Abstract
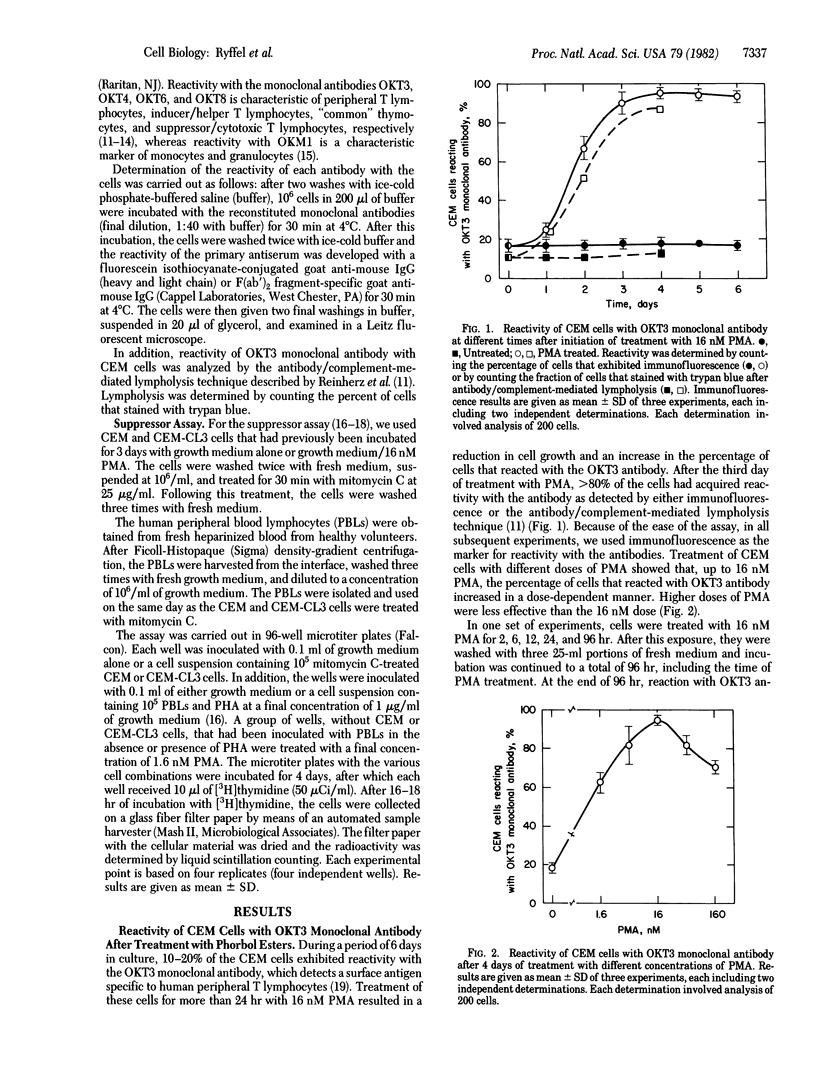
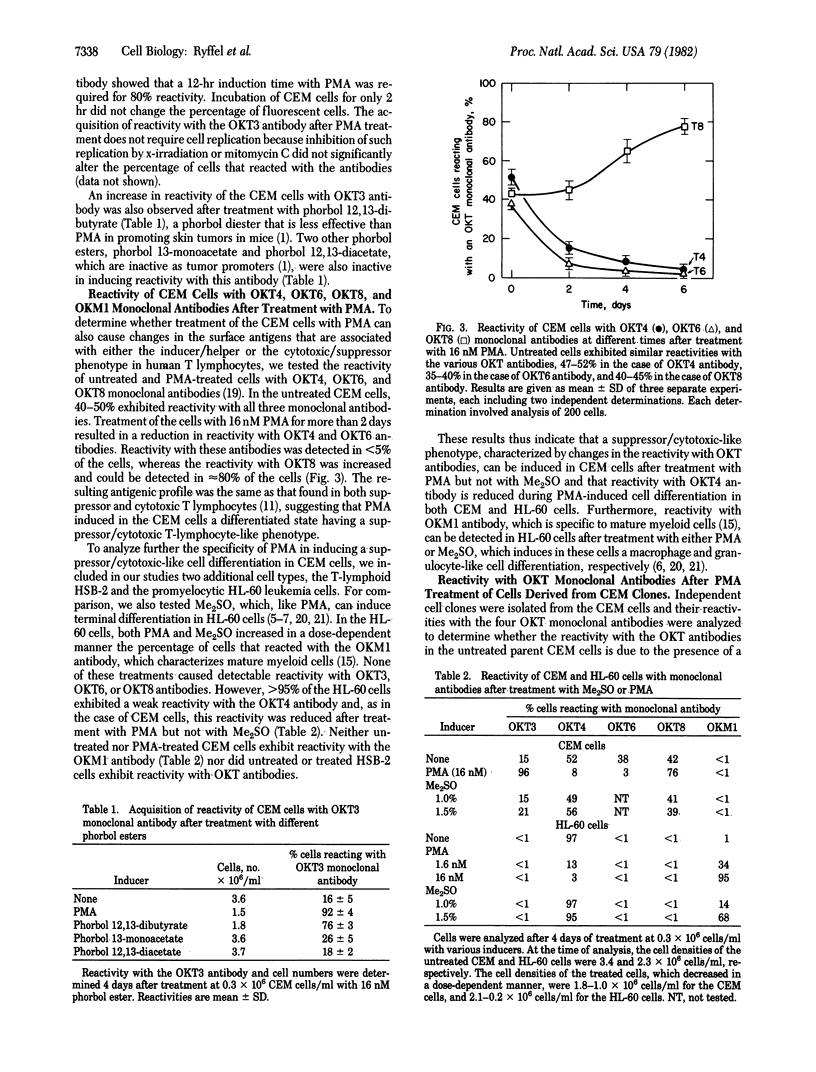
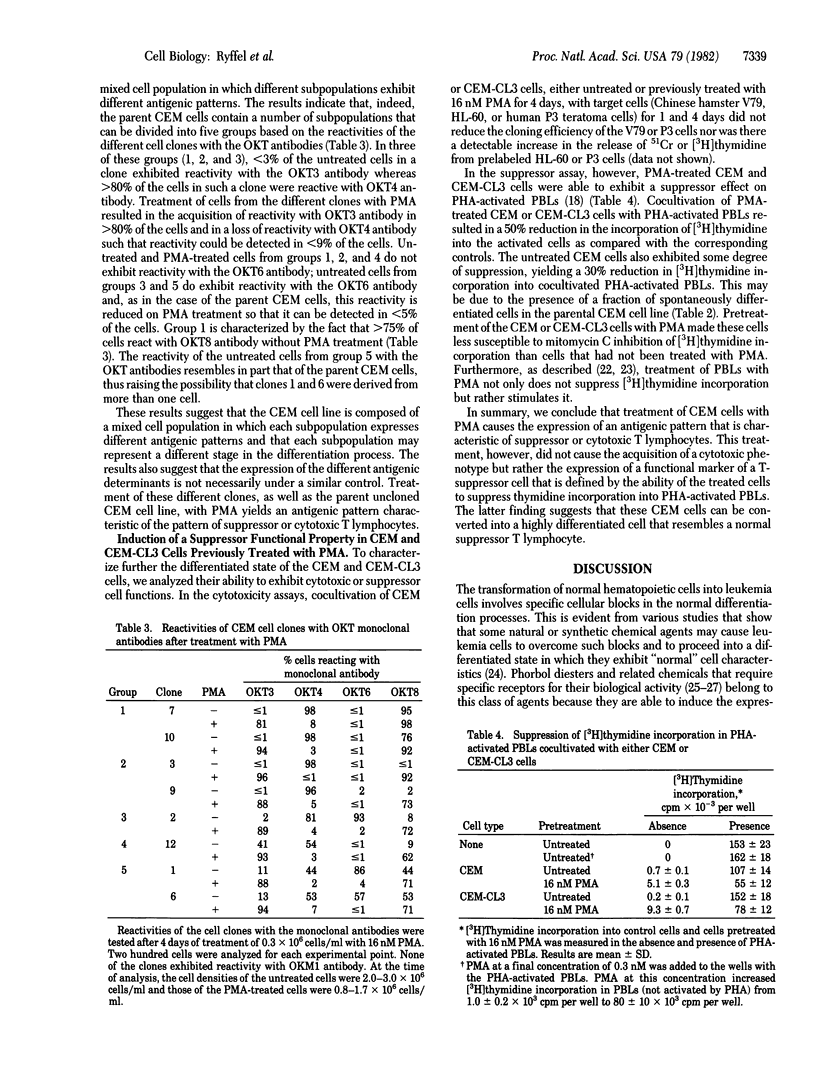
Treatment of cultured human T-lymphoid (CEM) leukemia cells with nanomolar concentrations of phorbol 12-myristate 13-acetate (PMA) resulted in a reduction in cell growth and in the acquisition of a surface antigenic pattern that is common to both suppressor and cytotoxic T lymphocytes. This antigenic pattern was detected by OKT monoclonal antibodies. PMA treatment did not cause the expression of a cytotoxic function but rather induced the expression of a suppressor cell marker. This marker was characterized by the ability of the treated CEM cells to suppress [3H]thymidine incorporation into phytohemagglutinin-activated peripheral blood lymphocytes. After 4 days of treatment of CEM cells from either cloned or the parental cell population with 16 nM PMA, 71-98% of the cells expressed reactivity with OKT3 and OKT8 antibodies whereas reactivity with OKT4 and OKT6 was detected in less than or equal to 1-8% of the cells. The CEM cells can be divided into five groups based on the antigenic patterns of cells from randomly isolated clones. The cells from four of these groups were characterized by either low or high reactivity with each of the four OKT antibodies. The antigenic pattern of the fifth group resembled that of the parent CEM cells. The acquisition of reactivity with the OKT3 antibody in the CEM cells after PMA treatment was dependent on both time and dose and did not require cell replication. Acquisition of reactivity with OKT3 antibody also occurred after treatment with phorbol 12,13-dibutyrate but not after treatment with phorbol 13-monoacetate, phorbol 12,13-diacetate, or dimethyl sulfoxide. These results indicate that treatment of CEM cells with PMA and related agents can cause the cells to express a phenotype that resembles that of a mature suppressor T lymphocyte.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abb J., Bayliss G. J., Deinhardt F. Lymphocyte activation by the tumor-promoting agent 12-O-tetradecanoylphorbol-13-acetate (TPA). J Immunol. 1979 May;122(5):1639–1642. [PubMed] [Google Scholar]
- Blumberg P. M. In vitro studies on the mode of action of the phorbol esters, potent tumor promoters, part 2. Crit Rev Toxicol. 1981 Jun;8(3):199–234. doi: 10.3109/10408448109109658. [DOI] [PubMed] [Google Scholar]
- Boutwell R. K. The function and mechanism of promoters of carcinogenesis. CRC Crit Rev Toxicol. 1974 Jan;2(4):419–443. doi: 10.3109/10408447309025704. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Braunwald A. D., Kuo A. L. Phorbol ester induction of leukemic cell differentiation is a membrane-mediated process. Proc Natl Acad Sci U S A. 1982 May;79(9):2865–2869. doi: 10.1073/pnas.79.9.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delia D., Greaves M. F., Newman R. A., Sutherland D. R., Minowada J., Kung P., Goldstein G. Modulation of T leukaemic cell phenotype with phorbol ester. Int J Cancer. 1982 Jan 15;29(1):23–31. doi: 10.1002/ijc.2910290106. [DOI] [PubMed] [Google Scholar]
- Eddie-Quartey A. C., Gross N. J. Phytohemagglutinin activation of lymphocytes: quantitative comparison of different preparations. Am J Clin Pathol. 1978 Mar;69(3):326–328. doi: 10.1093/ajcp/69.1.326. [DOI] [PubMed] [Google Scholar]
- Fisher M. S., Kripke M. L. Suppressor T lymphocytes control the development of primary skin cancers in ultraviolet-irradiated mice. Science. 1982 Jun 4;216(4550):1133–1134. doi: 10.1126/science.6210958. [DOI] [PubMed] [Google Scholar]
- Foon K. A., Schroff R. W., Gale R. P. Surface markers on leukemia and lymphoma cells: recent advances. Blood. 1982 Jul;60(1):1–19. [PubMed] [Google Scholar]
- Huberman E., Braslawsky G. R., Callaham M., Fugiki H. Induction of differentiation of human promyelocytic leukemia (HL-60) cells by teleocidin and phorbol-12-myristate-13-acetate. Carcinogenesis. 1982;3(1):111–114. doi: 10.1093/carcin/3.1.111. [DOI] [PubMed] [Google Scholar]
- Huberman E., Callaham M. F. Induction of terminal differentiation in human promyelocytic leukemia cells by tumor-promoting agents. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1293–1297. doi: 10.1073/pnas.76.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman E., Heckman C., Langenbach R. Stimulation of differentiated functions in human melanoma cells by tumor-promoting agents and dimethyl sulfoxide. Cancer Res. 1979 Jul;39(7 Pt 1):2618–2624. [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Krupen K., Araneo B. A., Brink L., Kapp J. A., Stein S., Wieder K. J., Webb D. R. Purification and characterization of a monoclonal T-cell suppressor factor specific for poly(LGlu60LAla30LTyr10). Proc Natl Acad Sci U S A. 1982 Feb;79(4):1254–1258. doi: 10.1073/pnas.79.4.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Regulation of normal differentiation in mouse and human myeloid leukemic cells by phorbol esters and the mechanism of tumor promotion. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5158–5162. doi: 10.1073/pnas.76.10.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa K., Howatson A., Mak T. W. Induction of human malignant T-lymphoblastic cell lines MOLT-3 and jurkat by 12-O-tetradecanoylphorbol-13-acetate: biochemical, physical, and morphological characterization. J Cell Physiol. 1981 Oct;109(1):181–192. doi: 10.1002/jcp.1041090120. [DOI] [PubMed] [Google Scholar]
- Nagasawa K., Mak T. W. Phorbol esters induce differentiation in human malignant T lymphoblasts. Proc Natl Acad Sci U S A. 1980 May;77(5):2964–2968. doi: 10.1073/pnas.77.5.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak W., Rosenstein R. W., Gershon R. K. Interactions between molecules (subfactors) released by different T cell sets that yield a complete factor with biological (suppressive) activity. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2375–2378. doi: 10.1073/pnas.79.7.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. Derivation of human T-cell leukemias. Cancer Res. 1981 Nov;41(11 Pt 2):4767–4770. [PubMed] [Google Scholar]
- Rovera G., Santoli D., Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs L. Control of normal cell differentiation and the phenotypic reversion of malignancy in myeloid leukaemia. Nature. 1978 Aug 10;274(5671):535–539. doi: 10.1038/274535a0. [DOI] [PubMed] [Google Scholar]
- Schroff R. W., Foon K. A., Billing R. J., Fahey J. L. Immunologic classification of lymphocytic leukemias based on monoclonal antibody-defined cell surface antigens. Blood. 1982 Feb;59(2):207–215. [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanki V., Slaga T. J., Callaham M., Huberman E. Down regulation of specific binding of [20-3H]phorbol 12,13-dibutyrate and phorbol ester-induced differentiation of human promyelocytic leukemia cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1722–1725. doi: 10.1073/pnas.78.3.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touraine J. L., Hadden J. W., Touraine F., Hadden E. M., Estensen R., Good R. A. Phorbol myristate acetate: a mitogen selective for a T-lymphocyte subpopulation. J Exp Med. 1977 Feb 1;145(2):460–465. doi: 10.1084/jem.145.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tötterman T. H., Nilsson K., Sundström C. Phorbol ester-induced differentiation of chronic lymphocytic leukaemia cells. Nature. 1980 Nov 13;288(5787):176–178. doi: 10.1038/288176a0. [DOI] [PubMed] [Google Scholar]



