroborated not only the role of GABAB receptors in this behavior but also the utility of several stereotypes, which contribute to a more precise description of the behavioral phenotype.
In summary, the data presented here confirmed that the CB1 receptor on GABAergic terminals is required for the anxiogenic-like behavior commonly observed in mice treated with a high dose of CP-55,940. In our hands, this behavior was also characterized by a reduction of exploration and an increase in fear-associated stereotypes. Paradoxically, the reduction in glutamate transmission caused by CP-55,940 at high doses was not sufficient to overcome the expected anxiogenic effects exerted at GABAergic terminals in WT mice. Under these circumstances, the cross-talk between the GABAergic and the glutamatergic tones appears to be a fundamental factor. In fact, a reduction in the GABAergic tone acting on glutamatergic terminals seems to be necessary for this response, because of the fact that a subthreshold positive modulation of GABAB receptors was able to counteract the anxiogenic effects of CP-55,940. On the other hand, the CB1 receptor on glutamatergic terminals can be considered as a requirement for the anxiolytic-like, pro-explorative and incautious behavior displayed by the mice under a treatment with a CB1 receptor agonist at low doses.
DISCUSSION
Our results give for the first time a mechanistic explanation for the biphasic effects of cannabinoids in anxiety behavior, whereby CB1 receptor activation on GABAergic neurons is required for developing anxiogenic-like behavior after a high dose of cannabinoids. Moreover, this high dose-dependent effect of CP-55,940 seems to rely at least partially in a reduced GABAergic signalling on GABAB receptors. On the other hand, an opposite function is observed on glutamatergic terminals, where the CB1 receptor mediates anxiolytic-like effects, when subjects were treated with a low dose of cannabinoids.
These results can be embedded into the context of the current knowledge of CB1 receptor function in the modulation of neurotransmission. To this end, in order to understand the role of the eCB system in the regulation of emotional homeostasis and corroborate the physiological relevance of this study, several features of the eCB system and CB1 receptor characteristics should be considered.
First, different neuronal subpopulations have been described based on their differential sensitivity of CB1 receptors towards agonist activation in hippocampal neurons. Ohno-Shosaku et al (2002) described that the CB1 receptor on glutamatergic terminals displays a lower sensitivity to the cannabinoid agonist WIN55,212-2 than the receptor on GABAergic terminals. Furthermore, the study by Lee et al (2010) demonstrated that inhibitory synapses that are sensitive to cannabinoid-induced depolarization-induced suppression of inhibition can be subdivided into two different groups. The group with high sensitivity is formed by perisomatic basket cells, whereas the dendritically projecting Schaffer-collateral associated cells belong to the group with low sensitivity to WIN55,212-2. Based on these experiments, a differential sensitivity of CB1 receptors towards cannabinoids can be associated to GABAergic (high sensitivity) and glutamatergic (low sensitivity) terminals. Despite the fact that these features cannot explain our observations by themselves, it is important to emphasize the relevance of the differential CB1 receptor sensitivity, because not every neuronal population is equally sensitive to CB1 receptor activation. On the other hand, in the case that only the sensitivity of the CB1 receptors would matter in cannabinoid action in vivo, the low dose of cannabinoid would be expected to activate the CB1 receptor on GABAergic interneurons at the first place, thereby reducing GABA transmission and exerting anxiogenic-like effects in the WT mouse. However, this is not the case, because our experiments clearly show that the anxiolytic-like effect of the low cannabinoid dose is mediated by the CB1 receptor on glutamatergic terminals. Consequently, additional considerations must be taken into account in order to clarify the role of CB1 receptors in anxiety.
Second, the tonic activity of the eCB system has been shown to be different depending on the neuronal subpopulation studied. In vitro experiments revealed that the application of CB1 receptor antagonist can enhance inhibitory and excitatory postsynaptic potentials, depending on the type of synapse (Roberto et al, 2010; Slanina and Schweitzer, 2005). The CB1 receptor antagonists used in these studies (SR141716 and AM251) were shown to promote also inverse agonist actions. However, it was found in a number of in vitro and in vivo experiments that both drugs exhibit greater potency in opposing effects induced by CB1 receptor agonists than in producing inverse effects at CB1 receptors by itself (Pertwee, 2005). This indicates that CB1 receptors are tonically activated under such conditions, and blockade of CB1 receptor relieves the continuous CB1 receptor-mediated suppression of neurotransmitter. The extent of tonic CB1 receptor-mediated inhibition appears to be stronger on GABAergic than on glutamateric terminals and has been more frequently described on GABAergic terminals (Roberto et al, 2010; Slanina and Schweitzer, 2005). Consistent with this notion is the proposition that the CB1 receptor on glutamatergic terminals acts as a stout guard when excessive glutamate transmission occurs and CB1 receptor will consequently be activated and suppress glutamatergic transmission (Marsicano et al, 2003; Katona and Freund, 2008). Therefore, this may imply that the CB1 receptor on glutamatergic terminals is predominately activated in a phasic (‘on-demand') manner being not much activated under basal states, whereas the CB1 receptor on GABAergic terminals displays a high tonic activation under basal states. These features may explain why a low dose of cannabinoids will first affect the CB1 receptor on glutamatergic neurons, thereby reducing glutamatergic transmission, leading to an anxiolytic-like effect.
Third, a possible mechanism underlying the anxiogenic-like effect at high cannabinoid dose is related with the cross-talk between GABAergic and glutamatergic neurotransmission. Despite of the fact that GABA transmission has been classically related with multiple neuropsychiatric disorders and that very effective drugs have been designed and extensively used targeting GABAA ionotropic receptors (ie, benzodiazepines), rather little is known about the role of metabotropic GABAB receptors in emotional homeostasis. In this context, GABAB receptors were found to be highly expressed in the limbic areas (McDonald et al, 2004). Additionally, GABAB receptors on glutamatergic terminals were shown to reduce glutamatergic transmission in the hippocampus (Gassmann et al, 2004). Further investigations confirmed the presynaptic localization of a considerable fraction of the GABAB receptors formed by GABAB1a and GABAB2 subunits (Biermann et al, 2010). Remarkably, genetic deletion of GABAB receptors leads to an anxiogenic phenotype. Consequently, GABAB receptors were postulated as a new target in the development of novel pharmacological strategies to treat mood disorders. The sedative and hypothermic effects of the prototypical GABAB receptor agonist baclofen have limited its use in behavioral pharmacological studies (Bowery et al, 2002). Thus, PAMs have recently been proposed as therapeutically superior drugs with respect to undesired side effects (Cryan et al, 2004; Koek et al, 2010). In fact, GS-39783 (novel GABAB receptor PAM) was shown to increase in vitro the GABAB receptor agonist affinity and potency, as well as the capacity of GABA to reduce cAMP production (Urwyler et al, 2005). In our hands, this compound exerted the same anxiolytic-like responses on the behavioral level as described elsewhere (Mombereau et al, 2004; Cryan et al, 2004; Jacobson and Cryan, 2008). Importantly, we found that a subthreshold dose of GS-39783 (2 mg/kg) was able to counteract to a large extent the anxiogenic effects of CP-55,940 at high dose (Figures 4a–f). It is noteworthy that this dose was ineffective under basal conditions (Supplementary Figures S8a–f) (with an appropriate GABA/glutamate balance) and became relevant only after cannabinoid administration (with a presumable GABA/glutamate unbalance). Consequently, it can be hypothesized that a reduction of GABA release mediated by the action of a high dose of CP-55,940 on the GABAergic CB1 receptor could indirectly increase glutamatergic tone via decreased activation of GABAB receptors on glutamatergic terminals. A similar mechanism, relying on excessive glutamate release, has recently been suggested for the amnesic effect of Δ9-THC in an object-recognition task (Puighermanal et al, 2009), where excessive activation of pyramidal neurons after Δ9-THC treatment leads to excessive new protein synthesis.
Remarkably, the administration of CP-55,940 had a significant effect in the total number of entries into the arms (Supplementary Figure S9a). However, despite of the fact that this parameter is usually considered as an indicator of general locomotion, it should not be a confounding factor for the interpretation of the anxiety-related results because of two reasons. First, the OA exploration described here is reported as a percentage of the total exploration. In other words, it is already normalized and consequently, the locomotion factor does not significantly interfere. Second, the other behavioral parameters presented here for locomotion (ie, total ambulation and rearing) were not affected in the mice treated only with CP-55,940. Thus, we believe that the effect of CP-55,940 on the total number of entries does not interfere with the interpretation of the results regarding anxiety regulation.
In our study, behavioral differences were not detected between KO and WT mice injected with vehicle solution. Other studies (Haller et al, 2004; Jacob et al, 2009; Kamprath et al, 2009) evidenced that the more stressful the experimental procedure is, the more activated the eCB system is, thus, allowing observable differences between CB1 receptor-deficient animals and their WT littermates. In reference to this point, our protocol aimed at achieving a biphasic effect. Therefore, the conditions established were predominantly similar to the basal activity of these mice, promoting a less aversive environment for the animals as possible. In agreement with these observations, stressful stimuli, as well as rewarding experiences, were reported to mediate changes in the expression level of the CB1 receptor specifically in GABAergic terminals (Rossi et al, 2008; De Chiara et al, 2010). Interestingly, the stress-mediated regulation of the GABAergic CB1 receptor was postulated as a compensatory mechanism required to restore the equilibrium between GABAergic and glutamatergic neurotransmission in emotional homeostasis (Ruehle et al, 2012). Therefore, a protective environment, which avoids any occasional alteration of the basal expression of the CB1 receptors, prevents the appearance of behaviorally observed differences between mutant mice and their WT littermates without cannabinoid treatment.
It is worth mentioning that the CB1 receptor has been shown to be 10–20 times more expressed in inhibitory terminals as compared with excitatory terminals in hippocampus and cerebellar cortex (Kawamura et al, 2006). Thus, another interesting issue is how the huge differences of CB1 receptor expression levels on the two neuronal subpopulations studied here (very high abundance in GABAergic neurons and low abundance in cortical glutamatergic neurons) can explain the differential responses to high and low doses of cannabinoids and the underlying molecular mechanisms discussed above. Differences in signalling pathways (eg, G-protein coupling) activated by CB1 receptors on the various neuronal subpopulations may be responsible for these distinctions at the molecular level (Steindel et al, 2008).
In summary, considering the differential sensitivity and basal activity of the CB1 receptors present either in glutamatergic or GABAergic terminals, together with the cross-talk between these two neurotransmitter systems, the biphasic effect of cannabinoids on anxiety can be viewed as a consequence of cannabinoid regulation of GABA/glutamate balance.
Finally, our findings evidenced for the first time a neurobiological substrate to explain the differential regulation of anxiety processing by cannabinoids, provided relevant original information regarding the interaction of the eCB system with the GABAergic and glutamatergic systems, and shed new light for the refinement of potential eCB-based therapies for anxiety disorders.
Acknowledgments
We wish to thank Yasmin Abassi, Krisztina Monory and Nadine Kaiser for discussion and excellent support in the experiments, Professor Bernhard Bettler for the helpful advices regarding the pharmacology of GABAB receptors, and Andrea Conrad and Danuta Dormann for the genotyping. This work was supported in part by the Hübner Foundation and the Deutschen Forschungsgemeinschaft (DFG) to BL (SPP1226; SFB TRR 58 TP A04).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Bellocchio L, Lafenêtre P, Cannich A, Cota D, Puente N, Grandes P, et al. Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci. 2010;13:281–283. doi: 10.1038/nn.2494. [DOI] [PubMed] [Google Scholar]
- Biermann B, Ivankova-Susankova K, Bradaia A, Abdel Aziz S, Besseyrias V, Kapfhammer JP, et al. The Sushi domains of GABAB receptors function as axonal targeting signals. J Neurosci. 2010;30:1385–1394. doi: 10.1523/JNEUROSCI.3172-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, et al. International Union of Pharmacology. XXXIII. Mammalian gamma-aminobutyric acid (B) receptors: structure and function. Pharmacol Rev. 2002;54:247–264. doi: 10.1124/pr.54.2.247. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kaupmann K. Don't worry ‘B' happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci. 2005;26:36–43. doi: 10.1016/j.tips.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kelly PH, Chaperon F, Gentsch C, Mombereau C, Lingenhoehl K, et al. Behavioral charcterizitaion of the novel GABAB receptor-positive modulator GS39783 (N,N′-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine): anxiolytic-like activity without side effects associated with baclofen or nezodiazepines. J Pharmacol Exp Ther. 2004;310:952–963. doi: 10.1124/jpet.104.066753. [DOI] [PubMed] [Google Scholar]
- De Chiara V, Errico F, Musella A, Rossi S, Matalauni G, Sacchetti L, et al. Voluntary exercise and sucrose consumption enhance cannabinoid CB1 receptor sensitivity in the striatum. Neuropsychopharmacology. 2010;35:374–387. doi: 10.1038/npp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Manira A, Kyriakatos A. The role of endocannabinoid signaling in motor control. Physiology. 2010;25:230–238. doi: 10.1152/physiol.00007.2010. [DOI] [PubMed] [Google Scholar]
- File SE, Wardill AG. Validity of head-dipping as a measure of exploration in a modified hole-board. Psychopharmacologia. 1975;44:53–59. doi: 10.1007/BF00421184. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Shaban H, Vigot R, Sansig G, Haller C, Barbieri S, et al. Redistribution of GABAB(1) protein and atypical GABAB responses in GABAB(2)-deficient mice. J Neurosci. 2004;24:6086–6097. doi: 10.1523/JNEUROSCI.5635-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genn RF, Tucci S, Marco EM, Viveros MP, File SE. Unconditioned and conditioned anxiogenic effects of the cannabinoid receptor agonist CP 55,940 in the social interaction test. Pharmacol Biochem Behav. 2004;77:567–573. doi: 10.1016/j.pbb.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Grewal SS, Shepherd JK, Bill DJ, Fletcher A, Dourish CT. Behavioural and pharmacological characterization of the canopy stretched attend posture test as a model of anxiety in mice and rats. Psychopharmacology. 1997;133:29–38. doi: 10.1007/s002130050367. [DOI] [PubMed] [Google Scholar]
- Haller J, Bakos N, Szirmay M, Ledent C, Freund TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur J Neurosci. 2002;16:1395–1408. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Varga B, Ledent C, Barna I, Freund TF. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur J Neurosci. 2004;19:1906–1912. doi: 10.1111/j.1460-9568.2004.03293.x. [DOI] [PubMed] [Google Scholar]
- Häring M, Kaiser N, Monory K, Lutz B. Circuit specific functions of cannabinoid CB1 receptor in the balance of investigatory drive and exploration. PLoS One. 2011;6:e26617. doi: 10.1371/journal.pone.0026617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Tristán R, Arévalo C, Canals S, Leret ML. The effects of acute treatment with Δ9-THC on exploratory behavior and memory in the rat. J Physiol Biochem. 2000;56:17–24. doi: 10.1007/BF03179772. [DOI] [PubMed] [Google Scholar]
- Jacob W, Yassouridis A, Marsicano G, Monory K, Lutz B, Wotjak CT. Endocannabinoids render exploratory behaviour largely independent of the test aversiveness: role of glutamatergic transmission. Genes Brain Behav. 2009;8:685–698. doi: 10.1111/j.1601-183X.2009.00512.x. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Cryan JF. Evaluation of the anxiolytic-like profile of the GABAB receptor positive allosteric modulator CGP7930 in rodents. Neuropharmacology. 2008;54:854–862. doi: 10.1016/j.neuropharm.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Kamprath K, Plendl W, Marsicano G, Deussing JM, Wurst W, Lutz B, et al. Endocannabinoids mediate acute fear adaptation via glutamatergic neurons independently of corticotropin-releasing hormone signaling. Genes Brain Behav. 2009;8:203–211. doi: 10.1111/j.1601-183X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. 2008;14:923–930. doi: 10.1038/nm.f.1869. [DOI] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, et al. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, France CP, Cheng K, Rice KC. GABAB receptor-positive modulators: enhancement of GABAB receptor agonist effects in vivo. J Pharmacol Exp Ther. 2010;335:163–171. doi: 10.1124/jpet.110.171116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafenêtre P, Chaouloff F, Marsicano G. Bidirectional regulation of novelty-induced behavioral inhibition by the endocannabinoid. Neuropharmacology. 2009;57:715–721. doi: 10.1016/j.neuropharm.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Lee SH, Foldy C, Soltesz I. Distinct endocannabinoid control of GABA release at perisomatic and dendritic synapses in the hippocampus. J Neurosci. 2010;30:7993–8000. doi: 10.1523/JNEUROSCI.6238-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology. 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Maldonado R. Study of cannabinoid dependence in animals. Pharmacol Ther. 2002;95:153–164. doi: 10.1016/s0163-7258(02)00254-1. [DOI] [PubMed] [Google Scholar]
- Marco EM, Pérez-Alvarez L, Borcel E, Rubio M, Guaza C, Ambrosio E, et al. Involvement of 5-HT1A receptors in behavioural effects of the cannabinoid receptor agonist CP 55,940 in male rats. Behav Pharmacol. 2004;15:21–27. doi: 10.1097/00008877-200402000-00003. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Massa F, Mancini G, Schmidt H, Steindel F, Mackie K, Angioni C, et al. Alterations in the hippocampal endocannabinoid system in diet-induced obese mice. J Neurosci. 2010;30:6273–6281. doi: 10.1523/JNEUROSCI.2648-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Muller JF. Immunocytochemical localization of GABABR1 receptor subunits in the basolateral amygdale. Brain Res. 2004;1018:147–158. doi: 10.1016/j.brainres.2004.05.053. [DOI] [PubMed] [Google Scholar]
- Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF. Genetic and pharmacological evidence of a role for GABAB receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology. 2004;29:1050–1062. doi: 10.1038/sj.npp.1300413. [DOI] [PubMed] [Google Scholar]
- Monory K, Blaudzun H, Massa F, Kaiser N, Lemberger T, Schütz G, et al. Genetic dissection of behavioural and autonomic effects of Δ9-tetrahydrocannabinol in mice. PLoS Biol. 2007;5:e269. doi: 10.1371/journal.pbio.0050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monory K, Massa F, Egertová M, Eder M, Blaudzun H, Westenbroek R, et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51:455–466. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Aguiar DC, Terzian AL, Guimarães FS, Wotjak CT. Cannabinoid type 1 receptors and transient receptor potential vanilloid type 1 channels in fear and anxiety-two sides of one coin. Neuroscience. 2012;204:186–192. doi: 10.1016/j.neuroscience.2011.08.046. [DOI] [PubMed] [Google Scholar]
- Navarro M, Hernández E, Muñoz RM, del Arco I, Villanúa MA, Carrera MR, et al. Acute administration of the CB1 cannabinoid receptor antagonist SR 141716A induces anxiety-like responses in the rat. Neuroreport. 1997;20:491–496. doi: 10.1097/00001756-199701200-00023. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci. 2002;22:3864–3872. doi: 10.1523/JNEUROSCI.22-10-03864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005;76:1307–1324. doi: 10.1016/j.lfs.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonado R, Ozaita A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci. 2009;12:1152–1158. doi: 10.1038/nn.2369. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz M, Bajo M, Siggins GR, Parsons LH, Schweitzer P. The endocannabinoid system tonically regulates inhibitory transmission and depresses the effect of ethanol in central amygdala. Neuropsychopharmacology. 2010;35:1962–1972. doi: 10.1038/npp.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, De Chiara V, Musella A, Kusayanagi H, Mataluni G, Bernardi G, et al. Chronic psychoemotional stress impairs cannabinoid-receptor control of GABA transmission in the striatum. J Neurosci. 2008;28:7284–7292. doi: 10.1523/JNEUROSCI.5346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Guidali C, Vigano D, Realini N, Valenti M, Massi P, et al. CB1 receptor activation in specific brain areas differently modulates anxiety-related behavior. Neuropharmacology. 2008;54:151–160. doi: 10.1016/j.neuropharm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Ruehle S, Aparisi Rey A, Remmers F, Lutz B. The endocannabinoid system in anxiety, fear memory and habituation. J Psychopharmacol. 2012;26:23–39. doi: 10.1177/0269881111408958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slanina KA, Schweitzer P. Inhibition of cyclooxygenase-2 elicits a CB1-mediated decrease of excitatory transmission in rat CA1 hippocampus. Neuropharmacology. 2005;49:653–599. doi: 10.1016/j.neuropharm.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Steindel F, Häring M, Marsicano B, Lutz B, Monory K.2008Differential coupling of G proteins to CB1 receptors in hippocampal glutamatergic and GABAergic neurons18th Annual Symposium on the Cannabinoids, Burlington, Vermont, International Cannabinoid Research Society. p. 119.
- Urwyler S, Gjoni T, jatiæ J, Dupuis DS. Mechanisms of allosteric modulation at GABAB receptors by CGP7930 and GS39783: effects on affinities and efficacies of orthosteric ligands with distinct intrinsic properties. Neuropharmacology. 2005;48:343–353. doi: 10.1016/j.neuropharm.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Urwyler S, Pozza MF, Lingenhoehl K, Mosbacher J, Lampert C, Froestl W, et al. N-N′-Dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine (GS39783) and structurally related compounds: novel allosteric enhancers of gamma-aminobutyric acid B receptor function. J Pharmacol Exp Ther. 2003;307:322–330. doi: 10.1124/jpet.103.053074. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, File SE. Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Behav. 2005;81:331–342. doi: 10.1016/j.pbb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Burston JJ, Leggett DC, Alekseeva OO, Razdan RK, Mahadevan A, et al. CB1 cannabinoid receptor-mediated modulation of food intake in mice. Br J Pharmacol. 2005;145:293–300. doi: 10.1038/sj.bjp.0706157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.