Abstract
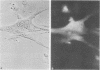
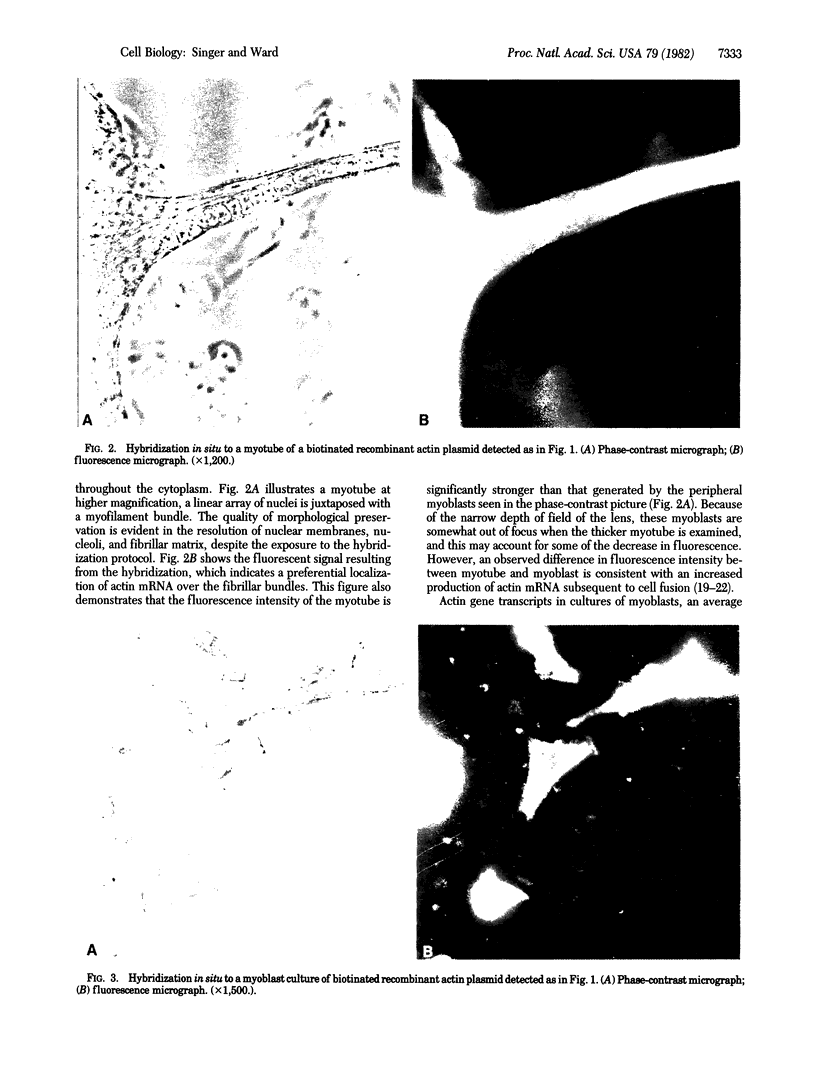
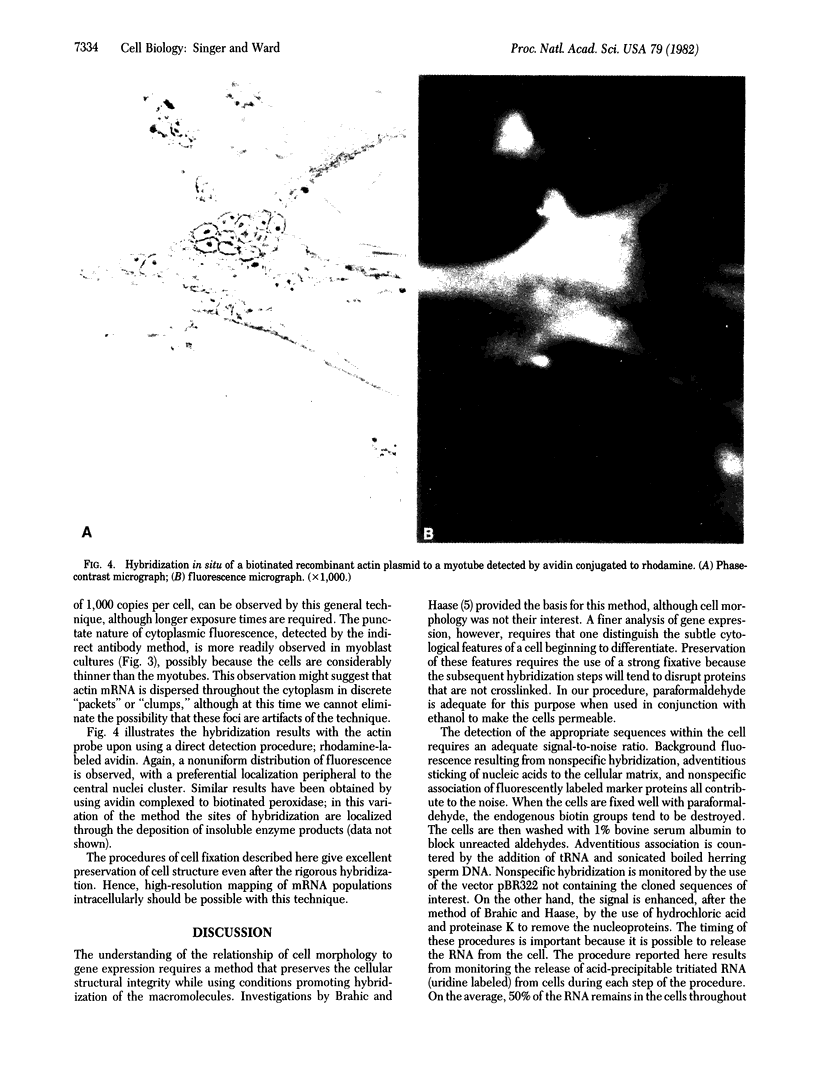
The chicken muscle tissue culture system has been used for visualizing actin gene expression after in situ hybridization. Cell differentiation is morphologically distinguishable in this system as the myoblasts fuse into myotubes. This differentiation involves the production of large amounts of actin required for myofibrils. The presence of actin mRNA has been observed in cells preserved with ethanol and paraformaldehyde by hybridizing a recombinant plasmid into which a biotinated analog of dUTP was incorporated by nick-translation. The biotin was then detected by using an anti-biotin antibody and a rhodamine-conjugated second antibody. Alternatively, avidin conjugated to rhodamine or avidin complexed to biotinated peroxidase has been used for mRNA detection. The procedure described preserved morphological detail yet is compatible with hybridization conditions and reveals the disposition of actin mRNA during gene expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerer L. M., Angerer R. C. Detection of poly A+ RNA in sea urchin eggs and embryos by quantitative in situ hybridization. Nucleic Acids Res. 1981 Jun 25;9(12):2819–2840. doi: 10.1093/nar/9.12.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman J. G., Wiegant J., van Duijn P. Cytochemical hybridization with fluorochrome-labeled RNA. I. Development of a method using nucleic acids bound to agarose beads as a model. J Histochem Cytochem. 1981 Feb;29(2):227–237. doi: 10.1177/29.2.6166653. [DOI] [PubMed] [Google Scholar]
- Bauman J. G., Wiegant J., van Duijn P. Cytochemical hybridization with fluorochrome-labeled RNA. II. Applications. J Histochem Cytochem. 1981 Feb;29(2):238–246. doi: 10.1177/29.2.6166654. [DOI] [PubMed] [Google Scholar]
- Berger S. L., Birkenmeier C. S. Inhibition of intractable nucleases with ribonucleoside--vanadyl complexes: isolation of messenger ribonucleic acid from resting lymphocytes. Biochemistry. 1979 Nov 13;18(23):5143–5149. doi: 10.1021/bi00590a018. [DOI] [PubMed] [Google Scholar]
- Brahic M., Haase A. T. Detection of viral sequences of low reiteration frequency by in situ hybridization. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6125–6129. doi: 10.1073/pnas.75.12.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capco D. G., Jeffery W. R. Differential distribution of poly(A)-containing RNA in the embryonic cells of Oncopeltus fasciatus. Analysis by in situ hybridization with a [3H]poly(U) probe. Dev Biol. 1978 Nov;67(1):137–151. doi: 10.1016/0012-1606(78)90305-6. [DOI] [PubMed] [Google Scholar]
- Cheung S. W., Tishler P. V., Atkins L., Sengupta S. K., Modest E. J., Forget B. G. Gene mapping by fluorescent in situ hybridization. Cell Biol Int Rep. 1977 May;1(3):255–262. doi: 10.1016/0309-1651(77)90050-9. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Clissold P. M., Arnstein H. R., Chesterton C. J. Quantitation of globin mRNA levels during erythroid development in the rabbit and discovery of a new beta-related species in immature erythroblasts. Cell. 1977 Jun;11(2):353–361. doi: 10.1016/0092-8674(77)90052-6. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate regulation of contractile protein synthesis during myoblast differentiation. Cell. 1978 Apr;13(4):599–611. doi: 10.1016/0092-8674(78)90211-8. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Ventura P., Gibbs C. J., Jr, Tourtellotte W. W. Measles virus nucleotide sequences: detection by hybridization in situ. Science. 1981 May 8;212(4495):672–675. doi: 10.1126/science.7221554. [DOI] [PubMed] [Google Scholar]
- Harding J. D., MacDonald R. J., Przybyla A. E., Chirgwin J. M., Pictet R. L., Rutter W. J. Changes in the frequency of specific transcripts during development of the pancreas. J Biol Chem. 1977 Oct 25;252(20):7391–7397. [PubMed] [Google Scholar]
- Hayashi S., Gillam I. C., Delaney A. D., Tener G. M. Acetylation of chromosome squashes of Drosophila melanogaster decreases the background in autoradiographs from hybridization with [125I]-labeled RNA. J Histochem Cytochem. 1978 Aug;26(8):677–679. doi: 10.1177/26.8.99471. [DOI] [PubMed] [Google Scholar]
- Jeffery W. R., Capco D. G. Differential accumulation and localization of maternal poly(A)-containing RNA during early development of the ascidian, Styela. Dev Biol. 1978 Nov;67(1):152–166. doi: 10.1016/0012-1606(78)90306-8. [DOI] [PubMed] [Google Scholar]
- John H. A., Patrinou-Georgoulas M., Jones K. W. Detection of myosin heavy chain mRNA during myogenesis in tissue culture by in vitro and in situ hybridization. Cell. 1977 Oct;12(2):501–508. doi: 10.1016/0092-8674(77)90126-x. [DOI] [PubMed] [Google Scholar]
- KONIGSBERG I. R. Clonal analysis of myogenesis. Science. 1963 Jun 21;140(3573):1273–1284. doi: 10.1126/science.140.3573.1273. [DOI] [PubMed] [Google Scholar]
- Kessler-Icekson G., Singer R. H., Yaffe D. The capacity of polyadenylated RNA from myogenic cells treated with actinomycin D to direct protein synthesis in a cell-free system. Eur J Biochem. 1978 Aug 1;88(2):403–410. doi: 10.1111/j.1432-1033.1978.tb12462.x. [DOI] [PubMed] [Google Scholar]
- Langer-Safer P. R., Levine M., Ward D. C. Immunological method for mapping genes on Drosophila polytene chromosomes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4381–4385. doi: 10.1073/pnas.79.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P. R., Waldrop A. A., Ward D. C. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenk R., Ransom L., Kaufmann Y., Penman S. A cytoskeletal structure with associated polyribosomes obtained from HeLa cells. Cell. 1977 Jan;10(1):67–78. doi: 10.1016/0092-8674(77)90141-6. [DOI] [PubMed] [Google Scholar]
- Neer A., Baran N., Manor H. In situ hybridization analysis of polyoma DNA replication in an inducible line of polyoma-transformed cells. Cell. 1977 May;11(1):65–71. doi: 10.1016/0092-8674(77)90317-8. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Bishop J. O. Changes in the mRNA population of chick myoblasts during myogenesis in vitro. Cell. 1977 Nov;12(3):751–765. doi: 10.1016/0092-8674(77)90275-6. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Yaffe D. Determination of actin messenger RNA in cultures of differentiating embryonic chick skeletal muscle. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4467–4471. doi: 10.1073/pnas.71.11.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudney J., Singer R. H. Electron microscopic visualization of the filamentous reticulum in whole cultured presumptive chick myoblasts. Am J Anat. 1979 Nov;156(3):321–336. doi: 10.1002/aja.1001560304. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rudkin G. T., Stollar B. D. High resolution detection of DNA-RNA hybrids in situ by indirect immunofluorescence. Nature. 1977 Feb 3;265(5593):472–473. doi: 10.1038/265472a0. [DOI] [PubMed] [Google Scholar]
- Venezky D. L., Angerer L. M., Angerer R. C. Accumulation of histone repeat transcripts in the sea urchin egg pronucleus. Cell. 1981 May;24(2):385–391. doi: 10.1016/0092-8674(81)90328-7. [DOI] [PubMed] [Google Scholar]