Abstract
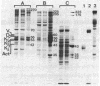
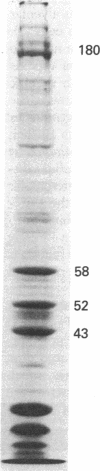
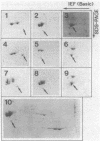
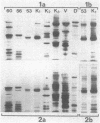
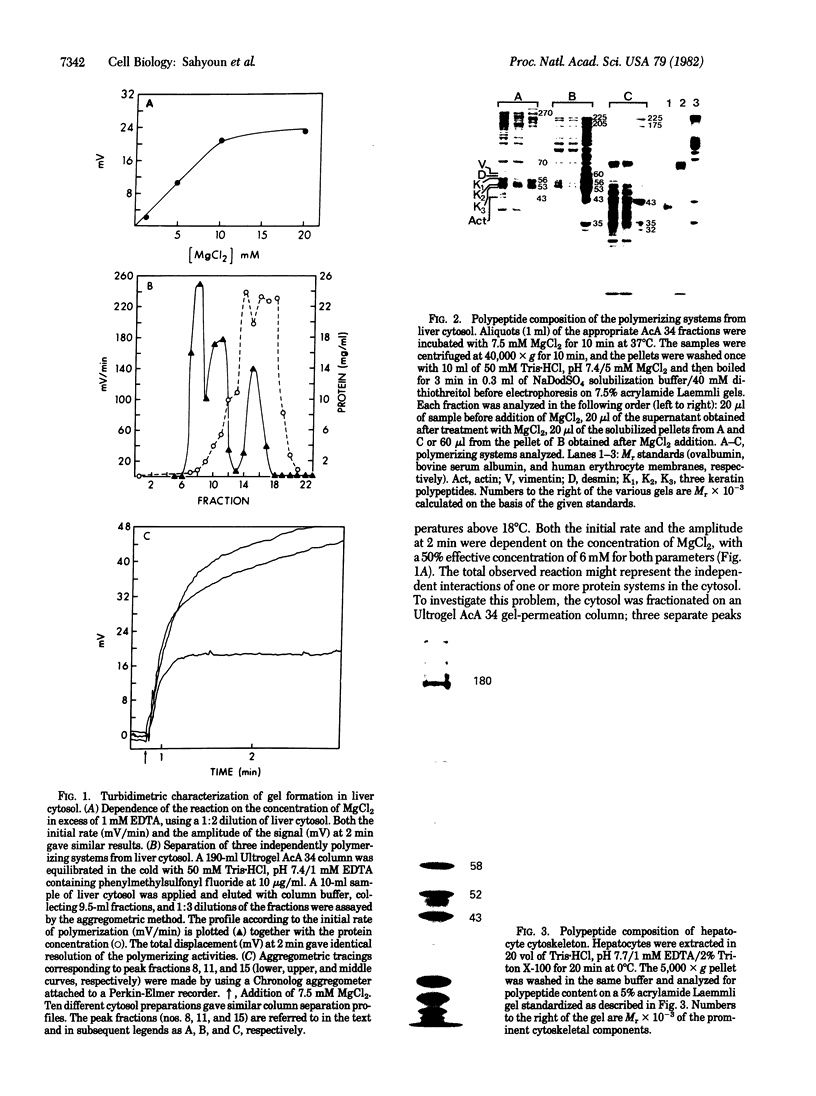
Liver cytosol forms a macroscopic fibrillary network in the presence of low concentrations of MgCl2. This process represents the generation of 3- to 11-nm filaments from soluble precursors, involving selectively at least 12 major polypeptides. Similar polypeptides are enriched in the detergent-insoluble fraction from hepatocytes, suggesting that they may be important constituents of the native cytoskeleton. AcA 34 gel-permeation chromatography resolves the cytosol into three independently "polymerizing" peaks: A, B, and C. The formation of filaments follows biphasic kinetics in peaks B and C, whereas peak A lacks the slow phase. Filament formation in all three systems is inhibited by 1-15 mM inorganic phosphate, 10 mM NaF, or 10 mM sodium molybdate. The polymerization of peak C only is inhibited by 0.2-2 mM ATP. CaCl2 (1-100 microM) has no apparent regulatory effect. Two-dimensional polypeptide analysis and peptide mapping show that actin is a major component of peak C, while peaks A and B contain prominent polypeptides that may be related to intermediate filament subunits. In addition, all three systems contain two or three high molecular weight (greater than 170,000) polypeptides that may participate in modulating and extending the filament network. The filaments from peaks A and B are soluble in 8 M urea and reform on removal of the urea in the presence of 5 mM MgCl2. The polypeptide composition remains constant through three such cycles.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandman E., Matsuda R., Strohman R. C. Myosin heavy chains from two different adult fast-twitch muscles have different peptide maps but identical mRNAs. Cell. 1982 Jun;29(2):645–650. doi: 10.1016/0092-8674(82)90180-5. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Villin is a major protein of the microvillus cytoskeleton which binds both G and F actin in a calcium-dependent manner. Cell. 1980 Jul;20(3):839–847. doi: 10.1016/0092-8674(80)90330-x. [DOI] [PubMed] [Google Scholar]
- Condeelis J. S., Taylor D. L. The contractile basis of amoeboid movement. V. The control of gelation, solation, and contraction in extracts from Dictyostelium discoideum. J Cell Biol. 1977 Sep;74(3):901–927. doi: 10.1083/jcb.74.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskum G., Craig S. W., Decker G. L., Lehninger A. L. The cytoskeleton of digitonin-treated rat hepatocytes. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3430–3434. doi: 10.1073/pnas.77.6.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Denk H., Kalt R., Schmid E. Biochemical and immunological identification of cytokeratin proteins present in hepatocytes of mammalian liver tissue. Exp Cell Res. 1981 Feb;131(2):299–318. doi: 10.1016/0014-4827(81)90234-2. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Freudenstein C., Appelhans B., Osborn M., Weber K., Keenan T. W. Intermediate-sized filaments of the prekeratin type in myoepithelial cells. J Cell Biol. 1980 Mar;84(3):633–654. doi: 10.1083/jcb.84.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Weber K., Osborn M., Schmid E., Freudenstein C. Antibody to prekeratin. Decoration of tonofilament like arrays in various cells of epithelial character. Exp Cell Res. 1978 Oct 15;116(2):429–445. doi: 10.1016/0014-4827(78)90466-4. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980 Apr;19(4):1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Gard D. L., Bell P. B., Lazarides E. Coexistence of desmin and the fibroblastic intermediate filament subunit in muscle and nonmuscle cells: identification and comparative peptide analysis. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3894–3898. doi: 10.1073/pnas.76.8.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard D. L., Lazarides E. The synthesis and distribution of desmin and vimentin during myogenesis in vitro. Cell. 1980 Jan;19(1):263–275. doi: 10.1016/0092-8674(80)90408-0. [DOI] [PubMed] [Google Scholar]
- Granger B. L., Lazarides E. Structural associations of synemin and vimentin filaments in avian erythrocytes revealed by immunoelectron microscopy. Cell. 1982 Aug;30(1):263–275. doi: 10.1016/0092-8674(82)90032-0. [DOI] [PubMed] [Google Scholar]
- Granger B. L., Lazarides E. Synemin: a new high molecular weight protein associated with desmin and vimentin filaments in muscle. Cell. 1980 Dec;22(3):727–738. doi: 10.1016/0092-8674(80)90549-8. [DOI] [PubMed] [Google Scholar]
- Gregolin C., Ryder E., Kleinschmidt A. K., Warner R. C., Lane M. D. Molecular characteristics of liver acetyl CoA carboxylase. Proc Natl Acad Sci U S A. 1966 Jul;56(1):148–155. doi: 10.1073/pnas.56.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. H., Stossel T. P. Structure of macrophage actin-binding protein molecules in solution and interacting with actin filaments. J Mol Biol. 1981 Jan 25;145(3):563–581. doi: 10.1016/0022-2836(81)90545-3. [DOI] [PubMed] [Google Scholar]
- Hellewell S. B., Taylor D. L. The contractile basis of ameboid movement. VI. The solation-contraction coupling hypothesis. J Cell Biol. 1979 Dec;83(3):633–648. doi: 10.1083/jcb.83.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huiatt T. W., Robson R. M., Arakawa N., Stromer M. H. Desmin from avian smooth muscle. Purification and partial characterization. J Biol Chem. 1980 Jul 25;255(14):6981–6989. [PubMed] [Google Scholar]
- Jahn W. The cytoskeleton of rat liver parenchymal cells. Naturwissenschaften. 1980 Nov;67(11):568–568. doi: 10.1007/BF00450674. [DOI] [PubMed] [Google Scholar]
- Jahn W. Visualization of a filamentous network in cryo sections of liver tissue. Eur J Cell Biol. 1980 Feb;20(3):301–304. [PubMed] [Google Scholar]
- Kane R. E. Actin polymerization and interaction with other proteins in temperature-induced gelation of sea urchin egg extracts. J Cell Biol. 1976 Dec;71(3):704–714. doi: 10.1083/jcb.71.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Maruta H., Korn E. D. Purification from Acanthamoeba castellanii of proteins that induce gelation and syneresis of F-actin. J Biol Chem. 1977 Jan 10;252(1):399–402. [PubMed] [Google Scholar]
- Mimura N., Asano A. Actin-related gelation of Ehrlich tumour cell extracts is reversibly inhibited by low concentrations of Ca2+. Nature. 1978 Mar 16;272(5650):273–276. doi: 10.1038/272273a0. [DOI] [PubMed] [Google Scholar]
- Mimura N., Asano A. Ca2+-sensitive gelation of actin filaments by a new protein factor. Nature. 1979 Nov 1;282(5734):44–48. doi: 10.1038/282044a0. [DOI] [PubMed] [Google Scholar]
- Nunnally M. H., Powell L. D., Craig S. W. Reconstitution and regulation of actin gel-sol transformation with purified filamin and villin. J Biol Chem. 1981 Mar 10;256(5):2083–2086. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Pollard T. D. Purification of a calcium-sensitive actin gelation protein from Acanthamoeba. J Biol Chem. 1981 Jul 25;256(14):7666–7670. [PubMed] [Google Scholar]
- Pollard T. D. The role of actin in the temperature-dependent gelation and contraction of extracts of Acanthamoeba. J Cell Biol. 1976 Mar;68(3):579–601. doi: 10.1083/jcb.68.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan R. A., Franke W. W. Heteropolymer filaments of vimentin and desmin in vascular smooth muscle tissue and cultured baby hamster kidney cells demonstrated by chemical crosslinking. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3452–3456. doi: 10.1073/pnas.79.11.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattilaro R. F., Dentler W. L., LeCluyse E. L. Microtubule-associated proteins (MAPs) and the organization of actin filaments in vitro. J Cell Biol. 1981 Aug;90(2):467–473. doi: 10.1083/jcb.90.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M. Proteins associated with cytoplasmic actin. Cell. 1981 Sep;25(3):587–590. doi: 10.1016/0092-8674(81)90166-5. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Cantieri J. S., Teller D. C., Lonsdale-Eccles J. D., Dale B. A. Characterization of a class of cationic proteins that specifically interact with intermediate filaments. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4097–4101. doi: 10.1073/pnas.78.7.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Hartwig J. H. Interactions of actin, myosin, and a new actin-binding protein of rabbit pulmonary macrophages. II. Role in cytoplasmic movement and phagocytosis. J Cell Biol. 1976 Mar;68(3):602–619. doi: 10.1083/jcb.68.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. T., Green H. Keratin filaments of cultured human epidermal cells. Formation of intermolecular disulfide bonds during terminal differentiation. J Biol Chem. 1978 Mar 25;253(6):2053–2060. [PubMed] [Google Scholar]
- Wang K., Singer S. J. Interaction of filamin with f-actin in solution. Proc Natl Acad Sci U S A. 1977 May;74(5):2021–2025. doi: 10.1073/pnas.74.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A. Actin-binding proteins--regulators of cell architecture and motility. Nature. 1982 Apr 29;296(5860):811–816. doi: 10.1038/296811a0. [DOI] [PubMed] [Google Scholar]
- Yin H. L., Hartwig J. H., Maruyama K., Stossel T. P. Ca2+ control of actin filament length. Effects of macrophage gelsolin on actin polymerization. J Biol Chem. 1981 Sep 25;256(18):9693–9697. [PubMed] [Google Scholar]
- Yin H. L., Zaner K. S., Stossel T. P. Ca2+ control of actin gelation. Interaction of gelsolin with actin filaments and regulation of actin gelation. J Biol Chem. 1980 Oct 10;255(19):9494–9500. [PubMed] [Google Scholar]
- Zahlten R. N., Rogoff T. M., Steer C. J. Isolated Kupffer cells, endothelial cells and hepatocytes as investigative tools for liver research. Fed Proc. 1981 Aug;40(10):2460–2468. [PubMed] [Google Scholar]