Abstract
Prior structural imaging studies of post-traumatic stress disorder (PTSD) have observed smaller volumes of the hippocampus and cingulate cortex, yet little is known about the integrity of white matter connections between these structures in PTSD samples. The few published studies using diffusion tensor imaging (DTI) to measure white matter integrity in PTSD have described individuals with focal trauma rather than chronically stressed individuals, which limits generalization of findings to this population; in addition, these studies have lacked traumatized comparison groups without PTSD. The present DTI study examined microstructural integrity of white matter tracts in a sample of highly traumatized African-American women with (n=25) and without (n=26) PTSD using a tract-based spatial statistical approach, with threshold-free cluster enhancement. Our findings indicated that, relative to comparably traumatized controls, decreased integrity (measured by fractional anisotropy) of the posterior cingulum was observed in participants with PTSD (p<0.05). These findings indicate that reduced microarchitectural integrity of the cingulum, a white matter fiber that connects the entorhinal and cingulate cortices, appears to be associated with PTSD symptomatology. The role of this pathway in problems that characterize PTSD, such as inadequate extinction of learned fear, as well as attention and explicit memory functions, are discussed.
Keywords: post-traumatic stress disorder, MRI, DTI, TBSS, cingulum, hippocampus
INTRODUCTION
Post-traumatic stress disorder (PTSD) is a debilitating condition that can develop in response to a traumatic stressor. In the past two decades, a burgeoning number of neuroimaging studies have found distinct differences in brain morphology between individuals with and without PTSD. A majority of this research has revealed structural differences in gray matter, with smaller cortical volumes frequently associated with a PTSD diagnosis (Karl et al, 2006; Tavanti et al, 2012). Volumetric differences between PTSD and psychopathology-free controls (measured through MRI) have been largely observed in specific structures, such as the anterior cingulate cortex (ACC) and orbitofrontal cortex (Thomaes et al, 2010); however, between-group differences have been particularly apparent for the hippocampus, a brain region that is especially sensitive to the effects of chronic stress (for a review, see Woon et al, 2010). Not surprisingly, hippocampal abnormalities have been linked to cognitive and affective disruptions that characterize PTSD (Bremner, 2006).
Considerably fewer studies have investigated PTSD-specific abnormalities in the white matter tracts that connect frontal and limbic structures. White matter fibers, which occupy >40% of total brain volume (Morell and Norton, 1980), represent the main routes of communication for cortical and sub-cortical structures. Consequently, insult to these structures, attributable to neurodegenerative disease (Drago et al, 2011), physical trauma (Johnson et al, 2012), or acute stress (Chen et al, 2011), can significantly disrupt behavior and cognition. The relatively recent advent of diffusion tensor imaging (DTI) has permitted investigations of microstructural integrity of white matter fibers, allowing measurement of the magnitude and direction of water diffusion within myelinated tracts. DTI quantifies subtle white matter differences in normal-appearing white matter on traditional MRI scans. Fractional anisotropy (FA), an index obtained through DTI, represents the degree of anisotropy, or strength of diffusion direction, of water molecules within these tracts, providing a reliable assessment of microstructural integrity of white matter fibers (Fox et al, 2012).
The extant research on white matter tract integrity in adults with PTSD is limited, and the findings have been variable with regard to the location and extent of between-group differences in FA. The majority of this research has targeted populations with acute and/or focal trauma, (Abe et al, 2006; Kim et al, 2005, 2006; Zhang et al, 2011), which may limit relevance to highly traumatized populations. In addition, given that only two DTI studies of PTSD samples included traumatized controls (TCs) (Abe et al, 2006; Schuff et al, 2011), most studies were not equipped to differentiate trauma vs PTSD-specific effects on white matter architecture.
Three studies found that PTSD was associated with lower FA values in various white matter tracts, predominantly tracts that are proximal to the cingulate gyrus (Kim et al, 2005, 2006; Schuff et al, 2011). One study of subway fire survivors found that, compared with non-TCs, participants with PTSD demonstrated decreased FA in white matter tracts near the cingulate gyrus (particularly, anterior aspects) and midbrain (Kim et al, 2005) in a brain-wide analysis. Lower FA in the left rostral, subgenual, and dorsal aspects of the cingulum bundle was observed in survivors, compared with controls, in a separate ROI analysis (Kim et al, 2006). A study of combat veterans observed that participants with PTSD had lower FA values in white matter tracts proximal to the ACC, prefrontal gyrus, precentral gyrus, and posterior angular gyrus (Schuff et al, 2011); however, these findings were complicated by the inclusion of blast-exposed/head-injured veterans, some of whom were taking psychotropic medications.
In contrast, two studies reported that higher FA values of the cingulum and superior frontal gyrus were associated with PTSD. Abe et al (2006) observed increased FA in anterior aspects of the cingulum bundle in individuals with PTSD involved in a sarin attack. Zhang et al (2011) found that participants with PTSD associated with a coal mine accident demonstrated higher FA in white matter tracts by the superior frontal gyrus, as compared with non-psychiatric controls.
To summarize, structural differences in gray matter have been frequently noted in the hippocampus and cingulate cortex in PTSD samples, but little is known about how a PTSD diagnosis may be associated with integrity of white matter connections between these structures. The few existing studies of white matter integrity in PTSD have yielded variable associations between PTSD and FA values, particularly within the cingulum, a tract that provides a connection between the cingulate cortex and entorhinal cortex. These studies have been either limited to populations of individuals with focal trauma exposure, or did not include traumatized non-PTSD control groups, which does not permit differentiation of PTSD vs trauma-specific effects. In addition, the findings of one study were complicated by the inclusion of individuals with significant head injury, some of whom were taking psychotropic medications (Schuff et al, 2011). Furthermore, a majority of these studies used cluster-based statistical methods, which make arbitrary assumptions about signal thresholds. Threshold-free cluster enhancement (TFCE) methods are more sensitive to the specific shapes and sizes of signal within the data set, reducing the need for spatial smoothing; this method offers a more robust way to measure group differences in FA. Thus, the goal of this study was to investigate the relationship between PTSD and white matter integrity in a population of highly traumatized African-American adults with and without PTSD, using DTI. We used tract-based spatial statistics (TBSSs) with a TFCE whole brain approach (Smith and Nichols, 2009) to test our hypothesis that individuals with PTSD would demonstrate significant differences in FA in white matter tracts connecting the hippocampus and cingulate cortex, compared with traumatized individuals without PTSD. In addition, we hypothesized that this difference in FA would not be better accounted for by trauma exposure or current depressive symptoms.
PARTICIPANTS AND METHODS
Participants
Study procedures were approved by the institutional review board of Emory University. A total of 51 African-American women aged 20–62 years were recruited through an ongoing study of risk factors for PTSD; they were approached in general medical clinics of a publicly funded hospital that serves low-income individuals in inner-city Atlanta. Patients seeking treatment at this hospital have been found to exhibit high rates of interpersonal trauma and post-traumatic symptoms that vary considerably in severity, as evidenced by previous studies sampling this population (Bradley et al, 2008; Gillespie et al, 2009; Schwartz et al, 2005).
Patients were deemed eligible for participation if they were able and willing to give informed consent and understand English, as determined by a study researcher. Participants were initially screened to assess for the presence of these exclusion criteria: current psychotropic medication use, current alcohol or substance abuse or dependence, medical or physical conditions that preclude MRI scanning (eg, metal implants), a history of schizophrenia or other psychotic disorder, medical conditions that contribute significantly to psychiatric symptoms (such as dementia), history of head injury or loss of consciousness for longer than 5 min (including concussion), or a history of neurological illness. Participants were also asked about past history of substance or alcohol abuse. They were given clinical assessments during a separate appointment. One day before scanning, participants were given a pregnancy test to confirm that they were not pregnant. Table 1 details sample demographics and clinical characteristics.
Table 1. Demographic and Clinical Characteristics.
| Trauma control | PTSD | F | |
|---|---|---|---|
|
(n=26) |
(n=25) |
||
| Mean (SD) | Mean (SD) | ||
| Age | 38 (12.8) | 34 (12.5) | 1.2 |
| PSS re-experiencing | 1.3 (1.7) | 5.1 (2.6) | 39.3** |
| PSS avoidance and numbing | 2.3 (3.1) | 10.4 (4.9) | 48.7** |
| PSS hyperarousal | 2.1 (2.7) | 7.8 (3.7) | 38.8** |
| PSS total | 5.7 (6.6) | 23.3 (8.8) | 65.0** |
| BDI total | 7.6 (6.8) | 16.8 (8.9) | 16.1** |
| TEI total | 4.2 (2.6) | 4.8 (2.0) | 0.73 |
| TEI adult trauma | 3.6 (2.3) | 3.8 (1.8) | 0.17 |
| CTQ total score | 37.2 (16.8) | 41.6 (15.4) | 0.92 |
| % | % | χ2 | |
| Education | 7.8 | ||
| <12th grade | 15.4 | 16 | |
| 12th grade/high school graduate | 30.8 | 32 | |
| GED | 3.8 | 0 | |
| Some college/technical school | 26.9 | 36 | |
| College/technical school graduate | 23.1 | 16 | |
| Monthly income | 2.56 | ||
| $0–249 | 4 | 8 | |
| $250–499 | 12 | 20 | |
| $500–999 | 44 | 24 | |
| $1000–1999 | 32 | 36 | |
| $2000+ | 8 | 12 |
Abbreviations: BDI, Beck Depression Inventory; CTQ, Childhood Trauma Questionnaire; PSS, PTSD Symptom Scale; TEI, Traumatic Events Inventory.
**p<0.01.
Trauma and Symptom Assessment
The traumatic events inventory (TEI) was administered to detail frequency and type of trauma(s) experienced; consistent with prior research (Binder et al, 2008; Gillespie et al, 2009), total level of trauma exposure was measured by a sum score reflecting the total number of different types of trauma (eg, car accident, sexual assault, and natural disaster) to which a participant had been exposed over the course of their life (TEI total score). The TEI also was used to measure trauma exposure in adulthood. The childhood trauma questionnaire (CTQ) was used to measure trauma exposure during childhood, specifically, maltreatment. The CTQ is a 28-item self-report measure that yields a total childhood trauma index used for the purposes of this study. The PTSD symptom scale (PSS; Falsetti et al, 1993), is an 18-item self-report questionnaire that provides a measure of re-experiencing, avoidance, and arousal symptoms that have occurred in the 2 weeks before test administration; it was administered to determine presence and severity of PTSD. Participants were classified as either TCs or participants with PTSD (PTSD+) based on DSM-IV criteria, consistent with earlier studies (Fani et al, 2012a, 2012b; Jovanovic et al, 2010). Participants were classified as PTSD+ if they endorsed the presence of one or more symptom in the re-experiencing cluster (items 1–4); three or more symptoms in the avoidance and numbing symptom cluster (items 5–11); two or more symptoms in the hyperarousal cluster (items 12–17); and symptom duration of 3 months or longer (as measured by question 18), in keeping with DSM-IV criterion for PTSD. The Beck depression inventory (BDI-II; Beck et al, 1996) was administered to measure current depression symptoms.
MRI Acquisition, Image Processing, and Statistical Analyses
Scanning took place on a research-dedicated Siemens 3-Tesla TIM-Trio scanner at Emory University Hospital. Diffusion-weighted images were acquired with maximum gradient strength of 40 m Tm−1 with the following parameters: 39 × 2.5 mm thick axial slices, matrix=128 × 128, field of view=220 × 220 mm, voxel size=1.72 × 1.72 × 2.5 mm. The diffusion weighting was isotropically distributed along 60 directions using a b-value of 1000 s/mm2. Four normalization images, with no diffusion encoding (b=0), were acquired and averaged for each direction using linear rigid body registration (FLIRT; Jenkinson and Smith, 2001). All image processing and analysis was conducted using FMRIB Software Library (FSL version 4.1; www.fmrib.ax.ac.uk/fsl; Smith et al, 2004).
Correction for head motion and eddy current distortion was performed for data from each individual participant using an automated affine registration algorithm. Normalization images were skull-stripped using the FSL brain extraction tool (Smith, 2002). FA maps were generated using the DTIfit in the FMRIB Diffusion Toolbox, and voxel-wise differences in DTI scalar indices were assessed using TBSS (version 1.2), an approach that increases the sensitivity and interpretability of the results compared with typical voxel-based approaches because it uses non-linear registration (Smith et al, 2006). All participants' FA maps were co-registered using the non-linear registration to the most ‘typical' participant's FA, then affine transformed into 1 × 1 × 1 mm MNI space. All transformed FA images were averaged to create a mean FA image, then thresholded by FA >0.2 to ensure that gray matter regions would be excluded from these analyses. Voxel values of each subject's FA map were projected onto the skeleton by searching the local maxima along the perpendicular direction from the skeleton. Voxel-wise t-tests were conducted on FA values across the overall skeleton to compare WM integrity between PTSD+ and control groups. A permutation algorithm (randomize, within FSL) was used for inference testing, and a total of 5000 permutations were conducted. Both uncorrected and family-wise error (FWE)-corrected p-value images were generated using a TFCE approach (Smith and Nichols, 2009). This method avoids arbitrary cluster thresholding and assumptions about signal extent, and is more sensitive to the specific shapes and sizes of signal within the data set, which reduces the need for spatial smoothing; thus, no smoothing was conducted. Statistically significant between-group differences in FA (p<0.05) were defined anatomically using the probabilistic Johns Hopkins University White Matter Atlas (Hua et al, 2008) provided by FSL. FA values were extracted from these regions using FSLmaths to examine potential associations with trauma exposure and current depressive symptoms.
RESULTS
Group Characteristics
A total of 26 participants were classified as TCs and 25 were classified as PTSD+. No statistically significant between-group differences (PTSD+ vs TC) were observed for age, education, or monthly income. Results from the TEI indicated that trauma rates were comparably high between PTSD and control groups (see Table 1); on average, participants had witnessed, experienced, or been confronted with four traumatic incidents of various types (eg, witnessing gun violence, being sexually assaulted). There were no significant between-group differences in trauma exposure (p>0.05), measured across the lifespan (TEI total score) or for adulthood (TEI adult trauma) or childhood (CTQ total score). As expected, there were significant between-group differences in total PTSD symptoms, re-experiencing, avoidance/numbing, and hyperarousal symptoms (all p<0.05), as well as depressive symptoms (BDI total score). There were no significant differences between PTSD and TC groups in self-reported history of substance and/or alcohol abuse (Pearson χ2=0.27; p>0.05); only two TCs and one PTSD+ participant reported having a significant drug or alcohol abuse history. Results below did not change appreciably when these participants were removed from analyses; thus, these participants were included in all analyses.
Tract-Based Spatial Statistics
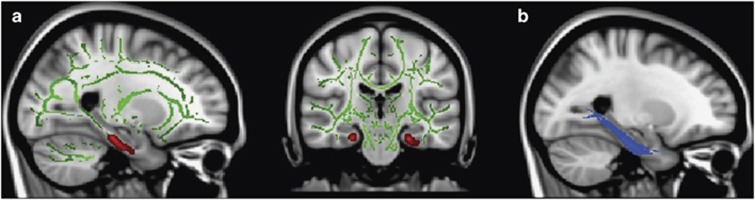
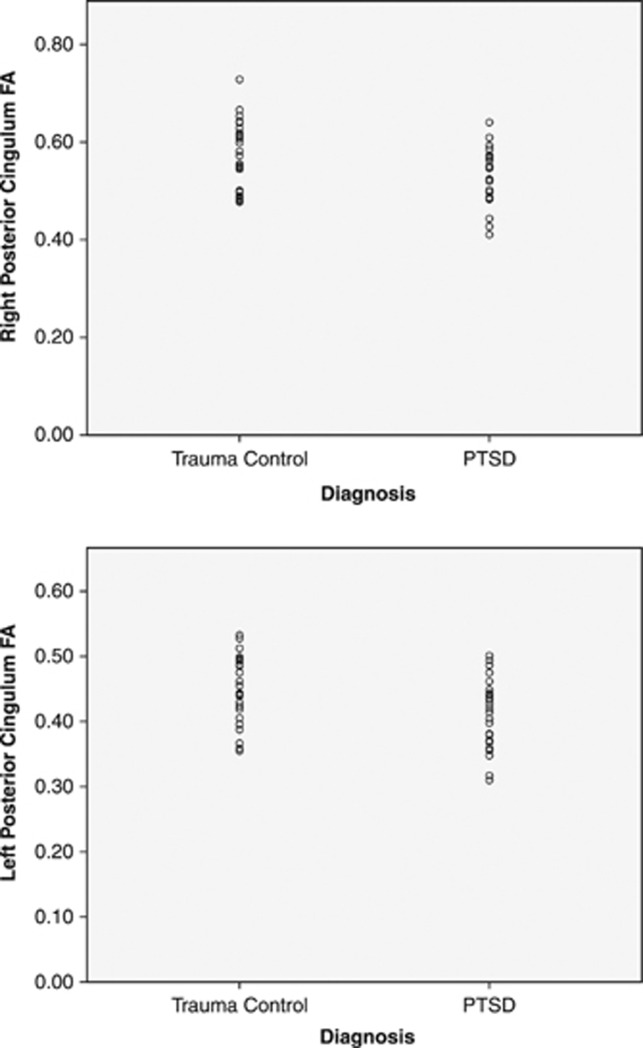
Voxel-wide t-test results indicated two large ‘clusters' of significantly lower FA in posterior regions of the cingulum, adjacent to the right and left hippocampi, in participants with PTSD, compared with TCs (cluster size >10 mm3; p<0.05uncorrected; see Table 2 and Figure 1a); medium-to-large effect sizes were observed for FA differences in the left cingulum (Cohen's d=0.63) and right cingulum (Cohen's d=0.85). A small (11 mm3) cluster indicating significant between-group differences in FA was also observed in the left superior longitudinal fasciculus (p<0.05uncorrected; see Table 2). For this brain-wide analysis, no other significant between-group differences in FA were observed. However, these differences were not statistically significant after applying FWE correction. Mean FA values were extracted from a mask of statistically significant clusters for this analysis. Distribution of extracted FA values met assumptions of normality, according to the Shapiro–Wilk statistic (p>0.05). There was no significant correlation between mean FA and depression symptoms (BDI total score; r(49)=−0.1, p>0.05) or trauma exposure, either for total exposure throughout the lifetime (TEI total; r(49)=1.12, p>0.05) or for adult (TEI adult; r(49)=−0.14, p>0.05), or childhood trauma exposure (CTQ total score; r(49)=0.04, p>0.05). FA values also were extracted from probabilistic masks of the left (illustrated in Figure 1b) and right posterior cingulum, provided by the Johns Hopkins University White Matter Tractography Atlas (Hua et al, 2008); the distribution of FA values for each group is illustrated in Figure 2. The findings from two univariate ANOVAs revealed between-group differences in FA for the left (F1,49=5.52; p<0.05) and right posterior cingulum (F1,49=4.07; p<0.05). However, correlations between posterior cingulum FA (for either the left or right hemisphere) and PTSD symptoms did not reach statistical significance within either of the two diagnostic groups (p>0.05).
Table 2. Anatomical Locations of Between-Group Differences (p<0.05uncorrected) in FA between PTSD+ and Trauma Control Participants.
| Anatomical location | Cluster size (mm3) | Z maxa | Z max xb | Z max yb | Z max zb |
|---|---|---|---|---|---|
| Controls>PTSD | |||||
| L. cingulum | 163 | 0.98 | −24 | −9 | −32 |
| R. cingulum | 63 | 0.98 | 25 | −27 | −19 |
| L. superior longitudinal fasciculus | 11 | 0.96 | −58 | −29 | −10 |
| PTSD>controls | |||||
| R. lateral occipital cortex | 13 | 0.96 | 29 | −87 | 16 |
Z max=maximum Z-value.
Location of maximum Z-value (Z max) in MNI standard space.
Figure 1.
(a) Voxel-wise t-test results indicating (in red) lower FA in the left and right posterior cingulum in PTSD+ participants compared with TC participants (p<0.05uncorrected). Results overlaid on MNI standard brain, displayed in radiological convention. Green voxels depict mean FA skeleton for the entire sample. Left panel, sagittal section highlights (in red) lower FA of the left cingulum in participants with PTSD vs TCs. (b) Probabilistic mask of the left posterior cingulum (as identified in the Johns Hopkins University White Matter Atlas; Hua et al, 2008), highlighted in blue.
Figure 2.
Distribution of FA values extracted from probabilistic masks of the left and right posterior cingulum (as identified in the Johns Hopkins University White Matter Atlas; Hua et al, 2008) within TC and PTSD+ groups.
PTSD+ participants, compared with controls, demonstrated greater FA in one small (13 mm3) voxel cluster in the right lateral occipital gyrus (p<0.05uncorrected; see Table 2). These between-group differences were not statistically significant after applying FWE correction, and evidenced a medium effect size (Cohen's d=0.61).
DISCUSSION
The findings from this study revealed that participants with PTSD demonstrated significantly lower white matter integrity in posterior regions of the cingulum bundle (proximal to bilateral hippocampi), relative to traumatized participants without PTSD. A PTSD diagnosis corresponded with lower FA within these regions, whereas no significant associations were observed between current depressive symptoms and cingulum FA. Notably, both groups had comparably high levels of trauma exposure, and trauma exposure in childhood or adulthood did not significantly correspond with variability in white matter integrity. These results suggest specific associations between PTSD symptomatology and altered microstructural integrity of a posterior region of the cingulum in a well-matched, traumatized group of African-American women.
Prior morphometric studies have documented associations between traumatic stress and white matter fiber volumes. For example, some findings indicated that, relative to non-maltreated peers, maltreated children demonstrated lower corpus callosum volumes (De Bellis et al, 2002; Jackowski et al, 2008; Teicher et al, 2004). Stress-related changes in white matter integrity have also been apparent in a few studies of adults; acute stress and/or focal trauma has been previously associated with lower FA in white matter tracts adjacent to the hippocampus in earthquake survivors without PTSD (Chen et al, 2011), and in the anterior cingulate in individuals with PTSD (Abe et al, 2006; Kim et al, 2005, 2006). However, it is plausible that the findings from these studies reflect white matter integrity differences related to stress or trauma exposure rather than PTSD symptomatology/diagnosis, given that these studies lacked a well-matched TC group. To our knowledge, this study is the first to observe PTSD-specific differences in white matter in adults with relatively high rates of trauma exposure, without concomitant head injury.
Consistent with our hypotheses, our findings indicated that a PTSD diagnosis was specifically associated with reduced FA in an association fiber that connects the cingulate gyrus to the entorhinal cortex: the cingulum bundle. Compromised cingulum integrity directly affects the quality of communication between the ACC and the hippocampus, two constituent regions that have frequently shown altered function in PTSD (for reviews, see Liberzon and Martis, 2006; Shin et al, 2006). The hippocampus actively participates in context-dependent learning, and is responsible for encoding and retrieval of trauma-related memories (Goosens, 2011). A functional hippocampus is also required for normal extinction of conditioned fear (Heldt et al, 2007; Ji and Maren, 2007; Radulovic and Tronson, 2010), a critical inhibitory process that appears to be disrupted in PTSD (Jovanovic and Ressler, 2010; Milad et al, 2009; Norrholm et al, 2011; Shin and Handwerger, 2009). The ventral ACC, a region known to be involved with response inhibition, is thought to interact with the hippocampus to extinguish a previously learned fear response (for a review, see Hartley and Phelps, 2010). Degraded communication between the hippocampus and ACC is likely to interfere with effective and appropriate extinction of fear response, and thus, may serve to create or maintain post-traumatic psychopathology.
These abnormalities may also give rise to impairments in selective attention and explicit memory, phenomena that characterize this disorder. Studies of older adults have consistently observed that FA reductions in posterior cingulum/parahippocampal regions are present in mildly cognitively impaired individuals (including those in the prodromal phase of Alzheimer's disease), compared with non-impaired, similar-aged peers (reviewed in Drago et al, 2011). Similarly, in healthy adults, lower FA in the posterior cingulum has corresponded with poorer performance on memory, attention, and executive function tasks (Kantarci et al, 2011). Thus, the lower FA we observed in the posterior cingulum in PTSD+ individuals may represent a mechanism through which attention and memory disruptions, as well as extinction deficiencies, may develop.
One potential explanation for the between-group differences we observed in cingulum white matter integrity relates to premorbid vulnerability. Although the cross-sectional nature of this study prohibits confirmation of this possibility, these FA abnormalities might have been present before trauma exposure. Architectural deficits in the cingulum could represent a diathesis for the development of psychopathology that may, in part, be mediated by genetic makeup. Consistent with this hypothesis, earlier studies of psychiatrically healthy youths with variable family histories of depression (Huang et al, 2011) and major depressive disorder (Keedwell et al, 2012), have revealed significantly lower cingulum FA in at-risk groups. Given the growing evidence indicating that white matter development is under the influence of genetic control (Chiang et al, 2009; Kochunov et al, 2010), it is possible that the lower cingulum FA observed in our sample of individuals with PTSD represents a biological marker of genetic risk for this disorder. Future prospective studies investigating genetic factors, white matter integrity, and psychopathology are warranted to confirm this possibility.
Although we included well-matched groups of traumatized African-American female adults, an under-represented group in the PTSD literature, some study limitations should be noted. First, given that we did not measure onset of PTSD symptoms, it was not possible to examine associations between tract integrity and disease onset and chronicity. Similarly, we did not use structured clinical interviews to confirm a diagnosis of PTSD, potentially introducing error variance related to response bias or demand characteristics. In addition, a non-traumatized comparison group would be ideal to further differentiate the potential effects of chronic trauma from PTSD in this sample. However, the primary concern across past studies of PTSD, and from our perspective a critical limitation in the extant literature, was the lack of a matched TC group. Our study was explicitly designed to differentiate those with and without PTSD given an equivalent level of lifetime trauma exposure. Although our findings are consistent with our a priori hypotheses and the existing literature, and moderate-to-large effect sizes were observed, these findings did not survive conservative FWE correction. Further, within-group correlations between left or right posterior cingulum FA and PTSD symptoms did not reach statistical significance. The two groups were distinct in terms of PTSD symptoms, with little variability in symptoms in the TC group (many of these participants' total PSS scores were at or near 0); this restricted range of values is likely to contribute to nonsignificant correlational findings. Another possibility for the lack of within-group correlational findings is insufficient statistical power. Therefore, it will be important for future research to replicate our findings in an independent sample of traumatized individuals with and without PTSD.
Notably, our findings of between-group differences in FA were not unique to one hemisphere. One possibility is that PTSD-specific abnormalities of the cingulum may tend to be bilateral, rather than unilateral, in nature. It is also noteworthy that we did not observe between-group differences in FA for the largest white matter connection between frontal and temporal regions, the uncinate fasciculus. Microstructural abnormalities of the uncinate fasciculus have been observed in social anxiety (Baur et al, 2011; Phan et al, 2009) and psychotic disorders, such as schizophrenia (Mandl et al, 2012; Price et al, 2008). One putative explanation for our lack of findings in this region is type I error; however, given that no prior DTI studies have implicated this region in PTSD, it is possible that architectural abnormalities of the posterior cingulum are more unique to this disorder.
Conclusions
In conclusion, structural MRI studies sampling PTSD populations have observed gray matter differences in ACC (Thomaes et al, 2010) and hippocampal volumes (Woon et al, 2010). Our study extends these observations to include the PTSD-specific abnormalities in the cingulum, the white matter fiber that connects the ACC and hippocampus. Lower posterior cingulum white matter integrity may represent a biological marker of PTSD, and is a worthy target for investigation in individuals at risk for developing this disorder, including individuals who hold genetic risk. Further studies are warranted to explore the relationships between cingulum integrity and genetic profiles, extinction/inhibition of fear response, selective attention, and explicit memory ability in PTSD populations.
Acknowledgments
We thank the participants who made this work possible, as well as the staff of the Grady Trauma Project, particularly Allen W Graham, Angelo Brown, Amreen Dharani, Jennifer S Davis, Jennifer Winkler, Sarah Spann, and William Holland. We thank Jaemin Shin for his valuable assistance with data analysis, and the Georgia State University-Georgia Institute of Technology Center for Advanced Brain Imaging for data support. This work was primarily supported by National Institutes of Mental Health (R01MH071537 to KJR) and (F32MH095456 to NF; co-mentors: TZK and KJR). Support was also received from PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources, and the Burroughs Wellcome Fund (KJR). Dr Fani and Dr Ressler's work was funded by the NIH, as noted above.
The authors declare no conflict of interest.
References
- Abe O, Yamasue H, Kasai K, Yamada H, Aoki S, Iwanami A, et al. Voxel-based diffusion tensor analysis reveals aberrant anterior cingulum integrity in posttraumatic stress disorder due to terrorism. Psychiatry Res. 2006;146:231–242. doi: 10.1016/j.pscychresns.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Baur V, Bruhl AB, Herwig U, Eberle T, Rufer M, Delsignore A, et al. 2011Evidence of frontotemporal structural hypoconnectivity in social anxiety disorder: a quantitative fiber tractography study Hum Brain Mapp(e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Beck AT, Steer RA, Brown G. Manual for Beck Depression Inventory II (BDI-II) Psychology: San Antonio, TX; 1996. [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci. 2006;8:445–461. doi: 10.31887/DCNS.2006.8.4/jbremner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Lui S, Wu QZ, Zhang W, Zhou D, Chen HF, et al. 2011Impact of acute stress on human brain microstructure: an MR diffusion study of earthquake survivors Hum Brain Mapp(e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Chiang MC, Avedissian C, Barysheva M, Toga AW, McMahon KL, de Zubicaray GI, et al. Extending genetic linkage analysis to diffusion tensor images to map single gene effects on brain fiber architecture. Med Image Comput Comput Assist Interv. 2009;12 (Pt 2:506–513. doi: 10.1007/978-3-642-04271-3_62. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan M, Shifflett H, Iyengar S, Beers SR, Hall J, et al. Brain structures in pediatric maltreatment-related PTSD: a sociodemographically matched study. Biol Psychiatry. 2002;52:1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- Drago V, Babiloni C, Bartres-Faz D, Caroli A, Bosch B, Hensch T, et al. Disease tracking markers for Alzheimer's disease at the prodromal (MCI) stage. J Alzheimers Dis. 2011;26 (Suppl 3:159–199. doi: 10.3233/JAD-2011-0043. [DOI] [PubMed] [Google Scholar]
- Falsetti SA, Resnick HS, Resick PA, Kilpatrick DG. The modified PTSD symptom scale: a brief self-report measure of posttraumatic stress disorder. Behav Ther. 1993;16:161–162. [Google Scholar]
- Fani N, Jovanovic T, Ely TD, Bradley B, Gutman D, Tone EB, et al. Neural correlates of attention bias to threat in post-traumatic stress disorder. Biol Psychol. 2012a;90:134–142. doi: 10.1016/j.biopsycho.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, Tone EB, Phifer J, Norrholm SD, Bradley B, Ressler KJ, et al. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol Med. 2012b;42:533–543. doi: 10.1017/S0033291711001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox RJ, Sakaie K, Lee JC, Debbins JP, Liu Y, Arnold DL, et al. A validation study of multicenter diffusion tensor imaging: reliability of fractional anisotropy and diffusivity values. AJNR Am J Neuroradiol. 2012;33:695–700. doi: 10.3174/ajnr.A2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, et al. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry. 2009;31:505–514. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA. Hippocampal regulation of aversive memories. Curr Opin Neurobiol. 2011;21:460–466. doi: 10.1016/j.conb.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35:136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Fan X, Williamson DE, Rao U. White matter changes in healthy adolescents at familial risk for unipolar depression: a diffusion tensor imaging study. Neuropsychopharmacology. 2011;36:684–691. doi: 10.1038/npp.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski AP, Douglas-Palumberi H, Jackowski M, Win L, Schultz RT, Staib LW, et al. Corpus callosum in maltreated children with posttraumatic stress disorder: a diffusion tensor imaging study. Psychiatry Res. 2008;162:256–261. doi: 10.1016/j.pscychresns.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17:749–758. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH.2012Axonal pathology in traumatic brain injury Exp Neurol(e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, et al. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety. 2010;27:244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry. 2010;167:648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Senjem ML, Avula R, Zhang B, Samikoglu AR, Weigand SD, et al. Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology. 2011;77:26–34. doi: 10.1212/WNL.0b013e31822313dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30:1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Chapman R, Christiansen K, Richardson H, Evans J, Jones DK.2012Cingulum white matter in young women at risk of depression: the effect of family history and anhedonia Biol Psychiatry(e-pub ahead of print). [DOI] [PubMed]
- Kim MJ, Lyoo IK, Kim SJ, Sim M, Kim N, Choi N, et al. Disrupted white matter tract integrity of anterior cingulate in trauma survivors. Neuroreport. 2005;16:1049–1053. doi: 10.1097/00001756-200507130-00004. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Jeong DU, Sim ME, Bae SC, Chung A, Kim MJ, et al. Asymmetrically altered integrity of cingulum bundle in posttraumatic stress disorder. Neuropsychobiology. 2006;54:120–125. doi: 10.1159/000098262. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Glahn DC, Lancaster JL, Winkler AM, Smith S, Thompson PM, et al. Genetics of microstructure of cerebral white matter using diffusion tensor imaging. Neuroimage. 2010;53:1109–1116. doi: 10.1016/j.neuroimage.2010.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. Ann NY Acad Sci. 2006;1071:87–109. doi: 10.1196/annals.1364.009. [DOI] [PubMed] [Google Scholar]
- Mandl RC, Rais M, van Baal GC, van Haren NE, Cahn W, Kahn RS, et al. 2012Altered white matter connectivity in never-medicated patients with schizophrenia Hum Brain Mapp(e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell P, Norton WT.1980Myelin Sci Am 24288–90.92, 96 passim. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, et al. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry. 2011;69:553–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Orlichenko A, Boyd E, Angstadt M, Coccaro EF, Liberzon I, et al. Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biol Psychiatry. 2009;66:691–694. doi: 10.1016/j.biopsych.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G, Cercignani M, Parker GJ, Altmann DR, Barnes TR, Barker GJ, et al. White matter tracts in first-episode psychosis: a DTI tractography study of the uncinate fasciculus. Neuroimage. 2008;39:949–955. doi: 10.1016/j.neuroimage.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J, Tronson NC. Molecular specificity of multiple hippocampal processes governing fear extinction. Rev Neurosci. 2010;21:1–17. doi: 10.1515/revneuro.2010.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Zhang Y, Zhan W, Lenoci M, Ching C, Boreta L, et al. Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: an MRI study. Neuroimage. 2011;54 (Suppl 1:S62–S68. doi: 10.1016/j.neuroimage.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AC, Bradley RL, Sexton M, Sherry A, Ressler KJ. Posttraumatic stress disorder among African Americans in an inner city mental health clinic. Psychiatr Serv. 2005;56:212–215. doi: 10.1176/appi.ps.56.2.212. [DOI] [PubMed] [Google Scholar]
- Shin LM, Handwerger K. Is posttraumatic stress disorder a stress-induced fear circuitry disorder. J Trauma Stress. 2009;22:409–415. doi: 10.1002/jts.20442. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann NY Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 (Suppl 1:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Tavanti M, Battaglini M, Borgogni F, Bossini L, Calossi S, Marino D, et al. Evidence of diffuse damage in frontal and occipital cortex in the brain of patients with post-traumatic stress disorder. Neurol Sci. 2012;33:59–68. doi: 10.1007/s10072-011-0659-4. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biol Psychiatry. 2004;56:80–85. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer N, de Ruiter MB, van Balkom AJ, Smit JH, et al. Reduced anterior cingulate and orbitofrontal volumes in child abuse-related complex PTSD. J Clin Psychiatry. 2010;71:1636–1644. doi: 10.4088/JCP.08m04754blu. [DOI] [PubMed] [Google Scholar]
- Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1181–1188. doi: 10.1016/j.pnpbp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang Y, Li L, Li Z, Li W, Ma N, et al. Different white matter abnormalities between the first-episode, treatment-naive patients with posttraumatic stress disorder and generalized anxiety disorder without comorbid conditions. J Affect Disord. 2011;133:294–299. doi: 10.1016/j.jad.2011.03.040. [DOI] [PubMed] [Google Scholar]