Abstract
Glutamatergic abnormalities in corticostriatal brain circuits are thought to underlie obsessive–compulsive disorder (OCD). Whether these abnormalities exist in adults with OCD is not clear. We used proton magnetic resonance spectroscopy (1H MRS) to test our hypothesis that unmedicated adults with OCD have reduced glutamate plus glutamine (Glx) levels in the medial prefrontal cortex (MPFC) compared with healthy controls. Levels of γ-aminobutyric acid (GABA) were also explored. Twenty-four unmedicated adults with OCD and 22 matched healthy control subjects underwent 1H MRS scans at 3.0 T. Resonances of both Glx and GABA were obtained using the standard J-editing technique and assessed as ratios relative to voxel tissue water (W) in the MPFC (the region of interest) and the dorsolateral prefrontal cortex (DLPFC) to explore the regional specificity of any finding. In the MPFC, Glx/W did not differ by diagnostic group (p=0.98) or sex (p=0.57). However, GABA/W was decreased in OCD (2.16±0.46 × 10−3) compared with healthy controls (2.43±0.45 × 10−3, p=0.045); moreover, age of OCD onset was inversely correlated with MPFC GABA/W (r=−0.50, p=0.015). MPFC GABA/W was higher in females than in males. In the DLPFC, there were no main effects of diagnosis or gender on Glx/W or GABA/W. These data indicate that unmedicated adults with OCD do not have Glx abnormalities in a MPFC voxel that includes the pregenual anterior cingulate cortex. However, they may have decreased MPFC GABA levels. How GABA abnormalities might contribute to corticostriatal dysfunction in OCD deserves further study.
Keywords: OCD, GABA, Glx, anterior cingulate cortex, dorsolateral prefrontal cortex, medial prefrontal cortex, magnetic resonance spectroscopy
INTRODUCTION
Dysfunction in corticostriatal circuits is thought to underlie symptoms of obsessive–compulsive disorder (OCD). Studies measuring blood flow or glucose metabolism by positron emission tomography and hemodynamic response by functional magnetic resonance imaging find increased activity in the orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), and striatum in people with OCD (reviewed in Maia et al, 2008). This increased activity may reflect primary abnormalities in glutamatergic neurotransmission (Carlsson, 2001; Pittenger et al, 2011; Rosenberg and Keshavan, 1998; Wu et al, 2012). At the same time, the inhibitory neurotransmitter γ-aminobutyric acid (GABA) modulates cortical glutamatergic neurons (Gonzalez-Burgos and Lewis, 2008), and abnormalities in cortical inhibitory processes have been reported in OCD (Greenberg et al, 2000; Richter et al, 2012). Alterations in GABA neurotransmission could also have a role in OCD (Rosenberg et al, 2001).
Proton magnetic resonance spectroscopy (1H MRS) is a noninvasive imaging technique that can measure brain levels of glutamate–glutamine (Glx) and GABA in vivo. Owing to increased magnetic susceptibility effects associated with nearby nasal cavity and frontal sinuses, MRS measurement in the OFC is technically challenging. Thus, three MRS studies of the cortex in OCD focused on the ACC. Using a 1.5 T MR system, Rosenberg et al (2004) found decreased Glx levels in 3.0-cm3 ACC voxels in 20 unmedicated pediatric OCD patients compared with 14 healthy controls. Using a 3.0T MR system, Yucel et al (2008) found no overall differences in Glx levels in 6.5-cm3 dorsal or rostral ACC voxels in 20 adults with OCD (some on medication) compared with 26 healthy controls. On the other hand, post-hoc analyses revealed that female OCD patients had significantly reduced Glx levels in the rostral ACC that included the voxel studied by Rosenberg et al (2004), and these Glx levels were correlated with symptom severity. Using a 1.5T MR system, Starck et al (2008) found no significant differences in Glx levels in 3.6-cm3 ACC voxels in 9 adults with OCD (some on medication) and 15 controls; their voxel was more dorsal and posterior.
Methodological differences, including sample size, subject age (eg, pediatric or adult), medication status, and MR methods and instrumentation (1.5 vs 3.0 T), may have contributed to these discrepant findings. Also, these studies did not measure Glx separately from GABA. As a result, it is unclear whether ACC Glx is increased, decreased or unchanged in unmedicated adults with OCD.
To address these issues, we used the J-editing technique (Rothman et al, 1993) at 3.0 T, which allows separate measurement of Glx and GABA in cortical regions. We used this method to measure cortical Glx and GABA in 24 unmedicated adults with OCD and 22 healthy control subjects. The region of interest was the medial prefrontal cortex (MPFC), which includes the pregenual (rostral) ACC, encompassing the voxel assessed by Rosenberg et al (2004) and overlapping that used by Yucel et al (2008). To assess regional specificity, we also measured Glx and GABA in the dorsolateral prefrontal cortex (DLPFC), a brain region not directly implicated in most OCD models. Following MRS findings in unmedicated pediatric OCD patients (2004) and consonant with current OCD models (Pittenger et al, 2011; Wu et al, 2012), we hypothesized that unmedicated adult OCD patients would have decreased Glx in the MPFC compared with healthy controls. Exploratory analyses examined whether there were group differences in MPFC GABA levels, and whether MPFC Glx or GABA levels were associated with OCD severity, OCD symptom dimensions or age of OCD onset.
MATERIALS AND METHODS
Subjects
The Institutional Review Board of the New York State Psychiatric Institute/Columbia University approved the study. Subjects were recruited by advertisements and word of mouth and provided written informed consent.
Eligible subjects were between the ages of 18 and 55 and had no significant medical problems and no current or past neurological disorder (other than tic disorder), and no current or past history of substance or alcohol abuse or dependence (with the exception of nicotine). Pregnant, nursing and postmenopausal women and those using hormonal contraceptives were excluded. OCD subjects fulfilled the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria for at least 1 year and had no other current Axis I psychiatric disorder, with the exception of specific phobia in one subject. This was achieved by recruiting OCD subjects from the community (eg, instead of relying solely upon psychiatrist referral). None were receiving OCD treatment; they were either naïve to cognitive-behavioral therapy (CBT) and psychoactive medications or free of both for at least 10 weeks before scanning. Healthy controls had no current or past DSM-IV Axis I disorder, and no exposure to psychoactive medications; none had a family history of OCD as assessed by the Family History Screen (Weissman et al, 2000). Healthy controls were recruited who matched the OCD subjects on age, sex, ethno-racial categories and cigarette-smoking status.
Diagnoses were made during a psychiatric evaluation by expert clinicians and confirmed by trained clinical raters using the Structured Clinical Interview for DSM-IV (First et al, 1996). Medical health was established by physical examination, blood and urine tests (including a urine drug screen), and electrocardiogram. OCD and depressive severity were assessed by trained clinician raters using the Yale-Brown Obsessive Compulsive Scale (Y-BOCS; Goodman et al, 1989a, 1989b) and the 17-item Hamilton Depression Rating Scale (HAM-D; Hamilton, 1960). The Y-BOCS checklist provided scores for each OCD patient along five symptom dimensions (contamination and cleaning, taboo thoughts, doubt and checking, symmetry and ordering, and hoarding), using the approach recommended by Pinto et al (2007, 2008, 2009).
MR Acquisition Procedures
Overview
Subjects underwent one scanning session on a research-dedicated General Electric 3.0T EXCITE MR system at the New York State Psychiatric Institute. The session lasted about 60 min and included a structural MRI and two MRS acquisitions.
Structural MRI
A three-plane, low-resolution, high-speed scout imaging series was obtained, followed by a series of high-resolution scans, consisting of standardized axial, coronal, and sagittal T1-, T2-, and spin density-weighted scans that were appropriately obliqued for prescribing the 1H MRS voxels. A T1-weighted spoiled gradient-recalled echo (SPGR) volumetric scan (TR/TE=30/8 ms, flip angle 45°, field of view 24 cm, 256 × 256 matrix, 124 coronal slices and a slice thickness of 1.0 mm) was acquired for brain tissue segmentation. A neuroradiologist confirmed that all MRI scans were free of gross structural abnormalities.
MRS scans
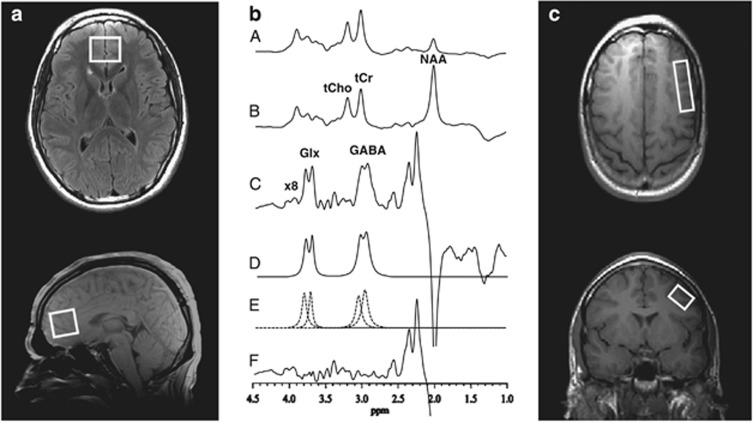
Glx and GABA MRS data were acquired with an 8-channel phased-array head coil using a standard PRESS sequence with volume-selective J-editing difference method (Rothman et al, 1993) as modified by Sailasuta et al (2001) and recently described (Geramita et al, 2011). MPFC measurements were acquired in 13 min from a 2.5 × 3.0 × 2.5 cm (18.8 cm3) voxel using TE/TR 68/1500 ms and 256 interleaved excitations (512 total) with the editing pulse on or off. The MPFC voxel was located anterior to the genu of the corpus callosum, oriented along the anterior–posterior commissure line and centered on the interhemispheric fissure, and included portions of Brodmann areas 24, 32, and 10 including a portion of the pregenual ACC (Figure 1a). DLPFC measurements were acquired in 26 min (512 interleaved excitations, 1024 total) from a 1.0 × 2.0 × 4.8 (9.6 cm3) voxel placed in the left middle frontal gyrus (Brodmann areas 9 and 46) angled parallel to the brain surface (Figure 1c, Kegeles et al, 2012).
Figure 1.
Medial prefrontal cortex (MPFC) and dorsolateral prefrontal cortex (DLPFC) Voxels and Sample 1H MR Spectral Data. (a) Axial (top) and sagittal localizer images showing MPFC voxel size and location. (b) PRESS 1H MR spectra acquired in 13 min from the MPFC voxel using TE/TR 68/1500 ms, and 256 interleaved excitations (total 512) with editing radiofrequency pulse on (A) or off (B). With the editing pulse off (B), a standard PRESS spectrum is obtained and yields high-quality spectra for NAA, tCr, and tCho. (C) The difference of the subspectra in (A) and (B) showing the detected GABA and Glx peaks. (D) The best-fit model curve. (E) Individual components of the fit. (F) The residual difference between the experimental and fitted curves. The fitting procedure yields areas under the GABA and Glx peaks, which are proportional to concentrations. (c) Axial (top) and coronal localizer images showing DLPFC voxel size and location. Spectra for the DLPFC were obtained as depicted in (b) for the MPFC, but in 26 min using 512 interleaved excitations (1024 total), and are not shown as they are visually indistinguishable from the sample provided in (b) for the MPFC.
GABA and Glx levels were measured by applying a frequency-selective inversion pulse that avoids excitation of the GABA C-3 peak at 1.9 p.p.m. on alternate scans. At TE 68 ms, the result is selective inversion of the outer lines of the C-4 GABA triplet resonance at 3.0 p.p.m. on alternate scans by, respectively, inhibiting and allowing its J-modulation (Figure 1b). Subtracting these two subspectra yields the desired GABA difference spectrum, consisting of the outer lines of the GABA C-4 triplet at 3.0 p.p.m., whereas the much stronger overlapping total creatine singlet resonance at 3.0 p.p.m. (which is not J-modulated) is eliminated. Optimized for GABA detection, this pulse sequence also achieves reliable detection of the combined resonances of Glx at 3.7 p.p.m. (Geramita et al, 2011; Kegeles et al, 2006) due to their high structural, chemical and magnetic similarities with GABA. This pulse sequence been used in other patient populations to separate Glx from GABA (Hasler et al, 2007; Kegeles et al, 2012). The Glx signal is sufficiently strong that the partially edited measurement has high reliability (Hasler et al, 2007; Kegeles et al, 2006, 2012), comparable to that of GABA itself.
The strong unsuppressed voxel tissue water signal, necessary for 8-channel coil data reconstruction and intensity normalization (vide infra), was automatically recorded in two excitations with the water suppression and J-editing pulses turned off, and then stored in separate memory blocks within the same raw data file as the neurotransmitter signals, which were recorded immediately thereafter and stored in subsequent memory blocks. The static magnetic field homogeneity was automatically adjusted by the host computer on the water proton signal, yielding typical linewidths at half maximum of 12–13 Hz, with 20 Hz being the upper limit of field homogeneity that was considered acceptable.
1H MRS Data Processing and Quantification
Data processing and quantification procedures have been described (Gabbay et al, 2011; Geramita et al, 2011). Briefly, the 8-channel phased-array coil data recorded were combined into single regular time-domain free-induction decay signals, using a C-language computer program that implemented a previously published algorithm (Brown, 2004), with the unsuppressed voxel tissue water signal from each receiver coil element providing the required relative array coil sensitivities. The two resulting free-induction decay signals for the editing pulses on/off scans were subtracted in the time domain to yield the difference signal, which was filtered with an exponential multiplication function corresponding to a 5-Hz linebroadening and then Fourier-transformed to obtain the GABA and Glx spectra (Figure 1b). The area under each neurotransmitter peak was obtained using a robust and highly optimized public-domain Levenberg–Marquardt nonlinear least-squares IDL minimization routine (Markwardt, 2009) to model the resonances in the frequency domain as a linear combination of pseudo-Voigt functions; this enables more precise analysis of line shapes that consist of mixtures of Lorentzian and Gaussian functions (Marshall et al, 2000), as is often the case for in vivo spectra. The IDL fitting procedure automatically reports the covariance matrix for the estimated set of MRS parameters, from which the parameter correlation matrix, statistical errors at one s.d., and uncertainties are computed as measures of goodness of fit (Markwardt, 2009). Detailed technical information on shim quality and goodness of fit is presented in Supplementary Information. To enable groupwise comparisons, the GABA and Glx peak areas were expressed as ratios relative to the area of the unsuppressed voxel tissue water (W).
Assessment of Voxel Tissue Heterogeneity
GABA differences have been reported between gray matter (GM) and white matter (WM, Choi et al, 2006; Jensen et al, 2005), whereas cerebral spinal fluid (CSF) concentrations are negligible in comparison with GM and WM (Petroff and Rothman, 1998). We thus performed volumetric MRI-based tissue segmentation to compare the proportions of GM, WM, or CSF in the voxels by diagnostic group. Segmentation and classification was based on the signal-intensity histogram obtained using the commercial software MEDx (Medical Numerics, Sterling, VA) from each subject's volumetric (SPGR) MRI. From the histogram, a segmentation mask of each voxel was generated and the proportions of GM, WM, and CSF computed.
Statistical Analyses
The final sample consisted of 24 OCD and 22 healthy control subjects, after excluding one OCD subject who declined imaging after signing consent, and another who was an extreme outlier (z-scores>5 for Glx/W concentrations in the MPFC and DLPFC). Demographic and clinical characteristics and proportion of tissue composition were compared across diagnostic groups using χ2 tests for categorical and independent sample t-tests for continuous variables.
Two-way analysis of variance (ANOVA) was used to test for metabolite differences between diagnostic groups. We hypothesized that MPFC Glx levels would be reduced in OCD subjects compared with controls. Exploratory analyses examined group differences in MPFC GABA levels. Neither Glx nor GABA was hypothesized to differ in the DLPFC. As sex effects on cortical GABA levels have been reported (Sanacora et al, 1999), a two-way ANOVA examined the effects of diagnostic group (OCD vs healthy control), sex (male vs female), and their interaction on Glx/W or GABA/W levels. The interaction term was not retained in the final model if not significant (p<0.05).
Within the OCD group, the relationship between MPFC Glx and GABA levels and OCD severity (measured by the Y-BOCS), age of OCD onset, and each of the five OCD symptom dimensions (contamination and cleaning, taboo thoughts, doubt, and checking, symmetry and ordering, and hoarding) was examined using Pearson's product-moment correlation. All statistical tests were two-tailed with level of significance α=0.05.
RESULTS
Sample Characteristics
Demographic and clinical characteristics of the 24 OCD subjects and 22 healthy control subjects with viable MRS data are provided in Table 1. There were no significant group differences in age (t=0.14, df=44, p=0.89), sex (χ2=0.32, df=1, p=0.57), race (caucasian vs noncausasian: χ2=0.03, df=1, p=0.86), or days since last menstrual period in females (t=0.82, df=19, p=0.42).
Table 1. Demographic and Clinical Characteristics.
| Parameter | OCD (n=24) | Healthy control (n=22) |
|---|---|---|
| Mean age in years (SD) | 29 (10) | 29 (10) |
| Number of males/females | 14/10 | 11/11 |
| Race-ethnicity | 18Cau/2His/3As/1O | 17Cau/2His/2As/1O |
| Mean age of OCD onset in years (SD) | 16 (7) | — |
| OCD in first-degree relative (by subject self-report) | 3 | 0 |
| Mean Y-BOCS (SD) | 26 (4) | — |
| Mean HAM-D (SD) | 5 (5) | — |
| DSM-IV axis I psychiatric comorbidity | ||
| None | 20 | 22 |
| Current specific phobia and history of MDD | 1 | — |
| History of MDD only | 3 | — |
| History of substance or alcohol abuse/dependencea | 0 | 0 |
| Chronic tic disorder | 2 | 0 |
| Treatment history | ||
| Any prior exposure to psychotropic medications | 8 | 0 |
| Prior exposure to SRIs | 8 | |
| Mean wks (SD, range) since last SRI dose | 41 (49, 10–156) | |
| Prior exposure to other medications | 3 | |
| Mean wks (SD, range) since last dose | 161 (130, 84–312) | |
| Prior CBT treatment | 2 | |
Abbreviations: As, Asian; Cau, Caucasian; HAM-D, Hamilton Depression Rating Scale (17-item); HIS, hispanic; MDD, major depressive disorder; O, other; OCD, obsessive-compulsive disorder; SRI, serotonin reuptake inhibitor; wks, weeks; Y-BOCS, Yale-Brown Obsessive Compulsive Scale.
Current nicotine use: two subjects in each group smoked ⩽10 cigarettes per day.
The OCD subjects had clinically significant symptoms, with a mean Y-BOCS score of 26 and a range from 20 (moderate OCD) to 36 (extreme OCD). All five OCD symptom dimensions were represented, with most patients exhibiting symptoms in more than one domain. Five had symptoms only in one domain (hoarding, n=1; contamination and cleaning symptoms, n=1; taboo thoughts, n=2; symmetry and ordering, n=1). Few had ever received OCD treatment. None were receiving treatment at the time of MRS scanning. As shown in Table 1, 16 of the 24 OCD subjects were naïve to all psychotropic medications. The other eight had previously received SRIs (weeks since last SRI: mean (SD)=41 (49) weeks; range: 10–156 weeks); two had also been exposed to buproprion and one to olanzapine (more than 84, 88, and 312 weeks before this study). Only two OCD subjects had ever received CBT consisting of exposure and response prevention (more than 2 and 5 years before this study).
Measures of tissue segmentation are provided in Table 2 (for MPFC) and Table 3 (for DLPFC). There were no significant group differences in tissue composition in either voxel (MPFC: all p-values >0.57; DLPFC: all p-values >0.10).
Table 2. MPFC Metabolite Levels and Tissue Composition.
| OCD (n=24) | Healthy control (n=22) | 95% CI for difference in means | |
|---|---|---|---|
| MRS metabolite | Mean (SD) | Mean (SD) | |
| Glx/Wa | 1.58 (0.29) × 10−3 | 1.57 (0.29) × 10−3 | −0.17 × 10−3, 0.17 × 10−3 |
| GABA/Wa | 2.16 (0.46) × 10−3 | 2.43 (0.45) × 10−3 | 0.01 × 10−3, 0.55 × 10−3 |
| Tissue composition | Mean percent (SD) | Mean percent (SD) | |
| Cerebral spinal fluid | 10.58 (3.22) | 10.32 (3.06) | −2.13, 1.61 |
| Gray matter | 53.23 (3.04) | 53.72 (2.90) | −1.27, 2.26 |
| White matter | 36.19 (3.93) | 35.95 (3.60) | −2.48, 2.01 |
Abbreviations: CI, confidence interval; GABA, γ-aminobutyric acid; Glx, glutamate plus glutamine; MPFC, medial prefrontal cortex (voxel includes the pregenual anterior cingulate cortex); OCD, obsessive–compulsive disorder; W, water.
Institutional units.
Table 3. DLPFC Metabolite Levels and Tissue Composition.
| OCD (n=24) | Healthy control (n=22) | 95% CI for difference in means | |
|---|---|---|---|
| MRS metabolite | Mean (SD) | Mean (SD) | |
| Glx/Wa | 3.29 (0.78) × 10−3 | 3.38 (0.77) × 10−3 | −0.37 × 10−3, 0.56 × 10−3 |
| GABA/Wa | 5.34 (1.04) × 10−3 | 5.17 (1.04) × 10−3 | −0.79 × 10−3, 0.45 × 10−3 |
| Tissue composition | Mean percent (SD) | Mean percent (SD) | |
| Cerebral spinal fluid | 16.46 (7.47) | 13.11(6.39) | −7.50, 0.80 |
| Gray matter | 51.42 (5.57) | 51.36 (7.86) | −4.08, 3.96 |
| White matter | 32.13 (5.57) | 35.53 (8.06) | −0.69, 7.49 |
Abbreviations: CI, confidence interval; DLPFC, dorsolateral prefrontal cortex; GABA, γ-aminobutyric acid; Glx, glutamate plus glutamine; OCD, obsessive–compulsive disorder; W, water.
Institutional units.
MPFC: Glx and GABA Levels
In the MPFC voxel that included the pregenual ACC, the interaction for Glx/W between diagnostic group and sex was not significant (F1,42=0.238, p=0.62). In the final model that included only main effects, there were no significant main effects of diagnostic group on Glx/W (F1,43=0.001, p=0.98) or of sex on Glx/W (F1,43=0.323, p=0.57). The estimated marginal means and 95% confidence intervals (CI) of the difference in the means by diagnostic group are provided in Table 2.
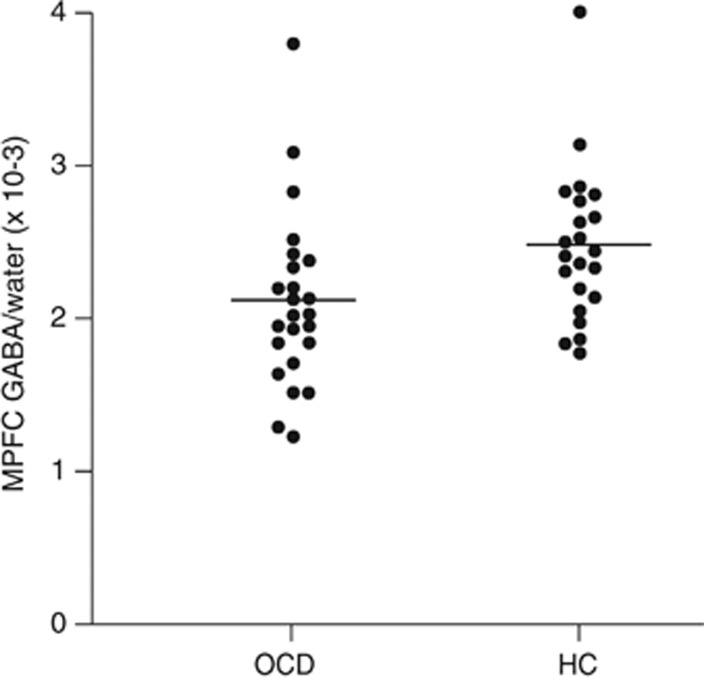
In the MPFC voxel, the interaction for GABA/W between diagnostic group and sex was not significant (F1,42=1.303, p=0.26). In the final model that included only main effects, there was a main effect of diagnostic group on MPFC GABA levels, with GABA/W significantly lower in OCD patients (F1,43=4.257, p=0.045). The estimated marginal means and 95% CIs of the difference in the means are listed in Table 2 by diagnostic group. A scatter plot of individual values is shown in Figure 2. There was also a main effect of sex on MPFC GABA levels, with GABA/W levels higher in all females (n=21) vs all males (n=25; mean (SD): 2.59 (0.45) × 10−3 vs 2.0 (0.46) × 10−3 institutional units; 95% CI of the difference in the means: 0.32 × 10−3, 0.86 × 10−3; F1,43=19.579, p<0.001).
Figure 2.
γ-Aminobutyric acid (GABA) levels in the medial prefrontal cortex (MPFC). Scatterplot of GABA/W in the MPFC for subjects with obsessive–compulsive disorder (OCD, n=24) and matched healthy controls (HC, n=22). OCD subjects had significantly lower levels of GABA/W (see Results). Mean values are denoted with the black line.
One OCD subject and one healthy control had MPFC GABA/W values three SD above their group means. If they are excluded, the results are qualitatively the same (eg, significant main effects of group (p=0.010) and of sex (p<0.001), no significant group by sex interaction (p=0.17)). There were no clinical or measurement factors justifying exclusion of these subjects from the sample, so they were included.
DLPFC: Glx and GABA Levels
In the DLPFC, the interaction between diagnostic group and sex was not significant for Glx/W (F1,42=0.303, p=0.58). In the final model that included only main effects, there were no main effects of diagnostic group on Glx/W (F1,43=0.177, p=0.67) or of sex on Glx/W (F1,43=0.98, p=0.32). The interaction between diagnostic group and sex was not significant for GABA/W (F1,42=2.46, p=0.12). In the final model, there were no main effects of diagnostic group on GABA/W (F1,43=0.310, p=0.33) or of sex on GABA/W (F1,43=0.951, p=0.33). The estimated marginal means and 95% CI of the difference in means by diagnostic group are shown in Table 3.
Clinical Correlations
In the OCD sample, Glx/W and GABA/W in the MPFC were not significantly correlated with OCD severity (all p-values>0.27) or any of the five OCD symptom dimensions (all p-values>0.26). There was a significant negative correlation between age of OCD onset and MPFC GABA/W (r=−0.50, p=0.015) but not Glx/W (p>0.94). The association was not statistically different between the men and women (z=1.06, p=0.28).
DISCUSSION
Contrary to our hypothesis, we did not find that unmedicated adult OCD patients had decreased Glx in the MPFC compared with healthy controls. Unexpectedly, we did find reduced MPFC GABA levels in OCD. To our knowledge, this is the first time that MPFC GABA abnormalities in OCD have been reported.
Our finding that OCD adults (ages 20–52) did not differ from healthy controls in MPFC Glx levels is in contrast to the findings of Rosenberg et al (2004). They found significant decreases (∼15%) in Glx levels (using a 1.5 T MR system) in pediatric OCD patients (ages 7–19) in an ACC voxel that our MPFC voxel encompassed. There are several possible explanations. One is that there are Glx reductions both in pediatric and adult OCD samples, but our larger voxel diluted a more localized Glx signal difference. However, use of this larger voxel did not prevent detection of significant MPFC Glx findings in other diagnostic groups using similar methods (Hasler et al, 2007; Kegeles et al, 2012). Another possibility is that there are reduced Glx levels in pediatric but not adult OCD patients in this brain region, suggesting that the pathophysiology of pediatric and adult OCD might differ. Finally, our GABA finding raises the possibility that there are GABA (and not Glx) reductions in the MPFC that may become more apparent with spectral editing and the improved spectral resolution available at higher field strength.
Using a 3.0 T MR system and fitting Glx by post-processing, Yucel et al (2008) also found no differences in ACC Glx levels in 20 OCD subjects compared with 26 healthy controls. However, in post-hoc analyses, Glx levels were reduced in OCD females (n=10) in a rostral ACC voxel that partially overlapped our MPFC voxel. We found no effect of diagnosis and no interaction between diagnosis and sex on MPFC Glx/W levels. Possible explanations for the different findings include: (1) anatomical differences between the voxels; (2) our use of J-editing to separate the GABA signal from Glx; and (3) the fact that more than half of their OCD sample was on serotonergic medication at the time of imaging. None of our OCD subjects were on medication, and many were drug-naïve.
Because we are the first to report decreased MPFC GABA levels in OCD, this serendipitous finding requires replication in an independent sample. Correcting for multiple comparisons would eliminate it, although at the risk of Type II error. At the same time, this finding could focus more attention on the GABA system and its potential contribution to corticostriatal dysfunction in OCD. The significant negative association that we found between age of OCD onset and MPFC GABA levels also warrants more study: although contrary to the idea that early onset of illness leads to greater abnormalities, it is consistent with other data, suggesting that the pathophysiology of early- and late-onset OCD differs (Busatto et al, 2001; Dickel et al, 2006, 2007; Huyser et al, 2009; Simpson et al, 2011). That DLPFC GABA levels did not differ between OCD and healthy controls suggests there may be some regional specificity to the MPFC finding.
If MPFC GABA levels are reduced in OCD, future research will need to clarify both the functional consequences on corticostriatal circuits and the clinical implications for patients. MRS measures total GABA levels, which predominantly reflect intracellular stores (Petroff, 2002). Thus, decreased GABA levels could reflect fewer GABAergic interneurons and result in reduced MPFC inhibitory tone, consistent with reports of cortical inhibitory dysfunction in OCD (Greenberg et al, 2000; Richter et al, 2012). Reduced MPFC inhibitory tone is also consistent with current OCD models that posit abnormal frontostriatal activation (Maia et al, 2008; Pittenger et al, 2011; Ting and Feng, 2011; Wu et al, 2012), as reduced MPFC inhibitory tone could generate abnormal striatal activation either directly due to ACC projections to the striatum or indirectly due to ACC projections to the OFC (Price and Drevets, 2010; Schmahmann and Pandya, 2006). On the other hand, acute psychological stress has recently been shown to decrease MPFC GABA levels in healthy controls (Hasler et al, 2010). Therefore, reduced MPFC GABA levels may not lead to OCD symptoms per se but simply reflect the emotional and stressed state of these patients. In either case, developing interventions that modulate MPFC inhibitory tone may prove therapeutic.
Abnormalities in MPFC GABA levels have been reported in some other psychiatric disorders. Although Hasler et al (2009) found no differences in MPFC GABA in unmedicated subjects with panic disorder or in unmedicated patients with major depressive disorder (MDD, Hasler et al, 2007), Kegeles et al (2012) found increased GABA levels in the MPFC of unmedicated patients with schizophrenia using identical methodology to ours. In more dorsal voxels, decreases in GABA levels were found in unmedicated patients with MDD (Hasler et al, 2007) and in recovered, medication-free patients with a history of MDD (Bhagwagar et al, 2008). Of note, when the four patients in our OCD sample with a history of MDD were removed, decreases in MPFC GABA levels were even more robust in OCD patients compared with controls (F1,39=8.079, p=0.007). In the depression studies, MPFC Glx levels were also reduced, and Glx and GABA levels were significantly correlated. Neither was the case in our OCD sample. Disruption of the normal positive correlation between GABA and Glx has previously been reported under conditions of abnormal GABA levels (Hasler et al, 2010; Sanacora et al, 2004; Yang and Shen, 2009).
In addition to finding significantly reduced MPFC GABA levels in OCD subjects, we also found that females had significantly higher levels of MPFC GABA than males. This was independent of the effect of OCD diagnosis as demonstrated by the two-way ANOVA. Although Sanacora et al. (1999) found that occipital GABA levels were significantly higher in females than in males, gender effects on cortical GABA levels have not been consistently reported. GABA levels have been shown to vary with the menstrual cycle in some cortical regions (Epperson et al, 2002), possibly contributing to the variable findings. Importantly, in our sample, OCD and control females did not significantly differ in days since last menstrual period.
Strengths of our study include the fact that we measured Glx in the largest sample of unmedicated adults with OCD to date; most of the OCD subjects were also treatment-naïve. This is also the first OCD study to apply J-editing to separately measure GABA. Limitations include the inability of the standard J-editing method to separate glutamate from glutamine, although the Glx resonance likely predominantly reflects glutamate levels, which are typically five to six times higher than those of glutamine (Kaiser et al, 2005). Moreover, the low tissue concentration of GABA necessitates relatively large voxels (eg, 10–20 cm3) to attain adequate signal-to-noise ratio for reliable quantification in a clinically tolerable scan time. Finally, the GABA MRS peak is an admixture of mobile macromolecule signal with that from GABA itself (Behar et al, 1994). We previously assessed the macromolecular contribution to the total peak in healthy volunteers under our experimental conditions and found no regional differences (Kegeles et al, 2007). However, it is not known whether this contribution might differ in OCD.
In conclusion, we did not find differences in Glx levels in adults with OCD in a MPFC voxel that included the pregenual ACC, but we did find decreased GABA levels. There was also a strong independent effect of gender on MPFC GABA levels. Given that GABA deficits in OCD were unexpected, replication studies are warranted. How GABA abnormalities might contribute to corticostriatal circuitry dysfunction in OCD deserves further study.
Acknowledgments
This study was supported by NIH (R01 MH073915 (HBS), K24 MH091555 (HBS) and R01 MH075895 (DCS)), and by the New York State Office of Mental Health. We thank staff of the Anxiety Disorders Clinic for help with study administration (Donna Vermes), clinical evaluations (Raphael Campeas), database management (Andrew Schmidt and Page Van Meter), and expert research assistance (Jessica McCarthy, Rena Staub, Liane Hunter). We thank Dr Holly Moore for comments on a prior version of this manuscript. These data were presented in part at the annual meetings of the International Society for Magnetic Resonance in Medicine, Stockholm, May 2010; the Society of Nuclear Medicine, Salt Lake City, June 2010, and the American College of Neuropsychopharmacology (ACNP), Miami, Florida, December 2010.
In the past five years, Dr Simpson has received medication at no-cost from Janssen Pharmaceuticals for another NIMH-funded study, consulting fees from Pfizer for advice regarding the medication Lyrica, and research funds from Neuropharm and from Transcept Pharmaceuticals to conduct clinical trials of investigational medications for OCD; Dr Shungu has received consulting fees from Hoffmann- La Roche; Dr Slifstein has received research funds from IntraCellular and Pierre-Fabre, and consulting fees from GlaxoSmithKline and Amgen; Dr Kegeles has received research funds from Amgen and Pfizer. All other authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Behar KL, Rothman DL, Spencer DD, Petroff OA. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magn Reson Med. 1994;32:294–302. doi: 10.1002/mrm.1910320304. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Boorman E, P MM, et al. Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication-free, recovered depressed patients. Int J Neuropsychopharmacol. 2008;11:255–260. doi: 10.1017/S1461145707007924. [DOI] [PubMed] [Google Scholar]
- Brown MA. Time-domain combination of MR spectroscopy data acquired using phased-array coils. Magn Reson Med. 2004;52:1207–1213. doi: 10.1002/mrm.20244. [DOI] [PubMed] [Google Scholar]
- Busatto GF, Buchpiguel CA, Zamignani DR, Garrido GE, Glabus MF, Rosario-Campos MC, et al. Regional cerebral blood flow abnormalities in early-onset obsessive-compulsive disorder: an exploratory SPECT study. J Am Acad Child Adolesc Psychiatry. 2001;40:347–354. doi: 10.1097/00004583-200103000-00015. [DOI] [PubMed] [Google Scholar]
- Carlsson ML. On the role of prefrontal cortex glutamate for the antithetical phenomenology of obsessive compulsive disorder and attention deficit hyperactivity disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:5–26. doi: 10.1016/s0278-5846(00)00146-9. [DOI] [PubMed] [Google Scholar]
- Choi IY, Lee SP, Merkle H, Shen J. In vivo detection of gray and white matter differences in GABA concentration in the human brain. Neuroimage. 2006;33:85–93. doi: 10.1016/j.neuroimage.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Dickel DE, Veenstra-VanderWeele J, Bivens NC, Wu X, Fischer DJ, Van Etten-Lee M, et al. Association studies of serotonin system candidate genes in early-onset obsessive-compulsive disorder. Biol Psychiatry. 2007;61:322–329. doi: 10.1016/j.biopsych.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Dickel DE, Veenstra-VanderWeele J, Cox NJ, Wu X, Fischer DJ, Van Etten-Lee M, et al. Association testing of the positional and functional candidate gene SLC1A1/EAAC1 in early-onset obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:778–785. doi: 10.1001/archpsyc.63.7.778. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Haga K, Mason GF, Sellers E, Gueorguieva R, Zhang W, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59:851–858. doi: 10.1001/archpsyc.59.9.851. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition. Biometrics Research Department, New York State Psychiatric Institute: New York, NY; 1996. [Google Scholar]
- Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, et al. Anterior cingulate cortex y-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch Gen Psychiatry. 2011;69:139–149. doi: 10.1001/archgenpsychiatry.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geramita M, van der Veen JW, Barnett AS, Savostyanova AA, Shen J, Weinberger DR, et al. Reproducibility of prefrontal gamma-aminobutyric acid measurements with J-edited spectroscopy. NMR Biomed. 2011;24:1089–1098. doi: 10.1002/nbm.1662. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, et al. The Yale-Brown Obsessive Compulsive Scale. II. Validity. Arch Gen Psychiatry. 1989a;46:1012–1016. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989b;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Ziemann U, Cora-Locatelli G, Harmon A, Murphy DL, Keel JC, et al. Altered cortical excitability in obsessive-compulsive disorder. Neurology. 2000;54:142–147. doi: 10.1212/wnl.54.1.142. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Geraci M, Shen J, Pine D, Drevets WC. Prefrontal cortical gamma-aminobutyric acid levels in panic disorder determined by proton magnetic resonance spectroscopy. Biol Psychiatry. 2009;65:273–275. doi: 10.1016/j.biopsych.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Grillon C, Drevets WC, Shen J. Effect of acute psychological stress on prefrontal GABA concentration determined by proton magnetic resonance spectroscopy. Am J Psychiatry. 2010;167:1226–1231. doi: 10.1176/appi.ajp.2010.09070994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- Huyser C, Veltman DJ, de Haan E, Boer F. Paediatric obsessive-compulsive disorder, a neurodevelopmental disorder? Evidence from neuroimaging. Neurosci Biobehav Rev. 2009;33:818–830. doi: 10.1016/j.neubiorev.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Jensen JE, Frederick Bde B, Renshaw PF. Grey and white matter GABA level differences in the human brain using two-dimensional, J-resolved spectroscopic imaging. NMR Biomed. 2005;18:570–576. doi: 10.1002/nbm.994. [DOI] [PubMed] [Google Scholar]
- Kaiser LG, Schuff N, Cashdollar N, Weiner MW. Age-related glutamate and glutamine concentration changes in normal human brain: 1H MR spectroscopy study at 4 T. Neurobiol Aging. 2005;26:665–672. doi: 10.1016/j.neurobiolaging.2004.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles L, Mao X, Gonzales J, Shungu D. Evaluation of anatomic variation in macromolecule contribution to the GABA signal using metabolite nulling and the J-editing technique at 3.0T. Proc Int Soc Magn Reson Med. 2007;15:1391. [Google Scholar]
- Kegeles LS, Mao X, Dyke J, Gonzales J, Soones T, Shungu D. Test-retest reliability of dorsolateral prefrontal cortical GABA measurement using an 8-channel phased-array head coil with the J-editing technique at 3T. Proc Int Soc Magn Reson Med. 2006;14:489. [Google Scholar]
- Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X, et al. Elevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69:449–459. doi: 10.1001/archgenpsychiatry.2011.1519. [DOI] [PubMed] [Google Scholar]
- Maia TV, Cooney RE, Peterson BS. The neural bases of obsessive-compulsive disorder in children and adults. Dev Psychopathol. 2008;20:1251–1283. doi: 10.1017/S0954579408000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwardt C.2009Non-linear least squares fitting in IDL with MPFIT Proceedings of Astronomical Data Analysis Software and Systems XVIII 2008vol 411Astronomical Society of the Pacific, San Francisco, CA; 251–254.The IDL fitting routine, ‘MPFIT', available at http://purl.com/net/mpfit Last modified on 2011–2012–2021 by Craig Markwardt.. [Google Scholar]
- Marshall I, Bruce SD, Higinbotham J, MacLullich A, Wardlaw JM, Ferguson KJ, et al. Choice of spectroscopic lineshape model affects metabolite peak areas and area ratios. Magn Reson Med. 2000;44:646–649. doi: 10.1002/1522-2594(200010)44:4<646::aid-mrm20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Petroff OA. GABA and glutamate in the human brain. Neuroscientist. 2002;8:562–573. doi: 10.1177/1073858402238515. [DOI] [PubMed] [Google Scholar]
- Petroff OA, Rothman DL. Measuring human brain GABA in vivo: effects of GABA-transaminase inhibition with vigabatrin. Mol Neurobiol. 1998;16:97–121. doi: 10.1007/BF02740605. [DOI] [PubMed] [Google Scholar]
- Pinto A, Eisen JL, Mancebo MC, Greenberg BD, Stout RL, Rasmussen SA. Taboo thoughts and doubt/checking: a refinement of the factor structure for obsessive-compulsive disorder symptoms. Psychiatry Res. 2007;151:255–258. doi: 10.1016/j.psychres.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A, Greenberg BD, Grados MA, Bienvenu OJ, III, Samuels JF, Murphy DL, et al. Further development of YBOCS dimensions in the OCD Collaborative Genetics study: symptoms vs. categories. Psychiatry Res. 2008;160:83–93. doi: 10.1016/j.psychres.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A, Greenberg BD, Murphy DL, Nestadt G, Rasmussen SA.2009Using individual items to clarify OCD symptom structure: the case for five factors Am J Psychiatry 166728–729.author reply 729–731. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Bloch MH, Williams K. Glutamate abnormalities in obsessive compulsive disorder: neurobiology, pathophysiology, and treatment. Pharmacol Ther. 2011;132:314–332. doi: 10.1016/j.pharmthera.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter MA, de Jesus DR, Hoppenbrouwers S, Daigle M, Deluce J, Ravindran LN, et al. Evidence for cortical inhibitory and excitatory dysfunction in obsessive compulsive disorder. Neuropsychopharmacology. 2012;37:1144–1151. doi: 10.1038/npp.2011.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, Keshavan MS. Toward a neurodevelopmental model of obsessive-compulsive disorder. Biol Psychiatry. 1998;43:623–640. doi: 10.1016/s0006-3223(97)00443-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, MacMillan SN, Moore GJ. Brain anatomy and chemistry may predict treatment response in paediatric obsessive--compulsive disorder. Int J Neuropsychopharmacol. 2001;4:179–190. doi: 10.1017/S1461145701002401. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Mirza Y, Russell A, Tang J, Smith JM, Banerjee SP, et al. Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls. J Am Acad Child Adolesc Psychiatry. 2004;43:1146–1153. doi: 10.1097/01.chi.0000132812.44664.2d. [DOI] [PubMed] [Google Scholar]
- Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci USA. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailasuta N, LeRoux P, Hurd R, Wang P, Sachs N, Ketter T. Detection of cerebral gamma-aminobutyric acid (GABA) in bipolar disorder patients and healthy volunteers at 3 T. Proc Intl Soc Magn Reson Med. 2001;6:1011. [Google Scholar]
- Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. Oxford University Press, Inc.: Oxford; 2006. [Google Scholar]
- Simpson HB, Slifstein M, Bender J, Xu X, Hackett E, Maher MJ, et al. Serotonin 2A receptors in obsessive-compulsive disoder: a positron emission tomography study with [11C] MDL 100907. Biol Psychiatry. 2011;70:897–904. doi: 10.1016/j.biopsych.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starck G, Ljungberg M, Nilsson M, Jonsson L, Lundberg S, Ivarsson T, et al. A 1H magnetic resonance spectroscopy study in adults with obsessive compulsive disorder: relationship between metabolite concentrations and symptom severity. J Neural Transm. 2008;115:1051–1062. doi: 10.1007/s00702-008-0045-4. [DOI] [PubMed] [Google Scholar]
- Ting JT, Feng G. Neurobiology of obsessive-compulsive disorder: insights into neural circuitry dysfunction through mouse genetics. Curr Opin Neurobiol. 2011;21:842–848. doi: 10.1016/j.conb.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M. Brief screening for family psychiatric history: the family history screen. Arch Gen Psychiatry. 2000;57:675–682. doi: 10.1001/archpsyc.57.7.675. [DOI] [PubMed] [Google Scholar]
- Wu K, Hanna GL, Rosenberg DR, Arnold PD. The role of glutamate signaling in the pathogenesis and treatment of obsessive-compulsive disorder. Pharmacol Biochem Behav. 2012;100:726–735. doi: 10.1016/j.pbb.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Shen J. Elevated endogenous GABA concentration attenuates glutamate-glutamine cycling between neurons and astroglia. J Neural Transm. 2009;116:291–300. doi: 10.1007/s00702-009-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Wood SJ, Wellard RM, Harrison BJ, Fornito A, Pujol J, et al. Anterior cingulate glutamate-glutamine levels predict symptom severity in women with obsessive-compulsive disorder. Aust N Z J Psychiatry. 2008;42:467–477. doi: 10.1080/00048670802050546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.