Abstract
Binge eating disorder is an addiction-like disorder characterized by episodes of rapid and excessive food consumption within discrete periods of time which occur compulsively despite negative consequences. This study was aimed at determining whether antagonism of Sigma-1 receptors (Sig-1Rs) blocked compulsive-like binge eating. We trained male wistar rats to obtain a sugary, highly palatable diet (Palatable group) or a regular chow diet (Chow control group), for 1 h a day under fixed ratio 1 operant conditioning. Following intake stabilization, we evaluated the effects of the selective Sig-1R antagonist BD-1063 on food responding. Using a light/dark conflict test, we also tested whether BD-1063 could block the time spent and the food eaten in an aversive, open compartment, where the palatable diet was offered. Furthermore, we measured Sig-1R mRNA and protein expression in several brain areas of the two groups, 24 h after the last binge session. Palatable rats rapidly developed binge-like eating, escalating the 1 h intake by four times, and doubling the eating rate and the regularity of food responding, compared to Chow rats. BD-1063 dose-dependently reduced binge-like eating and the regularity of food responding, and blocked the increased eating rate in Palatable rats. In the light/dark conflict test, BD-1063 antagonized the increased time spent in the aversive compartment and the increased intake of the palatable diet, without affecting motor activity. Finally, Palatable rats showed reduced Sig-1R mRNA expression in prefrontal and anterior cingulate cortices, and a two-fold increase in Sig-1R protein expression in anterior cingulate cortex compared to control Chow rats. These findings suggest that the Sig-1R system may contribute to the neurobiological adaptations driving compulsive-like eating, opening new avenues of investigation towards pharmacologically treating binge eating disorder.
Keywords: binge eating disorder, food intake, eating disorders, addiction, palatability, risk-taking behavior
INTRODUCTION
Binge eating disorder is a deadly disease that affects approximately 15 million people in the United States (Hudson et al, 2007) and very frequently occurs co-morbidly with obesity, diabetes, cardiovascular diseases, and certain psychiatric conditions, such as anxiety and depression (APA, 2000; Javaras et al, 2008; Wilfley et al, 2011; Yanovski, 2003). Binge eating episodes are characterized by excessive, rapid, and compulsive consumption of highly palatable foods (eg, food rich in sugars and/or fats) within short periods of time, and are followed by food restriction (APA, 2000; Avena et al, 2008; Corwin, 2006). The cyclic binge/restriction pattern of consumption of highly palatable foods has raised the question of whether binge eating disorder can be considered an addiction-‘like' disorder; however, the debate remains open (Corwin and Grigson, 2009). An effective pharmacological treatment for binge eating disorder is very much needed.
Accumulating evidence suggests that Sigma-1 receptors (Sig-1Rs) play a role in both the pathophysiology of neuropsychiatric diseases, and the mechanistic action of some therapeutic drugs (Hayashi et al, 2011). Sig-Rs were originally classified as members of the opioid receptor family (Martin et al, 1976) and high-affinity phencyclidine binding sites (Quirion et al, 1992). However, later findings have demonstrated that Sig-Rs are unique binding sites (Gundlach et al, 1986; Walker et al, 1990). Sig-1Rs have recently been proposed to be ligand-operated molecular chaperones expressed predominantly at the endoplasmic reticulum subdomain that apposes the mitochondria; under certain conditions, they translocate to loci close to the plasma membranes (Hayashi and Su, 2003, 2007), where they interact and regulate calcium flux, neurotransmitter release, neurotrophic factor signaling, and the opening of voltage-gated ion channels (Aydar et al, 2002; Hayashi and Su, 2008; Herrera et al, 2008). Several structurally unrelated molecules have been proposed as possible endogenous Sig-R ligands, including the neurosteroids dehydroepiandrosterone and progesterone, neuropeptide Y, calcitonin gene-related peptide, and the amine N,N-dimethyltryptamine (Fontanilla et al, 2009; Maurice et al, 1999; Su, 1993). Two different Sig-R subtypes are currently hypothesized, Sig-R1 and Sig-R2, of which only the Sig-R1 subtype has been cloned (Hanner et al, 1996; Hellewell and Bowen, 1990; Moebius et al, 1993). Sig-1Rs are found in several reward-related brain regions and growing evidence suggests a strong involvement of the Sig-1R system in the effects of drugs of abuse; Sig-R antagonists block several effects induced by psychostimulants and ethanol, suggesting that the activation of Sig-1R contributes to drug-induced motivational effects (Garces-Ramirez et al, 2011; Martin-Fardon et al, 2007; Matsumoto et al, 2003; Maurice et al, 2002; Nguyen et al, 2005; Sabino et al, 2009b, 2011).
While the role of Sig-Rs in drug abuse has been widely recognized (Hayashi and Su, 2008; Martin-Fardon et al, 2007; Matsumoto et al, 2003; Sabino et al, 2009c, 2011), whether this receptorial system plays a role in compulsive-like eating is still unknown. This series of studies was therefore aimed at investigating the role of Sig-1Rs in compulsive-like eating using a novel food self-administration approach and a combined behavioral, pharmacological, and molecular analysis. Specifically, we wanted to determine whether Sig-1R antagonists were able to block the addiction-like behavioral phenotype of compulsive-like eating rats.
For this purpose, we first developed an operant model of binge-like eating in male rats by providing a highly palatable diet under fixed ratio 1 operant conditioning for 1 h per day, and then tested the effects of the selective Sig-1R antagonist BD-1063 (Sabino et al, 2009b). Control rats received a standard chow diet in the same experimental conditions. This procedure allowed for a fine measurement of food responding, as well as rate and regularity of intake. It was hypothesized that rats under limited access conditions to the highly palatable diet would rapidly develop binge-like eating, and would increase the rate and the regularity of intake (Corwin, 2004; Cottone et al, 2007a, 2007b, 2008b); in addition, we hypothesized that blockade of Sig-1R would revert the maladaptive behaviors. We then provided control and bingeing rats with the standard chow diet and the highly palatable diet, respectively, in a bright, aversive compartment of a light/dark conflict box, and tested the effects of the selective Sig-1R antagonist BD-1063. Under these adverse environmental conditions, it was hypothesized that control rats would spend minimal time in the aversive compartment and would eat little to no food. Conversely, it was hypothesized that bingeing rats would significantly spend more time and eat more food than control rats. These changes were hypothesized to be blocked by BD-1063. Finally, we measured Sig-1R mRNA and protein expression in several brain areas involved in motivational/emotional processes 24 h after the last operant session, for both control and bingeing rats.
MATERIALS AND METHODS
Subjects
Male wistar rats (n=121), weighing 180–230 g and 41–47 days old (Charles River, Wilmington, MA), were housed in wire-topped, plastic cages (27 × 48 × 20 cm3) in a 12:12 h reverse light cycle (lights off at 1000 h), in a humidity- (60%) and temperature-controlled (22°C) vivarium. Upon arrival, rats had access to corn-based chow (Harlan Teklad LM-485 Diet 7012 (65% (kcal) carbohydrate, 13% fat, 21% protein, 341 cal/100 g); Harlan, Indianapolis, IN) and water ad libitum at all times. Procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication number 85-23, revised 1996) and the Principles of Laboratory Animal Care (http://www.nap.edu/readingroom/bookslabrats), and were approved by Boston University Institutional Animal Care and Use Committee (IACUC). All experimental procedures involved neither food nor water restriction/deprivation, unless otherwise specified.
Drugs
BD-1063 × 2HBr salt (1-[2-(3,4-dichlorophenyl)ethyl]-4-methylpiperazine dihydrobromide]) was synthesized as reported previously (de Costa et al, 1993). Doses of BD-1063 were calculated based on the base weight. BD-1063 was dissolved in isotonic saline and injected subcutaneously (2 ml/kg) 15 min before testing. DTG (1,3-di-(2-tolyl)guanidine) was purchased from Tocris Bioscience (Minneapolis, ME). DTG was suspended in isotonic saline with a few drops of Tween-20 and injected subcutaneously (2 ml/kg) 15 min before testing. These pretreatment intervals were chosen to ensure full compound activities throughout the entirety of the behavioral testing (Hiranita et al, 2010, 2011; Rawls et al, 2002; Sabino et al, 2009, 2011). For further details, see Supplementary Materials and Methods.
Development of an Operant Model of Binge-Like Eating in Rats
Baseline
After arrival, rats (n=42) were left to acclimate to the vivarium and fed the standard Harlan Teklad diet in the home cage for at least 1 week, which was then replaced with an AIN-76A-based diet, hereafter referred to as ‘Chow A/I' (5TUM diet formulated as 4–5 g extruded pellets, 65.5% (kcal) carbohydrate, 10.4% fat, 24.1% protein, 330 cal/100 g; TestDiet, Richmond, IN). After 1 week of maintenance on the Chow A/I diet, rats were trained to acquire operant self-administration for food (45-mg precision food pellets (Chow A/I)) and water (100 μl) in previously described test cages (Blasio et al, 2012; Cottone et al, 2009a), in which they could obtain nosepoke-contingent food and water on a fixed ratio 1 schedule of reinforcement (Cottone et al, 2009a). During operant training, 45-mg precision food pellets, identical to the home cage ∼5 g extruded diet, were delivered by a pellet dispenser. Therefore, in the operant chambers, rats were provided with a diet identical to the one received in the home cage to ensure that Chow rats' food intake during operant sessions was not influenced by any hedonic factor, but solely by energy homeostatic needs (Cottone et al, 2008a, 2009a). Pellet delivery was paired with a light cue (0.3 s) located above the nosepoke hole. The sessions were performed daily after dark cycle onset and were 1 h in duration.
Testing
After attaining stable baseline performances, rats were assigned to either a ‘Chow' control group, which in the operant boxes received the same 45-mg chow pellets offered in the training phase, or a ‘Palatable' group, which instead received a nutritionally complete, chocolate-flavored, high sucrose (50% kcal), AIN-76A-based diet, comparable in macronutrient composition and energy density to the chow diet (chocolate-flavored 5TUL: 66.7% (kcal) carbohydrate, 12.7% fat, 20.6% protein, 344 cal/100 g; TestDiet). Subjects were tested daily. For further details, see Supplementary Materials and Methods.
Rate and Regularity of Sustained Eating: Inter-Food Interval Analysis
To identify differences between Chow vs Palatable rats in the rate and regularity of sustained (not interrupted by drinking) eating, analysis of the ln-transformed duration of consecutive inter-food intervals was performed (Cottone et al, 2007a, 2007b). Mean and entropy are two variables inversely correlated with the rate and regularity of eating, respectively (Cottone et al, 2007a, 2007b, 2008a). Kurtosis and skewness are measures of distribution's ‘peakedness' and symmetry, respectively. For further details, see Supplementary Materials and Methods.
Effects of the Selective Sig-1R Antagonist BD-1063 on Operant Binge-Like Eating
A different cohort of rats (n=16) was trained for the binge-like eating procedure and, following food intake stabilization, was pretreated with BD-1063 (0, 3.75, 7.5, 15, and 30 mg/kg, subcutaneously) using a within-subject Latin square design. For further details, see Supplementary Materials and Methods.
Effects of the Selective Sig-1R Antagonist BD-1063 on High Rate of Responding for Chow A/I Induced by Food Restriction
A different cohort of rats (n=7) was trained to acquire operant self-administration for Chow A/I diet (see ‘Baseline' in ‘Development of an operant model of binge-like eating in rats' paragraph). To increase the rate of responding for Chow A/I during the operant self-administration sessions, rats were food restricted in their home cages (70% of a rat daily intake (home cage intake+food self-administered)). To assess the effects of BD-1063 (0 and 30 mg/kg, subcutaneously) on high rate of responding for the Chow A/I diet in food-restricted rats, subjects were injected using a within-subject Latin square design. For further details, see Supplementary Materials and Methods.
Effects of the Sig-R Agonist DTG on Operant Binge-Like Eating
A different cohort of rats (n=24) was trained for the binge-like eating procedure. Following food intake stabilization, 16 randomly selected subjects were pretreated with DTG (0, 15, and 30 mg/kg, subcutaneously) using a within-subject Latin square design. DTG, although very selective for Sig-Rs, does not discriminate between the two receptor subtypes; this agonist was chosen because it has been highly characterized in vivo and to ensure a complete Sig-R system activation, independently from the receptor subtypes. For further details, see Supplementary Materials and Methods.
Effects of the Selective Sig-1R Antagonist BD-1063 on Risk-Taking Behavior and Compulsive-Like Eating
The same rats used for the development of the binge-like eating procedure (n=42) were tested in a 10-min light/dark conflict test (Teegarden and Bale, 2007), where a pre-weighed amount of the same food received during self-administration (45-mg Chow A/I pellets for Chow rats or 45-mg chocolate pellets for Palatable rats) was positioned in the center of the light compartment. On the test day, following a 24-h withdrawal period from the last access to the highly palatable food (withdrawal here and henceforth strictly meaning a period in which the palatable food was not provided), rats were pretreated with BD-1063 (0 and 7.5 mg/kg, subcutaneously) 15 min before being placed into the light compartment, facing both the food cup and the doorway (Teegarden and Bale, 2007). The time spent in the open compartment and the amount of food eaten during the test were measured. The two dependent variables were then used to operationalize the constructs of ‘risk-taking behavior' and ‘compulsive-like eating'. Because of rats' innate fear for bright, aversive environments, the time spent exploring the light compartment of the light/dark box under normal, control conditions is minimal. An increased time spent in this compartment, as compared to control conditions, resulting from the presence of the highly palatable diet, was operationalized as ‘risk-taking behavior' (Colorado et al, 2006; Teegarden and Bale, 2007). Moreover, under normal, control conditions, eating behavior is typically suppressed when a rat faces adverse circumstances; a significant increase in food intake in spite of the adverse conditions, as compared to control conditions, was operationalized as a construct of ‘compulsive-like eating' (Belin et al, 2008; Davis et al, 2010; Heyne et al, 2009; Hopf et al, 2010; Johnson and Kenny, 2010). Water was not available during the 10-min test. For further details, see Supplementary Materials and Methods.
Effects of the Selective Sig-1R Antagonist BD-1063 on Motor Activity
A different cohort of rats (n=20) underwent the binge-like eating procedure and was used to test the effects of BD-1063 on motor activity. Motor activity of individually housed rats was measured in Plexiglas chambers (27 × 48 × 20 cm3) using an Opto-M3 activity system (Columbus Instruments, Columbus, OH). Testing was performed 24 h after the last binge-like eating session. Total activity and ambulatory activity were recorded by a computer using the Multi-Device Interface software over a 75-min period, which began right after rats were treated with BD-1063 (0 and 7.5 mg/kg, subcutaneously) (within-subject Latin square design). To better control for potential motor activity effects, the first 15 min post-injection were excluded from the analysis, because it represented the pretreatment time used for the behavioral tests of this study.
Sig-1R Gene Expression in Binge-Like Eating Rats: Quantitative Real-Time PCR
Two cohorts of Chow and Palatable rats were used for the quantification of the Sig-1R mRNA: a first cohort (n=12; randomly selected from rats used in the DTG dose–response experiment, following a washout period of 4–5 days) was killed 20–40 min after the end of the self-administration session. This time point was chosen to give enough time (80–100 min since the beginning of the session) for gene transcription changes to occur. A second cohort (n=16; randomly selected from rats used in the BD-1063 dose–response experiment, following a washout period) was killed 24 h following the last daily binge-like eating session. Procedures were performed as described previously (Sabino et al, 2009b). For further details, see Supplementary Materials and Methods.
Sig-1R Protein Levels in Binge-Like Eating Rats: Western Blotting
A different cohort of rats (n=12) underwent the binge-like eating procedure and, 24 h following the last daily self-administration session, was killed and brain areas were collected as described above. Western blotting procedure was performed using a slightly modified version of a previously described method (Hayashi and Su, 2007). For further details, see Supplementary Materials and Methods.
Statistical Analysis
The effects of diet alternation on self-administration intervals, the effects of BD-1063 or DTG on self-administration variables, motor activity variables, and light/dark test variables were analyzed using two-way analyses of variance (ANOVAs). Sigmoidal four-parameter logistic regression function was fit to 1 h food intake, inter-food intervals, entropy, and home cage intake (Hartz et al, 2001). The effects of BD-1063 treatment on high rate of response and the effects of Diet History on Sig-1R mRNA and protein levels were analyzed using Student's t-tests. Variables that failed the test for normality were analyzed as ranked (Akritas, 1990). The statistical packages used were Instat 3.0 (GraphPad, San Diego, CA) and Systat 11.0 (SPSS, Chicago, IL).
RESULTS
Development of an Operant Model of Binge-Like Eating in Rats
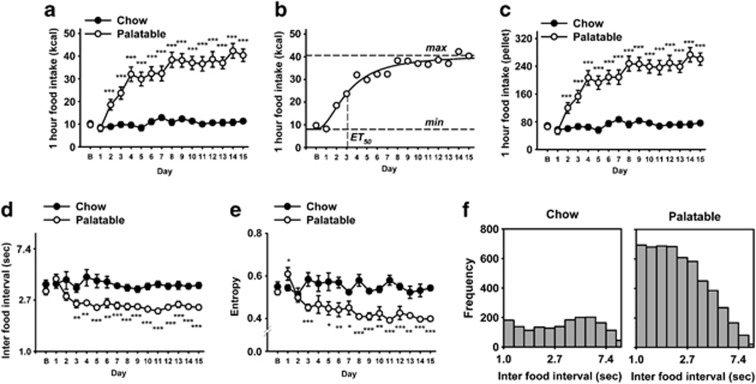
Rats allowed to self-administer for 1 h per day the sugary, highly palatable diet rapidly developed binge-like eating (Figure 1a; Diet History: F(1,40)=92.2, p<0.001). The development of operant binge-like eating was experience-dependent as indicated by the strong Diet History × Day interaction (F(14,560)=16.4, p<0.001) and by the excellent fit of intake to the sigmoidal associative learning function (Figure 1b and Supplemental Table S1; r=0.98, p<0.0001). Because of the very similar energy density between the Chow A/I and the sugary, highly palatable diet (330 vs 344 cal/100 g, respectively), the analysis of the number of pellets revealed a very similar outcome as the analysis of the kcal (Figure 1c; Diet History: F(1,40)=87.33, p<0.001; Diet History × Day (F(14,560)=16.1, p<0.001). In addition, Palatable rats progressively increased in both the rate and regularity of sustained food responding. Indeed, Palatable rats' eating rate increased after the exposure to the sugary diet, as revealed by the 60% reduction of inter-food intervals and by the shift to the left of their frequency distribution, compared to Chow control rats (Figures 1d and e and Table 1, and Supplementary Table S1; Diet History: F(1,40)=23.5, p<0.001; Diet History × Day: F(14,560)=3.6, p<0.001; r=0.92, p<0.0001). Palatable rats' food responding was more regular than that of Chow rats, as revealed by a highly significant decrease in the entropy of the inter-food interval frequency histogram (Figure 1f and Table 1, and Supplementary Table S1; Diet History: F(1,40)=54.1, p<0.001; Diet History × Day: F(14,560)=5.8, p<0.001; r=0.91, p<0.0001). Skewness and the kurtosis of the frequency histograms were not affected by the diet schedule (Table 1; t(40)=1.21, NS and t(40)=1.60, NS, respectively). Home cage food intake rapidly decreased for an energy-homeostatic compensatory mechanism or for devaluation of the less preferred diet (Supplementary Figure S1A) (Corwin, 2004; Cottone et al, 2008a, 2008b). Therefore, intermittent access to the palatable diet did not significantly affect cumulative food intake (Supplementary Figure S1C), absolute body weight (M±SEM: 461.8±8.0 vs 475.1±7.5; t(40)=1.03, NS, Chow vs Palatable, respectively), or body weight gain (M±SEM: 51.2±1.7 vs 53.8±3.2; t(40)=0.72, NS).
Figure 1.
Effects of daily 1-h self-administration of a highly palatable diet on food intake and eating rate in male wistar rats (n=20–22 per group). (a) Food intake; (b) sigmoidal regression of food intake in Palatable rats; (c) number of pellets; (d) inter-food intervals; (e) entropy; and (f) frequency histograms of inter food intervals of Chow and Palatable rats during the 15th test day. Panels represent M±SEM. *Differs from Chow p<0.05, **p<0.01, ***p<0.001 (unpaired Student's t-test).
Table 1. Rate and Regularity of Food Pellet Consumption in Chow and Palatable Rats.
| Parameter |
Food history |
|
|---|---|---|
| Chow | Palatable | |
| Mean | 1.29 (3.36)±0.21 | 0.86 (2.36)±0.07*** |
| Skewness | 1.08±0.25 | 0.71±0.16 |
| Kurtosis | 4.16±1.30 | 1.61±0.85 |
| Entropy | 0.54±0.01 | 0.40±0.01*** |
Effects of daily, 1-h self-administration of a highly palatable diet on the temporal structure of sustained food intake (not interrupted by drinking) during the 15th self-administration session, as determined from frequency histogram analysis (Cottone et al, 2007a, ). Data shown are mean (±SEM) measures of the rate and regularity of feeding as reflected in the frequency histogram of inter-food intervals in male wistar rats (n=20–22 per group). For the analysis, histograms were constructed from ln-transformed inter-food intervals that fell from e−1 to e3 s (∼0.34–20.1 s), with a bin width of e0.2. Parenthetical values reflect back-transformed (s) means of frequency histograms of inter-food interval duration.
***Differs from Chow p<0.001 (unpaired Student's t-test).
Effects of the Selective Sig-1R Antagonist BD-1063 on Operant Binge-Like Eating
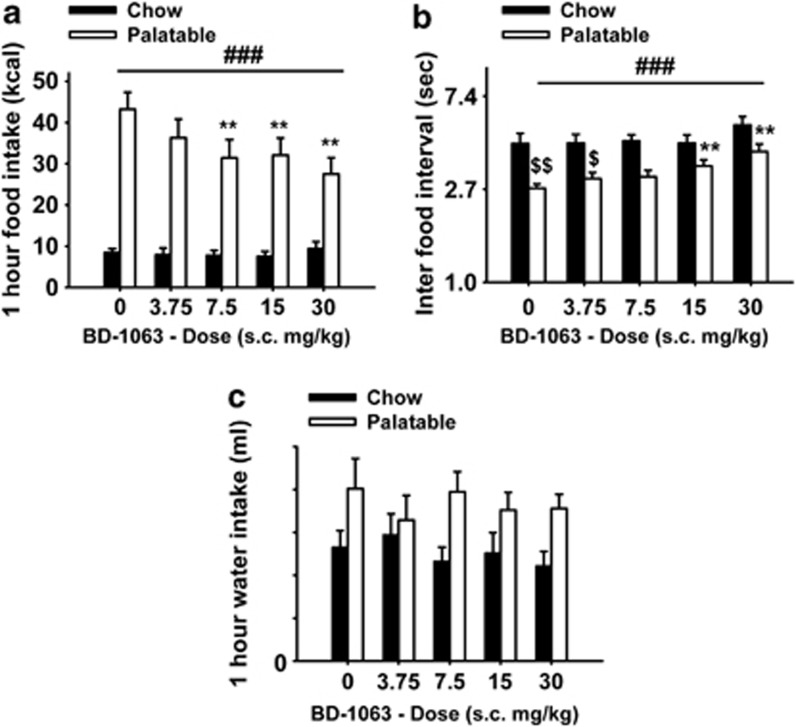
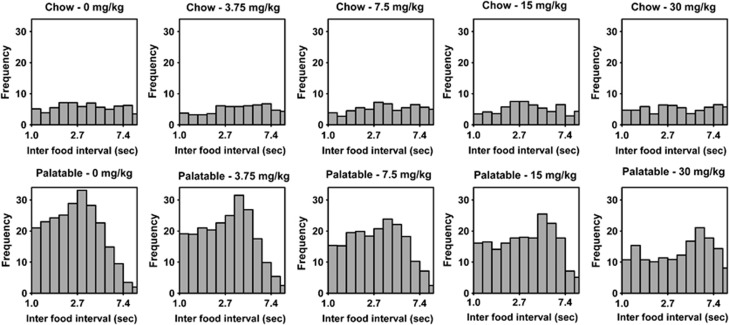
Pretreatment with the Sig-1R antagonist BD-1063 selectively and dose-dependently reduced binge-like eating in Palatable rats (Figure 2a; Treatment: F(4,56)=7.1, p<0.001; Diet History × Treatment: F(4,56)=7.9, p<0.001). Post hoc comparisons revealed that the 7.5, 15, and 30 mg/kg doses significantly reduced food self-administration in Palatable rats (36.3% reduction at the highest dose compared to vehicle condition). Drug treatment blocked increased eating rate in Palatable rats by increasing the inter-food interval (Figure 2b; Treatment: F(4,56)=9.5, p<0.001; Diet History × Treatment: F(4,56)=1.6, NS). Importantly, the effects of BD-1063 on food intake and eating rate were selective for Palatable rats, as drug treatment did not affect performance of Chow rats. Moreover, responding for water in both Palatable and Chow rats was not significantly affected by BD-1063 treatment (Figure 2c; Treatment: F(4,56)=0.7, NS; Diet History × Treatment: F(4,56)=1.5, NS). BD-1063 significantly reduced the regularity of sustained food responding by selectively increasing the entropy of the frequency histograms of inter-food intervals (Figure 3 and Table 2). Two-way ANOVA revealed an effect of the drug treatment on the skewness (Treatment: F(4,56)=3.4, p<0.05; Diet History × Treatment: F(4,56)=0.3, NS); however, individual one-way ANOVAs performed on Chow or Palatable values did not show any significant effect (Table 2). Furthermore, no effect of the drug treatment on the kurtosis was observed (Treatment: F(4,56)=2.4, NS; Diet History × Treatment: F(4,56)=0.3, NS). Finally, drug treatment did not affect body weight gain in either group (Treatment: F(4,56)=0.9, NS; Diet History × Treatment: F(4,56)=0.5, NS).
Figure 2.
Effects of pretreatment (−15 min) with the selective Sigma-1 receptor (Sig-1R) antagonist BD-1063 (0, 3.75, 7.5, 15, and 30 mg/kg, subcutaneously) on self-administration of food and water in male wistar rats (n=8 per group). (a) Food intake; (b) inter-food interval; and (c) water intake. Panels represent M±SEM. ###Main effect of treatment in Palatable rats p<0.001; **Differs from Palatable vehicle condition p<0.01 (Dunnett's test vs vehicle condition); $Differs from Chow vehicle condition p<0.05, $$p<0.01 (Bonferroni corrected Student's t-test).
Figure 3.
Effects of pretreatment (−15 min) with the selective Sigma-1 receptor (Sig-1R) antagonist BD-1063 (0, 3.75, 7.5, 15, and 30 mg/kg, subcutaneously) on the frequency histograms of inter-food intervals in male wistar rats (n=8 per group).
Table 2. Effects of Subcutaneous BD-1063 on the Rate and Regularity of Food Pellet Consumption in Chow and Palatable Rats.
| Parameter | Chow | Palatable |
|---|---|---|
| Mean ANOVA | ### | |
| 0 mg/kg | 1.49 (4.44)±0.11 | 1.01 (2.74)±0.05 |
| 3.75 mg/kg | 1.50 (4.46)±0.10 | 1.11 (3.04)±0.07 |
| 7.5 mg/kg | 1.52 (4.56)±0.07 | 1.13 (3.10)±0.07 |
| 15 mg/kg | 1.50 (4.46)±0.09 | 1.25 (3.48)±0.07** |
| 30 mg/kg | 1.69 (5.41)±0.09 | 1.40 (4.06)±0.08*** |
| Skewness ANOVA | ||
| 0 mg/kg | 1.48±0.37 | 1.49±0.49 |
| 3.75 mg/kg | 1.19±0.49 | 1.74±0.29 |
| 7.5 mg/kg | 1.14±0.50 | 1.52±0.48 |
| 15 mg/kg | 0.52±0.14 | 1.34±0.48 |
| 30 mg/kg | 0.15±0.23 | 0.57±0.28 |
| Kurtosis ANOVA | ||
| 0 mg/kg | 5.09±1.59 | 9.59±3.12 |
| 3.75 mg/kg | 5.33±2.63 | 8.96±2.05 |
| 7.5 mg/kg | 4.97±2.58 | 8.25±3.25 |
| 15 mg/kg | 0.54±0.56 | 7.79±4.05 |
| 30 mg/kg | −0.01±0.35 | 3.28±1.53 |
| Entropy ANOVA | ## | |
| 0 mg/kg | 0.57±0.02 | 0.41±0.01 |
| 3.75 mg/kg | 0.59±0.02 | 0.42±0.01 |
| 7.5 mg/kg | 0.59±0.02 | 0.44±0.02 |
| 15 mg/kg | 0.61±0.02 | 0.45±0.02 |
| 30 mg/kg | 0.59±0.01 | 0.48±0.02** |
Effects of subcutaneous pretreatment (−15 min) with BD-1063 on the temporal structure of sustained food intake (not interrupted by drinking), as determined by the frequency histogram analysis. Data shown are mean (±SEM) measures of the rate and regularity of feeding as reflected in the frequency histogram of inter-food intervals in male wistar rats (n=8 per group). Parameters are based on log-transformed (ln(s)) inter-food interval durations. Parenthetical values reflect back-transformed (s) means of frequency histograms of inter-food interval duration.
##Main effect of treatment in Palatable rats p<0.01.
###p<0.001.
**Differs from Chow p<0.01.
***p<0.001 (unpaired Student's t-test).
Effects of the Selective Sig-1R Antagonist BD-1063 on High Rate of Responding for Chow A/I Induced by Food Restriction
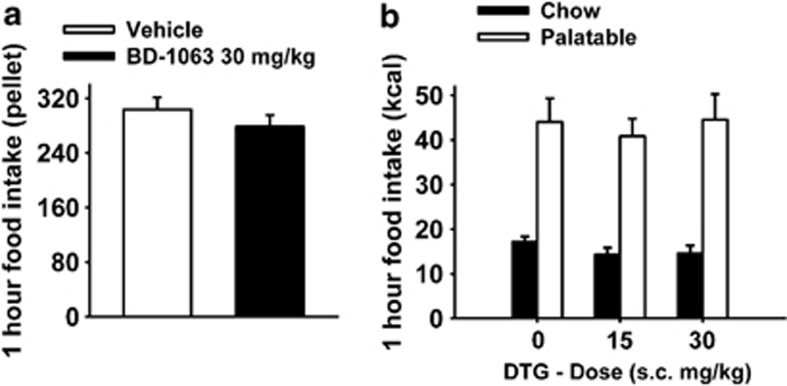
Rate of responding for the Chow A/I diet of food-restricted rats was comparable to the rate of responding for the highly palatable sugary diet of ad libitum-fed Palatable rats (food-restricted Chow A/I rats vs ad libitum-fed Palatable rats 303.6±17.5 vs 279.1±26.4; t(13)=0.75, p=0.47). Pretreatment with the Sig-1R antagonist BD-1063 (30 mg/kg, the highest dose used in the binge eating study) did not significantly affect high rate of responding for Chow A/I in rats that were food restricted (Figure 4a; t(6)=0.94, NS).
Figure 4.
(a) Effects of pretreatment (−15 min) with the selective Sig-1R antagonist BD-1063 (0 and 30 mg/kg, subcutaneously) on high rate of responding for Chow A/I induced by food restriction in male wistar rats (n=7 per group). (b) Effects of pretreatment with the Sig-R agonist DTG (0, 15, and 30 mg/kg, subcutaneously) on binge-like eating in male wistar rats (n=8 per group). Panels represent M±SEM.
Effects of the Sig-R Agonist DTG on Operant Binge-like Eating
Pretreatment with the Sig-R agonist DTG did not significantly affect food responding in Chow or Palatable rats at any tested dose (Figure 4b; Treatment: F(2,28)=0.42, NS; Diet History × Treatment: F(2,28)=0.70, NS).
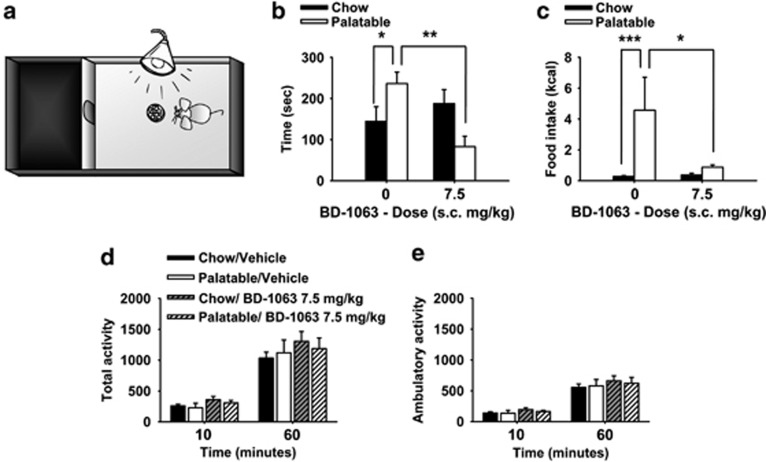
Effects of the Selective Sig-1R Antagonist BD-1063 on Risk-Taking Behavior and Compulsive-Like Eating
As shown in Figure 5b, vehicle-treated Palatable rats spent more time in the aversive light compartment, which was paired with the presence of the highly palatable food, compared to vehicle-treated Chow rats. However, pretreatment with 7.5 mg/kg of the selective Sig-1R antagonist BD-1063 (the lowest effective dose in the binge eating study) completely blocked the increased risk-taking behavior of Palatable rats. The BD-1063 effect was selective for Palatable rats as drug treatment did not influence the behavior in Chow rats (Diet History × Treatment (F(1,38)=9.7, p<0.005). In addition, Palatable rats compulsively consumed more food pellets in spite of the aversive environment, as compared to Chow rats; this effect was fully blocked by BD-1063 pretreatment (Figure 5c; Diet History × Treatment (F(1,38)=5.4, p<0.05).
Figure 5.
Effects of pretreatment (−15 min) with the selective Sigma-1 receptor (Sig-1R) antagonist BD-1063 (0 and 7.5 mg/kg, subcutaneously) on risk-taking behavior, compulsive-like eating, and motor activity in male wistar rats (n=9–11 per group). (a) Schematic of the light/dark test; (b) time spent in the open, aversive compartment; (c) food intake; (d) total activity; and (e) ambulatory activity. Panels represent M±SEM. *Differs from Palatable vehicle condition p<0.05, **p<0.01, ***p<0.001 (Fisher's least significant difference (LSD) test).
Effects of the Selective Sig-1R Antagonist BD-1063 on Motor Activity
BD-1063 altered neither total activity (Diet History × Treatment (F(1,18)=1.48, NS) nor ambulatory activity (Diet History × Treatment (F(1,18)=0.44, NS) when administered to either Chow or Palatable rats (Figure 5d and e).
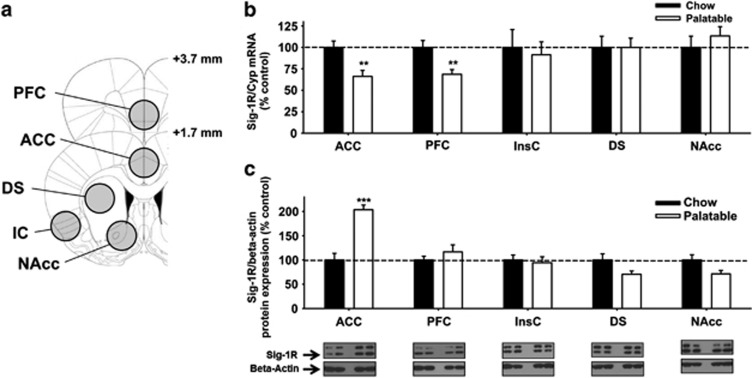
Sig-1R Gene Expression in Binge-Like Eating Rats: Quantitative Real-Time PCR
Quantitative real-time PCR showed (Figure 6b) that Sig-1R mRNA levels, 24 h after the last self-administration session, were significantly lower in the prefrontal cortex and the anterior cingulate cortex of Palatable rats, compared to Chow rats. No significant differences in Sig-1R mRNA expression between the two groups were observed in the insular cortex, nucleus accumbens, and dorsal striatum. The observed changes were dependent on highly palatable food withdrawal, as revealed by a separate analysis of the Sig-1R mRNA levels performed on brain areas of rats killed 20–40 min after the end of the binge-like eating session (Supplementary Figure S2).
Figure 6.
(a) Drawing of rat brain slices used for quantitative reverese transcription-polymerase chain reaction (RT-PCR) and western blotting studies. Circles show brain regions that were punched out: anterior cingulate cortex (ACC), prefrontal cortex (PFC), insular cortex (IC), dorsal striatum (DS), and nucleus accumbens (NAcc). (b) Sigma-1 receptor (Sig-1R) mRNA expression, and (c) Sig-1R protein expression following daily 1-h self-administration of a highly palatable diet in male wistar rats (n=4–8 per group). Rats were killed 24 h after the last binge-like eating session. Panels represent M±SEM expressed as the percent of Chow group. **p<0.01 vs Chow rats, ***p<0.001 (unpaired Student's t-test).
Sig-1R Protein Levels in Binge-Like Eating Rats: Western Blotting
Western blotting showed (Figure 6c) that Sig-1R protein levels, 24 h after the last self-administration session, increased two-fold in the anterior cingulate cortex of Palatable rats as compared to Chow rats. No significant differences in Sig-1R protein levels between the two groups were observed in the anterior cingulate cortex, insular cortex, nucleus accumbens, and dorsal striatum.
DISCUSSION
In this study, we show that daily intermittent availability of a highly palatable diet forced subjects to periods of 23 h of abstinence, inducing a progressive and dramatic escalation of operant responding for the sugary food (Corwin and Buda-Levin, 2004; Cottone et al, 2008b, 2009b). Indeed, Palatable rats quickly learned the time-limited availability of the preferred dietary option, and began eating significantly more than Chow rats after only the second 1-h access to the highly palatable diet. By the second week of self-administration, binge eaters reached a stable overeating ∼4 times higher than the intake of Chow control rats. The development of binge eating of the sugary, highly palatable diet was accompanied by a faster (10.7±1.1 to 21.0±0.8 pellets per min, M±SEM, Chow vs Palatable respectively), and more regular (ie decreased entropy) sustained food responding, as typically observed in palatability-driven processes (Cottone et al, 2007b; Yeomans, 1996). Therefore, the excessive food intake in such a short period of time, and the increased rate of sustained eating suggest an hedonic, rather than homeostatic, mechanism behind the observed behavioral adaptation (Cooper, 2004). The daily intermittent access to the highly palatable diet did not make subjects weigh more. This aspect is particularly relevant, because the disruption of feeding pattern could be studied independently from alterations associated with obesity (Corwin et al, 2011).
We show that the treatment with the selective Sig-1R antagonist BD-1063 dose-dependently decreased food responding in binge eating rats. Interestingly, BD-1063 did not affect water responding in bingeing rats or food and water responding in chow-fed control rats, suggesting selectivity of action and ruling out cognitive impairment or generalized behavioral deficit. Moreover, BD-1063 decreased the rate and the regularity of sustained food responding selectively in bingeing rats, but not in chow-fed control rats. Thus, BD-1063 dose-dependently and specifically reduced overeating and disrupted the ability of palatable food, but not regular chow, to sustain the strength of pellet-by-pellet responding (Cottone et al, 2007b). BD-1063, administered at the dose that maximally decreased binge-like eating of palatable diet (30 mg/kg), did not affect high rate of responding for Chow A/I in food-restricted animals. These results rule out the alternative hypothesis that the effects of BD-1063 treatment on binge-like eating were due to more general effects on high rates of responding.
These findings support and expand the view that Sig-R antagonists may potentially be employed as a pharmacological tool for the treatment of addictive disorders (Katz et al, 2011; Maurice and Su, 2009; Narayanan et al, 2011; Rodvelt and Miller, 2010; Sabino et al, 2009b). The selective Sig-1R antagonists BD-1063 and NE-100 have been shown to reduce ethanol self-administration in genetic (innate) and environmental (following chronic alcohol exposure) models of alcoholism (Sabino et al, 2009b, 2009c). Moreover, Sig-R antagonists block the c-Fos expression, locomotory stimulatory effects, place preference, and contextual reinstatement induced by cocaine in rodents (Martin-Fardon et al, 2007; Maurice and Romieu, 2004; McCracken et al, 1999; Menkel et al, 1991; Witkin et al, 1993). Finally, Sig-R antagonists reduce the locomotor stimulatory effects induced by 3,4-methylenedioxymethamphetamine and the behavioral sensitization induced by methamphetamine (Brammer et al, 2006; Takahashi et al, 2000).
In this study, acute treatment with the non-selective Sig-R agonist, DTG, did not affect binge-like eating. We have recently shown (Sabino et al, 2011) that an acute administration with DTG does not potentiate operant 30-min saccharin per sucrose self-administration in rats, while a chronic administration does. Therefore, a possible explanation for the lack of potentiation of binge-like eating following acute treatment with DTG is that a more prolonged activation (ie, chronic) of the Sig-R system is needed to observe an increase in responding for palatable food. Finally, all studies showing the ability of sigma receptor agonists to potentiate the effects of drugs of abuse in the conditioned place preference test were conducted in mice (Maurice and Romieu, 2004; Romieu et al, 2000, 2002), raising the potential issue of species differences in the effects of sigma receptor agonists. The only study performed in rats showing that acutely administered sigma receptor agonists potentiate the effects of cocaine was performed in rats experienced with intravenous cocaine self-administration, which per se might exert some effects on the Sig-R system (Hiranita et al, 2010).
A relevant point of discussion is the discrepancy between the findings shown here and the lack of effect the Sig-1R antagonist NE-100 had on sucrose consumption we reported recently (Sabino et al, 2009c). These different outcomes can be reconciled considering the much higher motivational settings of the current paradigm compared to the one published earlier: binge eating rats were trained under operant, limited access conditions and consumed as much as 13.5 g/kg of sucrose in 1-h sessions; rats in the previous study were provided with sucrose in the home cages unlimitedly, consuming only 0.125 g/kg (∼100 times less) during the first hour.
Our behavioral and pharmacological findings support the hypothesis that Sig-1Rs play a role in the loss of control and in the compulsiveness associated with binge-like eating. Indeed, bingeing rats, tested in a conflictual context following a 24 h withdrawal period from the last self-administration session, spent significantly more time in the open, aversive compartment where the highly palatable food was placed, and consumed ∼17 times more food compared to chow-fed rats, whose intake was almost completely abolished. These findings suggest that bingeing rats were highly motivated to eat compulsively the sugary diet even when facing the adverse context. Craving and risk-taking behavior for the highly desired substance in spite of known adverse consequences are typically observed in alcohol and drug addiction, and in certain forms of eating disorders and obesity (Hopf et al, 2010; Johnson and Kenny, 2010; Koob and Volkow, 2010; Teegarden and Bale, 2007). Pretreatment with the 7.5 mg/kg dose of the selective Sig-1R antagonist—the lowest effective dose in reducing binge-like eating—fully blocked both risk-taking behavior and compulsive-like eating driven by the palatable diet in bingeing rats. A not significant trend towards reduction in the time spent in the aversive compartment of BD-1063-treated Palatable rats compared to vehicle-treated Chow rats (p=0.19) could be observed. This trend could possibly be interpreted as an anxiogenic-like effect of BD-1063, although there is no definitive evidence that Sig-1Rs are involved in anxiety-like behavior (Hayashi et al, 2011; Sabino et al, 2009a). The lack of effects of the Sig-1R on motor activity confirmed that these effects were not due to a generalized behavioral deficit. Therefore, our results strongly suggest that the blockade of Sig-1R is effective in selectively reducing not only overeating and the rate and the regularity of food responding, but also the risk-taking behavior and compulsiveness associated with binge eating of palatable food.
A caveat must be added regarding the operationalization of ‘compulsive behavior' in the context of this work. There is still no absolute agreement about how compulsive behavior can be operationalized in preclinical research; the term ‘compulsivity' has been used frequently with different meanings and operationalized through a variety of experimental procedures. Among the divergent, but still widely accepted, definitions of compulsive behavior proposed, the two mostly frequently used are either the reward seeking/taking in spite of aversive/negative consequences (Belin et al, 2008; Davis et al, 2010; Hopf et al, 2010; Johnson and Kenny, 2010) or a behavior driven by a negatively reinforced mechanism (Cottone et al, 2009a; Koob, 2009). Within the former connotation, which embraces the eating behavior observed in this work, further complexity is added by the fact that multiple experimental conditions have been used to operationalize the ‘compulsivity' construct, including a variety of different rewards (food, alcohol, drug, or sex), and either unconditioned or conditioned (in turn, either classical or operant) aversive conditions (Belin et al, 2008; Davis et al, 2010; Hopf et al, 2010; Johnson and Kenny, 2010). Given such complexity, the question of whether, in this study, the use of the rats' innate fear for an open, bright compartment can be suitable as the aversive element of the ‘compulsive' construct may be raised, and caution should be exercised when interpreting these results.
Following 24 h of withdrawal from the highly palatable diet, bingeing rats showed a robust reduction in mRNA expression of Sig-1Rs, in both the prefrontal and the anterior cingulate cortices compared to chow-fed rats. Similar changes were not observed in other brain areas, suggesting regional specificity. At the same time point, Palatable rats showed a two-fold increase in the Sig-1R protein levels in the anterior cingulate cortex, but no difference between the two groups was observed in the prefrontal cortex. Future studies employing additional techniques will be needed to clarify the significance of the differential Sig-1R protein expression in these two brain regions. The opposite direction of the changes in mRNA and protein expression during withdrawal from the highly palatable diet can be explained by the cyclic pattern of the diet schedule and the differential temporal regulation of the two molecular processes, which do not always show concordant fluctuations (Fournier et al, 2010; Greenbaum et al, 2003). Possibly, the Sig-1R activity increases earlier than 24-h post-session and, therefore, the decreased gene expression may be the result of counteradaptive intracellular/intranuclear processes. Interestingly, the robust decrease in Sig-1R mRNA expression at the 24-h withdrawal time point is analogous to the decreased mRNA expression of Sig-1R observed in the nucleus accumbens of alcoholic rats (ie ethanol-naive Sardinian alcohol-preferring rats and 24-h withdrawn, alcohol-dependent wistar rats) that were also selectively responsive to the effects of BD-1063 in blocking the reinforcing effects of alcohol (Sabino et al, 2009b). Analogously to what we showed here, Sig-1R protein levels increased in the nucleus accumbens of ethanol-naive Sardinian alcohol-preferring rats (V Sabino et al, unpublished observations).
The increased fronto-cortical Sig-1R activity may, therefore, be responsible for the selective behavioral effects of BD-1063 observed here. A growing body of evidence supports the hypothesis that increased Sig-R activity may be responsible for the emergence of an addiction-like phenotype. Indeed, Sig-R agonists have been shown to facilitate the conditioned place preference induced by cocaine and ethanol (Maurice et al, 2003; Maurice and Romieu, 2004; Romieu et al, 2000, 2002), and the leftward shift in the dose–response curve for cocaine (Hiranita et al, 2009, 2010). Loss of function in the fronto-cortical system, involved in the cognitive control of conflict and in the regulation of emotional processes (Kalivas and Volkow, 2005; Perry et al, 2011; Robbins et al, 2008), has been postulated to be responsible for the poor decision-making and heightened incentive salience of reward in drug addiction, compulsive eating, and obesity (Boeka and Lokken, 2011; Koob and Volkow, 2010; Volkow et al, 2008). A heightened fronto-cortical Sig-R activity may be responsible for an increase in glutamate release (Dong et al, 2005, 2007), which, speculatively, may in turn promote compulsiveness for the highly palatable diet and diminished cognitive control (Kalivas and Volkow, 2005, 2011; Melendez and Kalivas, 2003; Melendez et al, 2005).
In summary, episodes of excessive, rapid, and compulsive-like consumption of highly palatable foods within brief periods of time are the criteria used for the diagnosis of binge eating disorder. Here we show that 1-h daily self-administration of a highly palatable diet induces a rat behavioral phenotype that mimics the appetitive and motivational aspects of binge eating disorder. These findings also suggest that Sig-1Rs participate in the mediation of the reinforcing and rewarding effects of the highly palatable diet in binge eating rats. Treatment with the Sig-1R antagonist BD-1063 reduced overeating and regularity of food responding, and fully blocked the increased rate of intake, risk taking behavior, and compulsive-like eating selectively in bingeing rats, supporting the hypothesis that Sig-1R activation contributes to neuroadaptive mechanisms driving compulsive-like eating. Our results also suggest that fronto-cortical regions of the brain are potential targets for the effects of the Sig-1R antagonist. Collectively, the results of this study propose Sig-1Rs as a new potential pharmacological target for the treatment of binge eating disorder.
Acknowledgments
We thank Stephen St Cyr, Aditi R Narayan, Esther Kim, Alexander Su, and Vamsee Neerukonda for the technical assistance. This publication was made possible by Grant numbers DA023680, DA030425, MH091945, MH093650A1, and AA016731 from the National Institute on Drug Abuse (NIDA), the National Institute of Mental Health (NIMH) and the National Institute on Alcohol Abuse and Alcoholism (NIAAA), and by the Peter Paul Career Development Professorship (PC). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Akritas MG. The rank transform method in some two-factor designs. J Am Statist Assoc. 1990;85:73–78. [Google Scholar]
- APA . Diagnostic and Statistical Manual of Mental Disorders (4th ed.—Text Revision. American Psychiatric Association: Washington, DC; 2000. [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasio A, Narayan AR, Kaminski BJ, Steardo L, Sabino V, Cottone P. A modified adjusting delay task to assess impulsive choice between isocaloric reinforcers in non-deprived male rats: effects of 5-HT(2A/C) and 5-HT (1A) receptor agonists. Psychopharmacology (Berl) 2012;219:377–386. doi: 10.1007/s00213-011-2517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeka AG, Lokken KL. Prefrontal systems involvement in binge eating. Eating Weight Disord. 2011;16:e121–e126. doi: 10.1007/BF03325317. [DOI] [PubMed] [Google Scholar]
- Brammer MK, Gilmore DL, Matsumoto RR. Interactions between 3,4-methylenedioxymethamphetamine and sigma1 receptors. Eur J Pharmacol. 2006;553:141–145. doi: 10.1016/j.ejphar.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colorado RA, Shumake J, Conejo NM, Gonzalez-Pardo H, Gonzalez-Lima F. Effects of maternal separation, early handling, and standard facility rearing on orienting and impulsive behavior of adolescent rats. Behav Process. 2006;71:51–58. doi: 10.1016/j.beproc.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Cooper SJ. Endocannabinoids and food consumption: comparisons with benzodiazepine and opioid palatability-dependent appetite. Eur J Pharmacol. 2004;500:37–49. doi: 10.1016/j.ejphar.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Corwin RL. Binge-type eating induced by limited access in rats does not require energy restriction on the previous day. Appetite. 2004;42:139–142. doi: 10.1016/j.appet.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Corwin RL. Bingeing rats: a model of intermittent excessive behavior. Appetite. 2006;46:11–15. doi: 10.1016/j.appet.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin RL, Avena NM, Boggiano MM. Feeding and reward: perspectives from three rat models of binge eating. Physiol Behav. 2011;104:87–97. doi: 10.1016/j.physbeh.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav. 2004;82:123–130. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Grigson PS. Symposium overview—food addiction: fact or fiction. J Nutr. 2009;139:617–619. doi: 10.3945/jn.108.097691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Nagy TR, Coscina DV, Zorrilla EP. Feeding microstructure in diet-induced obesity susceptible vs resistant rats: central effects of urocortin 2. J Physiol. 2007a;583 (Part 2:487–504. doi: 10.1113/jphysiol.2007.138867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, et al. CRF system recruitment mediates dark side of compulsive eating. Proc Natl Acad Sci USA. 2009a;106:20016–20020. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. FG 7142 specifically reduces meal size and the rate and regularity of sustained feeding in female rats: evidence that benzodiazepine inverse agonists reduce food palatability. Neuropsychopharmacology. 2007b;32:1069–1081. doi: 10.1038/sj.npp.1301229. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats. Am J Physiol. 2008a;295:R1066–R1076. doi: 10.1152/ajpregu.90309.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food. Neuropsychopharmacology. 2008b;33:524–535. doi: 10.1038/sj.npp.1301430. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Consummatory, anxiety-related and metabolic adaptations in female rats with alternating access to preferred food. Psychoneuroendocrinology. 2009b;34:38–49. doi: 10.1016/j.psyneuen.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JF, Loos M, Di Sebastiano AR, Brown JL, Lehman MN, Coolen LM. Lesions of the medial prefrontal cortex cause maladaptive sexual behavior in male rats. Biol Psychiatry. 2010;67:1199–1204. doi: 10.1016/j.biopsych.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Costa BR, He XS, Linders JT, Dominguez C, Gu ZQ, Williams W, et al. Synthesis and evaluation of conformationally restricted N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamines at sigma receptors. 2. Piperazines, bicyclic amines, bridged bicyclic amines, and miscellaneous compounds. J Med Chem. 1993;36:2311–2320. doi: 10.1021/jm00068a007. [DOI] [PubMed] [Google Scholar]
- Dong LY, Cheng ZX, Fu YM, Wang ZM, Zhu YH, Sun JL, et al. Neurosteroid dehydroepiandrosterone sulfate enhances spontaneous glutamate release in rat prelimbic cortex through activation of dopamine D1 and sigma-1 receptor. Neuropharmacology. 2007;52:966–974. doi: 10.1016/j.neuropharm.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Dong Y, Fu YM, Sun JL, Zhu YH, Sun FY, Zheng P. Neurosteroid enhances glutamate release in rat prelimbic cortex via activation of alpha1-adrenergic and sigma1 receptors. Cell Mol Life Sci. 2005;62:1003–1014. doi: 10.1007/s00018-005-5004-8. [DOI] [PubMed] [Google Scholar]
- Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier ML, Paulson A, Pavelka N, Mosley AL, Gaudenz K, Bradford WD, et al. Delayed correlation of mRNA and protein expression in rapamycin-treated cells and a role for Ggc1 in cellular sensitivity to rapamycin. Mol Cell Proteomics. 2010;9:271–284. doi: 10.1074/mcp.M900415-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garces-Ramirez L, Green JL, Hiranita T, Kopajtic TA, Mereu M, Thomas AM, et al. Sigma receptor agonists: receptor binding and effects on mesolimbic dopamine neurotransmission assessed by microdialysis. Biol Psychiatry. 2011;69:208–217. doi: 10.1016/j.biopsych.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003;4:117. doi: 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlach AL, Largent BL, Snyder SH. Autoradiographic localization of sigma receptor binding sites in guinea pig and rat central nervous system with (+)3H-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. J Neurosci. 1986;6:1757–1770. doi: 10.1523/JNEUROSCI.06-06-01757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, et al. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci USA. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz SM, Ben-Shahar Y, Tyler M. Logistic growth curve analysis in associative learning data. Anim Cogn. 2001;3:185–189. [Google Scholar]
- Hayashi T, Su TP. Intracellular dynamics of sigma-1 receptors (sigma binding sites) in NG108-15 cells. J Pharmacol Exp Ther. 2003;306:726–733. doi: 10.1124/jpet.103.051292. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER–mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. An update on the development of drugs for neuropsychiatric disorders: focusing on the sigma 1 receptor ligand. Expert Opin Therap Targets. 2008;12:45–58. doi: 10.1517/14728222.12.1.45. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Tsai SY, Mori T, Fujimoto M, Su TP. Targeting ligand-operated chaperone sigma-1 receptors in the treatment of neuropsychiatric disorders. Expert Opin Therap Targets. 2011;15:557–577. doi: 10.1517/14728222.2011.560837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellewell SB, Bowen WD. A sigma-like binding site in rat pheochromocytoma (PC12) cells: decreased affinity for (+)-benzomorphans and lower molecular weight suggest a different sigma receptor form from that of guinea pig brain. Brain Res. 1990;527:244–253. doi: 10.1016/0006-8993(90)91143-5. [DOI] [PubMed] [Google Scholar]
- Herrera Y, Katnik C, Rodriguez JD, Hall AA, Willing A, Pennypacker KR, et al. Sigma-1 receptor modulation of acid-sensing ion channel a (ASIC1a) and ASIC1a-induced Ca2+ influx in rat cortical neurons. J Pharmacol Exp Ther. 2008;327:491–502. doi: 10.1124/jpet.108.143974. [DOI] [PubMed] [Google Scholar]
- Heyne A, Kiesselbach C, Sahun I, McDonald J, Gaiffi M, Dierssen M, et al. An animal model of compulsive food-taking behaviour. Addict Biol. 2009;14:373–383. doi: 10.1111/j.1369-1600.2009.00175.x. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Tanda G, Katz JL. Reinforcing effects of sigma-receptor agonists in rats trained to self-administer cocaine. J Pharmacol Exp Ther. 2010;332:515–524. doi: 10.1124/jpet.109.159236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Tanda G, Katz JL. Lack of cocaine-like discriminative-stimulus effects of sigma-receptor agonists in rats. Behav Pharmacol. 2011;22:525–530. doi: 10.1097/FBP.0b013e328349ab22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Tanda G, Kopajtic TA, Newman AH, Katz JL.2009Reinforcing Effects of Sigma-Receptor Agonists in Cocaine-Experienced and Naive RatsProgram No 496.1.Neuroscience Meeting Planner Society for Neuroscience, Chicago, IL [Google Scholar]
- Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcohol Clin Exp Res. 2010;34:1565–1573. doi: 10.1111/j.1530-0277.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG., Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaras KN, Pope HG, Lalonde JK, Roberts JL, Nillni YI, Laird NM, et al. Co-occurrence of binge eating disorder with psychiatric and medical disorders. J Clin Psychiatry. 2008;69:266–273. doi: 10.4088/jcp.v69n0213. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16:974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JL, Su TP, Hiranita T, Hayashi T, Tanda G, Kopajtic T, et al. A role for sigma receptors in stimulant self administration and addiction. Pharmaceuticals (Basel) 2011;4:880–914. doi: 10.3390/ph4060880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56 (Suppl 1:18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Maurice T, Aujla H, Bowen WD, Weiss F. Differential effects of sigma1 receptor blockade on self-administration and conditioned reinstatement motivated by cocaine vs natural reward. Neuropsychopharmacology. 2007;32:1967–1973. doi: 10.1038/sj.npp.1301323. [DOI] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- Matsumoto RR, Liu Y, Lerner M, Howard EW, Brackett DJ. Sigma receptors: potential medications development target for anti-cocaine agents. Eur J Pharmacol. 2003;469:1–12. doi: 10.1016/s0014-2999(03)01723-0. [DOI] [PubMed] [Google Scholar]
- Maurice T, Casalino M, Lacroix M, Romieu P. Involvement of the sigma 1 receptor in the motivational effects of ethanol in mice. Pharmacol Biochem Behav. 2003;74:869–876. doi: 10.1016/s0091-3057(03)00002-9. [DOI] [PubMed] [Google Scholar]
- Maurice T, Martin-Fardon R, Romieu P, Matsumoto RR. Sigma(1) (sigma(1)) receptor antagonists represent a new strategy against cocaine addiction and toxicity. Neurosci Biobehav Rev. 2002;26:499–527. doi: 10.1016/s0149-7634(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Maurice T, Phan VL, Urani A, Kamei H, Noda Y, Nabeshima T. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn J Pharmacol. 1999;81:125–155. doi: 10.1254/jjp.81.125. [DOI] [PubMed] [Google Scholar]
- Maurice T, Romieu P. Involvement of the sigma1 receptor in the appetitive effects of cocaine. Pharmacopsychiatry. 2004;37 (Suppl 3:S198–S207. doi: 10.1055/s-2004-832678. [DOI] [PubMed] [Google Scholar]
- Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009;124:195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken KA, Bowen WD, de Costa BR, Matsumoto RR. Two novel sigma receptor ligands, BD1047 and LR172, attenuate cocaine-induced toxicity and locomotor activity. Eur J Pharmacol. 1999;370:225–232. doi: 10.1016/s0014-2999(99)00113-2. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Kalivas PW. Metabotropic glutamate receptor regulation of extracellular glutamate levels in the prefrontal cortex. Ann N Y Acad Sci. 2003;1003:443–444. doi: 10.1196/annals.1300.047. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Vuthiganon J, Kalivas PW. Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J Pharmacol Exp Ther. 2005;314:139–147. doi: 10.1124/jpet.104.081521. [DOI] [PubMed] [Google Scholar]
- Menkel M, Terry P, Pontecorvo M, Katz JL, Witkin JM. Selective sigma ligands block stimulant effects of cocaine. Eur J Pharmacol. 1991;201:251–252. doi: 10.1016/0014-2999(91)90355-t. [DOI] [PubMed] [Google Scholar]
- Moebius FF, Burrows GG, Hanner M, Schmid E, Striessnig J, Glossmann H. Identification of a 27-kDa high affinity phenylalkylamine-binding polypeptide as the sigma 1 binding site by photoaffinity labeling and ligand-directed antibodies. Mol Pharmacol. 1993;44:966–971. [PubMed] [Google Scholar]
- Narayanan S, Mesangeau C, Poupaert JH, McCurdy CR. Sigma receptors and cocaine abuse. Curr Top Med Chem. 2011;11:1128–1150. doi: 10.2174/156802611795371323. [DOI] [PubMed] [Google Scholar]
- Nguyen EC, McCracken KA, Liu Y, Pouw B, Matsumoto RR. Involvement of sigma (sigma) receptors in the acute actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacology. 2005;49:638–645. doi: 10.1016/j.neuropharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Perry JL, Joseph JE, Jiang Y, Zimmerman RS, Kelly TH, Darna M, et al. Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain Res Rev. 2011;65:124–149. doi: 10.1016/j.brainresrev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, et al. A proposal for the classification of sigma binding sites. Trends Pharmacol Sci. 1992;13:85–86. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Baron DA, Geller EB, Adler MW. Sigma sites mediate DTG-evoked hypothermia in rats. Pharmacol Biochem Behav. 2002;73:779–786. doi: 10.1016/s0091-3057(02)00903-6. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Rodvelt KR, Miller DK. Could sigma receptor ligands be a treatment for methamphetamine addiction. Curr Drug Abuse Rev. 2010;3:156–162. doi: 10.2174/1874473711003030156. [DOI] [PubMed] [Google Scholar]
- Romieu P, Martin-Fardon R, Maurice T. Involvement of the sigma1 receptor in the cocaine-induced conditioned place preference. NeuroReport. 2000;11:2885–2888. doi: 10.1097/00001756-200009110-00011. [DOI] [PubMed] [Google Scholar]
- Romieu P, Phan VL, Martin-Fardon R, Maurice T. Involvement of the sigma receptor in cocaine-induced conditioned place preference: possible dependence on dopamine uptake blockade. Neuropsychopharmacology. 2002;26:444–455. doi: 10.1016/S0893-133X(01)00391-8. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Blasio A, Iyer MR, Steardo L, Rice KC, et al. Activation of sigma-receptors induces binge-like drinking in sardinian alcohol-preferring rats. Neuropsychopharmacology. 2011;36:1207–1218. doi: 10.1038/npp.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Parylak SL, Steardo L, Zorrilla EP. Sigma-1 receptor knockout mice display a depressive-like phenotype. Behav Brain Res. 2009a;198:472–476. doi: 10.1016/j.bbr.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Zhao Y, Iyer MR, Steardo L, Jr, Steardo L, et al. The sigma-receptor antagonist BD-1063 decreases ethanol intake and reinforcement in animal models of excessive drinking. Neuropsychopharmacology. 2009b;34:1482–1493. doi: 10.1038/npp.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Zhao Y, Steardo L, Koob GF, Zorrilla EP. Selective reduction of alcohol drinking in Sardinian alcohol-preferring rats by a sigma-1 receptor antagonist. Psychopharmacology (Berl) 2009c;205:327–335. doi: 10.1007/s00213-009-1548-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP. Delineating biochemical and functional properties of sigma receptors: emerging concepts. Critl Rev Neurobiol. 1993;7:187–203. [PubMed] [Google Scholar]
- Takahashi S, Miwa T, Horikomi K. Involvement of sigma 1 receptors in methamphetamine-induced behavioral sensitization in rats. Neurosci Lett. 2000;289:21–24. doi: 10.1016/s0304-3940(00)01258-1. [DOI] [PubMed] [Google Scholar]
- Teegarden SL, Bale TL. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol Psychiatry. 2007;61:1021–1029. doi: 10.1016/j.biopsych.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. NeuroImage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JM, Bowen WD, Walker FO, Matsumoto RR, De Costa B, Rice KC. Sigma receptors: biology and function. Pharmacol Rev. 1990;42:355–402. [PubMed] [Google Scholar]
- Wilfley D, Berkowitz R, Goebel-Fabbri A, Hirst K, Ievers-Landis C, Lipman TH, et al. Binge eating, mood, and quality of life in youth with type 2 diabetes: baseline data from the today study. Diabet Care. 2011;34:858–860. doi: 10.2337/dc10-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Terry P, Menkel M, Hickey P, Pontecorvo M, Ferkany J, et al. Effects of the selective sigma receptor ligand, 6-[6-(4-hydroxypiperidinyl)hexyloxy]-3-methylflavone (NPC 16377), on behavioral and toxic effects of cocaine. J Pharmacol Exp Ther. 1993;266:473–482. [PubMed] [Google Scholar]
- Yanovski SZ. Binge eating disorder and obesity in 2003: could treating an eating disorder have a positive effect on the obesity epidemic. Int J Eat Disord. 2003;34 (Suppl:S117–S120. doi: 10.1002/eat.10211. [DOI] [PubMed] [Google Scholar]
- Yeomans MR. Palatability and the micro-structure of feeding in humans: the appetizer effect. Appetite. 1996;27:119–133. doi: 10.1006/appe.1996.0040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.